Simple Summary
Melanoma is the most aggressive type of skin cancer, and the prognosis for metastatic melanoma is poor. Despite the recent advancements in therapeutic options, the extension of life offered by these treatment strategies is only a matter of months due to the rapid development of resistance, which could lead to no response or a decreased response rate after the initial relief. The need for improved efficacy of current melanoma treatments calls for innovative strategies, such as the elucidation of combination therapies, continued discovery of novel therapeutic targets, and preclinical investigation of natural agents as adjuvant therapy. The antitumor potential and relatively low toxicities associated with the naturally occurring phytochemicals make them an attractive treatment option for metastatic melanoma. This review summarizes major plant-based compounds, such as curcumin, sulforaphane, epigallocatechin gallate, resveratrol, fucoxanthin, lycopene, and quercetin, that are most consumed through various foods and their role in the prevention and development of melanoma skin cancer.
Abstract
Melanoma, particularly the malignant type owing to its aggressive metastatic potential, is one of the most severe cancers. When detected early, the cure rate of melanoma is very promising and has a higher 5-year survival rate. However, it becomes very difficult to treat when it has spread to deeper layers of the skin and to other parts of the body. Despite the reduced mortality, the incidence of melanoma is on the rise due to exposure to various environmental factors, particularly UV radiation without protection. Naturally occurring dietary agents have been studied for their antitumor and chemopreventive potential against the development of various cancers including melanoma. The current review is aimed at compiling developments in the past ten years surrounding their preclinical and clinical relevance in the treatment and prevention of malignant melanoma. Various cellular pathways modulated by these phytochemicals are also examined to provide a comprehensive overview of their mechanisms involved in reducing tumor burden. Specifically, the review focuses on the most consumed foods across the world that are rich in such phytochemicals including curcumin, sulforaphane, resveratrol, quercetin and epigallocatechin gallate (EGCG) and their potential against the development of melanoma.
1. Introduction
Melanoma, an aggressive and deadly skin cancer, develops from cutaneous melanocytes that are present in the basal layer of the epidermis. The incidence of melanoma has increased steadily over the past three decades compared to other cancer types and is one of the most common cancers diagnosed among young adults in the United States. It is estimated that 104,960 new cases of melanoma will be diagnosed and 8450 people are expected to die from this devastating disease in 2025 [1]. Established risk factors for the development of melanoma include features, like light-colored skin, hair, and eyes, and exposure to ultraviolet radiation. In particular, the genetic background of individuals, the extent of UV radiation exposure, and blistering sunburns early in life have been shown to play a causal role [2,3]. Five to fifteen percent of individuals with cutaneous melanoma have been identified to have a family history of melanoma, but a direct association of hereditary melanoma due to a transmitted genetic mutation is less common [4,5]. Cyclin-dependent kinase inhibitor 2A [CDKN2A] gene and cyclin-dependent kinase 4 (CDK4) gene germline mutations, among other less commonly known mutations, are frequently identified genetic abnormalities in familial melanomas [5,6]. Xeroderma pigmentosum [XP], an autosomal recessive disease is also associated with an increased risk of skin cancers, including melanoma.
The management of melanoma is complex and typically includes surgery, immunotherapy, targeted therapy, and radiotherapy [7]. Surgery is curative for the majority of patients who have cutaneous melanomas of low thickness [8]. However, rates of survival drop predominantly with increased tumor thickness due to the increased risk of metastasis [8]. This transition from a mostly benign disease to one with a more serious prognosis occurs as melanoma progresses through the radial and vertical growth phases. The prognosis for metastatic melanoma is grim: 5-year survival ranges from 12–28%, depending on the location of the metastasis [8]. Traditional cytotoxic therapy and immunomodulatory agents have failed to demonstrate significant efficacy, with fewer than 5% of patients having complete responses at 5 years [8]. Fortunately, the therapeutic landscape of unresectable melanoma has been transformed by the advances in targeted therapies and immune therapies. The new approaches have offered significant outcomes with improved survival when compared with the traditional strategies [4]. Yet, despite these advancements, the extension of life offered by these strategies is only a matter of months due to the rapid development of resistance, which could lead to no response or a decreased response rate after the initial response. The agents also target a fraction of the oncogenic signaling pathways that play a role in melanoma. Limitations regarding toxicity and patient safety have shifted interests toward the exploration of natural compounds for the treatment of melanoma.
Natural products have received increasing attention in recent years as novel cancer preventive and therapeutic agents. Preclinical studies employing naturally occurring phytochemicals, both alone and in combination with traditional cytotoxic and targeted therapies, have yielded promising results [8]. The relatively low toxicities of these substances make the adjuvant use of natural agents an attractive treatment option for metastatic melanoma. The need for improved efficacy of current melanoma treatments calls for innovative strategies, such as the elucidation of combination therapies, continued discovery of novel therapeutic targets, and preclinical investigation of natural agents as adjuvant therapy. This review aims to critically appraise the current knowledge on the most consumed foods across the world that are rich in such phytochemicals including curcumin, sulforaphane, resveratrol, quercetin, and epigallocatechin gallate (EGCG) and their potential against the development of melanoma. By focusing on the role of these natural products, we seek to enrich our current understanding of their involvement against the development of melanoma. The information compiled could serve as a guide for future directions and offer insights into the development of diverse therapeutic regimens for melanoma. In this review, we compiled information by performing a literature search using the keywords “melanoma” and “Curcumin, sulforaphane, resveratrol, quercetin, EGCG, Fucoxanthin and Lycopene” from 2015 until August 2025 using PubMed and Google scholar. A search of ClinicalTrials.gov using the keywords “resveratrol or other phytochemicals” and “melanoma” was performed, along with a broader search of ClinicalTrials.gov using the terms “resveratrol or other phytochemicals” and “cancer” for studies in other cancer models to summarize available clinical trial data. Similarly, a search on PubMed [pubmed.ncbi.nlm.nih.gov] with the same keywords and filters applied for Adaptive Clinical Trial, Clinical Study, and all phases of Clinical Trials ([I–IV]) was also performed to obtain information on clinical studies.
1.1. Quercetin
Quercetin, a polyphenolic flavonoid present in fruits, vegetables, and tea, has demonstrated antioxidant, anti-inflammatory, and anti-cancer activity in various cancers, including melanoma [2]. Quercetin contains a low-toxicity profile while demonstrating inhibitory effects on the migration and invasion of melanoma [9]. This section reviews current literature on quercetin and its antitumor potential, focusing on the molecular mechanisms, discoveries from in vivo and in vitro studies, synergistic interactions with other agents, and current evidence from clinical studies.
Numerous in vitro studies have demonstrated quercetin’s inhibitory effects on melanoma cell proliferation. Quercetin induces apoptosis and suppresses proliferation in a concentration-dependent manner, primarily through the induction of cell cycle arrest at the G1 phase. This effect is mediated by downregulating key G0/G1 regulatory proteins including cyclin D, cyclin E, CDK4, and CDK6, thereby disrupting cell cycle progression and cellular replication [10]. Furthermore, quercetin has been found to modulate melanogenesis, the biosynthetic pathway of melanin that depends on tyrosinase and tyrosinase-related proteins TRP-1 and TRP-2 [11]. In murine B16-F10 melanoma cells, quercetin inhibited tyrosinase activity, leading to reduced melanin synthesis and decreased cancer cell viability [11]. These findings highlight quercetin’s role in attenuating both melanoma cell growth and melanogenesis-related pathways.
Quercetin has also been shown to modulate key signaling pathways that are frequently dysregulated in melanoma. Specifically, it downregulates the PI3K/Akt and MAPK/ERK pathways, both of which are central to melanoma cell growth, proliferation, and survival [12]. Treatment with quercetin reduces the level of phosphorylated Akt and ERK, decreasing cell survival and increasing pro-apoptotic signaling. In addition, quercetin induces mitochondrial-dependent apoptosis by suppressing the anti-apoptotic protein Bcl-2 and activating caspase-3 and caspase-9 [12,13].
In vivo studies have investigated the tumor-suppressive potential of quercetin in murine models of melanoma. Treatment of mice xenografted with B16-F10 or A375 melanoma cells with quercetin resulted in significant inhibition of tumor growth. Quercetin treatment was associated with upregulated IFN-α and IFN-β, which exert antiproliferative effects on melanoma cells [14]. Further studies have revealed quercetin’s enhanced antitumor immunity by increasing the percentage of immune effector cells, including CD8+ T lymphocytes, CD4+ T lymphocytes, and M1 macrophages, promoting phagocytosis and contributing to an immunogenic tumor microenvironment [15].
Quercetin has demonstrated synergistic effects when used in combination with conventional chemotherapeutic agents and natural bioactive compounds. For example, quercetin complexed with cerium ions has been shown to form a thermodynamically favorable structure that can enhance its bioactivity [16]. When applied to A375 melanoma and MDA-MB-231 breast cancer cell lines pre-treated with photodynamic red-light therapy, the quercetin-cerium complex significantly improved cellular uptake and cytotoxic efficacy in comparison to free quercetin, suggesting enhanced photodynamic potential of polyphenols in cancer treatment [16]. In another study, quercetin was reported to sensitize melanoma cells to recombinant human tumor necrosis factor-related apoptosis-inducing ligand [rhTRAIL], a therapeutic agent that has failed clinical translation due to resistance of cancer cells to rhTRAIL-induced apoptosis. Quercetin enhanced rhTRAIL efficacy by upregulating rhTRAIL-binding receptors DR4 and DR5 on the surface of cancer cells, increasing proteasome-mediated degradation of antiapoptotic proteins in cancer cells [17].
Additionally, quercetin has shown promising additive effects when used with other natural compounds. Administration of quercetin with curcumin led to a marked reduction in cell proliferation of several cancer cell lines, including A375 melanoma, A549 lung cancer, HCT116 colorectal cancer, and MCF7 breast cancer [18]. Further investigation realized that this finding was linked to a downregulation of Wnt/beta-catenin, DVL2, beta-catenin, cyclin D1, Cox2, and Axin2 [18]. Similarly, a multi-compound combination of quercetin, curcumin, green tea, cruciferex, and resveratrol was found to inhibit the growth of A2068 melanoma cells [19]. These findings highlight the potential of quercetin-based therapeutic combinations as promising adjuncts in the treatment of melanoma, leading to more effective and multifaceted anticancer strategies.
Despite compelling preclinical evidence, clinical data on the efficacy of quercetin for the treatment of melanoma and other cancers remain limited. A phase II/III clinical trial investigated the effects of isoquercetin on hypercoagulability in patients with unresectable or metastatic adenocarcinoma of the pancreas, stage IV colorectal cancer, or stage III/IV non–small cell lung cancer. The study demonstrated that administration of isoquercetin significantly reduced D-dimer plasma concentrations, suggesting improved markers of coagulation in advanced cancer patients [20]. However, no phase II or III trials have evaluated quercetin as a monotherapy or combination therapy for melanoma. This lack of clinical investigation highlights a critical gap in translating quercetin’s promising preclinical anti-cancer effects into clinical application and prompts the need for future clinical trials to evaluate its efficacy and safety in patients with melanoma. The biological effects of quercetin are summarized in Figure 1.
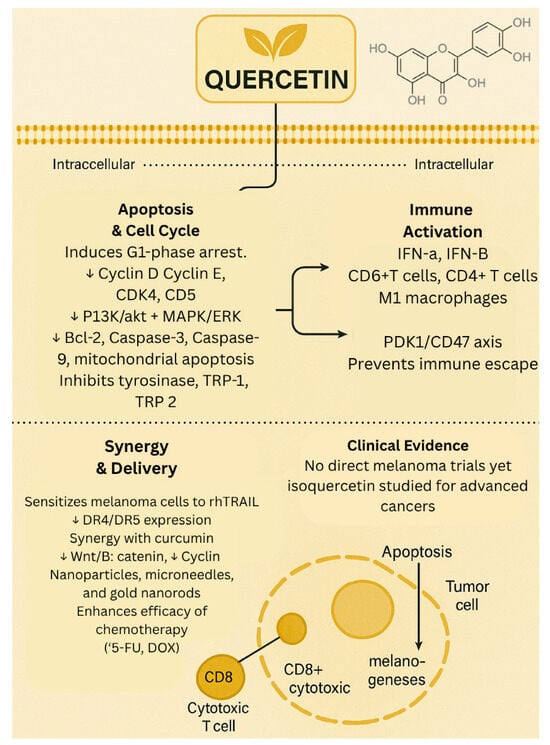
Figure 1.
Biological effects of quercetin. ↓—downregulated/decreased expression.
1.2. Sulforaphane
Sulforaphane [SFN], a phytochemical predominantly extracted from broccoli sprouts and other Brassica vegetables, has attracted a considerable amount of interest in medical research due to its antiproliferative and cytoprotective properties. This compound is produced secondary to the enzymatic hydrolysis of glucoraphanin, a glucosinolate found in Brassica species [21]. Notably, SFN carries anticancer activity while demonstrating a favorable safety profile with minimal side effects—an uncommon trait among chemotherapeutic agents. Given such properties, SFN has emerged as a promising candidate in cancer treatment and prevention. The unique pharmacological profile of SFN emphasizes the need for continued investigation into mechanisms and therapeutic application in the management of melanoma.
SFN has demonstrated potent pro-apoptotic and anti-proliferative effects in melanoma through multiple signaling pathways. One of the earliest reports by Misiewicz et al. showed that SFN and 2-oxohexyl isothiocyanate induced dose-dependent apoptosis in human melanoma (ME-18) and murine leukemia (L-1210) cells, confirmed by phosphatidylserine externalization and DNA fragmentation [22]. Subsequent studies using B16-F10 melanoma cells further revealed SFN-induced apoptosis marked by caspase-3 and -9 activation, upregulation of Bax and p53, and suppression of anti-apoptotic markers such as Bcl-2, Bid, NF-κB, and caspase-8 [23]. In addition to apoptotic induction, SFN treatment significantly downregulated pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12p40, and GM-CSF, in melanoma-bearing mice, indicating immunomodulatory potential [24]. Nuclear translocation of transcription factors such as NF-κB subunits, c-FOS, ATF-2, and CREB-1 was also inhibited, suggesting that SFN acts via a multi-pathway mechanism, further supporting its role in chemoprevention [23]. A broader evaluation of cruciferous-derived isothiocyanates (including SFN) demonstrated that SFN selectively induced cell cycle arrest at the G2/M phase in metastatic melanoma cells, accompanied by increased intracellular ROS and glutathione levels [25]. Importantly, melanoma cells showed heightened sensitivity to these effects compared to non-melanoma or normal skin cells, underscoring SFN’s tumor-selective cytotoxicity. Additionally, this highlights the synergistic potential of SFN with other agents.
Oxidative stress plays a critical role in UV-induced melanogenesis and melanoma progression. SFN activates the Keap1–Nrf2–ARE pathway, enhancing transcription of detoxifying and antioxidant enzymes such as NQO1, HO-1, and SOD. In keratinocytes and fibroblasts, SFN-mediated Nrf2 activation was associated with upregulation of IL-6, IL-8, and Bcl-2, promoting cytoprotection against UV-induced oxidative damage [26,27]. Dinkova-Kostova et al. reported that topical application of SFN in SKH-1 hairless mice led to a ~50% reduction in UV-induced tumor incidence, volume, and inflammation. In human subjects, topical SFN extract increased NQO1 activity 2–4.5-fold and reduced UVB-induced erythema by approximately 40%, highlighting its relevance as a chemopreventive agent [26]. Htut et al. further confirmed that SFN produces sustained Nrf2 activation and antioxidant response element (ARE) activity under UV stress conditions [28].
Beyond redox and immune modulation, SFN exhibits significant epigenetic regulatory activity. In B16 and S91 melanoma cells, SFN reduced viability and inhibited HDAC activity. In vivo, SFN-loaded albumin microspheres administered to C57BL/6 mice with B16 tumors resulted in significantly greater tumor volume reduction [~15% more] compared to free SFN and enhanced HDAC inhibition [30% vs. 15%, respectively] [29]. This controlled delivery approach enhanced histone H4 acetylation—a key modification associated with DNA repair and chromatin relaxation—demonstrating SFN’s potential as an epigenetic therapeutic [30]. Collectively, these studies affirm sulforaphane’s capacity to target melanoma through multi-dimensional mechanisms, involving oxidative defense activation, epigenetic remodeling, immunomodulation, and cell death induction.
SFN enhances the efficacy of other anti-cancer compounds, offering synergy through complementary mechanisms. When combined with quercetin, SFN significantly inhibited proliferation and migration of B16F10 melanoma cells more than either compound alone. In vivo, combination therapy suppressed melanoma tumor volume and weight effectively. Mechanistically, this combination suppressed MMP-9 expression and inhibited metastatic potential in melanoma cells [31]. SFN also potentiates the effects of epigenetic therapies. In one study, low-dose SFN paired with 5-aza-2′-deoxycytidine [DAC] enhanced suppression of melanoma cell growth and altered immune gene expression, such as CCL5 and IL-33, more than either agent alone, despite not inducing apoptosis individually [32].
Only a limited number of early-phase clinical studies have evaluated sulforaphane (SFN) in the context of melanoma. One notable investigation by Tahata et al. (2018) [33] involved a phase 0 randomized controlled trial in 17 melanoma survivors with at least two atypical nevi. Participants received oral broccoli sprout extract standardized to deliver between 50 and 200 µmol of SFN daily over 28 days [33]. The intervention was well-tolerated and demonstrated dose-dependent increases in both plasma and skin SFN levels. Importantly, treatment was associated with a reduction in pro-inflammatory cytokines, including MCP-1, IP-10, and MIP-1β, as well as an increase in decorin expression within nevus tissue, a potential biomarker of tumor suppression. These findings provide early clinical evidence supporting SFN’s bioavailability and biological activity in human skin. Beyond this study, additional early-phase trials in broader oncology populations that included melanoma patients further support SFN’s favorable safety and anti-inflammatory profile; however, melanoma-specific clinical outcomes beyond phase 0 remain largely unreported. The biological effects of sulforaphane are summarized in Figure 2.
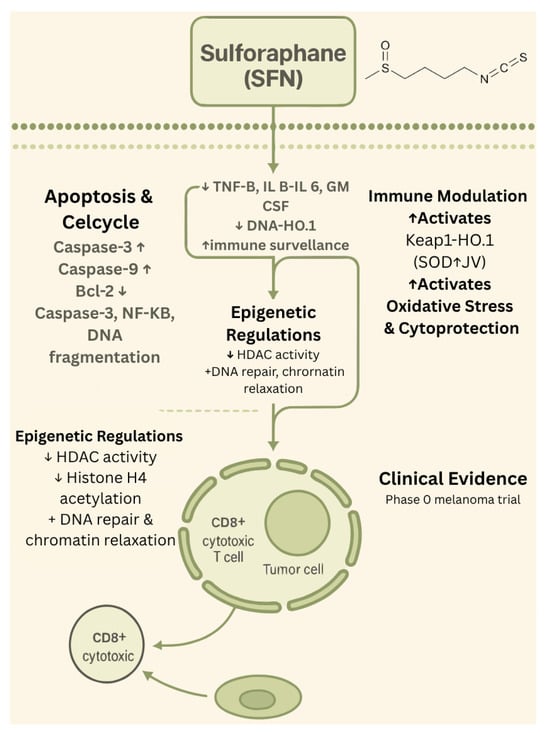
Figure 2.
Biological effects of sulforaphane. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
1.3. Curcumin
Curcumin [1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione], a lipophilic polyphenol derived from Curcuma longa Linn (turmeric) has been used traditionally as a spice, food colorant, and herbal medicine by various cultures for thousands of years [34]. The curcuminoids from turmeric, including curcumin, dimethoxy curcumin, and bisdemethoxycurcumin, are identified as the most bioactive compounds with various health benefits and are highly lipophilic, with increased solubility in various organic solvents and lower solubility in water. Orally administered turmeric compounds have poor bioavailability owing to their rapid metabolism and elimination from systemic circulation, and systemic concentrations of unconjugated curcumin do not exceed low micromolar concentrations in humans despite oral doses ≤12 g [35].
A large body of literature demonstrates the biological effects of curcumin, including cancer-preventive and therapeutic effects [36]. Evidence continues to add new knowledge, focusing on the different molecular and cellular pathways affected by curcumin in lowering the burden of melanoma pathogenesis [37]. A study by Liao et al. showed that curcumin induced apoptosis and caused inhibition of cell proliferation of A375 melanoma cells by regulating cell cycle arrest and cellular redox state. Specifically, the authors identified the role of oxidative stress in contributing to the biological activities of curcumin, which was reversed by the reactive oxygen species inhibitor, N-acetyl cysteine [38]. Similarly, Zhao et al. showed that curcumin induces autophagy and inhibits proliferation and invasion of A 375 and c8161 melanoma cells by downregulating the PI3k/AKT/mTOR signaling pathway [39]. The authors also showed that treatment of BALB/c mice inoculated with A 375 melanoma cells [to establish an explanted in vivo melanoma model] with curcumin for 21 days resulted in tumors that were smaller in size and weight compared to the DMSO-treated control mice [39]. Recently, curcumin was shown to inhibit proliferation, migration, and invasion ability of A 375 and HT144 melanoma cells through miR0222-3p to reduce SOX 10 expression and inactivate the Notch pathway [40]. The authors identified that the miR-222-3p inhibitor reversed the antiproliferative, antimigratory, and anti-invasive effects of curcumin in melanoma cells employed in the study [40].
In a systematic review and meta-analysis conducted by Teng et al. that included preclinical studies focusing on the effects of curcumin in animal models of melanoma, it was concluded that administration of curcumin was relatively safe and has no side effects, and reduced tumor burden by inhibiting growth and cell proliferation [41]. To summarize, various molecular mechanisms have been attributed to the antitumor potential of curcumin, including microRNAs, modulation of critical regulators of apoptotic cell death [caspases, Bax, mTOR, XIAP, Bcl-2 family], factors involved in cell cycle [cyclins, p21, p16], interactions with various growth factors and regulating kinases (TNF-α, IFN-γ, and IFN-α; JAK, MAPK, IKK, PI3K/Akt), transcription factors [NF-kB, STAT-3, p53, p21, p65, and AP-1], and markers involved in chemotaxis, angiogenesis, and metastasis (COX, VEGF, MMP, NOS, and LOX) [37,42].
Curcumin’s potential in overcoming drug resistance in cancer cells has been explored to increase the therapeutic efficacy of existing chemotherapeutic agents. For example, Chiu et al. showed that curcumin induces apoptosis and suppresses tumor proliferation by causing cell cycle arrest, ROS generation, and decreased mitochondrial membrane potential, in addition to downregulating the EGFR signaling pathway in vemurafenib-resistant A 375.S2 melanoma cells [43]. Similarly, curcumin exhibited synergistic anti-cancer effects in malignant melanoma cells [G 361 and SKmEL-2] in spheroid and 2D cultures when used in combination with binimetinib, a MEK1/2 inhibitor, through apoptosis, necroptosis, and ROS production [44]. Recently, curcumin, when used in combination with thymoquinone [a natural compound present in black cumin seeds], synergistically inhibited cell viability, induced apoptosis, and disrupted metabolic and oxidative homeostasis in A 375 melanoma cells [45]. In a different study, the combination of curcumin and disulfiram synergistically inhibited the growth of B16-F-10 mouse melanoma cells and induced apoptotic cell death. The authors also showed that the combination therapy [20 mg/kg curcumin + 60 mg/kg disulfiram] administered intraperitoneally for 15 days reduced tumor weight in an in vivo model employing C57BL/6 mice bearing B16-F10 melanoma cells when compared to the control group or the groups administered curcumin and disulfiram [46].
To increase the bioavailability of curcumin, various strategies/formulations have been developed and tested to evaluate their efficacy compared to the free form. For example, a nano-micellar formulation of curcumin prolonged the survival rates of mice with B16-F10 lung metastatic tumors by increasing the accumulation of activated T cells in the tumor, promoting apoptosis, controlling angiogenesis, and lowering regulatory T cell accumulation [47]. Similarly, oral administration of curcumin loaded into mucoadhesive chitosan-coated polycaprolactone nanoparticles also increased the efficacy of curcumin in preventing melanoma metastasis by inducing apoptosis [48]. Administration of cationic liposomal curcumin in combination with STAT3 siRNA resulted in decreased growth of B16-F10 mouse melanoma cells in culture and tumor reduction in C57BL/6 mice injected with B16-F10 cells when compared with the groups treated with the individual agents [49]. In another study, employing B16-F10 cells, it was shown that curcumin-based nanoformulation enhanced the cellular uptake and cytotoxic effects of the nano-formulated curcumin when compared to group treated with curcumin alone [50].
While much work has been conducted employing various preclinical models to establish the antitumor potential against melanoma, there are no published human trials testing the therapeutic potential of curcumin for melanoma. A search on ClinicalTrials.gov using the terms “curcumin” and “melanoma” resulted in no studies; however, using the terms “curcumin” and “cancer”, the search yielded 38 completed studies and 14 trials currently recruiting patients. Previously published phase I trials have shown that administration of curcumin is well tolerated at doses up to 12 g but has been identified to have poor bioavailability [51,52]. In a phase IIA clinical trial of curcumin for the prevention of colorectal neoplasia, Carroll et al. showed that curcumin at doses of 2 g and 4 g was well tolerated and decreased the number of aberrant crypt foci, potentially mediated by systemically delivered curcumin conjugates [53]. The biological effects of curcumin are presented in Figure 3.
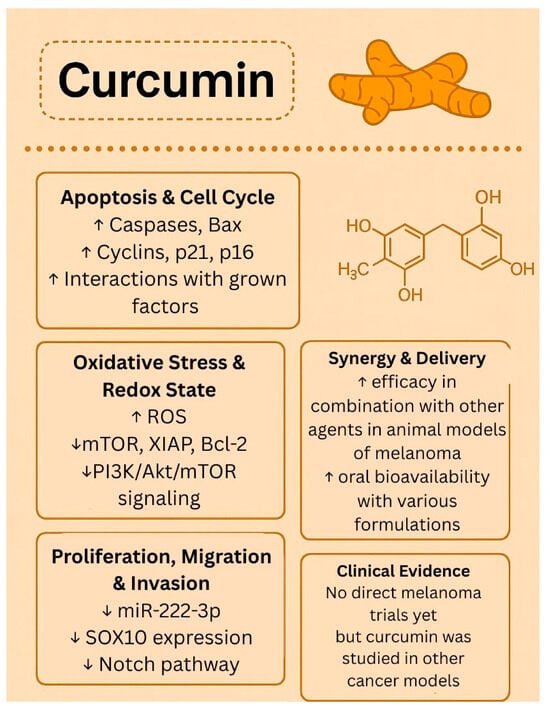
Figure 3.
Biological effects of curcumin. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
1.4. Epigallocatechin-3-Gallate (EGCG)
EGCG has garnered a lot of attention in cancer research owing to its role in modulating various signaling pathways associated with cancer growth and development. Instead of affecting a single pathway, EGCG is able to interact with many networks at a time, which can regulate cell survival, proliferation, and inflammation. This makes it a highly versatile compound. The PI3K/Akt/mTOR pathway is one of the most studied mechanisms in the field. In melanoma, this pathway promotes rapid cell division and contributes to resistance to apoptosis. Preclinical studies demonstrate that EGCG can inhibit this signaling cascade, leading to a reduction in tumor growth and an increase in programmed cell death [54]. Recent research has also linked EGCG with the induction of autophagy and cell cycle arrest, indicating that it interrupts cancer progression at multiple stages [55].
EGCG also affects immune-related pathways like JAK/STAT. The activation of this pathway is known to contribute to tumor immune evasion. This is achieved by promoting the expression of checkpoint proteins like PD-L1. Studies have shown that EGCG blocks STAT1 activity, which decreases PD-L1 and PD-L2 expression and restores CD8+ T-cell function in melanoma models [56]. These findings are relevant because they show signs of EGCG’s potential role in immunotherapies, particularly in patients who do not respond to PD-1/PD-L1 inhibitors. Beyond signaling, EGCG shows genetic effects that also contribute to its anticancer activity. EGCG generates DNA methyltransferases and histone deacetylases, leading to the activation of tumor suppressor genes [57]. This genetic finding demonstrates that EGCG has the capacity to act at both the molecular and genetic levels. While these findings represent a step in the right direction regarding EGCG as a potential candidate, more studies are needed to determine its stability and bioavailability. In its natural form, EGCG is known to rapidly be metabolized, which can affect its impact on cells. Some delivery methods, such as nanoparticle formulations, have been developed to overcome these limitations and have already been proven to improve anticancer activity in animal models [58]. These innovations suggest that EGCG may have the potential to become a new therapy.
Furthermore, apart from its effects on signaling pathways, EGCG plays an important role in regulating the tumor microenvironment and immune responses in melanoma cells. Tumors create immunosuppressive conditions that allow their uncontrollable growth. EGCG’s potential to counter the evasion of immune function by cancer cells is well documented. For example, it has been shown to contribute to the restoration of cytotoxic T-cell activity by decreasing the presence of regulatory T cells (Tregs) and myeloid-derived suppressor cells [MDSCs], both of which weaken antitumor immunity [57]. This sudden change in the tumor strengthens the body’s defense, leading to improved recognition of harm and a more rapid response. EGCG has also been found to be able to lower the expression of checkpoint proteins such as PD-L1 and PD-L2, which are usually used by tumors to avoid their detection [56]. By blocking STAT1 activation, EGCG decreases the checkpoint signals and increases CD8+ T-cell function, suggesting that it may work synergistically with immune checkpoint inhibitors [56]. EGCG’s effects on tumor microenvironments are also reflected in its metabolism. Cancer cells often reprogram glucose and lipid metabolism to promote cell growth and suppress immune responses. Administration of EGCG has been shown to reduce glucose uptake and lipid synthesis, ultimately depriving resources essential for tumor growth and development [57]. This also renders tumors more sensitive to immunotherapy and reduces drug resistance. These effects show the potential of EGCG in reshaping the tumor microenvironment and decrease tumor burden. By restoring immune surveillance and disrupting tumor-supportive metabolism, EGCG can strengthen the body’s own defenses.
EGCG’s rapid metabolism is a concern limiting its therapeutic potential against various cancer models [58]. To increase its bioavailability and systemic absorption, several systems have been explored to achieve better results. For example, nanoparticle formulations, liposomes, and polymer-based carriers have shown stronger anticancer activity compared to natural EGCG [59]. These discoveries have not only increased EGCG’s effectiveness but also reduced the required doses to achieve better therapeutic outcomes. Clinical studies, including animal models, have demonstrated that EGCG treatment reduces tumor growth and disrupts tumor-promoting pathways [60]. It has also been shown that EGCG may work synergistically with other treatments, such as immune checkpoint inhibitors or chemotherapy. While clinical trials remain scarce and further studies are required to determine its full effects on different subjects, the effects of EGCG have proven that it may be a promising candidate for cancer models and its therapeutic applications. Its ability to simultaneously interfere with multiple signaling pathways, regulate the tumor microenvironment, and enhance immune responses demonstrate the wide range of mechanisms that make EGCG a candidate for therapeutic treatments.
Although EGCG faces challenges in its natural form, such as its rapid metabolism and limited stability, new strategies such as nanoparticles, liposomes, and polymer-based carriers have helped overcome these barriers. Preclinical studies have supported EGCG’s role in reducing tumor growth, and evidence suggests the possibility of synergistic effects when combined with chemotherapy or immunotherapy. While clinical trials are still limited, the findings so far indicate that EGCG is a promising natural candidate for future melanoma therapies. The biological effects of EGCG are presented in Figure 4.
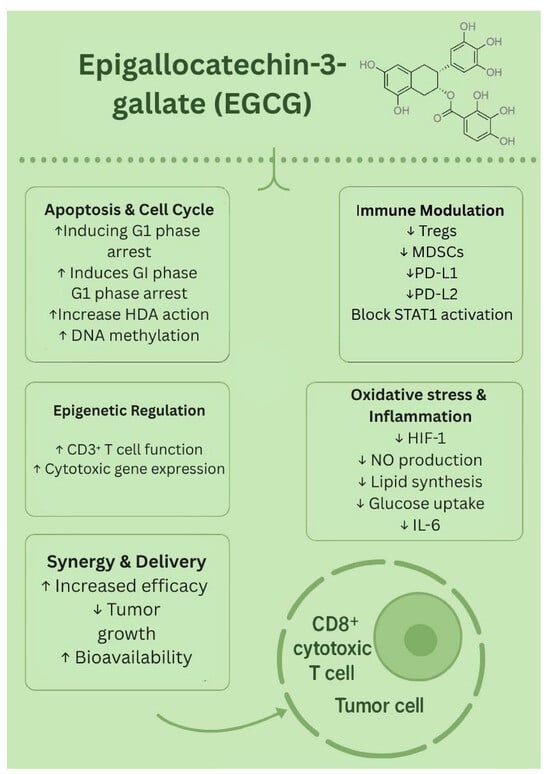
Figure 4.
Biological effects of EGCG. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
1.5. Resveratrol
Resveratrol [3,4′,5-trihydroxystilbene] is a naturally occurring polyphenolic compound that has gained significant attention for its potential health benefits. Numerous pre-clinical trials have demonstrated that resveratrol may confer protective and therapeutic effects against diseases such as diabetes, cancer, cardiovascular disorders, and infections through modulating multiple molecular targets and signaling pathways [61]. Perhaps most notable are the antineoplastic effects of resveratrol, which were discovered in 1997 [62].
Resveratrol is a small, lipophilic molecule belonging to the stilbene class of polyphenols [63]. Its molecular formula is C14H12O3, and the structure consists of two phenolic rings connected by a carbon–carbon double bond [64]. The polyphenol exists in both cis- and trans-conformations, with the trans-conformation being more stable and biologically active [65]. Plants synthesize resveratrol to protect themselves from environmental stress, such as microbial infections, ultraviolet light, and physical damage [66,67]. The stilbene is most abundant in the diet from Japanese knotweed (Polygonum cuspidatum) and the skin of red grapes [68]. It is particularly concentrated in red wine due to the extended fermentation process that involves grape skins [68]. Smaller amounts are also present in other foods, such as peanuts, pistachios, strawberries, blueberries, and mulberries [68].
Although resveratrol is well-known for its wide range of health benefits including antioxidant, cardioprotective, neuroprotective, anti-inflammatory, antimicrobial, and antidiabetic properties, its clinical use is hindered by its chemical instability and rapid degradation [65,69]. Trans-resveratrol is well absorbed after oral intake but has limited bioavailability due to rapid metabolism and elimination [70]. It undergoes rapid first-pass metabolism in the liver and intestines, converting mostly into less active sulfate and glucuronide forms [68]. As a result, despite good absorption, very little active resveratrol reaches the bloodstream, making it challenging to achieve effective clinical outcomes and determine appropriate dosing [68,71]. Resveratrol demonstrates promising pharmacological activity in inhibiting tumor growth, survival, and metastasis in melanoma. Preclinical studies, including both in vitro cellular assays and in vivo murine models, provide compelling evidence endorsing its efficacy as a chemopreventive and therapeutic agent against melanoma. A growing body of research highlights resveratrol’s ability to suppress melanoma cell proliferation and trigger apoptosis through diverse molecular pathways. One key pathway for this involves the extracellular signal-regulated kinase [ERK1/2] signaling cascade [72,73,74]. For instance, Yu et al. reported that resveratrol treatment reduced the expression of SHC binding and spindle-associated 1 [SHCBP1] in B16 melanoma cells, which caused decreased phosphorylation of ERK1/2 [72]. Given ERK1/2′s role in promoting G1/S cell cycle transition, this inhibition effectively suppressed melanoma cell proliferation [72,75]. Analogously, Zhao H et al. observed that resveratrol inhibited proliferation and induced apoptosis in MV3 and A375 human melanoma cell lines by downregulating the ERK/PKM2/Bcl-2 signaling pathway, which is critical for cell survival and metabolism [73,76]. This downregulation of ERK led to reduced PKM2 activation, decreased expression of the anti-apoptotic protein Bcl-2, and increased expression of the pro-apoptotic protein BAX, collectively promoting apoptosis in melanoma cells [73]. In addition, the study reported upregulation of p53 [a tumor suppressor gene], contributing further to growth inhibition [73].
Consistent with these findings, a 2018 in vitro study demonstrated that resveratrol triggered cell cycle arrest via the ROS-p38-p53 signaling axis. Expression of p53 and BAX was increased, while Bcl-2 expression levels decreased, thus leading to decreased growth and proliferation of melanoma cells [74]. Moriyama et al. also confirmed that resveratrol inhibited SK-MEL-28 melanoma cell proliferation by halting cell cycle progression at the G1/S phase, specifically by reducing ERK activation and increasing p21 expression [77]. Other studies have shown that resveratrol causes cell cycle arrest by downregulating multiple cyclins and cyclin-dependent kinases (CDKs) essential for cell cycle progression [77,78,79,80]. Yang et al. found that Cyclin D1, a key regulator of G1/S transition, was significantly reduced in A375 and A431 melanoma cells following resveratrol treatment, leading to cell cycle arrest and slowing melanoma cell growth [78,79]. In addition to the downregulation of cyclin D1, several studies have shown that resveratrol treatment also decreases the expression of cyclins A, E, and B1 in melanoma in vitro models, further corroborating the idea that resveratrol prevents melanoma cell proliferation by halting cell cycle progression [77,78,79]. Importantly, Nivelle et al. noted that while resveratrol effectively induced cell cycle arrest in in vitro studies of melanoma cells, it did not adversely affect healthy skin fibroblasts in the same model, highlighting resveratrol’s potential for safe therapeutic use [80]. Further emphasizing resveratrol’s impact on cell cycle regulation, one study found that resveratrol effectively suppressed melanoma cell growth by causing S-phase cell cycle arrest and reduced levels of microphthalmia-associated transcription factor (MITF), a key transcription factor essential for melanoma cell survival. Mokhamatam et al. pointed out that, although resveratrol promotes apoptosis through NF-κB inhibition, its main anti-melanoma effect is driven by the suppression of MITF transcription factor, supporting its promise as a selective therapeutic strategy [81].
Beyond apoptosis and cell cycle control, a review of the literature also identified two studies that reported resveratrol’s ability to reduce telomerase activity [82,83]. Both studies also demonstrated that trans-resveratrol binds selectively to cancer-related G-quadruplex DNA structures to inhibit melanoma cell proliferation [82,83]. G-quadruplexes are four-stranded DNA structures that can form in telomeric regions and suppress telomerase activity, thereby limiting the uncontrolled telomere elongation often seen in cancer cells [84]. Overall, the current literature demonstrates that resveratrol targets a wide range of cellular pathways to suppress melanoma cell growth and promote apoptosis in these cells—including suppression of the ERK signaling pathway, modulation of apoptotic proteins, induction of cell cycle arrest, downregulation of MITF, and inhibition of telomerase activity.
In addition to direct tumor cell cytotoxicity, resveratrol exerts immunomodulatory effects. A 2023 study by Morehead et al. reported that resveratrol increased MHC-I expression in vitro in B16-F10 and A375 melanoma cells, thereby promoting antigen presentation to CD8+ cytotoxic T cells [85]. Proteomic analysis in this investigation indicated that this effect may be driven by activation of the stimulator of interferon genes (STING) pathway in melanoma cells [85]. Resveratrol has also been shown to increase the activity of other immune lymphocytes. Lee Y et al. demonstrated that resveratrol significantly enhanced NK cell cytotoxicity and cytokine production, particularly interferon-gamma [IFN-γ], which contributed to suppressed tumor growth in mouse models of melanoma [86]. The same study reported that resveratrol also acted synergistically with interleukin-2(IL-2) to increase these antitumor effects, indicating resveratrol’s promising use for boosting NK cell activity in fighting tumor growth and metastasis in melanoma [86]. In a separate investigation, researchers also found that resveratrol reduced the growth of melanoma cells in vitro and significantly slowed lung metastasis in mice injected with melanoma cells by increasing immune-stimulating factors like CXCL10 and IFN-γ, reducing tumor angiogenesis, and limiting infiltration of immunosuppressive regulatory T cells [Tregs] in the lung [87]. Moreover, another study found that resveratrol reduced the expression of immune-modulating molecules [including IL1α, IL1β, IL6, IL8, TGFβ, MMP2, MMP3, uPA, uPAR, IL6R, IGF-1R, TGFβ-R2, and CXCR4], along with the transcription factor NF-κB in senescent fibroblasts [88]. This shift in the tumor microenvironment collectively inhibited melanoma cell proliferation and invasion, suggesting resveratrol’s broader role in promoting immune activation and contributing to melanoma prevention [88]. Together, these studies show that resveratrol boosts immune responses and alters the tumor environment to effectively inhibit melanoma progression and metastasis [85,86,87,88].
Several studies have also demonstrated that resveratrol influences epigenetic and transcriptional regulation in melanoma, contributing to suppressed tumor growth and progression. One key pathway involves the reversal of epigenetic silencing [89]. In melanoma cells, it has been found that the tumor suppressor gene RUNX3 is often downregulated due to promoter hypermethylation [89]. Resveratrol was observed to significantly reduce this hypermethylation of the RUNX3 promoter, resulting in an increase in RUNX3 protein expression, thereby restoring RUNX3 protein expression both in vitro and in xenograft models, highlighting its role in reactivating tumor suppressor genes [89]. Additionally, other research has shown that resveratrol targets epigenetic regulators such as HDAC1 and DNMT3a, recruiting them to the promoter of focal adhesion kinase [FAK], a protein involved in cell motility and metastasis [90]. By recruiting the epigenetic regulators to the FAK promoter, resveratrol increased promoter methylation and reduced FAK protein expression, thereby suppressing melanoma cell motility [89]. This suggests a mechanism through which resveratrol may prevent metastasis [90]. Another study also found resveratrol modulated gene expression through microRNA (miRNA) modulation. Specifically, Wu F et al. demonstrated in both in vitro melanoma cells and an in vivo mouse melanoma xenograft model that resveratrol downregulated the oncogenic miR-221 by inhibiting NF-κB signaling [91]. Inhibiting NF-κB signaling, in turn, reactivated expression of TFG, a tumor suppressor gene, contributing to resveratrol’s antitumor effects [91].
In addition to its therapeutic effects, resveratrol has shown promise in melanoma prevention. In the reviewed literature, one study developed a resveratrol-based topical gel to deliver resveratrol into the skin for melanoma prevention [92]. The gel demonstrated strong antioxidant properties and cytotoxic effects on melanoma cells [92]. When applied in a mouse model, the gel significantly inhibited tumor growth, indicating its potential utility as a preventive agent against melanoma development [92]. Another study focused on synthesizing novel derivatives inspired by resveratrol by triplicating the core resveratrol structure [93]. These compounds exhibited antioxidant activity, UV protection, and thermal stability, suggesting their possible application as natural, resveratrol-based sun-protective agents to reduce melanoma risk [93].
Collectively, the reviewed literature underscored the broad pharmacological potential of resveratrol in the prevention and treatment of melanoma. Through its ability to modulate key cellular pathways—including apoptosis, cell cycle progression, immune activation, and epigenetic regulation—resveratrol acts through multiple pathways to effectively suppress melanoma growth and progression. Due to its selective activity against melanoma cells and promising outcomes from topical formulations and novel resveratrol derivatives, resveratrol represents a compelling candidate for continued therapeutic development and comprehensive melanoma prevention strategies.
Given resveratrol’s well established anti-melanoma properties, recent studies have examined the synergistic effects of resveratrol when combined with established therapies and found promising results. Emerging literature has also reported the development of novel formulations, such as advanced delivery systems and structural derivatives, that significantly improve the bioavailability and pharmacological activity of resveratrol, thereby augmenting its pharmaceutical potential. In the context of melanoma, resveratrol has shown strong synergistic activity when combined with conventional chemotherapeutic agents, such as 5-fluorouracil (5-FU) [94,95]. Lee et al. utilized a murine model to demonstrate that the co-administration of 5-FU and resveratrol together synergistically decreased melanoma cell proliferation and angiogenesis [96]. Mechanistically, these findings were linked to the downregulation of tumor-promoting molecules, including cyclooxygenase-2 (COX-2), vascular endothelial growth factor [VEGF], and vasodilator-stimulated phosphoprotein (VASP), alongside an upregulation of AMP-activated protein kinase (AMPK) [96]. Thus, while 5-FU may be an effective treatment against melanoma, the literature shows that the combination was more effective than either treatment alone.
Thymidine Kinase/Ganciclovir (TK/GCV) is a suicide gene therapy used in cancer treatment [97]. Chen Y et al. studied the synergistic potential of combining resveratrol with TK/GCV therapy both in vitro and in vivo against B16 melanoma cells [98]. The results indicated that resveratrol improved the efficacy of TK/GCV treatment by upregulating connexin proteins, thus improving gap junction intracellular communication, and, when used in combination, the net effect was increased apoptosis in vitro and a reduction in tumor weight and volume in vivo [98].
Additionally, although several effective chemotherapy treatments are available, melanoma often acquires resistance to these drugs, posing a challenge for long-term efficacy of treatment. However, evidence from multiple studies suggests that resveratrol can help overcome resistance mechanisms in drug-resistant melanoma [94,95,99,100,101]. A 2016 in vitro study by Luo et al. examined the B-RAF (BRAF) inhibitor vemurafenib against melanoma cells resistant to the drug [94]. The researchers utilized cells harboring the BRAF V600E mutation—a common genetic alteration found in nearly 45% of melanomas [102]. Luo et al. concluded that, when added to the treatment regimen, resveratrol inhibited cancer cell proliferation and helped re-sensitize the resistant melanoma cells to vemurafenib treatment [94]. Corre et al. conducted a similar study in 2018 to explore the underlying mechanism by which BRAF V600E/K mutant melanoma cells develop resistance to BRAF inhibitors [95]. Researchers utilized patient-derived xenografts transplanted into in vivo mouse models to discover that the Aryl hydrocarbon Receptor (AhR), a transcription factor, becomes constitutively activated in these resistant melanoma cells, thereby driving treatment resistance [95]. It was further concluded that resveratrol blocks AhR activity, therefore restoring the efficacy of BRAF inhibitors. The combination of resveratrol and BRAF inhibitors was shown to delay tumor progression, highlighting the use of resveratrol as a promising strategy for overcoming drug resistance in melanoma treatment [95]. Resveratrol has also demonstrated synergistic effects when combined with all-trans retinoic acid (ATRA). ATRA is clinically established in the treatment of acute promyelocytic leukemia, but it has also been proposed as a differentiation therapy for targeting stem-like melanoma cells [99]. Stem-like melanoma cells are a subpopulation of tumor cells with stem cell–like properties, including capacity for self-renewal, differentiation, and enhanced resistance [100]. Stem-like melanoma cells are known to develop resistance to chemotherapeutic agents, such as docetaxel [99]. Notably, Kanai et al. reported that resveratrol can increase the effect of ATRA by promoting cellular differentiation, which in turn increases the susceptibility of stem-like melanoma cells to subsequent chemotherapeutic treatment with agents such as docetaxel [99]. Simply put, resveratrol enhances the effect of ATRA by promoting differentiation of resistant stem-like melanoma cells, making the cells more vulnerable to chemotherapy.
In summary, multiple studies have demonstrated that resveratrol not only boosts the effectiveness of conventional melanoma treatments but also helps to overcome common drug resistance challenges. Incorporating resveratrol into combination therapies could therefore offer a powerful approach to improving patient outcomes in melanoma care.
Shifting focus, given that resveratrol’s clinical application is limited by its poor bioavailability and rapid metabolism, researchers have explored strategies to enhance its effectiveness, including formulation with other compounds and incorporation into nanoparticle delivery systems. Extensive research has investigated the therapeutic potential of polyphenols against melanoma, including curcumin, as previously described in this study. However, a recent study developed a solid lipid nanoparticle delivery system designed for the topical co-administration of curcumin and resveratrol [103]. This formulation remained stable for two weeks, inhibited melanoma cell proliferation in vitro, and reduced cell migration, indicating potential to prevent metastasis [103]. Additionally, the formulation demonstrated localized retention, supporting its use as a targeted topical treatment. This research is valuable, as it demonstrates that resveratrol can be formulated into a stable, bioavailable topical treatment with potential efficacy against melanoma [103]. Furthermore, Sim et al. investigated the effects of the flavone compound 7,8-dihydroxyflavone (7,8-DHF) both alone and in combination with resveratrol [104]. They demonstrated that 7,8-DHF inhibited the growth of B16F10 melanoma cells without inducing cytotoxicity by downregulating microphthalmia-associated transcription factor (MITF) and its downstream targets [101]. However, the combination of 7,8-DHF with resveratrol produced enhanced inhibitory effects, highlighting the synergistic potential of these compounds in melanoma treatment [104].
Additionally, to address the limitations associated with resveratrol’s application in melanoma treatment, such as poor solubility, chemical instability, and limited bioavailability, numerous studies have developed nanotechnology-based delivery systems aimed at enhancing its therapeutic efficacy. Across these nine studies, researchers developed a range of resveratrol-based nanoformulations—including polymeric nanoparticles, nanocapsules, transfersomes, cubosomes, mesoporous silica, gold nanoflowers, and organogels—to improve resveratrol’s solubility, stability, and bioavailability [105,106,107,108,109,110,111,112,113]. These delivery systems enabled targeted or controlled drug release, enhanced skin penetration, or pH-responsive behavior, improving resveratrol’s anticancer activity against melanoma in both in vitro and in vivo models. Many studies showed enhanced tumor suppression, apoptosis induction, and reduced metastasis or toxicity compared to free resveratrol [105,106,107,108,109,110,111,112,113]. Overall, nanotechnology-driven delivery strategies present a promising direction for optimizing resveratrol’s therapeutic potential in melanoma treatment. Finally, Moon K et al. developed two glycosylated analogues of resveratrol that exhibited significantly enhanced solubility and stability compared to resveratrol [114]. These derivatives demonstrated improved cellular uptake, with evidence that they are metabolized back into resveratrol intracellularly [106]. This study demonstrates that resveratrol can be modified to have greater bioavailability and biological effectiveness as a drug [114]. The biological effects of resveratrol are shown in Figure 5.
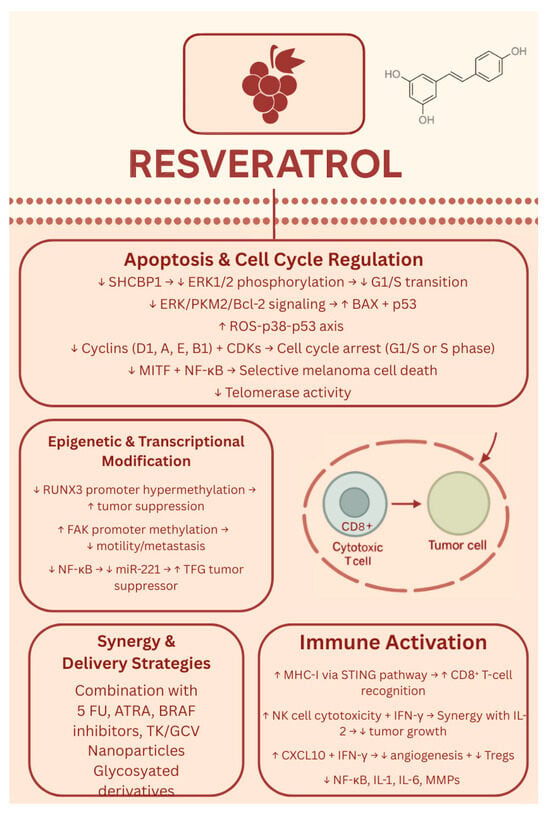
Figure 5.
Biological effects of resveratrol. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
As of August 2025, there have been no clinical trials evaluating the safety or efficacy of resveratrol as a treatment for melanoma. Most of the trials investigated resveratrol’s impact on various types of cancer [colon/colorectal, breast and ovarian, liver, multiple myeloma, lymphoma, and general cancer signaling mechanisms]. Several studies also explored utilizing resveratrol [alone or in combination] to improve hormonal balance, insulin sensitivity, and stress in women with PCOS. Two studies analyzed the safety and bioavailability of resveratrol. One study investigated the incorporation of resveratrol into a novel sunscreen formulation for ultraviolet [UV] protection; however, no results could be identified at the time of review. Overall, among the 18 registered clinical trials involving resveratrol, results and peer-reviewed publications were found for only nine; several trials were either withdrawn, terminated, or lacked publicly available outcome data, and no associated publications could be identified despite comprehensive database searches.
Of note from the clinical trial data reviewed, one trial evaluating a micronized formulation of resveratrol, administered alone or in combination with bortezomib in patients with multiple myeloma, was terminated prematurely due to safety concerns [115]. The published report documented five serious adverse events involving renal toxicity that occurred during resveratrol monotherapy. Although this finding is not directly related to melanoma, it warrants consideration that high supplemental doses of resveratrol may carry a risk of unexpected adverse effects [115].
However, interestingly, other human clinical trials investigating resveratrol supplementation have reported it to be safe [116,117]. One clinical trial investigated the effects of oral resveratrol [0.5 or 1.0 g daily] in 20 colorectal cancer patients prior to surgery, measuring its concentration in colorectal tissues and impact on tumor cell proliferation [116]. Resveratrol and its metabolites were detectable in both normal and cancerous tissues, and a 5% reduction in tumor cell proliferation was observed [116]. The study concluded that resveratrol reaches biologically active levels in the colon and no resveratrol-related adverse events were reported [116]. Furthermore, another clinical trial evaluated the safety and effects of SRT501, a micronized form of resveratrol, in colorectal cancer patients with hepatic metastases [117]. The micronized resveratrol was well tolerated, achieved significantly higher plasma levels than standard resveratrol, was detectable in liver tissue, and increased apoptosis in cancer cells [117]. Overall, current evidence from clinical trials presents conflicting data regarding the safety and efficacy of resveratrol. Additional research is warranted, though future investigations should be approached with caution given the variability in reported outcomes.
1.6. Other Phytochemicals
In addition to the well-studied anti-cancer agents discussed in this review, several other phytochemical components have been shown to be effective against various malignancies, but limited evidence is available on their role in the prevention and treatment of melanoma.
Fucoxanthin is an epoxycarotenol pigment found in brown seaweed. Although research on its anti-cancer effects against melanoma is still limited, emerging studies suggest that Fucoxanthin may help prevent melanoma and support skin health by promoting apoptosis, inhibiting malignant cell proliferation, and reducing metastasis. Specifically, Fucoxanthin has been shown to selectively induce apoptosis in melanoma cells by affecting mitochondrial function and activating the MAPK/COX-2/NF-κB signaling pathway [118]. Additionally, it can decrease melanoma cell viability and colony formation by inhibiting the JAK2/STAT3 pathway and downregulating BCL-xL, further triggering apoptotic cell death [119]. The biological properties of fucoxanthin identified in melanoma models are summarized in Figure 6.
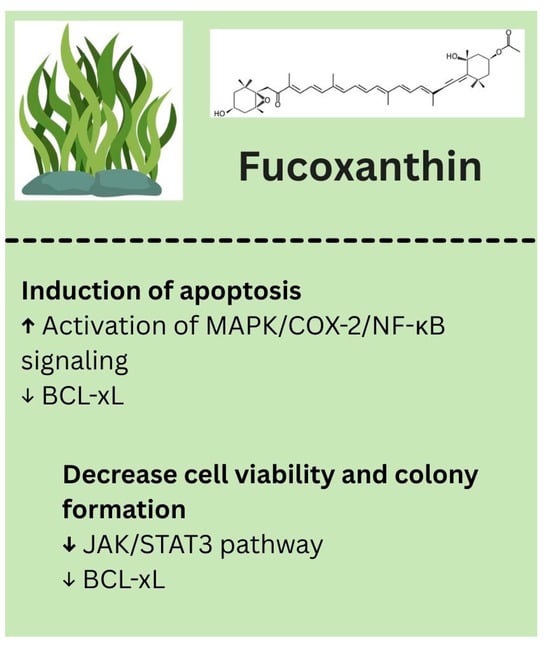
Figure 6.
Biological effects of fucoxanthin. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
Lycopene is another bioactive compound with potential anti-cancer properties, but research specifically examining its effects against melanoma has been limited over the past decade. In an in vivo study, lycopene was shown to inhibit cancer cell growth by activating Nrf2 through p62-mediated autophagic Keap1 degradation, though this evidence was demonstrated in chemically induced skin cancer, rather than melanoma [120]. Additionally, dietary lycopene has been reported to protect against UVB-induced skin damage and carcinogenesis in mice by reducing DNA damage, cell proliferation, and tumor growth; these effects, however, were also not studied in a melanoma-specific context [121]. Literature suggests potential chemopreventive effects in skin cancers, but further studies are needed to evaluate lycopene’s efficacy against melanoma. The biological properties of lycopene identified in melanoma models are summarized in Figure 7.
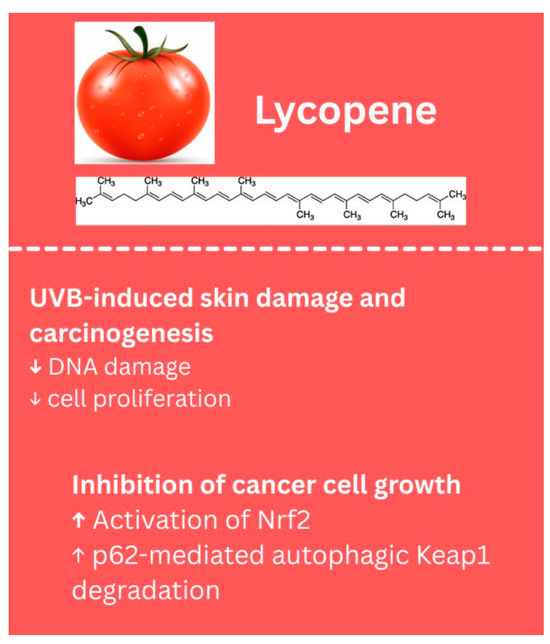
Figure 7.
Biological effects of lycopene. ↑—upregulated/increased expression. ↓—downregulated/decreased expression.
Altogether, although these lesser-studied phytochemicals show anti-cancer potential, current evidence is incredibly limited and largely preliminary. Future research is needed to investigate their effectiveness against melanoma, their bioavailability in humans against melanoma tumor cells, and their potential synergistic effects when used in combination with other well-studied compounds.
2. Conclusions
A wide variety of plant foods consumed across the world are known to have high levels of phytochemicals identified to modulate various biological functions and reduce the burden of developing various chronic diseases. The phytochemicals discussed in the current review have demonstrated health benefits in various preclinical models and clinical trials, including anti-inflammatory, antioxidant, chemopreventive, immunomodulatory, and antitumor properties, among others. In addition, it is their ability to serve as adjuvants and improve the outcomes of chemo- and radiation therapy that makes them an exciting option complementing the traditional therapies. For example, curcumin exhibited synergistic anti-cancer effects in malignant melanoma cells in spheroid and 2D cultures when used in combination with binimetinib, a MEK1/2 inhibitor, through apoptosis, necroptosis, and ROS production. Similarly, resveratrol, when used in combination with 5-FU, synergistically decreased melanoma cell proliferation and angiogenesis in a mouse model. In a different study, treatment of cells harboring the BRAFV600E mutation with resveratrol not only inhibited cellular growth but re-sensitized the resistant melanoma cells to vemurafenib. While these examples serve as a testament to the vast potential of these phytochemicals, there is much to be discovered regarding their therapeutic potential and clinical utility. One major drawback associated with phytochemicals is their lack of bioavailability, which serves as a major deterrent in establishing their role in promoting health benefits. Research strategies have focused on increasing their bioavailability by developing formulations that could potentially increase their availability when compared to the free form. For example, nanomicellar, chitosan-coated polycaprolactone nanoparticles and cationic liposomal formulations have been developed with different phytochemicals. These delivery systems enabled targeted or controlled phytochemical release, enhanced skin penetration, or pH-responsive behavior, improving their anticancer activity against melanoma in both in vitro and in vivo models. Many studies also showed enhanced tumor suppression, apoptosis induction, and reduced metastasis or toxicity compared to the free form. While these preclinical studies provide compelling evidence of the phytochemicals against the development of melanoma, future research should focus on human studies exploring their benefits not only in combating the malignancies but also in improving the quality of patients’ lives. Based on the results compiled, it is reasonable to conclude that the administration of dietary phytochemicals may offer protection against the development of malignant melanoma. Despite these promising cellular and preclinical animal models examined in this article, additional research is essential, focusing on the bioavailability, the development of novel derivatives, and determination of precise concentrations of these bioactive compounds to substantiate their health benefits.
Author Contributions
Conceptualization, A.B.; writing—original draft preparation, A.B., I.K., M.S., K.G., and V.C.; images, V.C., I.K., A.B., M.S., and K.G.; review and editing, V.C., I.K., A.B., M.S., and K.G.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge FAU SURF funds awarded to A.B. and V.C., and the Department of Biomedical Science, College of Medicine, FAU start-up funds awarded to A.B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed in this study. Data sharing is not applicable to this article and the original contributions discussed are included in the references.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-FU | 5-fluorouracil |
| 7,8-DHF | 7,8 dihydroxy flavone |
| AhR | aryl hydrocarbon receptor |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARE | antioxidant response element |
| ATRA | all-trans retinoic acid |
| Axin2 | axis inhibition protein 2 |
| BAX | Bcl-2–associated X protein [pro-apoptotic protein] |
| Bcl-2 B-cell lymphoma | 2 [anti-apoptotic protein] |
| B-RAF/BRAF V600E/BRAF V600E/K | B-Raf proto-oncogene mutations |
| CD4 | cluster of differentiation 4 |
| CD8 | cluster of differentiation 8 |
| CD8+ | cluster of differentiation 8 [cytotoxic T cell marker] |
| CDK6 | cyclin-dependent kinase 6 |
| CK1 | cysteine kinase 1 |
| COX-2 | cyclooxygenase-2 |
| CSC | cancer stem cells |
| CUR | curcumin |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCR4 | chemokine receptor type 4 |
| DATS | diallyl trisulfide |
| DC | destruction complex |
| DNA | deoxyribonucleic acid |
| DNMT3a | DNA methyltransferase 3 alpha |
| DR4 | death receptor 4 |
| DR5 | death receptor 5 |
| DVL2 | disheveled segment polarity protein 2 |
| EGCG | epigallocatechin gallate |
| EMT | epithelial–mesenchymal transition |
| ER | estrogen receptor |
| ERK/ERK1/2 | extracellular signal-regulated kinase [1 and 2] |
| FAK | focal adhesion kinase |
| GPCR | G-protein coupled receptor |
| GSK3β | glycogen synthase kinase 3β |
| HDAC | histone deacetylase |
| IFN-γ | interferon gamma |
| IGF-1R | insulin-like growth factor 1 receptor |
| IL1α, IL1β, IL6, IL8 | interleukin 1 alpha, 1 beta, 6, 8 |
| IL-2 | interleukin-2 |
| IL6R | interleukin-6 receptor |
| JAK | Janus kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MAPK/ERK | mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MDSC | myeloid-derived suppressor cell |
| MHC-I | major histocompatibility complex class I |
| miR-221 | microRNA-221 |
| MITF | microphthalmia-associated transcription factor |
| MMP2, MMP3 | matrix metalloproteinase 2 and 3 |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| p21 | cyclin-dependent kinase inhibitor p21 |
| p38 | p38 mitogen-activated protein kinase |
| p53 | tumor suppressor protein p53 |
| PCOS | polycystic ovary syndrome |
| PD-L1 | programmed death-ligand 1 |
| PD-L2 | programmed death-ligand 2 |
| PI3K | phosphoinositide 3-kinase |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| PKM2 | pyruvate kinase M2 |
| rhTRAIL | recombinant human tumor necrosis factor-related apoptosis-inducing ligand |
| p62 | sequestosome-1 (autophagy adaptor protein) |
| ROS | reactive oxygen species |
| RUNX3 | runt-related transcription factor 3 |
| S phase | synthesis phase of DNA replication |
| SHCBP1 | SHC binding and spindle-associated 1 |
| SRT501 | micronized form of resveratrol [drug formulation] |
| STAT | signal transducer and activator of transcription |
| STING | stimulator of interferon genes |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TFG | tropomyosin receptor kinase fused gene [tumor suppressor gene] |
| TGFβ/TGFβ-R2 | transforming growth factor beta/receptor 2 |
| TK/GCV | thymidine kinase/ganciclovir [suicide gene therapy] |
| Treg | regulatory T cell |
| uPA/uPAR | urokinase-type plasminogen activator/receptor |
| UV | ultraviolet |
| VASP | vasodilator-stimulated phosphoprotein |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene 2003, 22, 3099–3112. [Google Scholar] [CrossRef]
- Garibyan, L.; Fisher, D.E. How sunlight causes melanoma. Curr. Oncol. Rep. 2010, 12, 319–326. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Rossi, M.; Pellegrini, C.; Cardelli, L.; Ciciarelli, V.; Di Nardo, L.; Fargnoli, M.C. Familial Melanoma: Diagnostic and Management Implications. Dermatol. Pract. Concept. 2019, 9, 10–16. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet Lond. Engl. 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin. Cancer Res. 2006, 12, 2366s–2370s. [Google Scholar] [CrossRef] [PubMed]
- Strickland, L.R.; Pal, H.C.; Elmets, C.A.; Afaq, F. Targeting drivers of melanoma with synthetic small molecules and phytochemicals. Cancer Lett. 2015, 359, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.S.; Kim, J.S.; Lee, H.A.; Koo, D.B.; Choo, Y.K. uercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Seo, Y.; Yang, J.; Lee, H.; Kong, C.S. Quercetin 3-O-Galactoside Isolated from Limonium tetragonum Inhibits Melanogenesis by Regulating PKA/MITF Signaling and ERK Activation. Int. J. Mol. Sci. 2023, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bai, J.; Li, F.; Liu, J.; Wang, Y.; Li, N.; Wang, Y.; Xu, J.; Liu, W.; Xu, L.; et al. Investigation of the mechanism of the anti-cancer effects of Astragalus propinquus Schischkin and Pinellia pedatisecta Schott [A&P] on melanoma via network pharmacology and experimental verification. Front. Pharmacol. 2022, 13, 895738. [Google Scholar] [CrossRef]
- Khuanekkaphan, M.; Netsomboon, K.; Fristiohady, A.; Asasutjarit, R. Development of Quercetin Solid Dispersion-Loaded Dissolving Microneedles and In Vitro Investigation of Their Anti-Melanoma Activities. Pharmaceutics 2024, 16, 1276. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Sun, Y.; Sun, L.; Yin, Q.; Deng, S.; Niu, L.; Lou, F.; Wang, Z.; Xu, Z.; et al. Melanoma suppression by quercein is correlated with RIG-I and type I interferon signaling. Biomed. Pharmacother. 2020, 125, 109984. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Lin, B.; Li, L.; Deng, Q.; Wang, C.; Zhang, J.; Chen, Y.; Zhao, J.; Li, X.; et al. Quercetin Limits Tumor Immune Escape through PDK1/CD47 Axis in Melanoma. Am. J. Chin. Med. 2024, 52, 541–563. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Esfahani, H.S.; Habibi, M.; Hosseinzadeh, G. Preparation of cerium-curcumin and cerium-quercetin complexes and their LEDs irradiation assisted anticancer effects on MDA-MB-231 and A375 cancer cell lines. Photodiagnosis Photodyn. Ther. 2021, 34, 102326. [Google Scholar] [CrossRef]
- Turner, K.A.; Manouchehri, J.M.; Kalafatis, M. Sensitization of recombinant human tumor necrosis factor-related apoptosis-inducing ligand-resistant malignant melanomas by quercetin. Melanoma Res. 2018, 28, 277–285. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. CATIQ Investigators11. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, e125851. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misiewicz, I.; Skupinska, K.; Kasprzycka-Guttman, T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol. Rep. 2003, 10, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, T.P.; Thejass, P.; Kuttan, G. Induction of apoptosis by sulforaphane in highly metastatic B16F-10 melanoma cells. Drug Chem. Toxicol. 2011, 34, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Thejass, P.; Kuttan, G. Modulation of cell-mediated immune response in B16F-10 melanoma-induced metastatic tumor-bearing C57BL/6 mice by sulforaphane. Immunopharmacol. Immunotoxicol. 2007, 29, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Mitsiogianni, M.; Kyriakou, S.; Anestopoulos, I.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M.I. An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells. Antioxidants 2021, 10, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinkova-Kostova, A.T. Phytochemicals as protectors against ultraviolet radiation: Versatility of effects and mechanisms. Planta Medica 2008, 74, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Htut, N.W.; Onkoksoong, T.; Saelim, M.; Kueanjinda, P.; Sampattavanich, S.; Panich, U. Live-cell imaging Unveils stimulus-specific dynamics of Nrf2 activation in UV-exposed melanoma cells: Implications for antioxidant compound screening. Free Radic. Biol. Med. 2024, 211, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Do, D.P.; Pai, S.B.; Rizvi, S.A.; D’Souza, M.J. Development of sulforaphane-encapsulated microspheres for cancer epigenetic therapy. Int. J. Pharm. 2010, 386, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gursoy-Yuzugullu, O.; Parasuram, R.; Price, B.D. The tale of a tail: Histone H4 acetylation and the repair of DNA breaks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pradhan, S.J.; Mishra, R.; Sharma, P.; Kundu, G.C. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Exp. Ther. Med. 2010, 1, 915–920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiang, T.C.; Koss, B.; Su, L.J.; Washam, C.L.; Byrum, S.D.; Storey, A.; Tackett, A.J. Effect of Sulforaphane and 5-Aza-2’-Deoxycytidine on Melanoma Cell Growth. Medicines 2019, 6, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Q.H.; Jian, L.Y.; Hu, Y.; Wang, S. A comprehensive and systematic review of curcumin as a promising candidate for the inhibition of melanoma growth: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2024, 135, 156073. [Google Scholar] [CrossRef] [PubMed]
- Fança-Berthon, P.; Tenon, M.; Bouter-Banon, S.L.; Manfré, A.; Maudet, C.; Dion, A.; Chevallier, H.; Laval, J.; van Breemen, R.B. Pharmacokinetics of a single dose of turmeric curcuminoids depends on formulation: Results of a human crossover study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Liao, W.; Xiang, W.; Wang, F.F.; Wang, R.; Ding, Y. Curcumin inhibited growth of human melanoma A375 cells via inciting oxidative stress. Biomed. Pharmacother. 2017, 95, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via miR-222-3p/SOX10/Notch Axis. Dis. Markers 2022, 2022, 3129781. [Google Scholar] [CrossRef]
- Teng, L.; Li, W.; Shi, Y.; Qi, F. The Efficacy of Curcumin Application to Melanoma in Mice: A Systematic Review and Meta-analysis. Ann. Plast. Surg. 2024, 93, S75–S81. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Yang, J.S.; Tsai, F.J.; Chiu, H.Y.; Juan, Y.N.; Lo, Y.H.; Chiang, J.H. Curcumin suppresses cell proliferation and triggers apoptosis in vemurafenib-resistant melanoma cells by downregulating the EGFR signaling pathway. Environ. Toxicol. 2022, 37, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Heo, J.Y.; Kim, D.S.; Choi, Y.S.; Kim, S.; Nam, H.S.; Lee, S.H.; Cho, M.K. Curcumin Enhances the Anticancer Effects of Binimetinib on Melanoma Cells by Inducing Mitochondrial Dysfunction and Cell Apoptosis with Necroptosis. Ann. Dermatol 2023, 35, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Mohd, H.; Michniak-Kohn, B. Synergistic Anti-Cancer Effects of Curcumin and Thymoquinone Against Melanoma. Antioxidants 2024, 13, 1573. [Google Scholar] [CrossRef] [PubMed]
- Fontes, S.S.; Nogueira, M.L.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Vannier-Santos, M.A.; Bezerra, D.P. Combination Therapy of Curcumin and Disulfiram Synergistically Inhibits the Growth of B16-F10 Melanoma Cells by Inducing Oxidative Stress. Biomolecules 2022, 12, 1600. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef]
- Loch-Neckel, G.; Santos-Bubniak, L.; Mazzarino, L.; Jacques, A.V.; Moccelin, B.; Santos-Silva, M.C.; Lemos-Senna, E. Orally administered chitosan-coated poly-caprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J. Pharm. Sci. 2015, 104, 3524–3534. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective skin cancer treatment by topical co-delivery of curcumin and STAT3 siRNA using cationic liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Mardani, R.; Hamblin, M.R.; Taghizadeh, M.; Banafshe, H.R.; Nejati, M.; Mokhtari, M.; Borran, S.; Davoodvandi, A.; Khan, H.; Jaafari, M.R.; et al. Nanomicellar-Curcumin Exerts its Therapeutic Effects via Affecting Angiogenesis, Apoptosis, and T Cells in a Mouse Model of Melanoma Lung Metastasis. Pathol. Res. Pract. 2020, 216, 153082. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 6–10. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [PubMed]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Chopra, S.; Kaur, D.; Wang, H.; Li, M.; Chen, P.; Zhang, W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets 2020, 21, 1099–1104. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, C.; Liu, M.; Wang, J.; Qiao, S.; Jiang, P.; Sun, C.; Jiang, S. Epigallocatechin-3-gallate at the nanoscale: A new strategy for cancer treatment. Pharm. Biol. 2024, 62, 676–690. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Jang, M. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 445154, Resveratrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/resveratrol (accessed on 30 July 2025).
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption But Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2010, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, Z.; Nie, S.; Zhang, T.; Lu, H. Effects of Resveratrol on Mouse B16 Melanoma Cell Proliferation through the SHCBP1-ERK1/2 Signaling Pathway. Molecules 2023, 28, 7614. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Li, L. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. OncoTargets Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.; Hwang, K.; Kang, J.; Choi, K. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Martin–Vega, A.; Cobb, M.H. ERK1/2-MAPK signaling: Metabolic, organellar, and cytoskeletal interactions. Curr. Opin. Cell Biol. 2025, 95, 102526. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; De Melo, J.; Tang, D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int. J. Cell Biol. 2013, 2013, 242513. [Google Scholar] [CrossRef]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory Effects of Oligostilbenoids from the Bark of Shorea roxburghii on Malignant Melanoma Cell Growth: Implications for Novel Topical Anticancer Candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef]
- Yang, H.Z.; Zhang, J.; Zeng, J.; Liu, S.; Zhou, F.; Zhang, F.; Giampieri, F.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Ansary, J.; et al. Resveratrol inhibits the proliferation of melanoma cells by modulating cell cycle. Int. J. Food Sci. Nutr. 2019, 71, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef]
- Mokhamatam, R.B.; Sahoo, B.K.; Manna, S.K. Suppression of microphthalmia-associated transcription factor, but not NF-kappa B sensitizes melanoma specific cell death. Apoptosis 2016, 21, 928–940. [Google Scholar] [CrossRef]
- Platella, C.; Raucci, U.; Rega, N.; D’Atri, S.; Levati, L.; Roviello, G.N.; Fuggetta, M.P.; Musumeci, D.; Montesarchio, D. Shedding light on the interaction of polydatin and resveratrol with G-quadruplex and duplex DNA: A biophysical, computational and biological approach. Int. J. Biol. Macromol. 2020, 151, 1163–1172. [Google Scholar] [CrossRef]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour activity of resveratrol on human melanoma cells: A possible mechanism related to its interaction with malignant cell telomerase. Biochim. Biophys. Acta [BBA] Gen. Subj. 2017, 1861, 2843–2851. [Google Scholar] [CrossRef]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef]
- Morehead, L.C.; Koss, B.; Fil, D.; Heflin, B.; Garg, S.; Wallis, K.F.; Tackett, A.J.; Miousse, I.R. Resveratrol induces major histocompatibility complex class I antigen presentation in a STING-dependent and independent manner in melanoma. Mol. Immunol. 2023, 163, 188–195. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, H.; Kim, J. In vivo Anti-Cancer Effects of Resveratrol Mediated by NK Cell Activation. J. Innate Immun. 2020, 13, 94–106. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Menicacci, B.; Laurenzana, A.; Chillà, A.; Margheri, F.; Peppicelli, S.; Tanganelli, E.; Fibbi, G.; Giovannelli, L.; Del Rosso, M.; Mocali, A. Chronic Resveratrol Treatment Inhibits MRC5 Fibroblast SASP-Related Protumoral Effects on Melanoma Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1187–1195. [Google Scholar] [CrossRef]
- Kang, S.; Wang, Z.; Li, B.; Gao, X.; He, W.; Cao, S.; Cai, Y.; Chen, H. Anti-tumor effects of resveratrol on malignant melanoma is associated with promoter demethylation of RUNX3 gene. Pharmazie 2019, 74, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Xu, C.; Wang, J.; Zhu, B.; Huang, Q.; Chen, D.; Sheng, J.; Zou, Y.; Lee, Y.M.; et al. Comparative profiling of analog targets: A case study on resveratrol for mouse melanoma metastasis suppression. Theranostics 2018, 8, 3504–3516. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cui, L. Resveratrol suppresses melanoma by inhibiting NF-κB/miR-221 and inducing TFG expression. Arch. Dermatol. Res. 2017, 309, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Katariya, M.; Patil, S.; Tatke, P.; Pillai, R. Skin delivery of resveratrol encapsulated lipidic formulation for melanoma chemoprevention. J. Microencapsul. 2019, 36, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.S.; Corrêa, M.A.; Ribeiro, C.A.; Dos Santos, J.L. Synthesis and evaluation of 1,3,5-triazine derivatives as sunscreens useful to prevent skin cancer. Bioorg. Med. Chem. Lett. 2019, 29, 126755. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Masayo Umebayashi Doi, K.; Morisaki, T.; Senji Shirasawa Tsunoda, T. Resveratrol Overcomes Cellular Resistance to Vemurafenib Through Dephosphorylation of AKT in BRAF-mutated Melanoma Cells. PubMed 2016, 36, 3585–3589. [Google Scholar]
- Corre, S.; Tardif, N.; Mouchet, N.; Leclair, H.M.; Boussemart, L.; Gautron, A.; Bachelot, L.; Perrot, A.; Soshilov, A.; Rogiers, A.; et al. Sustained activation of the Aryl hydrocarbon Receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat. Commun. 2018, 9, 4775. [Google Scholar] [CrossRef]
- Lee, S.H.; Koo, B.S.; Park, S.Y.; Kim, Y.M. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef]
- Kokoris, M.S.; Black, M.E. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. A Publ. Protein Soc. 2002, 11, 2267–2272. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, G.; Wu, Y.; Xiao, J.; Liu, J.; Qiu, P.; Liu, X.; Sun, L.; Du, B.; et al. Synergistic inhibitory effect of resveratrol and TK/GCV therapy on melanoma cells. J. Cancer Res. Clin. Oncol. 2020, 146, 1489–1499. [Google Scholar] [CrossRef]
- Kanai, M.; Shinagawa, A.; Ota, M.; Virgona, N.; Yano, T. Resveratrol Can Differentiate Human Melanoma Stem-like Cells from Spheroids Treated With All-trans Retinoic Acid. Anticancer. Res. 2024, 44, 5283–5292. [Google Scholar] [CrossRef]
- Al Hmada, Y.; Brodell, R.T.; Kharouf, N.; Flanagan, T.W.; Alamodi, A.A.; Hassan, S.Y.; Shalaby, H.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. Mechanisms of Melanoma Progression and Treatment Resistance: Role of Cancer Stem-like Cells. Cancers 2024, 16, 470. [Google Scholar] [CrossRef]
- Islam, S.U.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. PRPF overexpression induces drug resistance through actin cytoskeleton rearrangement and epithelial-mesenchymal transition. Oncotarget 2017, 8, 56659–56671. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The Role of BRAF V600 Mutation in Melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Sim, D.Y.; Sohng, J.K.; Jung, H.J. Anticancer activity of 7,8-dihydroxyflavone in melanoma cells via downregulation of α-MSH/cAMP/MITF pathway. Oncol. Rep. 2016, 36, 528–534. [Google Scholar] [CrossRef]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Dourado, D.; Batista, F.P.R.; Philadelpho, B.O.; de Souza, M.L.; de Cerqueira E Silva, M.B.; de Grandis, R.A.; Miranda, P.A.; Colauto, N.B.; Pereira, D.T.; Formiga, F.R.; et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 12112. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, H.; Zhang, W.; Rong, L.; Zhang, D. Resveratrol-Coated Gold Nanoflowers for CT Imaging and Apoptosis/Photothermal Synergistic Therapy of Malignant Melanoma. ACS Omega 2023, 8, 34629–34639. [Google Scholar] [CrossRef]
- Gao, F.; Li, L.; Liu, L.; Li, G.; Zhang, J.; Zhan, W.; You, W.; Lin, X.; Liu, Y.; Wang, J.; et al. Novel Silicon-Based Fluorescent Nanocomposite Drug Carriers for Natural Compound Delivery in Melanoma Treatment. J. Fluoresc. 2025, 35, 8755–8767. [Google Scholar] [CrossRef]
- Yee, Y.J.; Benson, H.A.E.; Dass, C.R.; Chen, Y. Evaluation of novel conjugated resveratrol polymeric nanoparticles in reduction of plasma degradation, hepatic metabolism and its augmentation of anticancer activity in vitro and in vivo. Int. J. Pharm. 2022, 615, 121499. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. Formulation and evaluation of resveratrol loaded cubosomal nanoformulation for topical delivery. Curr. Drug Deliv. 2020, 18, 607–619. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, F.; Khan, F.; Chaudhary, S.C.; Samim, M. Enhancement of the cancer inhibitory effect of the bioactive food component resveratrol by nanoparticle based delivery. Food Funct. 2020, 11, 3213–3226. [Google Scholar] [CrossRef]
- Wu, P.S.; Li, Y.S.; Kuo, Y.C.; Tsai, S.J.; Lin, C.C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef]
- Moon, K.; Lee, S.; Park, H.; Cha, J. Enzymatic Synthesis of Resveratrol α-Glucoside by Amylosucrase of Deinococcus geothermalis. J. Microbiol. Biotechnol. 2021, 31, 1692–1700. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 [resveratrol] with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2012, 160, 714–717. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol [SRT501] in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Yao, T.; Dong, X.; Liu, Y.; Nakamura, Y.; Qi, H. Mechanism of inhibition of melanoma by fucoxanthin simulated in vitro digestion products in cell models constructed using human malignant melanoma cells (A375) and keratinocytes (HaCaT). Food Chem. 2025, 462, 141003. [Google Scholar] [CrossRef]
- Kuo, M.; Dai, W.; Chang, J.; Chang, J.; Lee, T.; Chang, C. Fucoxanthin induces human melanoma cytotoxicity by thwarting the JAK2/STAT3/BCL-xL signaling axis. Environ. Toxicol. 2024, 39, 3356–3366. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.Y.; Wang, X.; Shen, P.; Jia, Q.; Yu, S.; Wang, Y.; Li, X.; Chen, W.; Wang, A.; et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging 2020, 12, 8167–8190. [Google Scholar] [CrossRef]
- Zhou, X.; Burke, K.E.; Wang, Y.; Wei, H. Dietary Lycopene Protects SKH-1 Mice Against Ultraviolet B-Induced Photocarcinogenesis. J. Drugs Dermatol 2019, 18, 1244–1254. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).