Targeted Overexpression of Claudin 11 in Osteoblasts Increases Trabecular Bone Mass by Stimulating Osteogenesis at the Expense of Adipogenesis in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
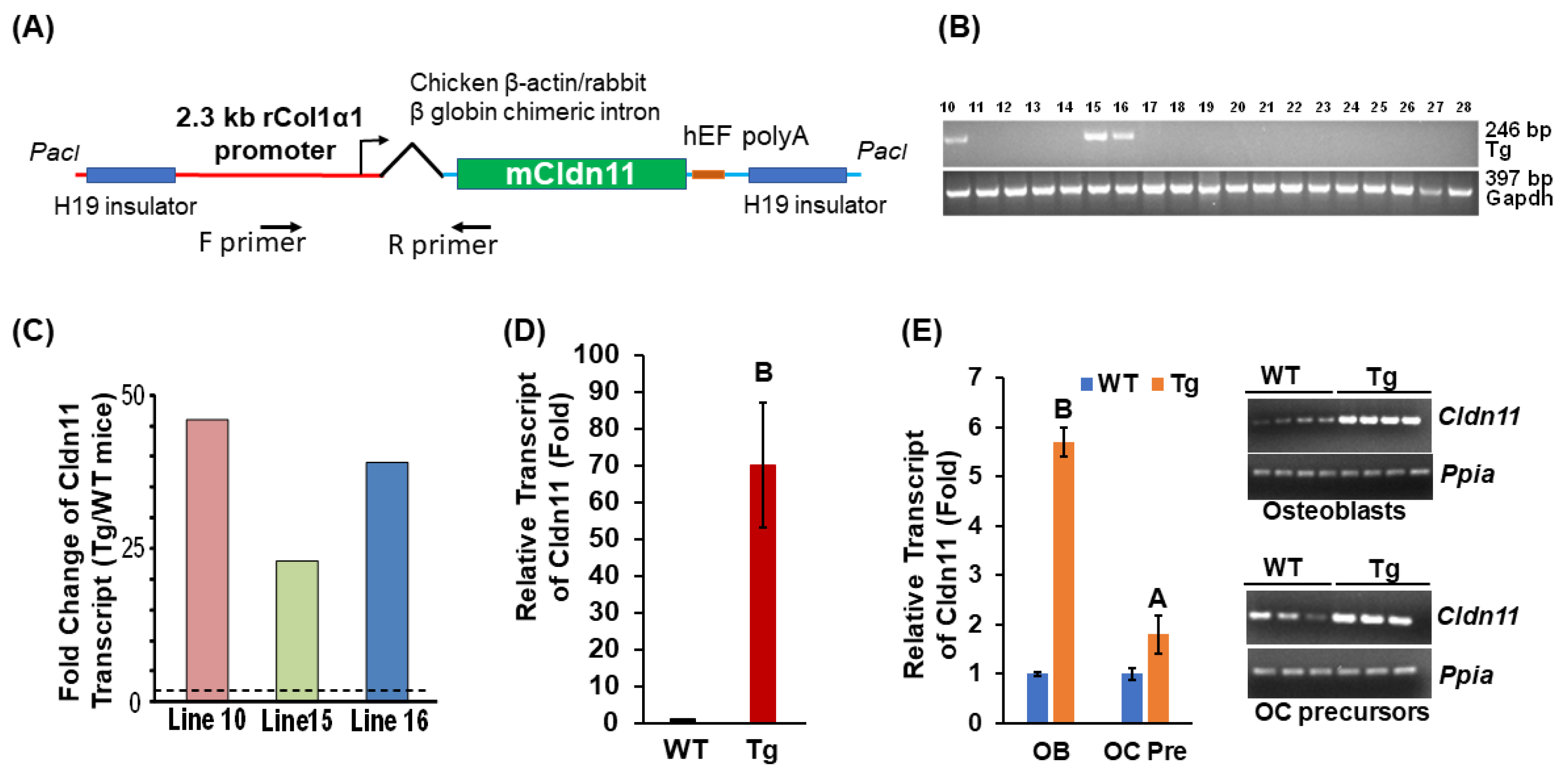
3. Results
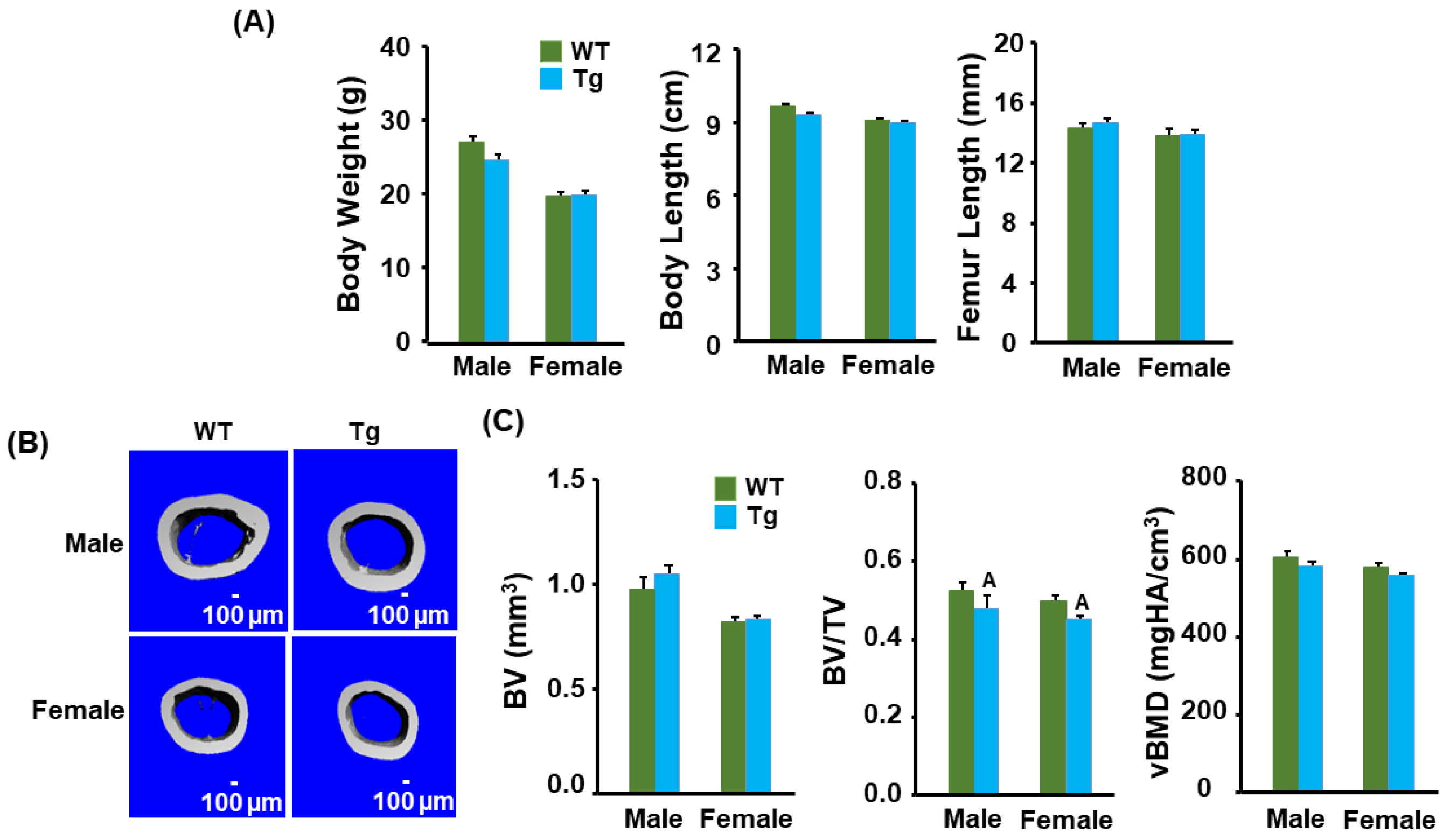
3.1. Transgenic Mice Have No Changes in Body Weight and Length but Display a Reduction in Cortical Bone Volume
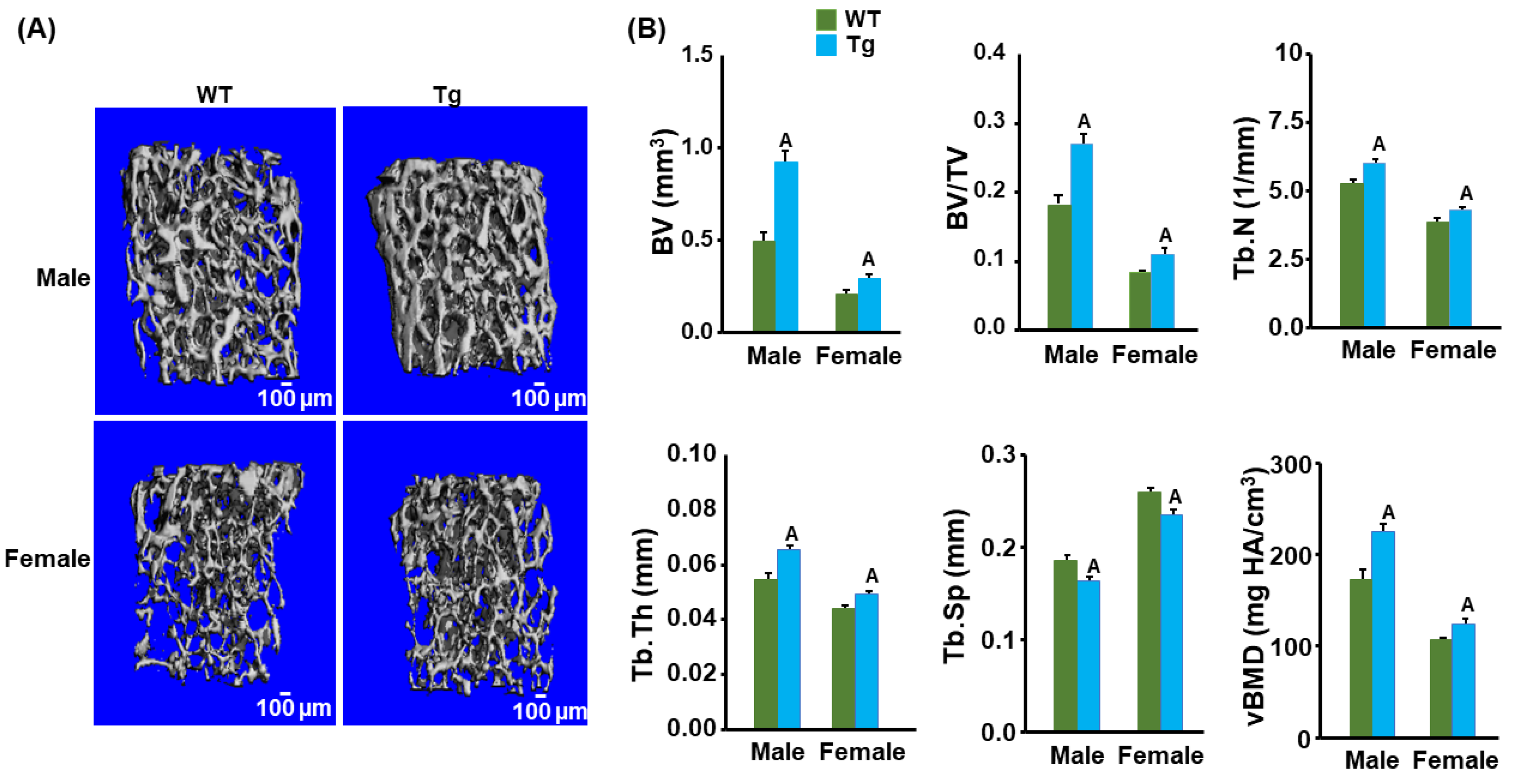
3.2. Cldn11 Transgenic Mice Exhibit an Increased Trabecular Bone Mass
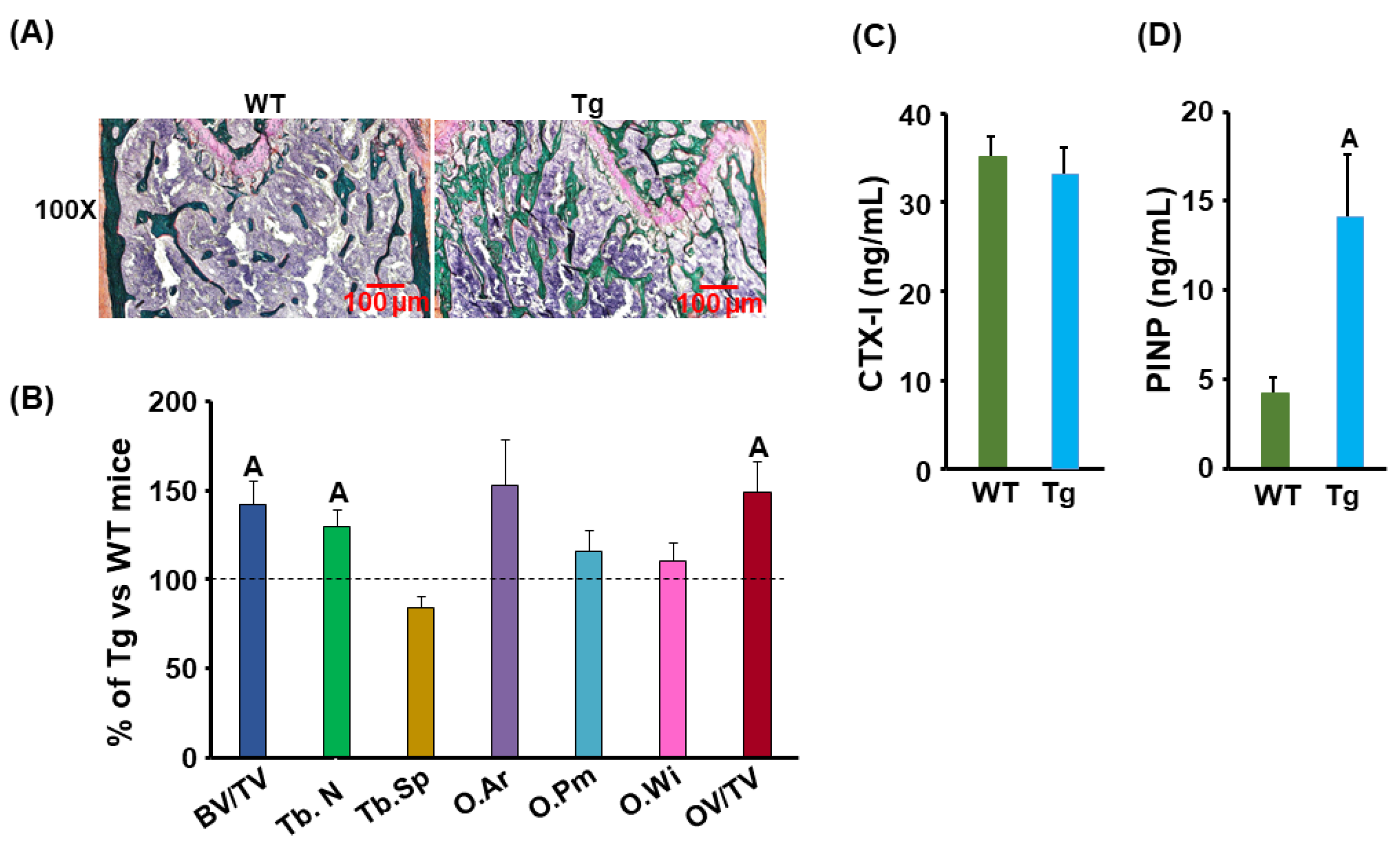
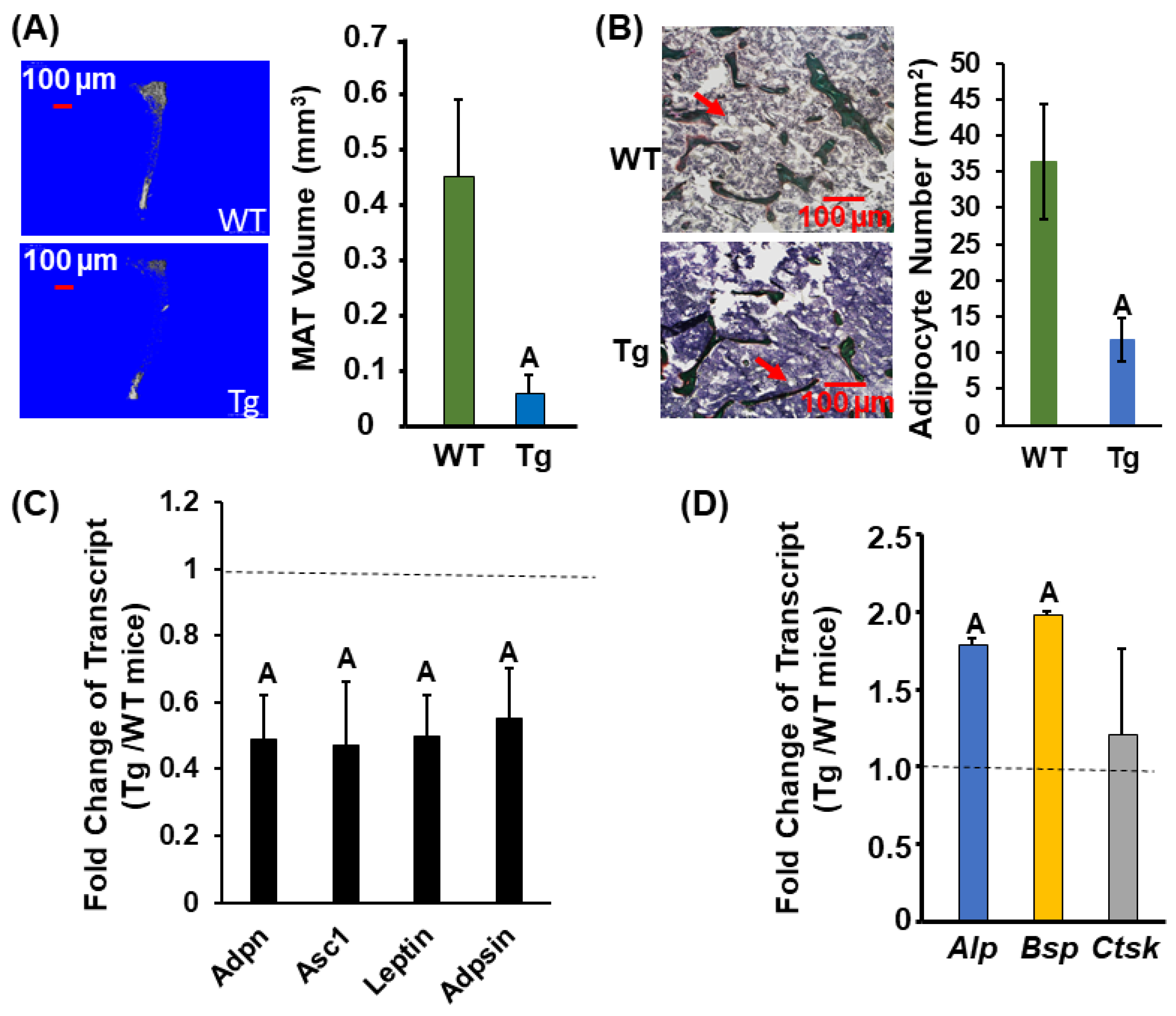
3.3. Bone Formation Was Increased but Marrow Adipose Tissue Was Reduced in the Cldn11 Transgenic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelow, S.; Ahlstrom, R.; Yu, A.S. Biology of claudins. Am. J. Physiol.-Ren. Physiol. 2008, 295, F867–F876. [Google Scholar] [CrossRef]
- Hagen, S.J. Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions. Tissue Barriers 2017, 5, e1327839. [Google Scholar] [CrossRef]
- Lal-Nag, M.; Morin, P.J. The claudins. Genome Biol. 2009, 10, 235. [Google Scholar] [CrossRef]
- Linares, G.R.; Brommage, R.; Powell, D.R.; Xing, W.; Chen, S.T.; Alshbool, F.Z.; Lau, K.H.; Wergedal, J.E.; Mohan, S. Claudin 18 is a novel negative regulator of bone resorption and osteoclast differentiation. J. Bone Miner. Res. 2012, 27, 1553–1565. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Devaux, J.; Gow, A. Tight junctions potentiate the insulative properties of small CNS myelinated axons. J. Cell Biol. 2008, 183, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.; Davies, C.; Southwood, C.M.; Frolenkov, G.; Chrustowski, M.; Ng, L.; Yamauchi, D.; Marcus, D.C.; Kachar, B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J. Neurosci. 2004, 24, 7051–7062. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Phosphorylation of tight junction transmembrane proteins: Many sites, much to do. Tissue Barriers 2018, 6, e1382671. [Google Scholar] [CrossRef]
- Lindsey, R.C.; Xing, W.; Pourteymoor, S.; Godwin, C.; Gow, A.; Mohan, S. Novel Role for Claudin-11 in the Regulation of Osteoblasts via Modulation of ADAM10-Mediated Notch Signaling. J. Bone Miner. Res. 2019, 34, 1910–1922. [Google Scholar] [CrossRef]
- Baek, J.M.; Cheon, Y.H.; Kwak, S.C.; Jun, H.Y.; Yoon, K.H.; Lee, M.S.; Kim, J.Y. Claudin 11 regulates bone homeostasis via bidirectional EphB4-EphrinB2 signaling. Exp. Mol. Med. 2018, 50, 1–18. [Google Scholar] [CrossRef]
- Dacic, S.; Kalajzic, I.; Visnjic, D.; Lichtler, A.C.; Rowe, D.W. Col1a1-driven transgenic markers of osteoblast lineage progression. J. Bone Miner. Res. 2001, 16, 1228–1236. [Google Scholar] [CrossRef]
- Scheller, E.L.; Troiano, N.; Vanhoutan, J.N.; Bouxsein, M.A.; Fretz, J.A.; Xi, Y.; Nelson, T.; Katz, G.; Berry, R.; Church, C.D.; et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014, 537, 123–139. [Google Scholar] [CrossRef]
- Xing, W.; Liu, J.; Cheng, S.; Vogel, P.; Mohan, S.; Brommage, R. Targeted disruption of leucine-rich repeat kinase 1 but not leucine-rich repeat kinase 2 in mice causes severe osteopetrosis. J. Bone Miner. Res. 2013, 28, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Larkin, D.; Pourteymoor, S.; Tambunan, W.; Gomez, G.A.; Liu, E.K.; Mohan, S. Lack of Skeletal Effects in Mice with Targeted Disruptionof Prolyl Hydroxylase Domain 1 (Phd1) Gene Expressed in Chondrocytes. Life 2022, 13, 106. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; Hoyland, J.A.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef]
- Wongdee, K.; Pandaranandaka, J.; Teerapornpuntakit, J.; Tudpor, K.; Thongbunchoo, J.; Thongon, N.; Jantarajit, W.; Krishnamra, N.; Charoenphandhu, N. Osteoblasts express claudins and tight junction-associated proteins. Histochem. Cell Biol. 2008, 130, 79–90. [Google Scholar] [CrossRef]
- Versnel, H.; Schoonhoven, R.; Prijs, V.F. Single-fibre and whole-nerve responses to clicks as a function of sound intensity in the guinea pig. Hear. Res. 1992, 59, 138–156. [Google Scholar] [CrossRef]
- Liu, P.; Ping, Y.; Ma, M.; Zhang, D.; Liu, C.; Zaidi, S.; Gao, S.; Ji, Y.; Lou, F.; Yu, F.; et al. Anabolic actions of Notch on mature bone. Proc. Natl. Acad. Sci. USA 2016, 113, E2152–E2161. [Google Scholar] [CrossRef]
- Zha, L.; Hou, N.; Wang, J.; Yang, G.; Gao, Y.; Chen, L.; Yang, X. Collagen1alpha1 promoter drives the expression of Cre recombinase in osteoblasts of transgenic mice. J. Genet. Genom. 2008, 35, 525–530. [Google Scholar] [CrossRef]
- Bogdanovic, Z.; Bedalov, A.; Krebsbach, P.H.; Pavlin, D.; Woody, C.O.; Clark, S.H.; Thomas, H.F.; Rowe, D.W.; Kream, B.E.; Lichtler, A.C. Upstream regulatory elements necessary for expression of the rat COL1A1 promoter in transgenic mice. J. Bone Miner. Res. 1994, 9, 285–292. [Google Scholar] [CrossRef]
- Xiang, G.; Huang, L.; Zhang, X.; Wang, N.; Wang, H.; Mu, Y.; Li, K.; Liu, Z. Molecular Characteristics and Promoter Analysis of Porcine COL1A1. Genes 2022, 13, 1971. [Google Scholar] [CrossRef]
- Boban, I.; Jacquin, C.; Prior, K.; Barisic-Dujmovic, T.; Maye, P.; Clark, S.H.; Aguila, H.L. The 3.6 kb DNA fragment from the rat Col1a1 gene promoter drives the expression of genes in both osteoblast and osteoclast lineage cells. Bone 2006, 39, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Hardouin, P.; Cotten, A.; Penel, G.; Cortet, B. The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians. J. Clin. Endocrinol. Metab. 2015, 100, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Subbalekha, K.; Sastravaha, P.; Pavasant, P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 2012, 36, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Longo, K.A.; Wright, W.S.; Suva, L.J.; Lane, T.F.; Hankenson, K.D.; MacDougald, O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 2005, 102, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Longo, K.A.; Wright, W.S.; Kang, S.; Gerin, I.; Chiang, S.H.; Lucas, P.C.; Opp, M.R.; MacDougald, O.A. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004, 279, 35503–35509. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bagchi, D.P.; Zhu, J.; Bowers, E.; Yu, H.; Hardij, J.; Mori, H.; Granger, K.; Skjaerlund, J.; Mandair, G.; et al. Constitutive bone marrow adipocytes suppress local bone formation. J. Clin. Investig. 2022, 7, e160915. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Wu, Y.; Zhao, S.; Bao, J.; Luo, Y.; Li, D. Structural insights into the committed step of bacterial phospholipid biosynthesis. Nat. Commun. 2017, 8, 1691. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Ppia | 5′-CCATGGCAAATGCTGGACCA-3′ | 5′-TCCTGGACCCAAAACGCTCC-3′ |
| Cldn11 | 5′- ACCTGCCGAAAAATGGACGA-3′ | 5′-TGCAGGGGAGAACTGTCAAC-3′ |
| Alp | 5′-ATGGTAACGGGCCTGGCTACA-3′ | 5′-AGTTCTGCTCATGGACGCCGT-3′ |
| Bsp | 5′-AACGGGTTTCAGCAGACAACC-3′ | 5′-TAAGCTCGGTAAGTGTCGCCA-3′ |
| Ctsk | 5′-GAACGAGAAAGCCCTGAAGAGA-3′ | 5′-TATCGAGTGCTTGCTTCCCTTC-3′ |
| Adpn | 5′-GATGCAGGTCTTCTTGGTCCTA-3′ | 5′-AGCGAATGGGTACATTGGGA-3′ |
| Asc1 | 5′-GGGTGGCACTCAAGAAAGAG-3′ | 5′-AGTGTTCCAGGACACCCTTG-3′ |
| Leptin | 5′-TGCTGCAGATAGCCAATGAC-3′ | 5′-GAGTAGAGTGAGGCTTCCAGGA-3′ |
| Adpsin | 5′-GCAGTGGGTGCTCAGTGCT-3′ | 5′-TCGTCATCCGTCACTCCATC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Pourteymoor, S.; Udayakumar, A.; Chen, Y.; Mohan, S. Targeted Overexpression of Claudin 11 in Osteoblasts Increases Trabecular Bone Mass by Stimulating Osteogenesis at the Expense of Adipogenesis in Mice. Biology 2024, 13, 108. https://doi.org/10.3390/biology13020108
Xing W, Pourteymoor S, Udayakumar A, Chen Y, Mohan S. Targeted Overexpression of Claudin 11 in Osteoblasts Increases Trabecular Bone Mass by Stimulating Osteogenesis at the Expense of Adipogenesis in Mice. Biology. 2024; 13(2):108. https://doi.org/10.3390/biology13020108
Chicago/Turabian StyleXing, Weirong, Sheila Pourteymoor, Anakha Udayakumar, Yian Chen, and Subburaman Mohan. 2024. "Targeted Overexpression of Claudin 11 in Osteoblasts Increases Trabecular Bone Mass by Stimulating Osteogenesis at the Expense of Adipogenesis in Mice" Biology 13, no. 2: 108. https://doi.org/10.3390/biology13020108
APA StyleXing, W., Pourteymoor, S., Udayakumar, A., Chen, Y., & Mohan, S. (2024). Targeted Overexpression of Claudin 11 in Osteoblasts Increases Trabecular Bone Mass by Stimulating Osteogenesis at the Expense of Adipogenesis in Mice. Biology, 13(2), 108. https://doi.org/10.3390/biology13020108







