GLP-1 Receptor Signaling and Oral Dysfunction: A Narrative Review on the Mechanistic Basis of Semaglutide-Related Oral Adverse Effects
Simple Summary
Abstract
1. Introduction
2. Method
3. Results of the Literature Review
4. Discussion
4.1. GLP-1: Origin and Function
4.2. GLP-1 Receptor (GLP-1R) in Salivary Glands
4.3. GLP-1 Receptor Agonists (GLP-1RAs)
4.4. GLP-1 Signaling Pathway
- Parasympathetic M3 muscarinic receptors (Gq-coupled) activate phospholipase C → IP3-mediated Ca2+ release → fluid (aqueous) secretion.
- Sympathetic β-adrenergic receptors (Gs-coupled) activate adenylyl cyclase → increase cAMP → PKA-dependent pathways that regulate protein-rich exocytotic secretion (e.g., amylase, mucins).
- Vesicle priming and exocytosis of salivary proteins (via cAMP signalling).
- Cellular protection and survival of acinar and ductal cells (via EGF).
- CREB-dependent transcription, possibly altering the synthesis of secretory proteins or ion transporters that regulate saliva composition.
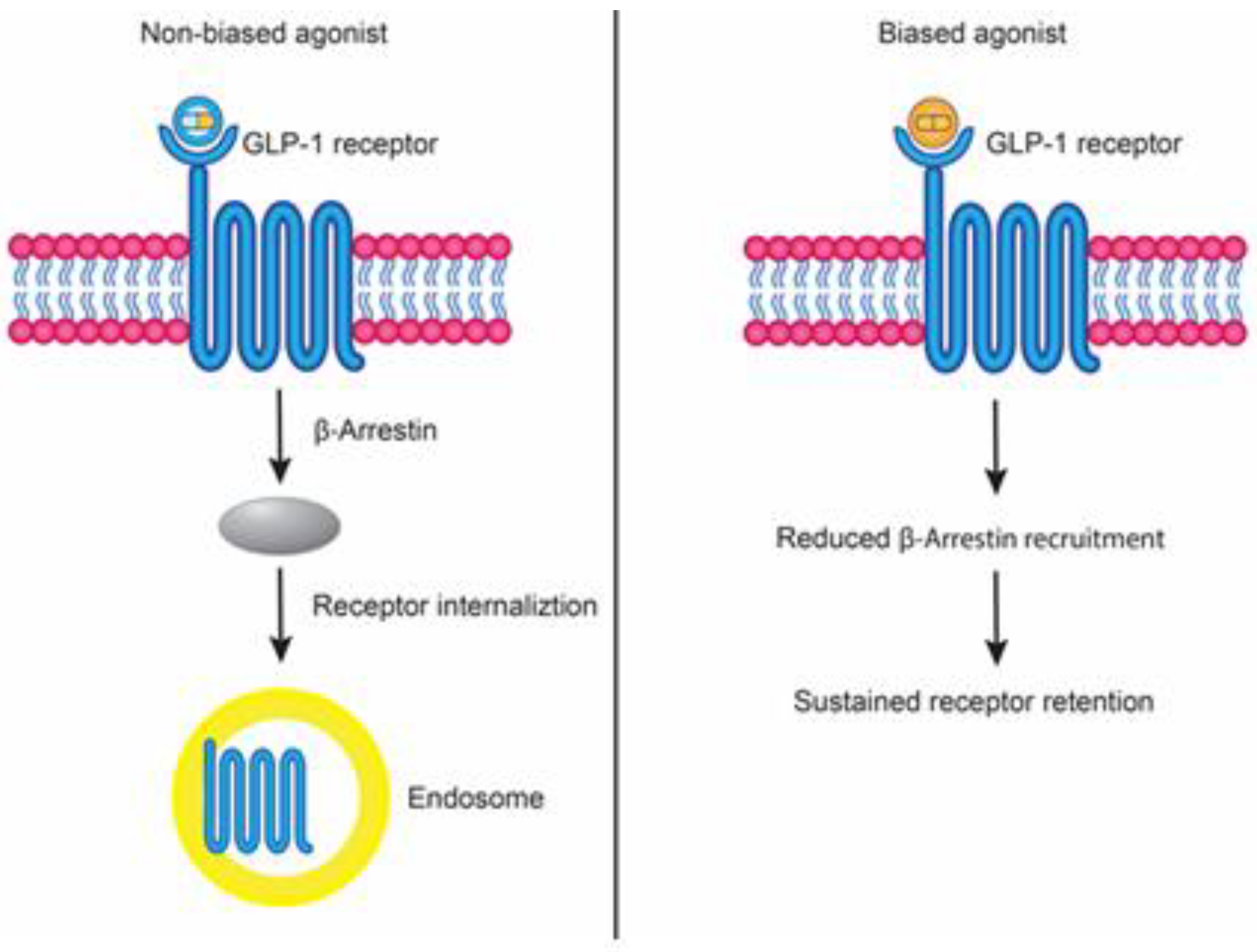
4.5. Biased Agonism of GLP-1R
4.6. Semaglutide as a “Not Clinically Confirmed as Biased” Agonist
4.7. β-Arrestins in Receptor Signaling and Adaptation
4.7.1. Desensitization and Receptor Trafficking
4.7.2. β-Arrestin-Dependent Signaling and Functional Duality
4.8. Albumin Binding as a Pharmacokinetic Determinant of Semaglutide Effects
4.9. Clinical Correlates: Oral Adverse Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLP-1 | glucagon-like peptide-1 |
| GLP-1R | glucagon-like peptide-1 receptor |
| GLP-1RAs | glucagon-like peptide-1 receptor (GLP-1R) agonists |
| GPCR | G-protein-coupled receptor |
| T2DM | type 2 diabetes mellitus |
| DPP-4 | enzyme dipeptidyl peptidase-4 |
| AEs | adverse effects |
| FDA | Food and Drug Administration’s |
| FAERS | FDA Adverse Effect Reporting System |
| PKA | protein kinase A |
| EPAC | exchange protein directly activated by cAMP |
| CREB | cAMP response element-binding protein |
| PI3K | phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| IRS | insulin receptor substrate |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| PIP3 | phosphatidylinositol-3,4,5-trisphosphate |
| GRK | GPCR kinase |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAPKs | mitogen-activated protein kinases |
| IP3 | Inositol 1,4,5-trisphosphate |
| HPA | Human protein atlas |
| GTEx | Genotype-tissue expression project |
| FANTOM5 | Functional Annotation of the Mammalian Genome 5 |
| EGF | Epidermal growth factor |
| RCTs | Randomized controlled trials |
| HbA1c | Glycated Hemoglobin A1c |
| Gly168Ser | Glycine at position 168 is replaced by Serine |
| ARRB1 | β-arrestin 1 |
| RNAi | Ribonucleic acid interference (RNA interference) |
| IRS-2 | Insulin Receptor Substrate 2 |
| KO | Knockout |
| PDE4D3 | Phosphodiesterase 4D, isoform 3 |
| PDE4D5 | Phosphodiesterase 4D, isoform 5 |
| β2AR | the β2-adrenergic receptor |
| PTH1R | Parathyroid Hormone 1 Receptor |
| PDE4 | Phosphodiesterase 4 |
| FC | Fragment crystallizable region (receptor) |
| IP3R | IP3 receptor |
| IBMX | 3-Isobutyl-1-methylxanthine |
| CAGE | Cap analysis of gene expression |
| nTPM | Normalized transcripts per million |
| GI | Gastrointestinal |
References
- Shu, Y.; He, X.; Wu, P.; Liu, Y.; Ding, Y.; Zhang, Q. Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system. Front. Public Health 2022, 10, 996179. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-week, open-label, randomized clinical trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Vaag, A.; Schmitz, O.; Sethi, B.K.; Lalic, N.; Antic, S.; Zdravkovic, M.; Ravn, G.M.; Simó, R. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): A randomised controlled trial. Diabetologia 2009, 52, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Hergarden, A.; Krishnan, S.; Morales, M.; Lam, D.; Tracy, T.; Tang, T.; Patton, A.; Lee, C.; Pant, A.; et al. Biased agonism of GLP-1R and GIPR enhances glucose lowering and weight loss, with dual GLP-1R/GIPR biased agonism yielding greater efficacy. Cell Rep. Med. 2025, 6, 102156. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of head-to-head clinical trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, Z.M.; Shao, C.S.; Zhang, Z.Y.; Li, M.W.; Wang, L.; Wang, H.; Zhao, G.H.; Wang, P. Rational design by structural biology of industrializable, long-acting antihyperglycemic GLP-1 receptor agonists. Pharmaceuticals 2022, 15, 740. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Khan, F.I.; Vazquez, S.G.; Mehdi, Z.; Somawardana, I.; Dongre, R.; Razmi, S.; Rashidi, K.; Shenoi, J.; Khan, N.; Dhanda, A.; et al. Otolaryngologic side effects of GLP-1 receptor agonists. Laryngoscope. 2025, 135, 2291–2298. [Google Scholar] [CrossRef]
- Mawardi, H.H.; Almazrooa, S.A.; Dakhil, S.A.; Aboalola, A.A.; Al-Ghalib, T.A.; Eshky, R.T.; Niyazi, A.A.; Mawardi, M.H. Semaglutide-associated hyposalivation: A report of case series. Medicine 2023, 102, e36730. [Google Scholar] [CrossRef]
- Zhang, T.; Perkins, M.H.; Chang, H.; Han, W.; de Araujo, I.E. An inter-organ neural circuit for appetite suppression. Cell 2022, 185, 2478–2494.e2428. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Kaihara, K.A.; Dickson, L.M.; Ellenbroek, J.H.; Orr, C.M.; Layden, B.T.; Wicksteed, B. PKA enhances the acute insulin response leading to the restoration of glucose control. Diabetes 2015, 64, 1688–1697. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Hameed, A.; Hafizur, R.M.; Hussain, N.; Raza, S.A.; Rehman, M.; Ashraf, S.; Ul-Haq, Z.; Khan, F.; Abbas, G.; Choudhary, M.I. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. Eur. J. Pharmacol. 2018, 820, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tengholm, A.; Gylfe, E. cAMP signalling in insulin and glucagon secretion. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 42–53. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51 (Suppl. 3), S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: A novel cardiometabolic therapeutic prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef]
- Miki, T.; Minami, K.; Shinozaki, H.; Matsumura, K.; Saraya, A.; Ikeda, H.; Yamada, Y.; Holst, J.J.; Seino, S. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes 2005, 54, 1056–1063. [Google Scholar] [CrossRef]
- Kaihara, K.A.; Dickson, L.M.; Jacobson, D.A.; Tamarina, N.; Roe, M.W.; Philipson, L.H.; Wicksteed, B. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes 2013, 62, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef]
- Glauser, D.A.; Schlegel, W. The emerging role of FOXO transcription factors in pancreatic β cells. J. Endocrinol. 2007, 193, 195–207. [Google Scholar] [CrossRef]
- Purwana, I.; Zheng, J.; Li, X.; Deurloo, M.; Son, D.O.; Zhang, Z.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.P.; et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef]
- Marzook, A.; Tomas, A.; Jones, B. The interplay of glucagon-like peptide-1 receptor trafficking and signalling in pancreatic beta cells. Front. Endocrinol. 2021, 12, 678055. [Google Scholar] [CrossRef] [PubMed]
- Lucey, M.; Ashik, T.; Marzook, A.; Wang, Y.; Goulding, J.; Oishi, A.; Broichhagen, J.; Hodson, D.J.; Minnion, J.; Elani, Y.; et al. Acylation of the incretin peptide exendin-4 directly impacts glucagon-like peptide-1 receptor signaling and trafficking. Mol. Pharmacol. 2021, 100, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Deganutti, G.; Liang, Y.L.; Zhang, X.; Khoshouei, M.; Clydesdale, L.; Belousoff, M.J.; Venugopal, H.; Truong, T.T.; Glukhova, A.; Keller, A.N.; et al. Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation. Nat. Commun. 2022, 13, 92. [Google Scholar] [CrossRef]
- Ma, H.; Huang, W.; Wang, X.; Zhao, L.; Jiang, Y.; Liu, F.; Guo, W.; Sun, X.; Zhong, W.; Yuan, D.; et al. Structural insights into the activation of GLP-1R by a small molecule agonist. Cell Res. 2020, 30, 1140–1142. [Google Scholar] [CrossRef]
- Cong, Z.; Zhao, F.; Li, Y.; Luo, G.; Mai, Y.; Chen, X.; Chen, Y.; Lin, S.; Cai, X.; Zhou, Q.; et al. Molecular features of the ligand-free GLP-1R, GCGR and GIPR in complex with G(s) proteins. Cell Discov. 2024, 10, 18. [Google Scholar] [CrossRef]
- Liu, T.M.; Ling, Y.; Woyach, J.A.; Beckwith, K.; Yeh, Y.Y.; Hertlein, E.; Zhang, X.; Lehman, A.; Awan, F.; Jones, J.A.; et al. OSU-T315: A novel targeted therapeutic that antagonizes AKT membrane localization and activation of chronic lymphocytic leukemia cells. Blood 2015, 125, 284–295. [Google Scholar] [CrossRef]
- Naylor, J.; Suckow, A.T.; Seth, A.; Baker, D.J.; Sermadiras, I.; Ravn, P.; Howes, R.; Li, J.; Snaith, M.R.; Coghlan, M.P.; et al. Use of CRISPR/Cas9-engineered INS-1 pancreatic β cells to define the pharmacology of dual GIPR/GLP-1R agonists. Biochem. J. 2016, 473, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Pamir, N.; Lynn, F.C.; Buchan, A.M.; Ehses, J.; Hinke, S.A.; Pospisilik, J.A.; Miyawaki, K.; Yamada, Y.; Seino, Y.; McIntosh, C.H.; et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E931–E939. [Google Scholar] [CrossRef] [PubMed]
- Quoyer, J.; Longuet, C.; Broca, C.; Linck, N.; Costes, S.; Varin, E.; Bockaert, J.; Bertrand, G.; Dalle, S. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem. 2010, 285, 1989–2002. [Google Scholar] [CrossRef]
- Buteau, J.; Foisy, S.; Joly, E.; Prentki, M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003, 52, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Fujita-Yoshigaki, J. Divergence and Convergence in Regulated Exocytosis: The characteristics of cAMP-dependent enzyme secretion of parotid salivary acinar cells. Cell. Signal. 1998, 10, 371–375. [Google Scholar] [CrossRef]
- Limesand, K.H.; Barzen, K.A.; Quissell, D.O.; Anderson, S.M. Synergistic suppression of apoptosis in salivary acinar cells by IGF1 and EGF. Cell Death Differ. 2003, 10, 345–355. [Google Scholar] [CrossRef]
- Fox, R.M.; Hanlon, C.D.; Andrew, D.J. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J. Cell Biol. 2010, 191, 479–492. [Google Scholar] [CrossRef]
- Wong, S.; Le, G.H.; Dri, C.E.; Teopiz, K.M.; McIntyre, R.S. Evaluating biased agonism of glucagon-like peptide-1 (GLP-1) receptors to improve cellular bioenergetics: A systematic review. Diabetes Obes. Metab. 2025, 27, 6105–6115. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.V.; Johnson, L.M.; Wootten, D.; Sexton, P.M.; Gellman, S.H. β-Arrestin-biased agonists of the GLP-1 receptor from β-amino acid residue incorporation into GLP-1 analogues. J. Am. Chem. Soc. 2016, 138, 14970–14979. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. G Protein-Coupled Receptor Signaling Through β-arrestin-dependent mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Moo, E.V.; Møller, T.C.; Sørensen, F.A.; Inoue, A.; Bräuner-Osborne, H. Arrestin-independent internalization of the GLP-1 receptor is facilitated by a GRK, clathrin, and caveolae-dependent mechanism. FEBS J. 2025, 292, 1675–1695. [Google Scholar] [CrossRef]
- Kuna, R.S.; Girada, S.B.; Asalla, S.; Vallentyne, J.; Maddika, S.; Patterson, J.T.; Smiley, D.L.; DiMarchi, R.D.; Mitra, P. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E161–E170. [Google Scholar] [CrossRef]
- Sonoda, N.; Imamura, T.; Yoshizaki, T.; Babendure, J.L.; Lu, J.C.; Olefsky, J.M. β-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6614–6619. [Google Scholar] [CrossRef]
- Bitsi, S.; El Eid, L.; Manchanda, Y.; Oqua, A.I.; Mohamed, N.; Hansen, B.; Suba, K.; Rutter, G.A.; Salem, V.; Jones, B.; et al. Divergent acute versus prolonged pharmacological GLP-1R responses in adult β cell-specific β-arrestin 2 knockout mice. Sci. Adv. 2023, 9, eadf7737. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.; O’Brien, S.L.; Bernecker, M.; Grandl, G.; Kleinert, M.; Knerr, P.J.; Stemmer, K.; Klingenspor, M.; Zeigerer, A.; DiMarchi, R.; et al. Spatiotemporal GLP-1 and GIP receptor signaling and trafficking/recycling dynamics induced by selected receptor mono- and dual-agonists. Mol. Metab. 2021, 49, 101181. [Google Scholar] [CrossRef]
- Dawed, A.Y.; Mari, A.; Brown, A.; McDonald, T.J.; Li, L.; Wang, S.; Hong, M.G.; Sharma, S.; Robertson, N.R.; Mahajan, A.; et al. Pharmacogenomics of GLP-1 receptor agonists: A genome-wide analysis of observational data and large randomised controlled trials. Lancet Diabetes Endocrinol. 2023, 11, 33–41. [Google Scholar] [CrossRef]
- Salvador, R.; Moutinho, C.G.; Sousa, C.; Vinha, A.F.; Carvalho, M.; Matos, C. Semaglutide as a GLP-1 Agonist: A breakthrough in obesity treatment. Pharmaceuticals 2025, 18, 399. [Google Scholar] [CrossRef]
- Jensen, L.; Kupcova, V.; Arold, G.; Pettersson, J.; Hjerpsted, J.B. Pharmacokinetics and tolerability of semaglutide in people with hepatic impairment. Diabetes Obes. Metab. 2018, 20, 998–1005. [Google Scholar] [CrossRef]
- Hinds, C.E.; Peace, E.; Chen, S.; Davies, I.; El Eid, L.; Tomas, A.; Tan, T.; Minnion, J.; Jones, B.; Bloom, S.R. Abolishing β-arrestin recruitment is necessary for the full metabolic benefits of G protein-biased glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2024, 26, 65–77. [Google Scholar] [CrossRef]
- Jones, B.; Buenaventura, T.; Kanda, N.; Chabosseau, P.; Owen, B.M.; Scott, R.; Goldin, R.; Angkathunyakul, N.; Corrêa, I.R., Jr.; Bosco, D.; et al. Targeting GLP-1 receptor trafficking to improve agonist efficacy. Nat. Commun. 2018, 9, 1602. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by β-arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.J.; Baillie, G.S.; Kohout, T.A.; McPhee, I.; Magiera, M.M.; Ang, K.L.; Miller, W.E.; McLean, A.J.; Conti, M.; Houslay, M.D.; et al. Targeting of cyclic AMP degradation to β 2-adrenergic receptors by β-arrestins. Science 2002, 298, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S.; Downey, W.E., 3rd; Colapietro, A.M.; Barak, L.S.; Ménard, L.; Caron, M.G. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 1996, 271, 363–366. [Google Scholar] [CrossRef]
- Goodman, O.B., Jr.; Krupnick, J.G.; Santini, F.; Gurevich, V.V.; Penn, R.B.; Gagnon, A.W.; Keen, J.H.; Benovic, J.L. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 1996, 383, 447–450. [Google Scholar] [CrossRef]
- Han, S.O.; Kommaddi, R.P.; Shenoy, S.K. Distinct roles for β-arrestin2 and arrestin-domain-containing proteins in β2 adrenergic receptor trafficking. EMBO Rep. 2013, 14, 164–171. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Snyder, J.C.; Shenoy, S.K. Chapter One—Ubiquitination and deubiquitination of G protein-coupled receptors. Prog. Mol. Biol. Transl. Sci. 2016, 141, 1–55. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Miller, W.E. Arrestins as regulators of kinases and phosphatases. Prog. Mol. Biol. Transl. Sci. 2013, 118, 115–147. [Google Scholar] [CrossRef]
- Whalen, E.J.; Rajagopal, S.; Lefkowitz, R.J. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends. Mol. Med. 2011, 17, 126–139. [Google Scholar] [CrossRef]
- Luttrell, L.M. Minireview: More than just a hammer: Ligand “bias” and pharmaceutical discovery. Mol. Endocrinol. 2014, 28, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: A long march to therapeutic successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Vilardaga, J.P.; Gardella, T.J.; Wehbi, V.L.; Feinstein, T.N. Non-canonical signaling of the PTH receptor. Trends Pharmacol. Sci. 2012, 33, 423–431. [Google Scholar] [CrossRef]
- Tohgo, A.; Choy, E.W.; Gesty-Palmer, D.; Pierce, K.L.; Laporte, S.; Oakley, R.H.; Caron, M.G.; Lefkowitz, R.J.; Luttrell, L.M. The stability of the G protein-coupled receptor-β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003, 278, 6258–6267. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Zomer, H.D.; Cooke, P.S. Advances in drug treatments for companion animal obesity. Biology 2024, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Wahl, A.M.; Huang, K.-T.; Narita, T.; Rugis, J.; Sneyd, J.; Yule, D.I. Highly localized intracellular Ca2+ signals promote optimal salivary gland fluid secretion. eLife 2021, 10, e66170. [Google Scholar] [CrossRef]
- Rajagopal, S.; Shenoy, S.K. GPCR desensitization: Acute and prolonged phases. Cell. Signal. 2018, 41, 9–16. [Google Scholar] [CrossRef]
- Mosser, V.A.; Jones, K.T.; Hoffman, K.M.; McCarty, N.A.; Jackson, D.A. Differential role of beta-arrestin ubiquitination in agonist-promoted down-regulation of M1 vs M2 muscarinic acetylcholine receptors. J. Mol. Signal. 2008, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Imbery, J.F.; Bhattacharya, S.; Khuder, S.; Weiss, A.; Goswamee, P.; Iqbal, A.K.; Giovannucci, D.R. cAMP-dependent recruitment of acidic organelles for Ca2+ signaling in the salivary gland. Am. J. Physiol. Cell Physiol. 2016, 311, C697–C709. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Inoue, H.; Kida, S.; Masushige, S.; Nishiyama, T.; Mishima, K.; Saito, I. Involvement of cAMP response element-binding protein activation in salivary secretion. Pathobiology 2006, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Hirono, C.; Sugita, M.; Iwasa, Y.; Shiba, Y. Suppression of carbachol-induced oscillatory Cl− secretion by forskolin in rat parotid and submandibular acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G738–G747. [Google Scholar] [CrossRef]
- Jones, B.; Bloom, S.R.; Buenaventura, T.; Tomas, A.; Rutter, G.A. Control of insulin secretion by GLP-1. Peptides 2018, 100, 75–84. [Google Scholar] [CrossRef]
- Tamayo-Trujillo, R.; Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Paz-Cruz, E.; Zambrano-Villacres, R.; Simancas-Racines, D.; Zambrano, A.K. Molecular mechanisms of semaglutide and liraglutide as a therapeutic option for obesity. Front. Nutr. 2024, 11, 1398059. [Google Scholar] [CrossRef]

| Source | Method | Average Expression | Range/Details | Interpretation |
|---|---|---|---|---|
| HPA RNA-seq | RNA sequencing (HPA samples) | ~2.1 nTPM | 1.3–3.3 nTPM across donors (ages 21–68) | Low–moderate expression, mainly in glandular and ductal cells |
| GTEx | RNA-seq (minor salivary glands, 162 samples) | ~0.1 nTPM | 0.0–1.0 nTPM | Very low expression, often near detection limit |
| FANTOM5 CAGE | CAGE (Cap Analysis of Gene Expression) | 1.8–3.4 TPM | Parotid: 1.8; Submandibular: 2.6; Unspecified gland: 3.4 | Confirms low–moderate expression in major salivary glands |
| Aspect | GLP1R Signalling (General) | Semaglutide Specifics |
|---|---|---|
| Principal signaling pathway | Gs → ↑ cAMP → PKA & EPAC2 | Strong, sustained cAMP signaling |
| Complementary signaling pathway | PI3K/Akt, MAPK, β-arrestin | Reduced β-arrestin recruitment and receptor internalization compared to endogenous GLP-1~“not clinically confirmed as biased” agonist |
| Tissue distribution | Pancreas, brain, heart, kidney, GI, salivary ducts | Same, but sustained exposure may alter receptor availability (e.g., salivary ducts) |
| Pharmacokinetics | Native GLP-1 t½ ~2 min | Modified for albumin binding → t½~7 days |
| Receptor dynamics | Pulsatile exposure (short-acting agonists) | Continuous exposure → adaptive downregulation possible |
| Clinical efficacy | Improves glucose and weight | Greater HbA1c reduction and weight loss |
| Side effects | GI upset, rare pancreatitis | Increased hypoaesthesia and xerostomia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barać, M.; Roganović, J. GLP-1 Receptor Signaling and Oral Dysfunction: A Narrative Review on the Mechanistic Basis of Semaglutide-Related Oral Adverse Effects. Biology 2025, 14, 1650. https://doi.org/10.3390/biology14121650
Barać M, Roganović J. GLP-1 Receptor Signaling and Oral Dysfunction: A Narrative Review on the Mechanistic Basis of Semaglutide-Related Oral Adverse Effects. Biology. 2025; 14(12):1650. https://doi.org/10.3390/biology14121650
Chicago/Turabian StyleBarać, Milena, and Jelena Roganović. 2025. "GLP-1 Receptor Signaling and Oral Dysfunction: A Narrative Review on the Mechanistic Basis of Semaglutide-Related Oral Adverse Effects" Biology 14, no. 12: 1650. https://doi.org/10.3390/biology14121650
APA StyleBarać, M., & Roganović, J. (2025). GLP-1 Receptor Signaling and Oral Dysfunction: A Narrative Review on the Mechanistic Basis of Semaglutide-Related Oral Adverse Effects. Biology, 14(12), 1650. https://doi.org/10.3390/biology14121650






