Simple Summary
The corneal epithelium is a protective barrier and refractive structure in the eye maintained through a complex regenerative process involving the lacrimal gland, tear film, and corneal nerves. This review explores the insulin-like growth factor (IGF) system and its role in corneal epithelium homeostasis. Emphasis is placed on the significance of limbal epithelial stem cells and potential therapeutic applications targeting the system components.
Abstract
The corneal epithelium, comprising three layers of cells, represents the outermost portion of the eye and functions as a vital protective barrier while concurrently serving as a critical refractive structure. Maintaining its homeostasis involves a complex regenerative process facilitated by the functions of the lacrimal gland, tear film, and corneal nerves. Crucially, limbal epithelial stem cells located in the limbus (transitional zone between the cornea and the conjunctiva) are instrumental for the corneal epithelium integrity by replenishing and renewing cells. Re-epithelialization failure results in persistent defects, often associated with various ocular conditions including diabetic keratopathy. The insulin-like growth factor (IGF) system is a sophisticated network of insulin and other proteins essential for numerous physiological processes. This review examines its role in maintaining the corneal epithelium homeostasis, with a special focus on the interplay with corneal limbal stem cells and the potential therapeutic applications of the system components.
1. Introduction
The corneal epithelium is the outermost part of the cornea and consists of three cellular layers: the superficial layer, middle wing layer, and the innermost basal cell layer with Bowman’s membrane in humans separating it from the corneal stroma []. It plays the role of a protective barrier as well as a refractive structure due to its avascular character. Its homeostasis is maintained through a complex regenerative process which takes about 10 days and involves proliferation and migration of epithelial cells []. In addition, the lacrimal gland function, tear film, and corneal nerves are pivotal in maintaining the health and integrity of the corneal epithelium [].
The XYZ hypothesis, as proposed by Thoft and Friend, describes three phases of epithelial recovery, which include proliferation and stratification of limbal basal cells, their subsequent centripetal migration, and finally, desquamation of superficial cells []. Notably, in some mammalian species, the entire ocular surface serves as a source of epithelial stem cells []. A study by Chang et al. further suggests that human epithelial cells in the central cornea can also contribute to wound healing []. However, the failure of re-epithelialization may result in persistent corneal epithelial defects, which can be caused by limbal stem cell deficiency and keratopathy related to corneal exposure, surgical and non-surgical injuries, prior infections, diabetes, and neurotrophic and dry eye changes [].
Limbal epithelial stem cells (LESCs) are instrumental in maintaining a healthy epithelium by continuously replenishing damaged and aging cells []. The limbus is a 1–2 mm transitional zone that separates the epithelium from the conjunctiva and constitutes a niche for LESCs []. This region provides a barrier, preventing the conjunctiva from invading the cornea and, as a consequence, reducing its transparency due to conjunctivalization. In a study from 1945, Mann indirectly demonstrated the presence of LESCs by describing the migration of pigmented basal cells in rabbit corneas []. Typically, they divide in an asymmetric pattern, generating two cells: a new LESC, which maintains its renewal capacity, and an early transient amplifying cell undergoing further differentiation and centripetal migration []. Recent studies suggest that LESC populations may vary in terms of their activity (active and quiescent pools), distribution in the limbus (outer vs. inner limbus), and roles in regenerative processes [,,,]. Unlike in the mouse limbus with a uniform LESC distribution [], in humans, LESCs reside in the basal layer of numerous fibrovascular ridges, termed the Palisades of Vogt, and other tangentially and circumferentially extending structures such as crypts and pits [,]. This arrangement provides protection from the external environment and reduces the risk of damage from detrimental factors, such as ultraviolet radiation, chemicals, thermal burns, and pathogens []. At the same time, the LESC niche creates a microenvironment enabling interactions with biochemical mediators, including growth factors, cytokines, and chemokines.
The insulin-like growth factor (IGF) system is a complex network of hormones and proteins that play crucial roles in cell growth, development, and metabolism []. It consists of insulin, insulin-like growth factor 1 and -2 (IGF-1, IGF-2), their receptors: insulin receptor (INSR), IGF type 1 and 2 receptors (IGF-1R, IGF-2R), as well as several IGF-binding proteins (IGFBPs), Figure 1.
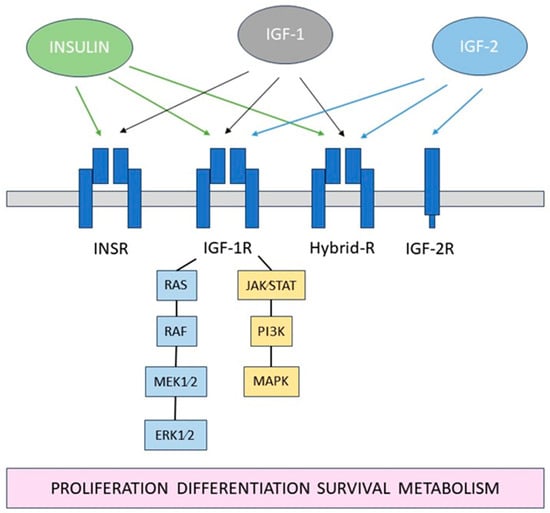
Figure 1.
Schematic diagram of the IGF system.
Dysregulation of this system has been implicated in various diseases including diabetes, which profoundly impacts the eye. The IGF system is also pivotal for corneal epithelium homeostasis, influencing critical cellular processes and potentially serving as a target in therapeutic applications (Table 1).

Table 1.
The overview of the key roles of the IGF system components in the corneal epithelium.
2. The Role of Insulin and Effect of Diabetes
Insulin is a polypeptide hormone consisting of two A and two B chains, produced by pancreatic beta cells and secreted in response to a high blood glucose level []. It has metabolic effects and plays a role in various phases of the cell cycle, from growth to apoptosis. The presence of INSR in the cornea was first demonstrated by Naeser []. Alternative splicing of INSR occurs at exon 11, leading to the generation of two distinct isoforms: INSRA and INSRB []. An immunohistochemical analysis by Rocha et al. demonstrated that INSR is expressed in the corneal epithelium []. In their study of human corneas, INSR was predominantly localized within the cytoplasm and plasma membrane in the wing and superficial cell layers, with noticeable variability in its expression across the basal and intermediate suprabasal cells (Table 2).

Table 2.
Summary of the IGF system receptors and their localization in the corneal epithelium.
Diabetes mellitus, a chronic metabolic disorder characterized by hyperglycemia, results from a deficiency in insulin secretion, impaired insulin action, or a combination of both []. The two primary forms of diabetes, type 1 and type 2, differ in their pathophysiology but share the common feature of dysregulated glucose metabolism. In type 1 diabetes, an autoimmune response leads to the destruction of pancreatic beta cells, resulting in insufficient insulin production, while type 2 diabetes involves a combination of insulin resistance and relative insulin deficiency. Diabetes can significantly impact the eye, including the ocular surface, leading to dysfunction of epithelium and development of diabetic keratopathy.
Unlike most tissues, where insulin stimulates glucose uptake through the glucose transporter-4 (GLUT4), corneal epithelium is insulin-independent []. Glucose uptake in epithelial cells occurs through constitutively active glucose transporters, GLUT1, which undergo upregulation in case of a high metabolic demand, such as wound healing, similarly in diabetic and non-diabetic corneas.
Culture studies with human corneal epithelial cells demonstrated that insulin induces phosphorylation of extracellular signal regulated kinase (ERK 1/2), PI3-kinase [] and epidermal growth factor receptor (EGFR), thereby promoting cell migration and wound healing []. Interestingly, increased expression of PI3K pathway kinases occurs in canine corneal cells following insulin treatment, contrasting with the observed lack of analogous effects in vitro in human corneal cells []. Within the corneal epithelium, insulin influences PTEN-induced kinase 1 (PINK-1)-mediated mitophagy and the mitochondrial accumulation of insulin receptor (INSR). Interactions between INSR and the voltage-dependent anion channel-1 (VDAC1) prevent fragmentation and altered polarization of mitochondria, as well as facilitate PINK-1-mediated mitophagy []. In diabetic rats, the process of histone H3 acetylation is reduced in corneal epithelial cells, resulting in compacted chromatin organization in nuclei characterized by increased size and elevated DNA ploidy [].
A study by Song et al. showed that insulin can normalize the circadian rhythm of corneal cell mitosis via five main clock genes (Clock, Bmal1, Per2, Cry1, and Rev-erbα) whose expression is affected in diabetes []. Moreover, innate-like lymphocytes, such as γ δ T-cells expressing chemotactic factor IL-17 for neutrophils and monocytes, were found to be recruited to the corneal limbus in a diurnal pattern. In diabetes, the limbal cell migration is increased, potentially leading to the inflammatory state delaying wound healing, but is restored upon systemic insulin administration.
Insulin is secreted from the lacrimal gland and present in the tear film at a mean concentration of 0.404 ± 0.129 ng/mL, which is reduced in fasted individuals and shows no difference related to gender []. In diabetes, its secretion is reduced due to damage to the lacrimal gland and reduced corneal sensation, caused by hyperglycemia and oxidative stress []. In rat models of diabetes, histological analysis showed an increased lipofuscin level and higher malonaldehyde as well as peroxidase activity in the lacrimal glands compared to healthy animals []. Insulin signaling in rat lacrimal glands becomes impaired in the fourth week of diabetes, with the lacrimal gland serving as an extra pancreatic source of insulin for at least 4–7 weeks [,].
Several studies reported ocular surface abnormalities in patients with diabetes, such as reduced tear breakup time, lower tears secretion, and increased level of inflammatory markers: NPY, STAT-5 ICAM-1, and TNF-α [,,]. Clinically, diabetic ocular surface complications include reduced corneal sensitivity and delayed epithelialization leading to dry eye syndrome, punctate corneal epitheliopathy, recurrent erosions, persistent epithelial defects, and neurotrophic keratopathy [,]. On a histological level, reduction in basal epithelial cell density and size, and increased intercellular space as well as increased corneal epithelial basement membrane thickness and irregularity were demonstrated in diabetic corneas [,,,,]. It is postulated that diabetic ocular changes might be in part explained by the dysregulation of a pathway involving the opioid growth factor (OGF), i.e., [Met5]- enkephalin binding to nuclear-associated receptor (OGFr) []. In diabetes, serum OGF levels are elevated, and insulin may affect the OGF-OGFr axis [,,].
A fundamentally negative impact of diabetes on the corneal epithelium is closely linked to its effect on LESC functioning []. A significant reduction in Palisades of Vogt in all four limbus quadrants was demonstrated in patients with type 2 diabetes using in vivo confocal microscopy []. In the same study, a higher risk of stem cell damage was noted in those with a high-density lipoprotein, triglycerides, and total cholesterol level above 1.215 mmol/L, 1.59 mmol/L, and 4.75 mmol/L, respectively.
Immunohistochemistry analysis of corneas from diabetic patients revealed a reduction in putative limbal stem cell markers, including ATP-binding cassette superfamily G member 2 protein (ABCG2), N-cadherin, ΔNp63α, K15, K17, K19, and β1 integrin []. This decrease in marker expression was associated with lower immunoreactivity and a diminished number of detected cells, indicating potential depletion or dysfunction of LESCs. Moreover, the ex vivo diabetic limbus was characterized by irregular epithelial basement membrane and reduced expression of laminin γ3 and fibronectin.
In another study, a reduction in expression of putative stem cell markers K15 and ΔNp63α was demonstrated in limbal epithelial stem cell (LESC)-enriched cultures obtained from the corneoscleral rims in diabetic patients with changes reversed by adenoviral gene therapy []. In an animal model of type 2 diabetes, reduced expression of corneal stem/progenitor cell markers, including Hes1, Keratin15, and p75, was observed in mice corneas []. Similarly, in mice with type 1 diabetes, the expression of putative LESC markers K15, ∆Np63α, and glycoprotein hormone alpha-2 (GPHA2) was reduced in the limbus [].
A study employing a new method of objective quantification of immunofluorescence, aiming to overcome limitations of manual grading, observed a reduction in the putative LESC marker K14 in the limbus of diabetic mice []. Furthermore, reduced expression of putative limbal epithelial stem cell markers, such as paired box protein-6 (PAX6), ∆Np63α, K15, K17, and membrane transporter ABCG2, was demonstrated in cultured diabetic human limbal epithelial cells, which was associated with slower corneal epithelial wound healing []. It is crucial to note, however, that several cell markers for LESCs can also be identified in other parts of the eye, and their use has limitations in discriminating pure stem cells from early transient amplifying cells [,].
The nervous system plays an instrumental role in maintaining the homeostasis of the corneal epithelium. Nerve branches of the sub-basal corneal nerve plexus are considered an important source of substances contributing to ocular surface health. Importantly, corneal innervation impacts a stem cells niche [], with a limbal stem cells reduction reported following the destruction of the ophthalmic branch of the trigeminal nerve [].
Detrimental effects of diabetes on corneal sensitivity, nerve fiber length, and density in both humans [,,,,] and animals [,,,,,,] have been reported in numerous studies and reviewed elsewhere [,,]. Recently, a 3D tissue model of the human cornea was employed to demonstrate the degenerative effects of hyperglycemia on corneal nerves []. On a cellular level, pannexin1 channels present in corneal synaptosomes were found to be more glycosylated, characterized by enhanced membrane localization and leading to increased ATP release in diabetic subjects compared to non-diabetic controls []. Animal studies showed that insulin stimulates corneal nerve regeneration and expression of a limbal stem cell marker (DNp63) via Wnt signaling [], and when applied topically, it exerts neuroprotective properties in diabetic rats []. In patients with type 2 diabetes, a nerve regenerative process can be limited by insulin resistance []. Interestingly, a prediabetic state is characterized by increased parameters of intra-epithelial corneal basal nerves, which could be attributed to the neurotrophic effect of higher insulin levels [].
More recently, the overactivation of the ocular sympathetic nervous system adjacent to the limbus, mediated through the β2-adrenoceptor NE-Adrb2- sonic hedgehog (Shh) signaling pathway, was reported in type 1 diabetic mice []. This overactivation led to dysfunction and reduced proliferation of limbal stem/progenitor cells in response to a chemical injury. Additionally, the interplay between the nervous and immune system may impact limbal stem cells [,]. Immune cells residing in the cornea, such as T cells, interact with dendritic corneal cells and sensory nerves, influencing a response to acute and chronic stimuli []. A study with a mouse model of prediabetes demonstrated that dysfunction of corneal nerves, upregulation of inflammatory mediators, and reduction in neutrophil numbers in the limbus may precede a state of hyperglycemia []. In contrast, mechanical epithelium damage was associated with the accumulation of neutrophils in the limbus, possibly explained by reduced migratory capabilities of inflammatory cells, resulting in a slower healing response in diabetes.
MicroRNAs (miRNAs) are 18–25 nucleotides long non-coding RNAs that downregulate the expression of genes at a post-transcription level by binding complementary mRNAs []. They are involved in numerous cellular processes and can modulate multiple genes, making them a valuable research focus in regenerative medicine []. Studies suggest that they also play a role in LESC-associated processes, such as macropinocytosis, autophagy, and the expression of putative stem cell markers [,]. Funari at al. reported on the dysregulation of miRNA expression in human autopsy diabetic corneas, which was associated with abnormal wound healing []. Microarray analysis demonstrated that among the 29 miRNA studied, miR-146a, 21, and 424 were the most upregulated, while miR-509-3p and 143 were expressed at the lowest level. This is in line with other reports, which indicate that overexpression of miR-146a in the diabetic limbus may result in a reduced corneal inflammatory and healing response [,,]. In another study, genome-wide sequencing was applied, identifying differences in expression profiles of 20 miRNA between normal and diabetic human corneas []. Results showed that miR-10b was upregulated in the diabetic limbus, with a higher increase observed in type 1 compared to type 2 diabetes. Moreover, altered expression of miRNA in exosomes have been demonstrated in diabetic limbal stem cells [].
Overall, diabetes may affect the function of LESCs through various mechanisms and can potentially lead to severe, persistent corneal erosions. However, despite the detrimental effect on the cornea, the limbal barrier tends to be preserved, with no development of conjunctivalization or neovascularization characteristic for limbal stem cell deficiency [].
Animal studies have demonstrated the beneficial effect of insulin treatment on epithelial wound healing in diabetic animals [,]. Klocek et al. found that corneal abrasions in type 1 diabetic mice, treated with topical insulin, reduced in size by 29% compared to the controls after 16 h []. Moreover, Zagon et al. reported a comparable rate of corneal healing in diabetic rats with an insulin implant and normal animals [].
The first therapeutic use of topical insulin in humans was described by Aynsely in 1945 in a case series of patients with corneal ulcers []. Successful treatment with topical insulin for corneal conditions involving the epithelium has also been reported in subsequent studies summarized in Table 3.

Table 3.
Published studies evaluating results of a treatment of corneal conditions with epithelial defects with a topical insulin.
Interestingly, a recent culture study with ocular surface cells showed that drops from plasma rich in growth factors were superior to topical insulin at two different concentrations (1 and 0.2 IU/m) in promoting wound healing and reducing the fibrosis process []. Currently, no universal protocol exists regarding treatment with topical insulin, as studies apply different preparation methods, dosages, concentrations, and types of drops [,].
Several studies have demonstrated novel ways of delivering insulin to the eye. A new delivery system, containing chitosan/poloxamer gel loaded with chitosan microparticles, has been reported to increase the local bioavailability of topical insulin in the treatment of the ocular surface in diabetic rats []. Additionally, electrospun fiber mats were shown to be effective in delivering insulin to the porcine cornea []. Recently, a convolutional neural network statistical analysis was applied to demonstrate the therapeutic effect of insulin liposomes on corneal epithelial defects in rats []. In a study of alkali-burned corneal models, a nano-system combining liposomes and trimethyl chitosan was used to deliver insulin and vascular endothelial growth factor small interfering RNA []. This combined therapy proved effective in reducing oxidative stress, increasing epithelialization, and inhibiting corneal neovascularization. Metabolomic analysis demonstrated that the therapeutic effect was possibly linked to insulin inhibiting the ferroptosis signaling pathway.
3. The Role of Insulin-like Growth Factor-1 (IGF-1) and -2 (IGF-2)
IGF-1 is a 7649 Daltons peptide hormone composed of seventy amino acids and produced mainly in the liver as a result of stimulation by human growth hormone []. IGF-1 binds to the IGF-1 receptor (IGF-1R) with 8 and 300 times higher affinity than IGF-2 and insulin, respectively []. Upon binding, it activates mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) []. IGF binding proteins (IGFBPs), which compete with IGF-1R, may prolong the half-life of free IGF-1 to several hours []. The concentration of IGF-1 in the tear film is comparable in males and females but declines with age []. Notably, the tear concentration of IGF-1 in diabetic mice was found to be reduced immediately after an alkaline chemical injury, as well as three and seven days later [].
IGF-1 plays a significant role in cell growth, proliferation, and migration. A study involving a three-dimensional culture of human embryonic stem cells demonstrated that IGF-1 signaling is essential in the development of corneal epithelial and stromal cells, as evidenced by CK19 and vimentin markers []. Additionally, IGF-1 stimulates the expression of IGF receptors in limbal stem cells and promotes their differentiation into the epithelium []. This process is enhanced in the case of corneal injury, resulting in the downregulation of IGF-1R receptors in corneal cells and higher penetration of remaining IGF-1 into the limbal niche. Notably, IGF-1 was found to prevent the reduction in corneal stem/progenitor cells markers, such as Hes1, Keratin15, and p75, while also increasing nerve density in diabetic mice [].
IGF-1 promotes the differentiation of murine mesenchymal stem cells into epithelia-like cells []. It is also speculated that the proliferation of human corneal epithelial cells facilitated by amniotic membrane occurs via IGF-1 []. Notably, corneal epithelial cells were demonstrated to release IGF-1, which in turn, increased the expression of N-cadherin (an adherens-junction protein) in corneal fibroblasts, enabling cell interactions to maintain tissue homeostasis []. IGF-1 has also been found to form complexes with vitronectin, resulting in enhanced corneal epithelium cell migration []. In addition, the migration of cultured human epithelial corneal cells is mediated by IGF-1 which increases the production of the matrix protein Ln-5 and its receptor β1 integrin via the PI3-K/AKT pathway []. Furthermore, it has been demonstrated that the protective effect of histatins (anti-microbial and anti-fungal proteins present in human saliva) in a cell model of UV-induced damage in human corneal epithelium occurs through the upregulated expression of IGF-1 and Bcl-2 [].
IGF-1 treatment following laser in situ keratomileusis in rabbit eyes showed an association with a higher number of epithelial microvilli and a faster rate of nerve regeneration []. More recently, promising therapeutic outcomes were achieved by combining modified mRNA (modRNA) technologies with stem cells to treat corneas in mice subjected to alkali burns []. IGF-1 modRNA-engineered adipose-derived mesenchymal stem cells (ADSCs) showed superiority over normal ADSCs and IGF-1 protein eyedrops in promoting corneal epithelium healing, stimulating trigeminal ganglion cells activity, and maintaining stemness of limbal stem cells.
The IGF-1R is present in all layers of the cornea []. Its highest concentration has been observed in proximity to cellular nuclei within actively differentiating corneal epithelial cells, forming complexes with E-cadherins to augment cellular adhesion processes []. The IGF-1R and the INSR are transmembrane glycoproteins characterized by two extracellular alpha sub-units constituting the ligand-binding domain and two transmembrane beta subunits with inherent tyrosine kinase activity []. These receptors exhibit an amino acid sequence homology exceeding 50%. The shared structural features between the two receptors facilitate the formation of insulin and IGF-1 hybrid receptors (Hybrid-R). The precise mechanisms driving this formation remain elusive, with hypotheses suggesting a potential influence of the IGF-1R to INSR ratio or developmental factors. Moreover, Hybrid-R has been observed to exhibit a greater affinity for binding with IGF-1 compared than insulin, and its nuclear localization has been demonstrated in human corneal epithelial cells []. It is suggested that IGF-1R and INSR might be present in the nucleus of corneal epithelial cells only as Hybrid-R and as a result of upregulated expression of IGF-1R and INSR in the absence of insulin [].
The synergistic effect of substance P (SP) and IGF-1 in promoting cell migration and, consequently, corneal epithelium healing, has been widely reported [,,,,,], mediated by p38 MAP kinase [] and protein kinase C pathways []. Peptide FGLM-amide has been identified as the minimal component of SP capable of promoting corneal wound closure in combination with IGF-1 []. The combined action of SP and IGF-1 has proven effective in preventing superficial punctate keratopathy after cataract surgery in patients with diabetes [] and treating persistent epithelial defects [,,,]. Moreover, this combination reduces epithelial healing time by 70 h on average in a rabbit eye after PRK []. When applied topically, SP (or the SP-derived peptide FGLM-amide) with IGF-1 improves corneal epithelial barrier function and enhances the healing process in rat models of neurotrophic keratopathy [,,]. However, in dogs, no benefit of adding topical IGF-1 to SP was demonstrated when treating spontaneous chronic corneal epithelial defects [].
Insulin-like growth factor-2 (IGF-2) and its receptor (IGF-2R) have been found in corneal epithelial cells and demonstrated to play a role in corneal regeneration [,]. Unlike insulin but similar to IGF-1, the IGF-2 structure has no D domain []. It binds to IGF-2R, which is a monomeric transmembrane protein comprising 15 different domains in its extracellular region []. Following injury, there is a significant increase in the expression of IGF-2 and its receptor in corneal peripheral cells. Moreover, IGF-2 has been shown to stimulate LESC differentiation, evidenced by the expression of K12 cell markers []. Pterygium, a common degenerative condition resulting in conjunctival overgrowth which may extend beyond the limbus and involve the cornea [], was found to exhibit overexpression of IGF-2 and IGF-1R compared to normal conjunctiva, as revealed by immunohistochemical analysis [].
4. The Role of IGF-Binding Proteins (IGFBPs)
The IGF-binding proteins (IGFBPs) regulate IGFs availability and activity, due to their equal or greater affinity than the IGF-1 receptor []. In the circulation, they increase the half-life of IGFs and block them from binding to the insulin receptor. While the IGFBP family members exhibit notable sequence homology, each possesses distinct structural features and functions.
IGFBP-2, a protein with a molecular weight of 36 kDa [], plays a role in the growth and development of eye structures, with the highest ocular concentration measured in the cornea [,]. Its presence in the corneal germinal epithelium was demonstrated in developing rat eyes []. In chicken corneal epithelium, IGFB-2 was detected as early as at 3.5 days of embryonic development [].
Insulin-like growth factor binding protein-3 (IGFBP3) is an N-linked glycosylated, phosphorylated, secretory protein [] found in the cornea, including the cytoplasm of basal and suprabasal limbal epithelial cells []. It is a pleiotropic protein involved in cell survival by blocking IGF-1 from activating IGF-1R with hyperosmolar stress, reducing its expression [,]. IGFBP-3 acts as a molecular regulator of mitochondrial structure and function in epithelial cells []. The interplay between IGF-1R and IGFBP-3 contributes to corneal epithelium homeostasis, independently of P13K/Akt pathway []. Notably, IGFBP-3 promotes nuclear translocation of IGF-1R via SUMOylation by SUMO 2/3. Sirtuin (silent mating type information regulation 2 homolog) 1 (SIRT1), a class III histone deacetylase, inhibits IGFBP-3 via decreased acetylation of p53 which results in the activation of the IGF-1R/AKT pathway and promotes corneal epithelial wound healing []. IGFBP-3 was found in tears and at 2.8–3.5 higher concentration in diabetics compared to non-diabetic controls [,,]. Moreover, in diabetes, its tear level shows a negative correlation with the length of nerve fibers and the density of nerve branches []. However, it is not fully clear if it is secondary to increased secretion or release from damaged corneal epithelium. In HSV-1 infected corneas, the immunofluorescence staining revealed the cytosolic accumulation and nuclear localization of IGFBP-3 within the infected corneal epithelial cells [].
Insulin-like growth factor binding protein-5 (IGFBP-5) has been identified in the cornea, with upregulated expression demonstrated in diabetic rats []. A recent study showed that the expression of IGFBP-5 can be inhibited by microRNA miR-203 present in tears, resulting in reduced viability of corneal epithelial cells [].
Insulin-like growth factor binding protein-7 (IGFBP-7) weighs 27 kDa and shows 94.4% similarity between human and mouse proteins []. It has high affinity for insulin and low affinity for IGF-1 as well as IGF-2 []. IGFBP-7 plays a role in angiogenesis, constitutes a target for transforming growth factor (TGF)-β1 [], and is considered a biomarker of conjunctivalization in limbal stem cell deficiency [].
5. Conclusions
In summary, the intricate interplay between the corneal epithelium and the surrounding microenvironment is essential for maintaining ocular health and function. Notably, the limbal epithelial stem cells, strategically located in the limbal region, serve as central players in ensuring the integrity of the corneal epithelium through their remarkable ability to replenish and renew cells.
The IGF system is involved in the regulation of corneal epithelial physiology, contributing to essential processes, such as wound healing, with implications for the activity of limbal epithelial stem cells. Insulin, particularly due to its pivotal association with diabetes, assumes a central role within the system. Diabetes represents a significant public health challenge, given its widespread prevalence and significant impact on the eye, including the corneal epithelium.
Further research is warranted to uncover the complexity of the interplay between the IGF system components, other signaling pathways, and the corneal epithelium. Elucidating the molecular mechanisms that govern their interactions will not only deepen our understanding of corneal epithelium homeostasis, but also pave the way for more targeted therapeutic interventions. In the future, advances in gene therapy and regenerative medicine involving limbal epithelial stem cells may offer promising avenues for manipulating these intricate processes to treat corneal disorders.
Author Contributions
Conceptualization, M.W., H.R. and P.S.; methodology, M.W.; writing—original draft preparation, M.W.; writing—review and editing, M.W., H.R. and P.S.; visualization, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vaidyanathan, U.; Hopping, G.C.; Liu, H.Y.; Somani, A.N.; Ronquillo, Y.C.; Hoopes, P.C.; Moshirfar, M. Persistent Corneal Epithelial Defects: A Review Article. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2019, 8, 163–176. [Google Scholar]
- Tarvestad-Laise, K.E.; Ceresa, B.P. Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis. Cells 2023, 12, 2730. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. IGFBP-3 Functions as a Molecular Switch That Mediates Mitochondrial and Metabolic Homeostasis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22062. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z Hypothesis of Corneal Epithelial Maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoudé, G.A.; Barrandon, Y. Oligopotent Stem Cells Are Distributed throughout the Mammalian Ocular Surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Green, C.R.; McGhee, C.N.J.; Sherwin, T. Acute Wound Healing in the Human Central Corneal Epithelium Appears to Be Independent of Limbal Stem Cell Influence. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Rafii, M.; Sullivan, D.A.; Morelli, J.; Ding, J. Novel Therapy to Treat Corneal Epithelial Defects: A Hypothesis with Growth Hormone. Ocul. Surf. 2015, 13, 204–212.e1. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N. Stem Cells of the Human Cornea. Br. Med. Bull. 2011, 100, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Zieske, J.D. The Corneal Epithelial Stem Cell Niche. Ocul. Surf. 2005, 3, 15–26. [Google Scholar] [CrossRef]
- Mann, I. A Study of Epithelial Regeneration in the Living Eye. Br. J. Ophthalmol. 1944, 28, 26–40. [Google Scholar] [CrossRef]
- Di Girolamo, N. “Eyeing” Corneal Stem Cell Identity, Dynamics, and Compartmentalization. Cell Stem Cell 2021, 28, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, O.; Suzuki-Horiuchi, Y.; Brewster, M.; Kuri, P.; Huang, S.; Rice, G.; Bae, H.; Xu, J.; Dentchev, T.; Lee, V.; et al. Two-Photon Live Imaging of Single Corneal Stem Cells Reveals Compartmentalized Organization of the Limbal Niche. Cell Stem Cell 2021, 28, 1233–1247.e4. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete Limbal Epithelial Stem Cell Populations Mediate Corneal Homeostasis and Wound Healing. Cell Stem Cell 2021, 28, 1248–1261.e8. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wang, Q.; Qi, X.; Zhang, B.; Jiang, H.; Chen, S.; Duan, H.; Lu, Y.; Dong, J.; Cao, Y.; et al. Molecular Identity of Human Limbal Heterogeneity Involved in Corneal Homeostasis and Privilege. Ocul. Surf. 2021, 21, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N. Moving Epithelia: Tracking the Fate of Mammalian Limbal Epithelial Stem Cells. Prog. Retin. Eye Res. 2015, 48, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, V.A.; Foster, T.; Kulkarni, B.B.; Hopkinson, A.; Gray, T.; Powe, D.G.; Lowe, J.; Dua, H.S. Morphological Characteristics of the Limbal Epithelial Crypt. Br. J. Ophthalmol. 2007, 91, 514–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldberg, M.F.; Bron, A.J. Limbal Palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar]
- Townsend, W.M. The Limbal Palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1991, 89, 721–756. [Google Scholar]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Shanley, L.J.; McCaig, C.D.; Forrester, J.V.; Zhao, M. Insulin, Not Leptin, Promotes in Vitro Cell Migration to Heal Monolayer Wounds in Human Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1088–1094. [Google Scholar] [CrossRef]
- Lyu, J.; Lee, K.-S.; Joo, C.-K. Transactivation of EGFR Mediates Insulin-Stimulated ERK1/2 Activation and Enhanced Cell Migration in Human Corneal Epithelial Cells. Mol. Vis. 2006, 12, 1403–1410. [Google Scholar]
- Titone, R.; Robertson, D.M. Insulin Receptor Preserves Mitochondrial Function by Binding VDAC1 in Insulin Insensitive Mucosal Epithelial Cells. FASEB J. 2020, 34, 754–775. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xue, Y.; Dong, D.; Liu, J.; Fu, T.; Xiao, C.; Wang, H.; Lin, C.; Liu, P.; Zhong, J.; et al. Insulin Restores an Altered Corneal Epithelium Circadian Rhythm in Mice with Streptozotocin-Induced Type 1 Diabetes. Sci. Rep. 2016, 6, 32871. [Google Scholar] [CrossRef] [PubMed]
- Nureen, L.; Di Girolamo, N. Limbal Epithelial Stem Cells in the Diabetic Cornea. Cells 2023, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Hattori, T.; Kumagai, Y.; Suzuki, N.; Ueno, S.; Takagi, H. Alterations in the Corneal Nerve and Stem/Progenitor Cells in Diabetes: Preventive Effects of Insulin-like Growth Factor-1 Treatment. Int. J. Endocrinol. 2014, 2014, 312401. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Li, Y.; Sun, D.; Chen, R.; Dou, S.; Liu, T.; Zhang, S.; Zhou, Q.; Xie, L. Interference of Sympathetic Overactivation Restores Limbal Stem/Progenitor Cells Function and Accelerates Corneal Epithelial Wound Healing in Diabetic Mice. Biomed. Pharmacother. 2023, 161, 114523. [Google Scholar] [CrossRef] [PubMed]
- Kramerov, A.A.; Saghizadeh, M.; Ljubimov, A.V. Adenoviral Gene Therapy for Diabetic Keratopathy: Effects on Wound Healing and Stem Cell Marker Expression in Human Organ-Cultured Corneas and Limbal Epithelial Cells. J. Vis. Exp. 2016, 110, e54058. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Soleymani, S.; Harounian, A.; Bhakta, B.; Troyanovsky, S.M.; Brunken, W.J.; Pellegrini, G.; Ljubimov, A.V. Alterations of Epithelial Stem Cell Marker Patterns in Human Diabetic Corneas and Effects of C-Met Gene Therapy. Mol. Vis. 2011, 17, 2177–2190. [Google Scholar] [PubMed]
- Kramerov, A.A.; Saghizadeh, M.; Maguen, E.; Rabinowitz, Y.S.; Ljubimov, A.V. Persistence of Reduced Expression of Putative Stem Cell Markers and Slow Wound Healing in Cultured Diabetic Limbal Epithelial Cells. Mol. Vis. 2015, 21, 1357–1367. [Google Scholar]
- Mellough, C.B.; Collin, J.; Khazim, M.; White, K.; Sernagor, E.; Steel, D.H.W.; Lako, M. IGF-1 Signaling Plays an Important Role in the Formation of Three-Dimensional Laminated Neural Retina and Other Ocular Structures from Human Embryonic Stem Cells. Stem Cells 2015, 33, 2416–2430. [Google Scholar] [CrossRef]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The Key Role of Insulin-like Growth Factor I in Limbal Stem Cell Differentiation and the Corneal Wound-Healing Process. Stem Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, S.L.; Barnard, Z.; Upton, Z.; Harkin, D.G. Vitronectin Supports Migratory Responses of Corneal Epithelial Cells to Substrate Bound IGF-I and HGF, and Facilitates Serum-Free Cultivation. Exp. Eye Res. 2006, 83, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Hyung, K.L.; Lee, J.H.; Kim, M.; Kariya, Y.; Miyazaki, K.; Eung, K.K. Insulin-like Growth Factor-1 Induces Migration and Expression of Laminin-5 in Cultured Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 873–882. [Google Scholar] [CrossRef]
- Jiang, Y.; Ju, Z.; Zhang, J.; Liu, X.; Tian, J.; Mu, G. Effects of Insulin-like Growth Factor 2 and Its Receptor Expressions on Corneal Repair. Int. J. Clin. Exp. Pathol. 2015, 8, 10185–10191. [Google Scholar] [PubMed]
- Bohnsack, R.N.; Warejcka, D.J.; Wang, L.; Gillespie, S.R.; Bernstein, A.M.; Twining, S.S.; Dahms, N.M. Expression of Insulin-like Growth Factor 2 Receptor in Corneal Keratocytes during Differentiation and in Response to Wound Healing. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7697–7708. [Google Scholar] [CrossRef] [PubMed]
- Schoen, T.J.; Bondy, C.A.; Zhou, J.; Dhawan, R.; Mazuruk, K.; Arnold, D.R.; Rodriguez, I.R.; Waldbillig, R.J.; Beebe, D.C.; Chader, G.J. Differential Temporal and Spatial Expression of Insulin-like Growth Factor Binding Protein-2 in Developing Chick Ocular Tissues. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2652–2662. [Google Scholar]
- Burren, C.P.; Berka, J.L.; Batch, J.A. Localization Studies of IGFBP-2 and IGFBP-5 in the Anterior Compartment of the Eye. Curr. Eye Res. 1997, 16, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Titone, R.; Zhu, M.; Robertson, D.M. Mutual Regulation between IGF-1R and IGFBP-3 in Human Corneal Epithelial Cells. J. Cell. Physiol. 2019, 234, 1426–1441. [Google Scholar] [CrossRef]
- Nakagawa, A.; Nakajima, T.; Azuma, M. Tear MiRNA Expression Analysis Reveals MiR-203 as a Potential Regulator of Corneal Epithelial Cells. BMC Ophthalmol. 2021, 21, 377. [Google Scholar] [CrossRef]
- Park, M.; Mazalo, J.; Di Girolamo, N. Insulin-like Growth Factor Binding Protein-7: A Marker of Conjunctivalization in an Animal Model of Limbal Stem Cell Deficiency. Ocul. Surf. 2019, 17, 447–457. [Google Scholar] [CrossRef]
- Naeser, P. Insulin Receptors in Human Ocular Tissues. Immunohistochemical Demonstration in Normal and Diabetic Eyes. Ups. J. Med. Sci. 1997, 102, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Cunha, D.A.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Identification of Insulin in the Tear Film and Insulin Receptor and IGF-1 Receptor on the Human Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2002, 43, 963–967. [Google Scholar]
- Robertson, D.M.; Zhu, M.; Wu, Y.-C. Cellular Distribution of the IGF-1R in Corneal Epithelial Cells. Exp. Eye Res. 2012, 94, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Zhu, M.; Robertson, D.M. Novel Nuclear Localization and Potential Function of Insulin-like Growth Factor-1 Receptor/Insulin Receptor Hybrid in Corneal Epithelial Cells. PLoS ONE 2012, 7, e42483. [Google Scholar] [CrossRef] [PubMed]
- Tests, D.; Diabetes, F.O.R. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohara, K.; Ohmura, T.; Takahashi, R.; Zieske, J.D. Glucose Transporter 1 Expression in Corneal Wound Repair under High Serum Glucose Level. Jpn. J. Ophthalmol. 2000, 44, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Chandler, H.L. Insulin Facilitates Corneal Wound Healing in the Diabetic Environment through the RTK-PI3K/Akt/MTOR Axis in Vitro. Mol. Cell. Endocrinol. 2022, 548, 111611. [Google Scholar] [CrossRef]
- Herencia-Bueno, K.E.; Aldrovani, M.; Crivelaro, R.M.; Thiesen, R.; Barros-Sobrinho, A.A.F.; Claros-Chacaltana, F.D.Y.; Padua, I.R.M.; Santos, D.M.; Laus, J.L. Reduction in Histone H3 Acetylation and Chromatin Remodeling in Corneas of Alloxan-Induced Diabetic Rats. Cornea 2018, 37, 624–632. [Google Scholar] [CrossRef]
- de Cássia Alves, M.; Carvalheira, J.B.; Módulo, C.M.; Rocha, E.M. Tear Film and Ocular Surface Changes in Diabetes Mellitus. Arq. Bras. Oftalmol. 2008, 71, 96–103. [Google Scholar] [CrossRef]
- Módulo, C.M.; Jorge, A.G.; Dias, A.C.; Braz, A.M.; Bertazolli-Filho, R.; Jordão, A.A.J.; Sérgio Marchini, J.; Rocha, E.M. Influence of Insulin Treatment on the Lacrimal Gland and Ocular Surface of Diabetic Rats. Endocrine 2009, 36, 161–168. [Google Scholar] [CrossRef]
- Cunha, D.A.; de Alves, M.C.; Stoppiglia, L.F.; Jorge, A.G.; Módulo, C.M.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A.; Rocha, E.M. Extra-Pancreatic Insulin Production in RAt Lachrymal Gland after Streptozotocin-Induced Islet Beta-Cells Destruction. Biochim. Biophys. Acta 2007, 1770, 1128–1135. [Google Scholar] [CrossRef]
- Rocha, E.M.; Lima, M.H.d.M.; Carvalho, C.R.; Saad, M.J.; Velloso, L.A. Characterization of the Insulin-Signaling Pathway in Lacrimal and Salivary Glands of Rats. Curr. Eye Res. 2000, 21, 833–842. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Coassin, M.; Micera, A.; Mori, T.; De Piano, M.; Scartozzi, L.; Sgrulletta, R.; Bonini, S. Ocular Surface Diabetic Disease: A Neurogenic Condition? Ocul. Surf. 2021, 19, 218–223. [Google Scholar] [CrossRef]
- Manchikanti, V.; Kasturi, N.; Rajappa, M.; Gochhait, D. Ocular Surface Disorder among Adult Patients with Type II Diabetes Mellitus and Its Correlation with Tear Film Markers: A Pilot Study. Taiwan J. Ophthalmol. 2021, 11, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Çakır, B.K.; Katırcıoğlu, Y.; Ünlü, N.; Duman, S.; Üstün, H. Ocular Surface Changes in Patients Treated with Oral Antidiabetic Drugs or Insulin. Eur. J. Ophthalmol. 2016, 26, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, I.; Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Ocular Surface Complications in Diabetes: The Interrelationship between Insulin and Enkephalin. Biochem. Pharmacol. 2021, 192, 114712. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, M.J.; Popper, M.; Morgado, A.M.; Murta, J.N.; Van Best, J.A. Diabetes and Corneal Cell Densities in Humans by in Vivo Confocal Microscopy. Cornea 2006, 25, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Kimsey, R.A. Corneal Epithelial Basement Membrane Changes in Diabetes. Investig. Ophthalmol. Vis. Sci. 1981, 20, 548–553. [Google Scholar]
- Qu, J.H.; Li, L.; Tian, L.; Zhang, X.Y.; Thomas, R.; Sun, X.G. Epithelial Changes with Corneal Punctate Epitheliopathy in Type 2 Diabetes Mellitus and Their Correlation with Time to Healing. BMC Ophthalmol. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, L.; Latour, G.; Bourges, J.-L.; Savoldelli, M.; Jeanny, J.-C.; Plamann, K.; Schanne-Klein, M.-C.; Behar-Cohen, F. Multimodal Highlighting of Structural Abnormalities in Diabetic Rat and Human Corneas. Transl. Vis. Sci. Technol. 2013, 2, 3. [Google Scholar] [CrossRef]
- Ishibashi, F.; Kawasaki, A.; Yamanaka, E.; Kosaka, A.; Uetake, H. Morphometric Features of Corneal Epithelial Basal Cells, and Their Relationship with Corneal Nerve Pathology and Clinical Factors in Patients with Type 2 Diabetes. J. Diabetes Investig. 2013, 4, 492–501. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; Purushothaman, I.; McLaughlin, P.J. Blockade of OGFr Delays the Onset and Reduces the Severity of Diabetic Ocular Surface Complications. Exp. Biol. Med. 2021, 246, 629–636. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; Purushothaman, I.; McLaughlin, P.J. Dysregulation of the OGF–OGFr Pathway Correlates with Elevated Serum OGF and Ocular Surface Complications in the Diabetic Rat. Exp. Biol. Med. 2020, 245, 1414–1421. [Google Scholar] [CrossRef]
- Purushothaman, I.; Zagon, I.S.; Sassani, J.W.; Zhou, S.; McLaughlin, P.J. Sex Differences in the Magnitude of Diabetic Ocular Surface Complications: Role of Serum OGF. Physiol. Behav. 2021, 237, 113436. [Google Scholar] [CrossRef]
- Chen, D.; Wang, L.; Guo, X.; Zhang, Z.; Xu, X.; Jin, Z.-B.; Liang, Q. Evaluation of Limbal Stem Cells in Patients With Type 2 Diabetes: An In Vivo Confocal Microscopy Study. Cornea 2024, 43, 67–75. [Google Scholar] [CrossRef]
- Mort, R.L.; Douvaras, P.; Morley, S.D.; Dorà, N.; Hill, R.E.; Collinson, J.M.; West, J.D. Stem Cells and Corneal Epithelial Maintenance: Insights from the Mouse and Other Animal Models. Results Probl. Cell Differ. 2012, 55, 357–394. [Google Scholar] [CrossRef]
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of Corneal Stem/Progenitor Cells on Ocular Surface Innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872. [Google Scholar] [CrossRef]
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Zirie, M.A.; Qazi, M.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; et al. Painful Diabetic Neuropathy Is Associated with Increased Nerve Regeneration in Patients with Type 2 Diabetes Undergoing Intensive Glycemic Control. J. Diabetes Investig. 2021, 12, 1642–1650. [Google Scholar] [CrossRef]
- Mahelková, G.; Burdová, M.Č.; Malá, Š.; Hoskovcová, L.; Dotřelová, D.; Štechová, K. Higher Total Insulin Dose Has Positive Effect on Corneal Nerve Fibers in DM1 Patients. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3800–3807. [Google Scholar] [CrossRef]
- Issar, T.; Tummanapalli, S.S.; Kwai, N.C.G.; Chiang, J.C.B.; Arnold, R.; Poynten, A.M.; Markoulli, M.; Krishnan, A. V Associations between Acute Glucose Control and Peripheral Nerve Structure and Function in Type 1 Diabetes. Diabet. Med. 2020, 37, 1553–1560. [Google Scholar] [CrossRef]
- He, J.; Bazan, H.E.P. Mapping the Nerve Architecture of Diabetic Human Corneas. Ophthalmology 2012, 119, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Slater, J.A.; McGhee, C.N.J.; Pradhan, M.; Braatvedt, G.D. Corneal Confocal Microscopy in Type 1 Diabetes Mellitus: A Six-Year Longitudinal Study. Transl. Vis. Sci. Technol. 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of Diet-Induced Obesity or Type 1 or Type 2 Diabetes on Corneal Nerves and Peripheral Neuropathy in C57Bl/6J Mice. J. Peripher. Nerv. Syst. 2015, 20, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Lupachyk, S.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of Glycemic Control on Corneal Nerves and Peripheral Neuropathy in Streptozotocin-Induced Diabetic C57Bl/6J Mice. J. Peripher. Nerv. Syst. 2014, 19, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.P.; Coppey, L.J.; Kardon, R.H.; Yorek, M.A. Differences and Similarities in Development of Corneal Nerve Damage and Peripheral Neuropathy and in Diet-Induced Obesity and Type 2 Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1222–1230. [Google Scholar] [CrossRef]
- Chen, D.K.; Frizzi, K.E.; Guernsey, L.S.; Ladt, K.; Mizisin, A.P.; Calcutt, N.A. Repeated Monitoring of Corneal Nerves by Confocal Microscopy as an Index of Peripheral Neuropathy in Type-1 Diabetic Rodents and the Effects of Topical Insulin. J. Peripher. Nerv. Syst. 2013, 18, 306–315. [Google Scholar] [CrossRef]
- Chan, K.; Badanes, Z.; Ledbetter, E.C. Decreased Corneal Subbasal Nerve Fiber Length and Density in Diabetic Dogs with Cataracts Using in Vivo Confocal Microscopy. Vet. Ophthalmol. 2023, 26, 524–531. [Google Scholar] [CrossRef]
- Machet, J.; Park, M.; Richardson, A.; Carnell, M.; Mouat, M.A.; Smith, N.J.; Turner, N.; Cochran, B.J.; Rye, K.A.; Di Girolamo, N. Type 2 Diabetes Influences Intraepithelial Corneal Nerve Parameters and Corneal Stromal-Epithelial Nerve Penetration Sites. J. Diabetes Investig. 2023, 14, 591–601. [Google Scholar] [CrossRef]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The Impact of Diabetes on Corneal Nerve Morphology and Ocular Surface Integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, A.; Lo, A.C.Y.; Kwok, J.S.W.J. Diabetic Corneal Neuropathy: Pathogenic Mechanisms and Therapeutic Strategies. Front. Pharmacol. 2022, 13, 816062. [Google Scholar] [CrossRef]
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. [Google Scholar] [CrossRef]
- Deardorff, P.M.; McKay, T.B.; Wang, S.; Ghezzi, C.E.; Cairns, D.M.; Abbott, R.D.; Funderburgh, J.L.; Kenyon, K.R.; Kaplan, D.L. Modeling Diabetic Corneal Neuropathy in a 3D In Vitro Cornea System. Sci. Rep. 2018, 8, 17294. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Qin, L.; Wang, L.; Huang, Y. Increased Membrane Localization of Pannexin1 in Human Corneal Synaptosomes Causes Enhanced Stimulated ATP Release in Chronic Diabetes Mellitus. Medicine 2016, 95, e5084. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Zhang, Z.; Dan, J.; Zhou, Q.; Wang, X.; Li, W.; Zhou, L.; Yang, L.; Xie, L. Insulin Promotes Corneal Nerve Repair and Wound Healing in Type 1 Diabetic Mice by Enhancing Wnt/β-Catenin Signaling. Am. J. Pathol. 2020, 190, 2237–2250. [Google Scholar] [CrossRef]
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Zirie, M.A.; Al-Mohannadi, S.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; et al. Insulin Resistance Limits Corneal Nerve Regeneration in Patients with Type 2 Diabetes Undergoing Intensive Glycemic Control. J. Diabetes Investig. 2021, 12, 2002–2009. [Google Scholar] [CrossRef]
- Hargrave, A.; Courson, J.A.; Pham, V.; Landry, P.; Magadi, S.; Shankar, P.; Hanlon, S.; Das, A.; Rumbaut, R.E.; Wayne Smith, C.; et al. Corneal Dysfunction Precedes the Onset of Hyperglycemia in a Mouse Model of Diet-Induced Obesity. PLoS ONE 2020, 15, e0238750. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Zhang, X.; Wu, M.; Karunaratne, S.; Loi, J.K.; Senthil, K.; Arshad, S.; Bertram, K.; Cunningham, A.L.; Carnt, N.; et al. Redefining the Human Corneal Immune Compartment Using Dynamic Intravital Imaging. Proc. Natl. Acad. Sci. USA 2023, 120, e2217795120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Peng, H.; Katsnelson, J.; Yang, W.; Kaplan, N.; Dong, Y.; Rappoport, J.Z.; He, C.; Lavker, R.M. MicroRNAs-103/107 Coordinately Regulate Macropinocytosis and Autophagy. J. Cell Biol. 2016, 215, 667–685. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Chen, Q.; Wei, Y.; Wei, Z.; Jin, Z.-B.; Liang, Q. Extracellular Vesicle MicroRNAs From Corneal Stromal Stem Cell Enhance Stemness of Limbal Epithelial Stem Cells by Targeting the Notch Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 42. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.A.; Winkler, M.; Brown, J.; Dimitrijevich, S.D.; Ljubimov, A.V.; Saghizadeh, M. Differentially Expressed Wound Healing-Related MicroRNAs in the Human Diabetic Cornea. PLoS ONE 2013, 8, e84425. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.A.; Dib, C.; Ljubimov, A.V.; Saghizadeh, M. Targeting MiR-146a to Treat Delayed Wound Healing in Human Diabetic Organ-Cultured Corneas. PLoS ONE 2014, 9, e114692. [Google Scholar] [CrossRef]
- Poe, A.J.; Shah, R.; Khare, D.; Kulkarni, M.; Phan, H.; Ghiam, S.; Punj, V.; Ljubimov, A.V.; Saghizadeh, M. Regulatory Role of MiR-146a in Corneal Epithelial Wound Healing via Its Inflammatory Targets in Human Diabetic Cornea: MiR-146a Inflammatory Role in Diabetic Corneal Epithelial Wound Healing. Ocul. Surf. 2022, 25, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Leszczynska, A.; Wei, G.; Winkler, M.A.; Tang, J.; Funari, V.A.; Deng, N.; Liu, Z.; Punj, V.; Deng, S.X.; et al. Genome-Wide Analysis Suggests a Differential MicroRNA Signature Associated with Normal and Diabetic Human Corneal Limbus. Sci. Rep. 2017, 7, 3448. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from Normal and Diabetic Human Corneolimbal Keratocytes Differentially Regulate Migration, Proliferation and Marker Expression of Limbal Epithelial Cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J.; Zagon, I.S. Naltrexone and Insulin Are Independently Effective but Not Additive in Accelerating Corneal Epithelial Healing in Type I Diabetic Rats. Exp. Eye Res. 2009, 89, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Insulin Treatment Ameliorates Impaired Corneal Reepithelialization in Diabetic Rats. Diabetes 2006, 55, 1141–1147. [Google Scholar] [CrossRef]
- Aynsley, T.R. The use of insulin in the treatment of corneal ulcers. Br. J. Ophthalmol. 1945, 29, 361–363. [Google Scholar] [CrossRef]
- Fai, S.; Ahem, A.; Mustapha, M.; Mohd Noh, U.K.; Bastion, M.-L.C. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia-Pac. J. Ophthalmol. 2017, 6, 418–424. [Google Scholar] [CrossRef]
- Dasrilsyah, A.M.; Wan Abdul Halim, W.H.; Mustapha, M.; Tang, S.F.; Kaur, B.; Ong, E.Y.; Catherine Bastion, M.L. Randomized Clinical Trial of Topical Insulin Versus Artificial Tears for Healing Rates of Iatrogenic Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Cornea 2023, 42, 1395–1403. [Google Scholar] [CrossRef]
- Balal, S.; Din, N.; Ashton, C.; Ahmad, S. Healing of Chemical Injury-Related Persistent Corneal Epithelial Defects With Topical Insulin. Cornea 2022, 42, 1000–1004. [Google Scholar] [CrossRef]
- Diaz-Valle, D.; Burgos-Blasco, B.; Gegundez-Fernandez, J.A.; Garcia-Caride, S.; Puebla-Garcia, V.; Peña-Urbina, P.; Benitez-del-Castillo, J.M. Topical Insulin for Refractory Persistent Corneal Epithelial Defects. Eur. J. Ophthalmol. 2021, 31, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Ibrahim, M.; Nage, S. Efficacy of Topical Insulin for Recurrent Epithelial Corneal Erosions. Ir. J. Med. Sci. 2023, 192, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.J.D.S.M.; Arêde, C.; Sousa Neves, F.; da Silva Fernandes, J.; Cunha Ferreira, C.; Sequeira, J. Topical Insulin-Utility and Results in Refractory Neurotrophic Keratopathy in Stages 2 and 3. Cornea 2022, 41, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valle, D.; Burgos-Blasco, B.; Rego-Lorca, D.; Puebla-Garcia, V.; Perez-Garcia, P.; Benitez-Del-Castillo, J.M.; Herrero-Vanrell, R.; Vicario-de-la-Torre, M.; Gegundez-Fernandez, J.A. Comparison of the Efficacy of Topical Insulin with Autologous Serum Eye Drops in Persistent Epithelial Defects of the Cornea. Acta Ophthalmol. 2022, 100, e912–e919. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Weinlander, E.; Metcalf, B.M.; Barney, N.P.; Gamm, D.M.; Nehls, S.M.; Struck, M.C. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 2017, 36, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Bastion, M.L.C.; Ling, K.P. Topical Insulin for Healing of Diabetic Epithelial Defects?: A Retrospective Review of Corneal Debridement during Vitreoretinal Surgery in Malaysian Patients. Med. J. Malays. 2013, 68, 208–216. [Google Scholar]
- Burgos-Blasco, B.; Diaz-Valle, D.; Rego-Lorca, D.; Perez-Garcia, P.; Puebla-Garcia, V.; Fernandez-Vigo, J.I.; Benitez-Del-Castillo, J.M.; Gegundez-Fernandez, J.A. Topical Insulin, a Novel Corneal Epithelial Regeneration Agent in Dry Eye Disease. Eur. J. Ophthalmol. 2023, 11206721231206790. [Google Scholar] [CrossRef]
- Serrano-Giménez, R.; Contreras-Macías, E.; García-Bernal, A.; Fobelo-Lozano, M.J. Insulina Tópica En El Tratamiento de Úlcera Corneal Refractaria En Un Paciente No Diabético: A Propósito de Un Caso. Farm. Hosp. 2020, 44, 297–299. [Google Scholar] [CrossRef]
- Tong, C.M.; Iovieno, A.; Yeung, S.N. Topical Insulin for Neurotrophic Corneal Ulcers. Can. J. Ophthalmol. 2020, 55, e170–e172. [Google Scholar] [CrossRef]
- Galvis, V.; Niño, C.A.; Tello, A.; Grice, J.M.; Gómez, M.A. Topical Insulin in Neurotrophic Keratopathy after Resection of Acoustic Neuroma. Arch. Soc. Esp. Oftalmol. 2019, 94, 100–104. [Google Scholar] [CrossRef]
- Giannaccare, G.; Coco, G.; Rossi, C.; Borselli, M.; Lucisano, A.; Vaccaro, S.; Verdiglione, M.; Scorcia, V. Combined Use of Therapeutic Hyper-CL Soft Contact Lens and Insulin Eye Drops for the Treatment of Recalcitrant Neurotrophic Keratopathy. Cornea 2023, 43, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Khilji, M.; Tanveer, S.; Khan, F.Z.; Yazdan, D.A.; Khilji, A. Neurotrophic Keratopathy and Topical Insulin Therapy: A Case Report. Cureus 2023, 15, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Sánchez-Ávila, R.M.; de la Sen-Corcuera, B.; Merayo-Lloves, J.; Muruzábal, F. Beneficial Effects of Plasma Rich in Growth Factors (PRGF) Versus Autologous Serum and Topical Insulin in Ocular Surface Cells. Curr. Eye Res. 2023, 48, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Castro Mora, M.P.; Palacio Varona, J.; Perez Riaño, B.; Laverde Cubides, C.; Rey-Rodriguez, D.V. Effectiveness of Topical Insulin for the Treatment of Surface Corneal Pathologies. Arch. La Soc. Española Oftalmol. 2023, 98, 220–232. [Google Scholar] [CrossRef]
- Jaworski, M.; Lorenc, A.; Leszczyński, R.; Mrukwa-Kominek, E. Topical Insulin in Neurotrophic Keratopathy: A Review of Current Understanding of the Mechanism of Action and Therapeutic Approach. Pharmaceutics 2023, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cazarim, E.L.C.; Cazarim, M.S.; Ogunjimi, A.T.; Petrilli, R.; Rocha, E.M.; Lopez, R.F. V Prospective Insulin-Based Ophthalmic Delivery Systems for the Treatment of Dry Eye Syndrome and Corneal Injuries. Eur. J. Pharm. Biopharm. 2019, 140, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Voronova, A.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M.; Sanyal, A.; Demir, B.; Hubert, T.; Plaisance, V.; Pawlowski, V.; Vignoud-Despond, S.; et al. Photothermal Activatable Mucoadhesive Fiber Mats for On-Demand Delivery of Insulin via Buccal and Corneal Mucosa. ACS Appl. Bio. Mater. 2022, 5, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jiang, H.; Du, Y.; Xiong, X.; Zhang, Y.; Du, Z. Using Convolutional Neural Network as a Statistical Algorithm to Explore the Therapeutic Effect of Insulin Liposomes on Corneal Inflammation. Comput. Intell. Neurosci. 2022, 2022, 1169438. [Google Scholar] [CrossRef]
- Xiong, X.; Jiang, H.; Liao, Y.; Du, Y.; Zhang, Y.; Wang, Z.; Zheng, M.; Du, Z. Liposome–Trimethyl Chitosan Nanoparticles Codeliver Insulin and SiVEGF to Treat Corneal Alkali Burns by Inhibiting Ferroptosis. Bioeng. Transl. Med. 2023, 8, e10499. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Silkiss, R.Z. The Role of Insulin-like Growth Factor-1 and Its Receptor in the Eye: A Review and Implications for IGF-1R Inhibition. Ophthal. Plast. Reconstr. Surg. 2023, 39, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chikama, T.I.; Nishida, T. Characterization of Insulin-like Growth Factor-1 Receptors in Rabbit Corneal Epithelial Cells. Exp. Eye Res. 2000, 70, 199–204. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Sex Hormones, Growth Hormone, and the Cornea. Cells 2022, 11, 224. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Patel, R.; Zhu, M.; Robertson, D.M. Shifting the IGF-Axis: An Age-Related Decline in Human Tear IGF-1 Correlates with Clinical Signs of Dry Eye. Growth Horm. IGF Res. 2018, 40, 69–73. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Wong, H.L.; Kwok, S.S.; Lo, A.C.-Y.; Chan, J.Y.-K.; Ng, A.L.-K.; Chan, T.C.-Y.; Jhanji, V.; Tong, L. The Association between Altered Intestinal Microbiome, Impaired Systemic and Ocular Surface Immunity, and Impaired Wound Healing Response after Corneal Alkaline-Chemical Injury in Diabetic Mice. Front. Immunol. 2023, 14, 1063069. [Google Scholar] [CrossRef] [PubMed]
- Trosan, P.; Javorkova, E.; Zajicova, A.; Hajkova, M.; Hermankova, B.; Kossl, J.; Krulova, M.; Holan, V. The Supportive Role of Insulin-Like Growth Factor-I in the Differentiation of Murine Mesenchymal Stem Cells into Corneal-Like Cells. Stem Cells Dev. 2016, 25, 874–881. [Google Scholar] [CrossRef]
- Lee, J.H.; Ik, H.R.; Eung, K.K.; Jong, E.L.; Hong, S.W.; Hyung, K.L. Induced Expression of Insulin-like Growth Factor-1 by Amniotic Membrane-Conditioned Medium in Cultured Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 864–872. [Google Scholar] [CrossRef]
- Ko, J.-A.; Yanai, R.; Nishida, T. IGF-1 Released by Corneal Epithelial Cells Induces up-Regulation of N-Cadherin in Corneal Fibroblasts. J. Cell. Physiol. 2009, 221, 254–261. [Google Scholar] [CrossRef]
- Huang, G.Q.; Yi, G.G.; Wu, L.W.; Feng, S.F.; Wu, W.; Peng, L.; Yi, R.W.; Ma, W.; Lu, X. Protective Effect of Histatin 1 against Ultraviolet-Induced Damage to Human Corneal Epithelial Cells. Exp. Ther. Med. 2018, 15, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, Y.; Pan, S.; Li, L. Effect of Insulin-like Growth Factor-1 on Corneal Surface Ultrastructure and Nerve Regeneration of Rabbit Eyes after Laser in Situ Keratomileusis. Neurosci. Lett. 2014, 558, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gong, D.; Yan, D.; Wang, H.; Witman, N.; Lu, Y.; Fu, W.; Fu, Y. Enhanced Adipose-Derived Stem Cells with IGF-1-Modified MRNA Promote Wound Healing Following Corneal Injury. Mol. Ther. 2023, 31, 2454–2471. [Google Scholar] [CrossRef] [PubMed]
- Titone, R.; Zhu, M.; Robertson, D.M. Insulin Mediates de Novo Nuclear Accumulation of the IGF-1/Insulin Hybrid Receptor in Corneal Epithelial Cells. Sci. Rep. 2018, 8, 4378. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Nakamura, M.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic Effects of Substance P with Insulin-like Growth Factor-1 on Epithelial Migration of the Cornea. J. Cell. Physiol. 1996, 169, 159–166. [Google Scholar] [CrossRef]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. Combined Effects of Substance P and Insulin-like Growth Factor-1 on Corneal Epithelial Wound Closure of Rabbit in Vivo. Curr. Eye Res. 1997, 16, 275–278. [Google Scholar] [CrossRef]
- Ofuji, K.; Nakamura, M.; Nishida, T. Signaling Regulation for Synergistic Effects of Substance P and Insulin-like Growth Factor-1 or Epidermal Growth Factor on Corneal Epithelial Migration. Jpn. J. Ophthalmol. 2000, 44, 1–8. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Inui, M.; Nishida, T. Sensitizing Effect of Substance P on Corneal Epithelial Migration Induced by IGF-1, Fibronectin, or Interleukin-6. Investig. Ophthalmol. Vis. Sci. 2005, 46, 833–839. [Google Scholar] [CrossRef]
- Nakamura, M.; Chikama, T.; Nishida, T. Synergistic Effect with Phe-Gly-Leu-Met-NH2 of the C-Terminal of Substance P and Insulin-like Growth Factor-1 on Epithelial Wound Healing of Rabbit Cornea. Br. J. Pharmacol. 1999, 127, 489–497. [Google Scholar] [CrossRef]
- Nakamura, M.; Ofuji, K.; Chikama, T.; Nishida, T. The NK1 Receptor and Its Participation in the Synergistic Enhancement of Corneal Epithelial Migration by Substance P and Insulin-like Growth Factor-1. Br. J. Pharmacol. 1997, 120, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chikama, T.-I.; Nishida, T. Participation of P38 MAP Kinase, but Not P44/42 MAP Kinase, in Stimulation of Corneal Epithelial Migration by Substance P and IGF-1. Curr. Eye Res. 2005, 30, 825–834. [Google Scholar] [CrossRef]
- Chikamoto, N.; Chikama, T.-I.; Yamada, N.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Efficacy of Substance P and Insulin-like Growth Factor-1 Peptides for Preventing Postsurgical Superficial Punctate Keratopathy in Diabetic Patients. Jpn. J. Ophthalmol. 2009, 53, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Matsuda, R.; Morishige, N.; Yanai, R.; Chikama, T.I.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Open Clinical Study of Eye-Drops Containing Tetrapeptides Derived from Substance P and Insulin-like Growth Factor-1 for Treatment of Persistent Corneal Epithelial Defects Associated with Neurotrophic Keratopathy. Br. J. Ophthalmol. 2008, 92, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Chikama, T.I.; Morishige, N.; Yanai, R.; Yamada, N.; Saito, J. Persistent Epithelial Defects Due to Neurotrophic Keratopathy Treated with a Substance P-Derived Peptide and Insulin-like Growth Factor 1. Jpn. J. Ophthalmol. 2007, 51, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Chalam, K.V.; Gupta, S.K.; Vinjamaram, S.; Shah, V. A Clinicopathologic Reports, Case Reports, and Small Case Series. Arch. Ophthalmol. 2006, 119, 409–410. [Google Scholar]
- Benitez-Del-Castillo, J.M.; Rodríguez-Bayo, S.; Fontan-Rivas, E.; Martinez-de-la-Casa, J.M.; Garcia-Sanchez, J. Treatment of Recurrent Corneal Erosion with Substance P-Derived Peptide and Insulin-like Growth Factor I. Arch. Ophthalmol. 2005, 123, 1445–1447. [Google Scholar] [CrossRef][Green Version]
- Ghiasi, Z.; Gray, T.; Tran, P.; Dubielzig, R.; Murphy, C.; McCartney, D.L.; Reid, T.W. The Effect of Topical Substance-P Plus Insulin-like Growth Factor-1 (IGF-1) on Epithelial Healing After Photorefractive Keratectomy in Rabbits. Transl. Vis. Sci. Technol. 2018, 7, 12. [Google Scholar] [CrossRef]
- Nakamura, M.; Kawahara, M.; Nakata, K.; Nishida, T. Restoration of Corneal Epithelial Barrier Function and Wound Healing by Substance P and IGF-1 in Rats with Capsaicin-Induced Neurotrophic Keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2937–2940. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kawahara, M.; Morishige, N.; Chikama, T.; Nakata, K.; Nishida, T. Promotion of Corneal Epithelial Wound Healing in Diabetic Rats by the Combination of a Substance P-Derived Peptide (FGLM-NH2) and Insulin-like Growth Factor-1. Diabetologia 2003, 46, 839–842. [Google Scholar] [CrossRef]
- Nagano, T.; Nakamura, M.; Nakata, K.; Yamaguchi, T.; Takase, K.; Okahara, A.; Ikuse, T.; Nishida, T. Effects of Substance P and IGF-1 in Corneal Epithelial Barrier Function and Wound Healing in a Rat Model of Neurotrophic Keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3810–3815. [Google Scholar] [CrossRef]
- Murphy, C.J.; Marfurt, C.F.; McDermott, A.; Bentley, E.; Abrams, G.A.; Reid, T.W.; Campbell, S. Spontaneous Chronic Corneal Epithelial Defects (SCCED) in Dogs: Clinical Features, Innervation, and Effect of Topical SP, with or without IGF-1. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2252–2261. [Google Scholar]
- Nishida, T.; Inui, M.; Nomizu, M. Peptide Therapies for Ocular Surface Disturbances Based on Fibronectin-Integrin Interactions. Prog. Retin. Eye Res. 2015, 47, 38–63. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M. Update on Overview of Pterygium and Its Surgical Management. J. Popul. Ther. Clin. Pharmacol. 2022, 29, e30–e45. [Google Scholar] [CrossRef] [PubMed]
- Maxia, C.; Isola, M.; Grecu, E.; Cuccu, A.; Scano, A.; Orrù, G.; Di Girolamo, N.; Diana, A.; Murtas, D. Synergic Action of Insulin-like Growth Factor-2 and MiRNA-483 in Pterygium Pathogenesis. Int. J. Mol. Sci. 2023, 24, 4329. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kang, H.S.; Park, J.H.; Bae, J.H.; Song, D.K.; Im, S.S. Recent Insights into Insulin-Like Growth Factor Binding Protein 2 Transcriptional Regulation. Endocrinol. Metab. 2017, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.R.; Moshayedi, P.; Schoen, T.J.; Jones, B.E.; Chader, G.J.; Waldbillig, R.J. Distribution of IGF-I and -II, IGF Binding Proteins (IGFBPs) and IGFBP MRNA in Ocular Fluids and Tissues: Potential Sites of Synthesis of IGFBPs in Aqueous and Vitreous. Exp. Eye Res. 1993, 56, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Ho, S.-I.; Hansen, B.S.; Petroll, W.M.; Cavanagh, H.D. Insulin-like Growth Factor Binding Protein-3 Expression in the Human Corneal Epithelium. Exp. Eye Res. 2007, 85, 492–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stuard, W.L.; Guner, M.K.; Robertson, D.M. IGFBP-3 Regulates Mitochondrial Hyperfusion and Metabolic Activity in Ocular Surface Epithelia during Hyperosmolar Stress. Int. J. Mol. Sci. 2022, 23, 4066. [Google Scholar] [CrossRef]
- Bogdan, E.D.; Stuard, W.L.; Titone, R.; Robertson, D.M. IGFBP-3 Mediates Metabolic Homeostasis During Hyperosmolar Stress in the Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Shi, D.; Chen, P.; Yu, Y.; Yang, L.; Xie, L. Overexpression of SIRT1 Promotes High Glucose-Attenuated Corneal Epithelial Wound Healing via P53 Regulation of the IGFBP3/IGF-1R/AKT Pathway. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3806–3814. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. Tear Levels of IGFBP-3: A Potential Biomarker for Diabetic Nerve Changes in the Cornea. Eye Contact Lens 2020, 46, 319–325. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. Tear Levels of Insulin-Like Growth Factor Binding Protein 3 Correlate With Subbasal Nerve Plexus Changes in Patients With Type 2 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6105–6112. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Buckner, B.R.; Zhu, M.; Cavanagh, H.D.; Robertson, D.M. Elevated IGFBP3 Levels in Diabetic Tears: A Negative Regulator of IGF-1 Signaling in the Corneal Epithelium. Ocul. Surf. 2012, 10, 100–107. [Google Scholar] [CrossRef][Green Version]
- Rao, P.; Suvas, P.K.; Jerome, A.D.; Steinle, J.J.; Suvas, S. Role of Insulin-like Growth Factor Binding Protein-3 in the Pathogenesis of Herpes Stromal Keratitis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 46. [Google Scholar] [CrossRef]
- Bergman, P.B.; Moravski, C.J.; Edmondson, S.R.; Russo, V.C.; Bach, L.A.; Wilkinson-Berka, J.L.; Werther, G.A. Expression of the IGF System in Normal and Diabetic Transgenic (MRen-2)27 Rat Eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Nagalla, S.R.; Yamanaka, Y.; Kim, H.S.; Wilson, E.; Rosenfeld, R.G. Synthesis and Characterization of Insulin-like Growth Factor-Binding Protein (IGFBP)-7. Recombinant Human Mac25 Protein Specifically Binds IGF-I and -II. J. Biol. Chem. 1996, 271, 30322–30325. [Google Scholar] [CrossRef] [PubMed]
- Pen, A.; Moreno, M.J.; Durocher, Y.; Deb-Rinker, P.; Stanimirovic, D.B. Glioblastoma-Secreted Factors Induce IGFBP7 and Angiogenesis by Modulating Smad-2-Dependent TGF-Beta Signaling. Oncogene 2008, 27, 6834–6844. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).