Predicting Habitat Suitability and Range Dynamics of Three Ecologically Important Fish in East Asian Waters Under Projected Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data
2.2. Environmental Data
2.3. Ecological Niche Modeling (ENM)
2.4. Projecting Distribution Shift Under Different Climate Scenarios
2.5. Conservation Gap Analysis
3. Results
3.1. Model Performance
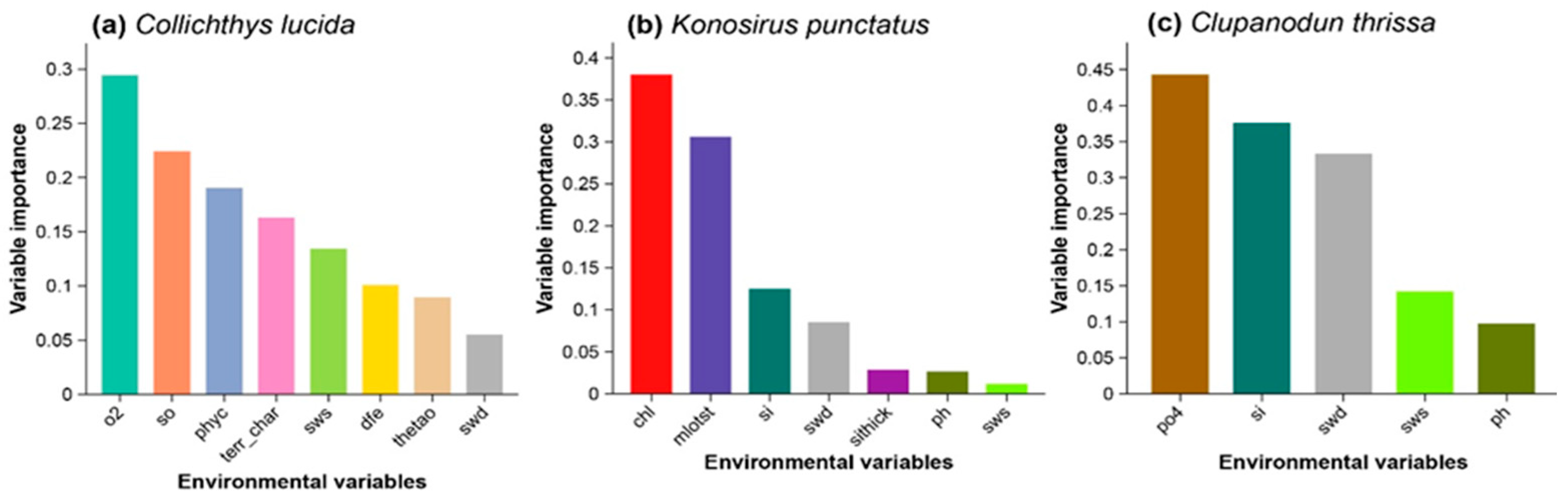
3.2. The Relative Contribution of Environmental Predictors
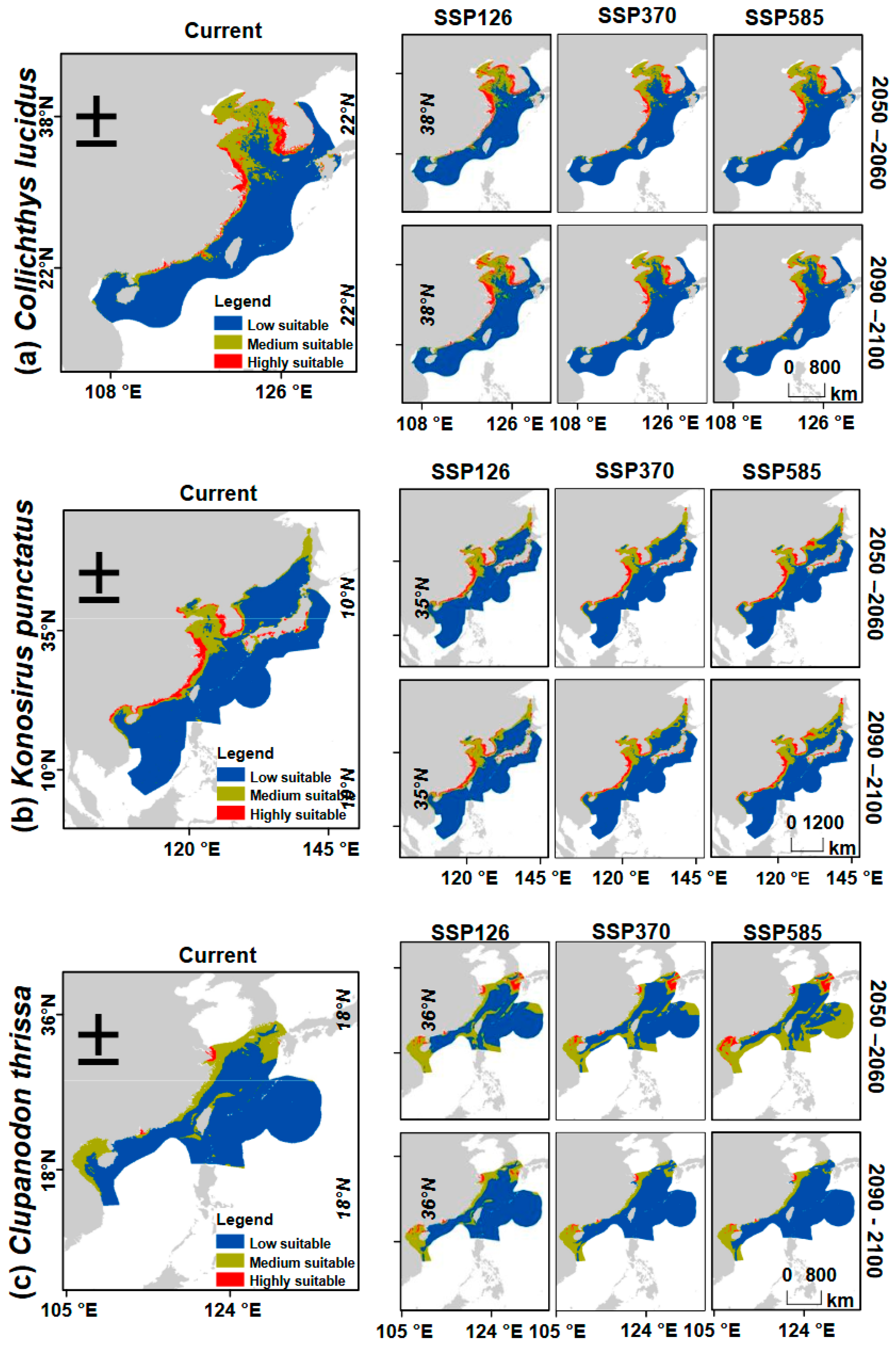
3.3. Potential Distribution Ranges Under the Current Climatic Conditions
3.4. Predicted Shifts in Potential Distribution Ranges Under Climate Change Scenarios
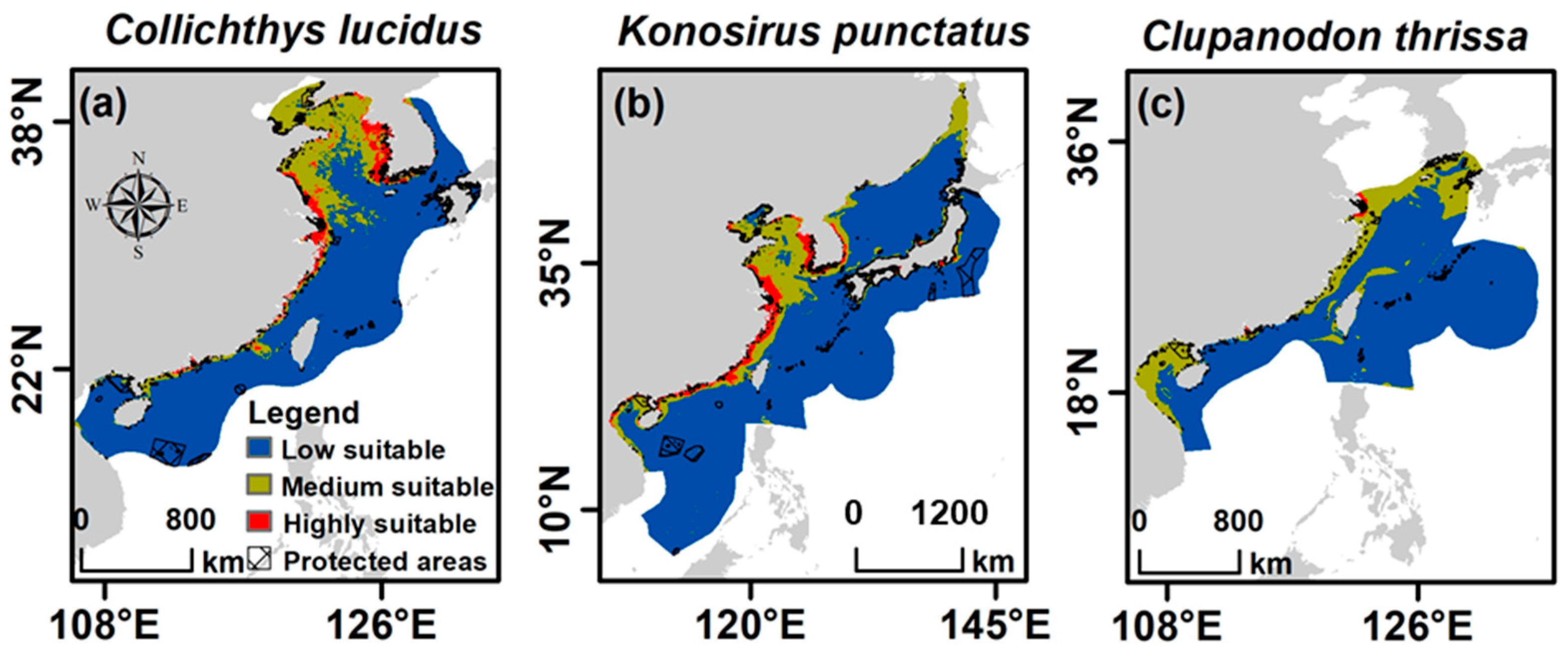
3.5. Conservation Status of the Focal Species Due to Climate Change Impacts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Han, G.; Zhang, Q. Impacts of environmental change and human activities on aquatic ecosystems. Water 2025, 17, 1669. [Google Scholar] [CrossRef]
- Poff, N.L.; Brinson, M.M.; Day, J.W., Jr. Aquatic Ecosystems & Global Climate Change: Potential Impacts on Inland Freshwater and Coastal Wetland Ecosystems in the United States; Pew Center on Global Climate Change: Arlington, VA, USA, 2002. [Google Scholar]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Xenopoulos, M.A.; Lodge, D.M.; Alcamo, J.; Märker, M.; Schulze, K.; Van Vuuren, D.P. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Glob. Change Biol. 2005, 11, 1557–1564. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Change 2017, 7, 718–722. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of historical warming on marine fisheries production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.W.Y.; Allison, E.H.; Bell, J.D.; Blythe, J.; Cheung, W.W.L.; Frölicher, T.L.; Gasalla, M.A.; Sumaila, U.R. Climate change, tropical fisheries and prospects for sustainable development. Nat. Rev. Earth Environ. 2020, 1, 440–454. [Google Scholar] [CrossRef]
- Murdoch, A.; Power, M. The effect of lake morphometry on thermal habitat use and growth in Arctic charr populations: Implications for understanding climate-change impacts. Ecol. Freshw. Fish 2013, 22, 453–466. [Google Scholar] [CrossRef]
- Tao, J.; He, D.K.; Kennard, M.J.; Ding, C.Z.; Bunn, S.E.; Liu, C.L.; Jia, Y.T.; Che, R.X.; Chen, Y.F. Strong evidence for changing fish reproductive phenology under climate warming on the Tibetan Plateau. Glob. Change Biol. 2018, 24, 2093–2104. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Yan, Y.; Xiang, X.; Chu, L.; Zhan, Y.; Fu, C. Influences of local habitat and stream spatial position on fish assemblages in a dammed watershed, the Qingyi Stream, China. Ecol. Freshw. Fish 2011, 20, 199–208. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Oberdorff, T.; Cornu, J.-F.; Beauchard, O.; Brosse, S.; Dürr, H.H.; Grenouillet, G.; Leprieur, F.; Tisseuil, C.; Zaiss, R.; et al. A scenario for impacts of water availability loss due to climate change on riverine fish extinction rates. J. Appl. Ecol. 2013, 50, 1105–1115. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater eco-systems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Carozza, D.A.; Bianchi, D.; Galbraith, E.D. Metabolic impacts of climate change on marine ecosystems: Implications for fish communities and fisheries. Glob. Ecol. Biogeogr. 2019, 28, 158–169. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Dahe, Q., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; p. 582. [Google Scholar]
- Oppenheimer, M.; Glavovic, B.C.; Hinkel, J.; van de Wal, R.; Magnan, A.K.; Abd-Elgawad, A.; Cai, R.; Cifuentes-Jara, M.; DeConto, R.M.; Ghosh, T.; et al. Sea Level Rise and implications for low-lying islands, coasts and communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 321–445. [Google Scholar] [CrossRef]
- Asian Development Bank. Rising Seas: Building Resilience Against Coastal Flooding in Asia and the Pacific; Development Asia: Manila, PA, USA, 2024; Available online: https://development.asia/insight/rising-seas-building-resilience-against-coastal-flooding-asia-and-pacific (accessed on 1 August 2025).
- You, Q.; Jiang, Z.; Yue, X.; Guo, W.; Liu, Y.; Cao, J.; Li, W.; Wu, F.; Cai, Z.; Zhu, H.; et al. Recent frontiers of climate changes in East Asia at global warming of 1.5 °C and 2 °C. NPJ Clim. Atmos. Sci. 2022, 5, 80. [Google Scholar] [CrossRef]
- Zeng, Z.; Cheung, W.W.L.; Li, S.; Hu, J.; Wang, Y. Effects of climate change and fishing on the Pearl River Estuary ecosystem and fisheries. Rev. Fish Biol. Fish. 2019, 29, 861–875. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Freitas, C.; Villegas-Ríos, D.; Moland, E.; Olsen, E.M. Sea temperature effects on depth use and habitat selection in a marine fish community. J. Anim. Ecol. 2021, 90, 1787–1800. [Google Scholar] [CrossRef]
- Teh, L.C.L.; Pauly, D. Who brings in the fish? The relative contribution of small-scale and industrial fisheries to food security in Southeast Asia. Front. Mar. Sci. 2018, 5, 44. [Google Scholar] [CrossRef]
- Yu, D.; Chen, M.; Zhou, Z.; Eric, R.; Tang, Q.; Liu, H. Global climate change will severely decrease potential distribution of the East Asian coldwater fish Rhynchocypris oxycephalus (Actinopterygii, Cyprinidae). Hydrobiologia 2013, 700, 23–32. [Google Scholar] [CrossRef]
- Barros, N.B.; Jefferson, T.A.; Parsons, E.C.M. Feeding habits of Indo-Pacific humpback dolphins (Sousa chinensis) stranded in Hong Kong. Aquat. Mamm. 2004, 30, 179–188. [Google Scholar] [CrossRef]
- Li, W.; Lei, L.; Zhu, J.; Chen, H.; Chen, C.; Wang, Y.; Geng, L.; Chen, F.; Zhu, X. Simple and rapid method for molecular identification of Konosirus punctatus and Clupanodon thrissa. Conserv. Genet. Resour. 2022, 14, 163–165. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Zhu, K.; Shafi, M.; Gong, L.; Jiang, L.; Liu, L.; Muhammad, F.; Lü, Z. Population genetics of Konosirus punctatus in Chinese coastal waters inferred from two mtDNA genes (COI and cytb). Front. Mar. Sci. 2020, 7, 534. [Google Scholar] [CrossRef]
- Lin, W.; Karczmarski, L.; Zhou, R.; Mo, Y.; Guo, L.; Yiu, S.K.F.; Ning, X.; Wai, T.-C.; Wu, Y. Prey decline leads to diet shift in the largest population of Indo-Pacific humpback dolphins? Integr. Zool. 2021, 16, 548–574. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Huang, H.; Shen, X.; Zhu, J.; Yang, J. Estuarine dependency in Collichthys lucidus of the Yangtze River Estuary as revealed by the environmental signature of otolith strontium and calcium. Environ. Biol. Fishes 2015, 98, 165–172. [Google Scholar] [CrossRef]
- Zhuang, P. Fishes of the Yangtze Estuary, 2nd ed.; China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Xuan, W.; Zhang, H.; Zhang, H.; Wu, T.; Zhou, Y.; Zhu, W. Distribution characteristics and driving factors of Collichthys lucidus species in offshore waters of Zhejiang Province, China. Fishes 2024, 9, 83. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. 2011. Available online: https://www.fishbase.se (accessed on 15 July 2025).
- Xu, Q.; Jiang, Y.; Fang, L.P.; Liu, M.; Jiang, X.B. Reproductive dynamics of three important clupeiform food Fishes in the Min River estuary and its adjacent nearshore waters, China. Mar. Coast. Fish. 2021, 13, 679–692. [Google Scholar] [CrossRef]
- Xia, J.; Hu, H.; Gao, X.; Kan, J.; Gao, Y.; Li, J. Phytoplankton diversity, spatial patterns, and photosynthetic characteristics under environmental gradients and anthropogenic influence in the Pearl River estuary. Biology 2024, 13, 550. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Sharifian, S.; Mortazavi, M.S.; Mohebbi Nozar, S.L. Projected habitat preferences of commercial fish under different scenarios of climate change. Sci. Rep. 2024, 14, 10177. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S.S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Change Biol. 2023, 29, 5170–5188. [Google Scholar] [CrossRef]
- Kay, S.; Avillanosa, A.L.; Cheung, V.V.; Dao, H.N.; Gonzales, B.J.; Palla, H.P.; Praptiwi, R.A.; Queirós, A.M.; Sailley, S.F.; Sumeldan, J.D.C.; et al. Projected effects of climate change on marine ecosystems in Southeast Asian seas. Front. Mar. Sci. 2023, 10, 1082170. [Google Scholar] [CrossRef]
- Robinson, N.M.; Nelson, W.A.; Costello, M.J.; Sutherland, J.E.; Lundquist, C.J. A systematic review of marine-based species distribution models (SDMs) with recommendations for best practice. Front. Mar. Sci. 2017, 4, 421. [Google Scholar] [CrossRef]
- Karp, M.A.; Cimino, M.; Craig, J.K.; Crear, D.P.; Haak, C.; Hazen, E.L.; Kaplan, I.; Kobayashi, D.R.; Moustahfid, H.; Muhling, B.; et al. Applications of species distribution modeling and future needs to support marine resource management. ICES J. Mar. Sci. 2025, 82, fsaf024. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Assis, J.; Fernández Bejarano, S.J.; Salazar, V.W.; Schepers, L.; Gouvêa, L.; Fragkopoulou, E.; Leclercq, F.; Vanhoorne, B.; Tyberghein, L.; Serrão, E.A.; et al. Bio-ORACLE v3.0. Pushing marine data layers to the CMIP6 Earth System Models of climate change research. Glob. Ecol. Biogeogr. 2024, 33, e13813. [Google Scholar] [CrossRef]
- Luna, S.; Peña-Peniche, A.; Mendoza-Alfaro, R. Species distribution model accuracy is strongly influenced by the choice of calibration area. Biodivers. Inform. 2024, 18, 43–45. [Google Scholar] [CrossRef]
- Diaz-Carballido, P.L.; Mendoza-González, G.; Yañez-Arenas, C.A.; Chiappa-Carrara, X. Evaluation of shifts in the potential future distributions of carcharhinid sharks under different climate change scenarios. Front. Mar. Sci. 2022, 8, 745501. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Rathore, M.K.; Sharma, L.K. Efficacy of species distribution models (SDMs) for ecological realms to ascertain biological conservation and practices. Biodivers. Conserv. 2023, 32, 3053–3087. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. blockCV: An R package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods Ecol. Evol. 2019, 10, 225–232. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Romera, R.; Gaertner, M.Á.; Sánchez, E.; Domínguez, M.; González-Alemán, J.J.; Miglietta, M.M. Climate change projections of medicanes with a large multi-model ensemble of regional climate models. Glob. Planet. Change 2017, 151, 134–143. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. SDM: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package “dismo”. Circles 2017, 9, 1–68. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Mambo, W.W.; Zhu, G.-F.; Milne, R.I.; Wambulwa, M.C.; Oyebanji, O.O.; Ngarega, B.K.; Carver, D.; Liu, J. Shrinking horizons: Climate-induced range shifts and conservation status of hickory trees (Carya Nutt.). Ecol. Inform. 2024, 84, 102910. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R. biomod2: Ensemble Platform for Species Distribution Modelling, version 2; The R Project for Statistical Computing: Vienna, Austria, 2014.
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- UNEP-WCMC and IUCN. Protected Planet: The World Database on Protected Areas (WDPA); UNEP-WCMC and IUCN: Cambridge, UK, 2025. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Keeling, R.E.; Körtzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Chávez, F.P.; Messié, M. A comparison of Eastern Boundary upwelling Ecosystems. Prog. Oceanogr. 2009, 83, 80–96. [Google Scholar] [CrossRef]
- Carr, M.-E.; Kearns, E.J. Production regimes in four Eastern Boundary Current systems. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 3199–3221. [Google Scholar] [CrossRef]
- Bakun, A.; Black, B.A.; Bograd, S.J.; García-Reyes, M.; Miller, A.J.; Rykaczewski, R.R.; Sydeman, W.J. Anticipated effects of climate change on coastal upwelling ecosystems. Curr. Clim. Change Rep. 2015, 1, 85–93. [Google Scholar] [CrossRef]
- Wang, D.; Gouhier, T.C.; Menge, B.A.; Ganguly, A.R. Intensification and spatial homogenization of coastal upwelling under climate change. Nature 2015, 518, 390–394. [Google Scholar] [CrossRef]
- Wiens, J.J.; Zelinka, J. How many species will Earth lose to climate change? Glob. Change Biol. 2024, 30, e17125. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Burrows, M.T.; García Molinos, J. Temperature tracking by North Sea benthic invertebrates in response to climate change. Glob. Change Biol. 2015, 21, 117–129. [Google Scholar] [CrossRef]
- Jones, M.C.; Cheung, W.W.L. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- Rincón-Díaz, M.P.; Svendsen, G.M.; Venerus, L.A.; Villanueva-Gomila, L.; Lattuca, M.E.; Vanella, F.A.; Cuesta Núñez, J.; Galván, D.E. Traits related to distributional range shifts of marine fishes. J. Fish Biol. 2025, 106, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Beaugrand, G.; Kirby, R.R. How do marine pelagic species respond to climate change? Theories and observations. Annu. Rev. Mar. Sci. 2018, 10, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllmann, C.; Pinnegar, J.K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009, 66, 1570–1583. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Worm, B.; Fogarty, M.J.; Sarmiento, J.L.; Levin, S.A. Marine taxa track local climate velocities. Science 2013, 341, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Gaines, S.D.; Costello, C.; Owashi, B.; Mangin, T.; Bone, J.; Molinos, J.G.; Burden, M.; Dennis, H.; Halpern, B.S.; Kappel, C.V.; et al. Improved fisheries management could offset many negative effects of climate change. Sci. Adv. 2018, 4, eaao1378. [Google Scholar] [CrossRef]
- Wilson, K.L.; Tittensor, D.P.; Worm, B.; Lotze, H.K. Incorporating climate change adaptation into marine protected area planning. Glob. Change Biol. 2020, 26, 3251–3267. [Google Scholar] [CrossRef]
- White, J.W.; Hopf, J.K.; Arafeh-Dalmau, N.; Ban, N.C.; Bates, A.E.; Claudet, J.; Lopazanski, C.; Sunday, J.M.; Caselle, J.E. Measurements, mechanisms, and management recommendations for how marine protected areas can provide climate resilience. Mar. Policy 2025, 171, 106419. [Google Scholar] [CrossRef]
- Morelli, T.L.; Barrows, C.W.; Ramirez, A.R.; Cartwright, J.M.; Ackerly, D.D.; Eaves, T.D.; Ebersole, J.L.; Krawchuk, M.A.; Letcher, B.H.; Mahalovich, M.F.; et al. Climate-change refugia: Biodiversity in the slow lane. Front. Ecol. Environ. 2020, 18, 228–234. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Mantua, N.J. Emerging adaptation approaches for climate-ready fisheries management. Oceanography 2014, 27, 146–159. Available online: https://www.jstor.org/stable/24862219 (accessed on 4 August 2025). [CrossRef]
- Moudrý, V.; Bazzichetto, M.; Remelgado, R.; Devillers, R.; Lenoir, J.; Mateo, R.G.; Lembrechts, J.J.; Sillero, N.; Lecours, V.; Cord, A.F.; et al. Optimising occurrence data in species distribution models: Sample size, positional uncertainty, and sampling bias matter. Ecography 2024, 2024, e07294. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]


| Species | Environment | No | AUC | TSS |
|---|---|---|---|---|
| Collichthys lucidus | Benthic zone | 92 | 0.888 | 0.725 |
| Clupanodon thrissa | Pelagic zone | 19 | 0.960 | 0.920 |
| Konosirus punctatus | Pelagic zone | 355 | 0.922 | 0.756 |
| Species | Period | Area (km2) | ||
|---|---|---|---|---|
| Highly Suitable | Medium Suitable | Low Suitable | ||
| Collichthys lucidus | Current | 92,393 | 311,007 | 1,193,992 |
| 2050–2060 | ||||
| SSP126-2050 | 120,840 | 283,224 | 1,193,328 | |
| SSP370-2050 | 87,548 | 283,803 | 1,226,126 | |
| SSP585-2050 | 86,198 | 283,632 | 1,227,563 | |
| 2090–2100 | ||||
| SSP126-2090 | 120,390 | 282,903 | 1,194,100 | |
| SSP370-2090 | 81,289 | 256,321 | 1,259,782 | |
| SSP585-2090 | 78,823 | 250,790 | 1,267,778 | |
| Konosirus punctatus | Current | 243,330 | 707,654 | 3,695,314 |
| 2050–2060 | ||||
| SSP126-2050 | 266,332 | 813,638 | 3,566,328 | |
| SSP370-2050 | 245,495 | 772,350 | 3,628,453 | |
| SSP585-2050 | 281,016 | 830,937 | 3,534,345 | |
| 2090–2100 | ||||
| SSP126-2090 | 283,632 | 862,857 | 3,499,810 | |
| SSP370-2090 | 258,486 | 850,295 | 3,537,517 | |
| SSP585-2090 | 277,565 | 960,030 | 3,408,703 | |
| Clupanodon thrissa | Current | 10,869 | 331,993 | 1,420,066 |
| 2050–2060 | ||||
| SSP126-2050 | 55,179 | 556,545 | 1,151,204 | |
| SSP370-2050 | 58,373 | 562,118 | 1,142,437 | |
| SSP585-2050 | 93,529 | 947,382 | 722,016 | |
| 2090–2100 | ||||
| SSP126-2090 | 30,698 | 376,946 | 1,355,284 | |
| SSP370-2090 | 17,385 | 310,985 | 1,434,557 | |
| SSP585-2090 | 17,814 | 276,150 | 1,468,964 | |
| Species | Time Change | Area Change km2 | ||||
|---|---|---|---|---|---|---|
| Range Expansion | Range Contraction | Stable | % Gain | % Loss | ||
| Collichthys lucidus | 2050–2060 | |||||
| Current → SSP126 | 12,069 | 42,659 | 270,512 | 4 | 14 | |
| Current → SSP370 | 3623 | 25,639 | 287,533 | 1 | 8 | |
| Current → SSP585 | 4180 | 28,640 | 284,532 | 1 | 9 | |
| 2090–2100 | ||||||
| Current → SSP126 | 13,334 | 26,582 | 286,590 | 4 | 8 | |
| Current → SSP370 | 7353 | 51,449 | 261,723 | 2 | 16 | |
| Current → SSP585 | 8939 | 57,772 | 255,399 | 3 | 18 | |
| Konosirus punctatus | 2050–2060 | |||||
| Current → SSP126 | 197,734 | 13,998 | 536,887 | 36 | 3 | |
| Current → SSP370 | 174,496 | 18,864 | 532,021 | 32 | 3 | |
| Current → SSP585 | 270,641 | 12,476 | 538,409 | 49 | 2 | |
| 2090–2100 | ||||||
| Current → SSP126 | 242,902 | 7481 | 543,404 | 44 | 1 | |
| Current → SSP370 | 249,547 | 19,165 | 531,721 | 45 | 3 | |
| Current → SSP585 | 377,590 | 10,697 | 540,188 | 69 | 2 | |
| Clupanodon thrissa | 2050–2060 | |||||
| Current → SSP126 | 131,815 | 22,466 | 268,068 | 45 | 8 | |
| Current → SSP370 | 276,279 | 49,133 | 241,401 | 95 | 17 | |
| Current → SSP585 | 668,574 | 36,164 | 254,370 | 230 | 12 | |
| 2090–2100 | ||||||
| Current → SSP126 | 77,923 | 38,736 | 251,798 | 27 | 13 | |
| Current → SSP370 | 106,048 | 76,272 | 214,262 | 37 | 26 | |
| Current → SSP585 | 155,889 | 77,280 | 213,254 | 54 | 27 | |
| Species | Area (km2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Highly Suitable | Outside PA | Inside PA | Medium Suitable | Outside PA | Inside PA | Low Suitable | Outside PA | Inside PA | |
| Collichthys lucidus | 92,393 | 72,817 (79%) | 19,576 (21%) | 311,007 | 283,609 (91%) | 27,398 (9%) | 1,193,992 | 1,132,198 (95%) | 61,794 (5%) |
| Konosirus punctatus | 243,330 | 199,061 (82%) | 44,269 (18%) | 707,654 | 654,273 (92%) | 53,381 (8%) | 3,695,314 | 3,526,489 (95%) | 168,825 (5%) |
| Clupanodon thrissa | 10,869 | 6681 (61%) | 4188 (39%) | 331,993 | 304,494 (92%) | 27,499 (8%) | 1,420,066 | 1,408,885 (99%) | 11,181 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nneji, I.C.; Mambo, W.W.; Zheng, Z.; Oladipo, S.O.; Zhao, H.; Lu, W.; Nneji, L.M.; Lin, J.; Liu, W. Predicting Habitat Suitability and Range Dynamics of Three Ecologically Important Fish in East Asian Waters Under Projected Climate Change. Biology 2025, 14, 1476. https://doi.org/10.3390/biology14111476
Nneji IC, Mambo WW, Zheng Z, Oladipo SO, Zhao H, Lu W, Nneji LM, Lin J, Liu W. Predicting Habitat Suitability and Range Dynamics of Three Ecologically Important Fish in East Asian Waters Under Projected Climate Change. Biology. 2025; 14(11):1476. https://doi.org/10.3390/biology14111476
Chicago/Turabian StyleNneji, Ifeanyi Christopher, Winnie Wanjiku Mambo, Zhao Zheng, Segun Olayinka Oladipo, Hancheng Zhao, Wentao Lu, Lotanna Micah Nneji, Jianqing Lin, and Wenhua Liu. 2025. "Predicting Habitat Suitability and Range Dynamics of Three Ecologically Important Fish in East Asian Waters Under Projected Climate Change" Biology 14, no. 11: 1476. https://doi.org/10.3390/biology14111476
APA StyleNneji, I. C., Mambo, W. W., Zheng, Z., Oladipo, S. O., Zhao, H., Lu, W., Nneji, L. M., Lin, J., & Liu, W. (2025). Predicting Habitat Suitability and Range Dynamics of Three Ecologically Important Fish in East Asian Waters Under Projected Climate Change. Biology, 14(11), 1476. https://doi.org/10.3390/biology14111476







