Repurposing ProTAME for Bladder Cancer: A Combined Therapeutic Approach Targeting Cell Migration and MMP Regulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Cell Culture and Reagents
2.3. Drug Dose–Response Assays
2.4. Scratch-Wound Healing Assay
2.5. Real-Time qRT-PCR
2.6. Statistical Analysis
3. Results
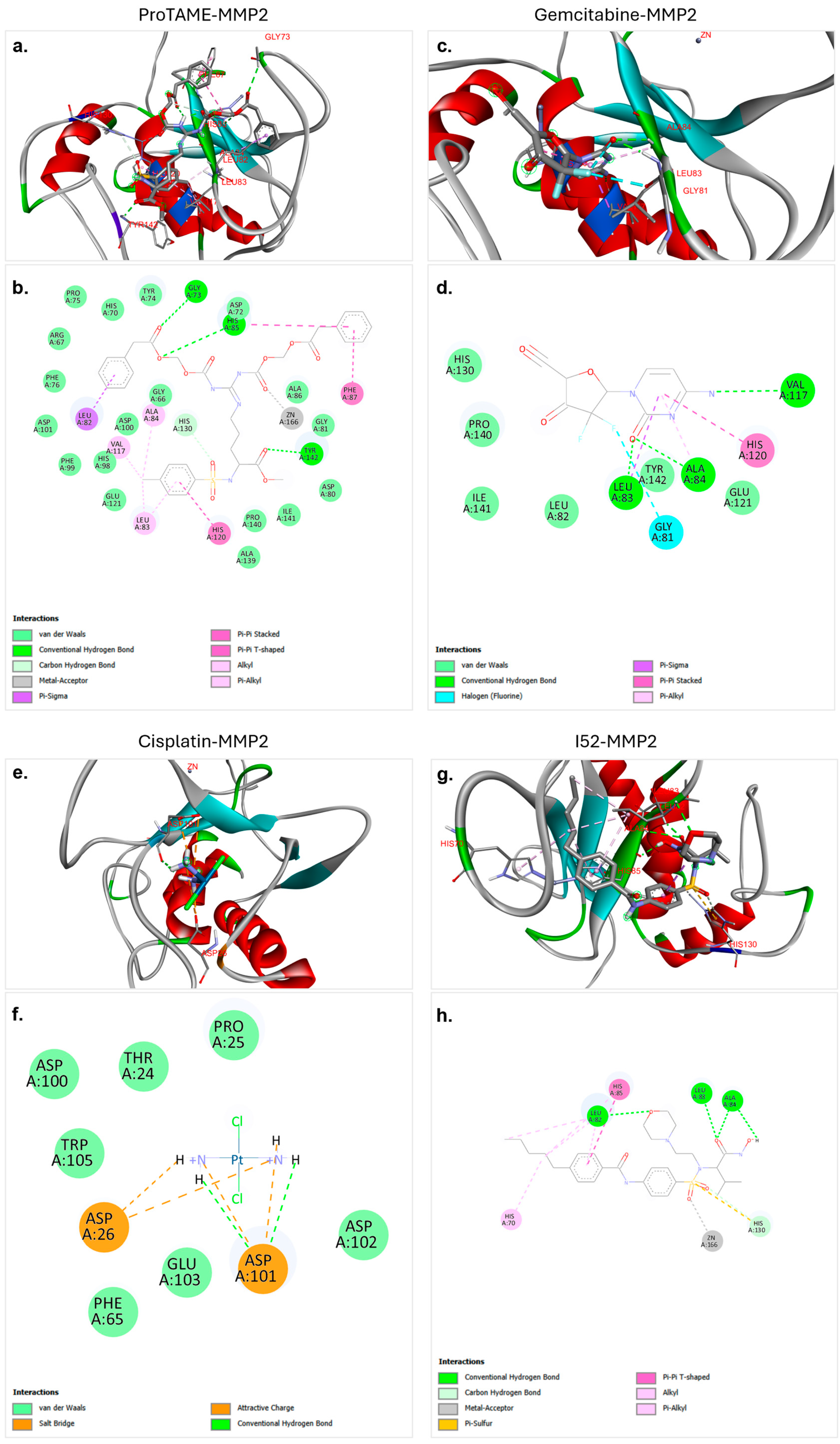
3.1. Molecular Docking of ProTAME, Gemcitabine, and Cisplatin with MMP2 and MMP9
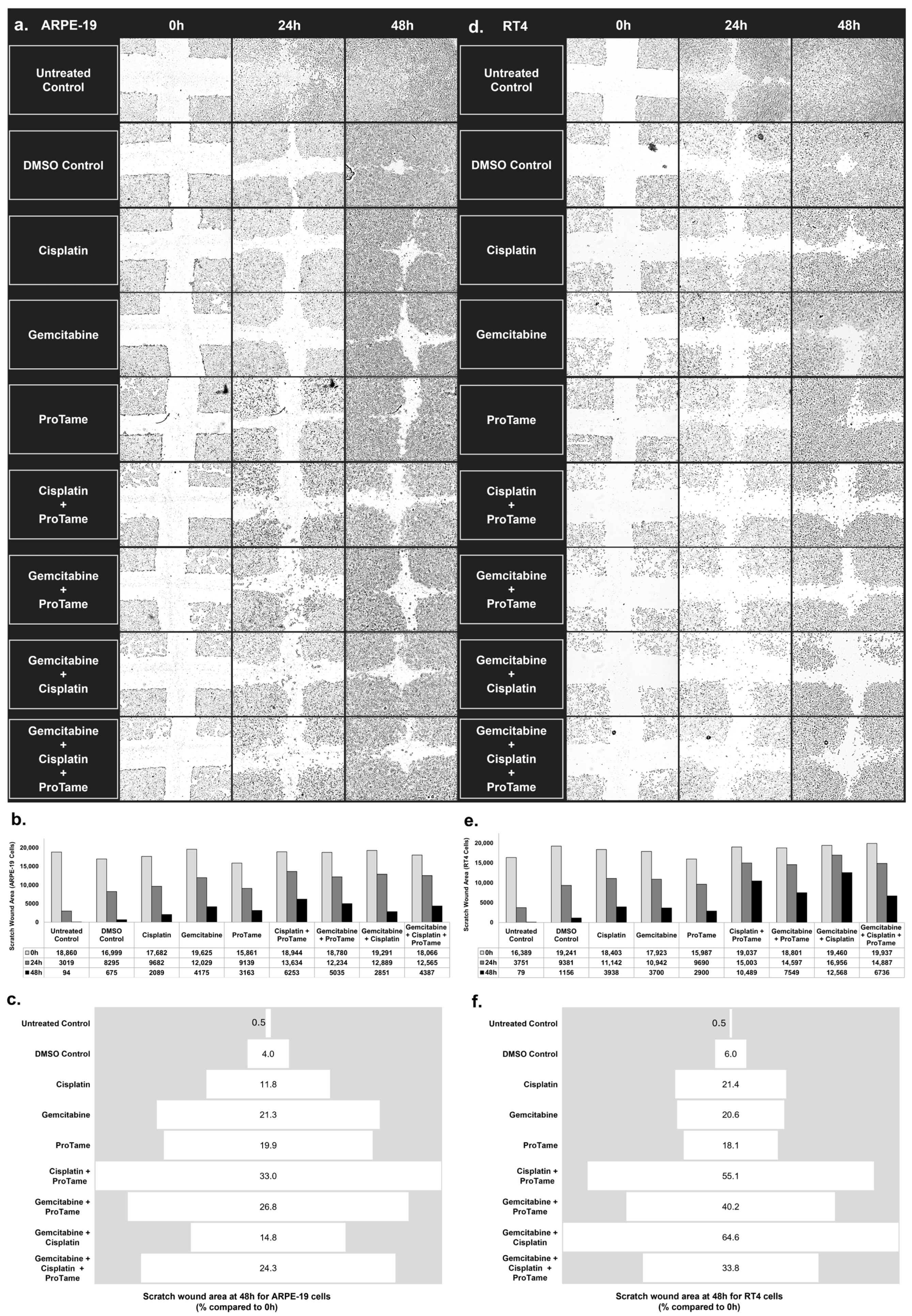
3.2. Inhibition of Cell Migration by Cisplatin, Gemcitabine, and ProTAME in Scratch-Wound Healing Assay
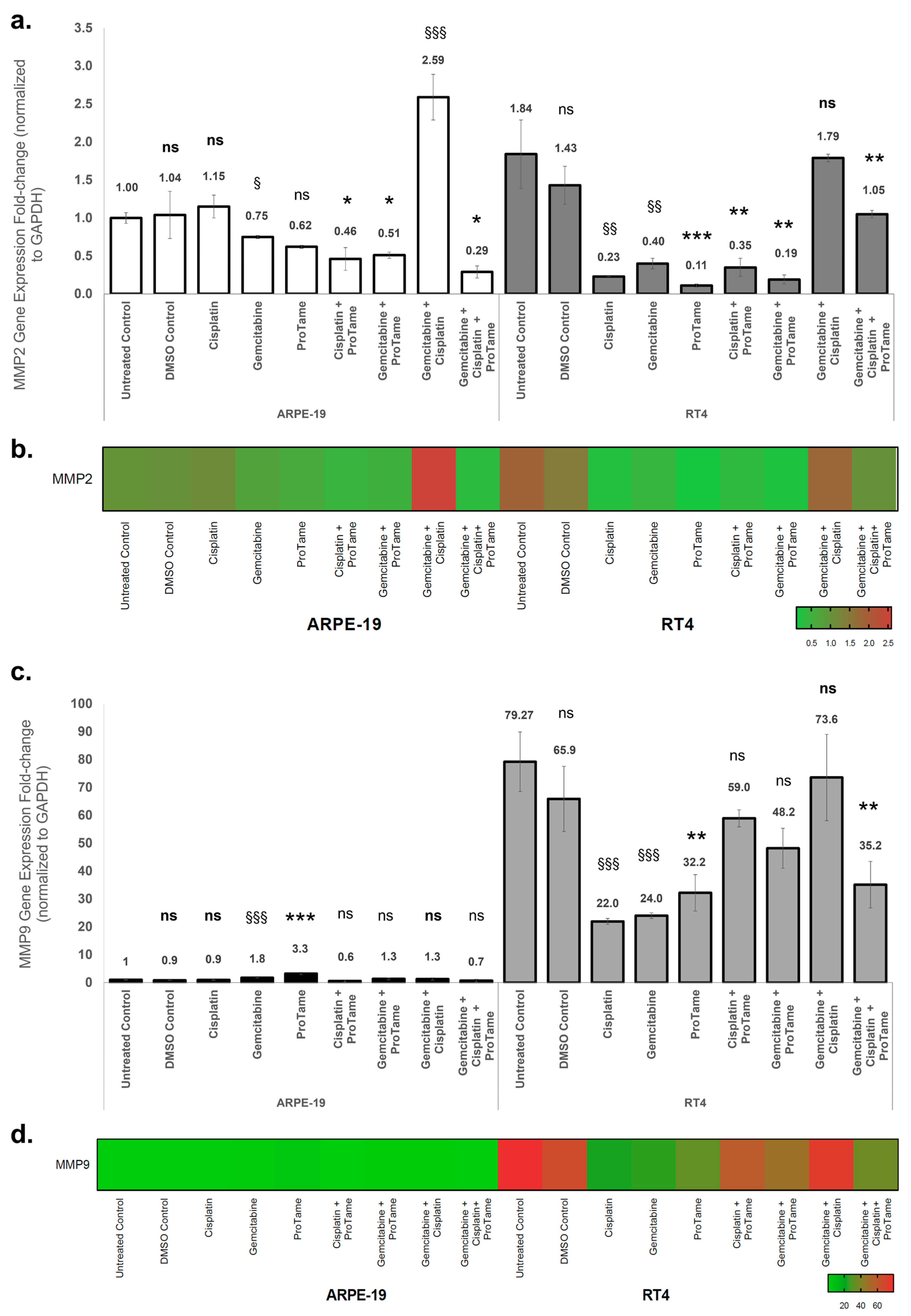
3.3. Modulation of MMP2 and MMP9 Gene Expression by Cisplatin, Gemcitabine, and ProTAME in ARPE-19 and RT4 Cells
4. Discussion
4.1. Background and Clinical Relevance
4.2. Effects of Treatments on MMP Expression
4.3. Differential Cellular Responses
4.4. Molecular Docking Insights
4.5. Mechanistic Insights and APC/C Inhibition
4.6. Therapeutic Implications
4.7. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Beltran, A.; Cookson, M.S.; Guercio, B.J.; Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ 2024, 384, e076743. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, screening, and prevention of bladder cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; Olivera, P.; Dueñas-Gonzalez, A.; Pérez-Pastenes, M.A.; Zárate, A.; Maldonado, V.; Meléndez-Zajgla, J. Gemcitabine activity in cervical cancer cell lines. Cancer Chemother. Pharmacol. 2001, 48, 488–492. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Sedighi, M.; Namdari, M.; Mahmoudi, P. An overview of angiogenesis and chemical and physiological angiogenic factors: Short review. J. Chem. Health Risks 2023, 411–422. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Zhou, Z.; He, M.; Shah, A.A.; Wan, Y. Insights into APC/C: From cellular function to diseases and therapeutics. Cell Div. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Wan, L.; Zhou, X.; Wang, Z.; Wei, W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol. Ther. 2015, 151, 141–151. [Google Scholar] [CrossRef]
- Zeng, X.; Sigoillot, F.; Gaur, S.; Choi, S.; Pfaff, K.L.; Oh, D.-C.; Hathaway, N.; Dimova, N.; Cuny, G.D.; King, R.W. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 2010, 18, 382–395. [Google Scholar] [CrossRef]
- Sevim Nalkiran, H.; Biri, I.; Nalkiran, I.; Uzun, H.; Durur, S.; Bedir, R. CDC20 and CCNB1 overexpression as prognostic markers in bladder cancer. Diagnostics 2024, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, Y.; Chen, J.; Li, H.; Yao, K.; Liu, Y.; Liu, Q.; Lu, J. The oncogenic role of APC/C activator protein CDC20 by an integrated pan-cancer analysis in human tumors. Front. Oncol. 2021, 11, 721797. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Likos, J.J.; Zhu, L.; Woodward, H.; Munie, G.; McDonald, J.J.; Stevens, A.M.; Howard, C.P.; De Crescenzo, G.A.; Welsch, D.; et al. Solution structure and backbone dynamics of the catalytic domain of matrix metalloproteinase-2 complexed with a hydroxamic acid inhibitor. Biochim. Biophys. Acta Proteins Proteom. 2002, 1598, 10–23. [Google Scholar] [CrossRef]
- Rowsell, S.; Hawtin, P.; Minshull, C.A.; Jepson, H.; Brockbank, S.M.; Barratt, D.G.; Slater, A.M.; McPheat, W.L.; Waterson, D.; Henney, A.M.; et al. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J. Mol. Biol. 2002, 319, 173–181. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sevim Nalkiran, H.; Akcora Yildiz, D.; Saydam, F.; Guzel, A.I.; Nalkiran, I. Targeting the anaphase-promoting complex/cyclosome (APC/C) enhanced antiproliferative and apoptotic response in bladder cancer. Saudi J. Biol. Sci. 2023, 30, 103564. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Muto, S.; Horie, S. Molecular biomarkers in bladder cancer: Novel potential indicators of prognosis and treatment outcomes. Disease Markers 2016, 2016, 8205836. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Lobo, A.; Mishra, S.K.; Cheng, L. Precision medicine in bladder cancer: Present challenges and future directions. J. Pers. Med. 2023, 13, 756. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chan, K.; Chang, S.; Friedlander, T. NCCN Guidelines® insights: Bladder cancer, version 2.2022: Featured updates to the NCCN guidelines. J. Natl. Compr. Canc Netw. 2022, 20, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Guercio, B.J.; Iyer, G.; Rosenberg, J.E. Developing mrecision Medicine for bladder cancer. Hematol. Oncol. Clin. N. Am. 2021, 35, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Kardoust Parizi, M.; Shariat, S.F.; Margulis, V.; Mori, K.; Lotan, Y. Value of tumour-infiltrating immune cells in predicting response to intravesical BCG in patients with non-muscle-invasive bladder cancer: A systematic review and meta-analysis. BJU Int. 2021, 127, 617–625. [Google Scholar] [CrossRef]
- Flaig, T.W.; Tangen, C.M.; Daneshmand, S.; Alva, A.; Lerner, S.P.; Lucia, M.S.; McConkey, D.J.; Theodorescu, D.; Goldkorn, A.; Milowsky, M.I.; et al. A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin. Cancer Res. 2021, 27, 2435–2441. [Google Scholar] [CrossRef]
- Singh, R.; Singh Banipal, R.P.; Goyal, L.D. Cisplatin versus gemcitabine as concurrent chemoradiotherapy in squamous cell carcinoma cervix: A comparative study of clinical response and toxicities. J. Cancer Res. Ther. 2022, 18, 1518–1524. [Google Scholar] [CrossRef]
- Crino, L.; Scagliotti, G.; Marangolo, M.; Figoli, F.; Clerici, M.; De Marinis, F.; Salvati, F.; Cruciani, G.; Dogliotti, L.; Pucci, F. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: A phase II study. J. Clin. Oncol. 1997, 15, 297–303. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Hitt, R.; Castellano, D.; Hidalgo, M.; Garcia-Carbonero, R.; Pena, M.; Brandariz, A.; Millan, J.; Vincent, J.A.; Cortes-Funes, H. Phase II trial of cisplatin and gemcitabine in advanced squamous-cell carcinoma of the head and neck. Ann. Oncol. 1998, 9, 1347–1349. [Google Scholar] [CrossRef]
- Xin, Y.; Ning, S.; Zhang, L.; Cui, M. CDC27 facilitates gastric cancer cell proliferation, invasion and metastasis via twist-induced epithelial-mesenchymal transition. Cell Physiol. Biochem. 2018, 50, 501–511. [Google Scholar] [CrossRef]
- Ashour, A.E.; Jamal, S.; Cheryan, V.T.; Muthu, M.; Zoheir, K.M.; Alafeefy, A.M.; Abd-Allah, A.R.; Levi, E.; Tarca, A.L.; Polin, L.A.; et al. CARP-1 functional mimetics: A novel class of small molecule inhibitors of medulloblastoma cell growth. PLoS ONE 2013, 8, e66733. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Grubb, T.M.; Zalenski, A.A.; Pfaff, K.E.; Pal, D.; Majumder, S.; Summers, M.K.; Venere, M. Hyperphosphorylation of CDH1 in glioblastoma cancer stem cells attenuates APC/CCDH1 activity and pharmacologic inhibition of APC/CCDH1/CDC20 compromises viability. Mol. Cancer Res. 2019, 17, 1519–1530. [Google Scholar] [CrossRef]
- Raab, M.; Sanhaji, M.; Zhou, S.; Rödel, F.; El-Balat, A.; Becker, S.; Strebhardt, K. Blocking mitotic exit of ovarian cancer cells by pharmaceutical inhibition of the anaphase-promoting complex reduces chromosomal instability. Neoplasia 2019, 21, 363–375. [Google Scholar] [CrossRef]
- Maes, A.; Maes, K.; De Raeve, H.; De Smedt, E.; Vlummens, P.; Szablewski, V.; Devin, J.; Faict, S.; De Veirman, K.; Menu, E. The anaphase-promoting complex/cyclosome: A new promising target in diffuse large B-cell lymphoma and mantle cell lymphoma. Br. J. Cancer 2019, 120, 1137–1146. [Google Scholar] [CrossRef]
- Lub, S.; Maes, A.; Maes, K.; De Veirman, K.; De Bruyne, E.; Menu, E.; Fostier, K.; Kassambara, A.; Moreaux, J.; Hose, D. Inhibiting the anaphase promoting complex/cyclosome induces a metaphase arrest and cell death in multiple myeloma cells. Oncotarget 2016, 7, 4062. [Google Scholar] [CrossRef]
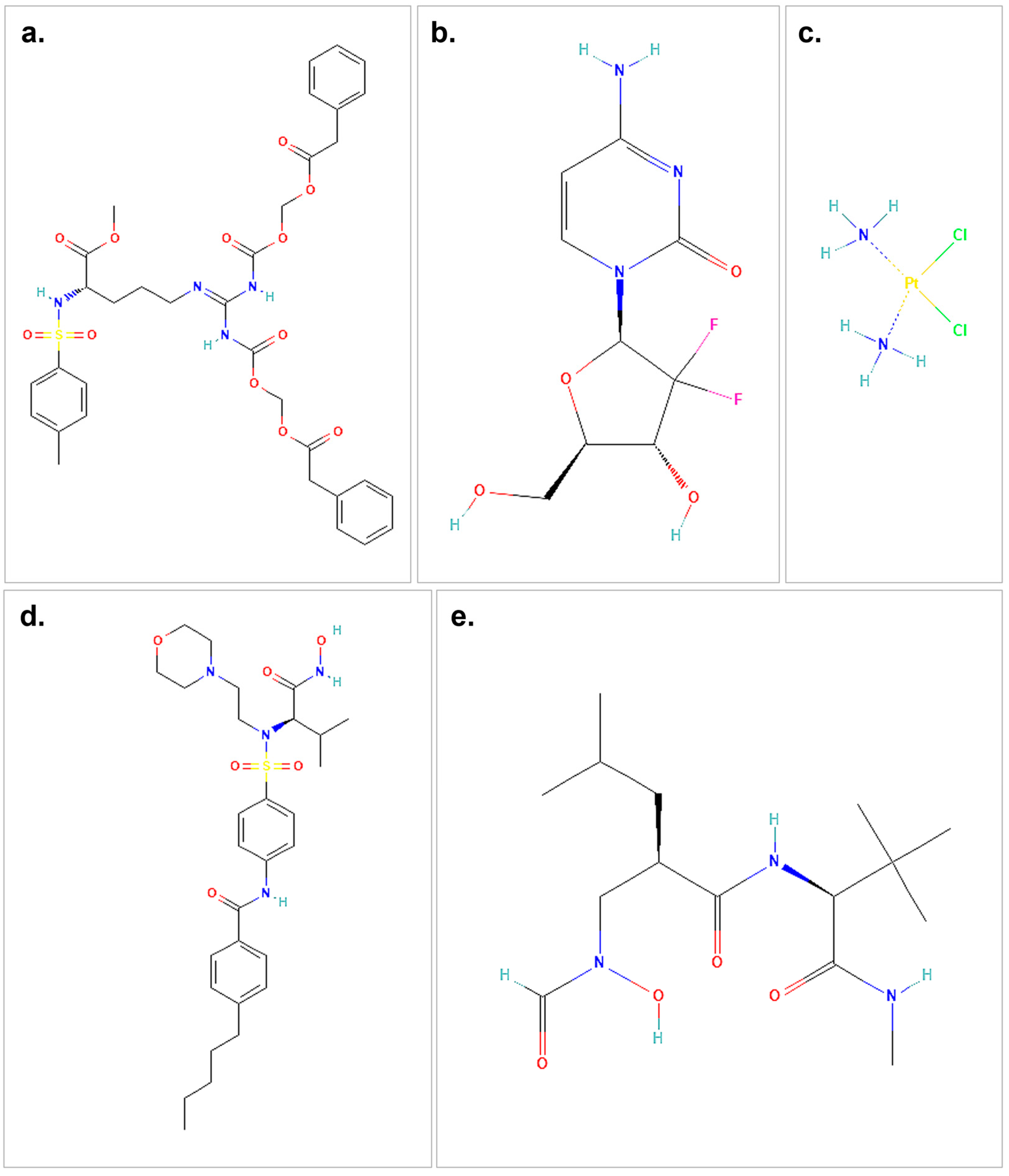

| Protein | Center at (X, Y, Z) | Dimension (Å) |
|---|---|---|
| MMP2 | X: 8.587, Y: 20.237, Z: 12.663 | 40 Å × 40 Å × 40 Å |
| MMP9 | X: 54.532, Y: 21.247, Z: 129.543 | 40 Å × 40 Å × 40 Å |
| Protein/Ligand | Minimum Binding Affinity (Kcal/mol) | No. of Conventional Hydrogen Bonds | Hydrogen Bond Distance (Å) | Key Structural Amino Acid Residues |
|---|---|---|---|---|
| MMP2–proTAME | −9.2 | 3 | 2.74 | GLY73 |
| 2.79 | HIS85 | |||
| 2.16 | TYR142 | |||
| MMP2–gemcitabine | −7.0 | 3 | 2.02 | LEU83 |
| 2.54 | ALA84 | |||
| 3.15 | VAL117 | |||
| MMP2–cisplatin | −3.2 | 2 | 2.43 | ASP101 |
| 1.98 | ASP101 | |||
| MMP2–I52 | −8.1 | 4 | 2.51 | LEU82 |
| 2.10 | LEU83 | |||
| 3.02 | ALA84 | |||
| 2.14 | ALA84 |
| Protein/Ligand | Minimum Binding Affinity (Kcal/mol) | No. of Conventional Hydrogen Bonds | Hydrogen Bond Distance (Å) | Key Structural Amino Acid Residues |
|---|---|---|---|---|
| MMP9–proTAME | −8.7 | 3 | 3.07 | GLY186 |
| 1.83 | LEU188 | |||
| 2.62 | TYR423 | |||
| MMP9–gemcitabine | −8.6 | 1 | 3.21 | GLU416 |
| MMP9–cisplatin | −3.7 | 6 | 2.20 | ARG424 |
| 2.12 | MET422 | |||
| 2.78 | LEU418 | |||
| 2.86 | TYR420 | |||
| 2.64 | TYR420 | |||
| 2.88 | LEU418 | |||
| MMP9–NFH | −6.2 | 4 | 1.83 | LEU188 |
| 2.51 | TYR423 | |||
| 2.30 | PRO421 | |||
| 2.06 | GLY186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalkiran, I.; Sevim Nalkiran, H. Repurposing ProTAME for Bladder Cancer: A Combined Therapeutic Approach Targeting Cell Migration and MMP Regulation. Biology 2025, 14, 263. https://doi.org/10.3390/biology14030263
Nalkiran I, Sevim Nalkiran H. Repurposing ProTAME for Bladder Cancer: A Combined Therapeutic Approach Targeting Cell Migration and MMP Regulation. Biology. 2025; 14(3):263. https://doi.org/10.3390/biology14030263
Chicago/Turabian StyleNalkiran, Ihsan, and Hatice Sevim Nalkiran. 2025. "Repurposing ProTAME for Bladder Cancer: A Combined Therapeutic Approach Targeting Cell Migration and MMP Regulation" Biology 14, no. 3: 263. https://doi.org/10.3390/biology14030263
APA StyleNalkiran, I., & Sevim Nalkiran, H. (2025). Repurposing ProTAME for Bladder Cancer: A Combined Therapeutic Approach Targeting Cell Migration and MMP Regulation. Biology, 14(3), 263. https://doi.org/10.3390/biology14030263





