Simple Summary
Zebrafish form an ideal model for studying a wide range of ophthalmological and neurological conditions. The optokinetic reflex (OKR) assays in zebrafish models are proven to be a valuable tool for investigating these conditions. Despite its increasing popularity in recent years, the field lacks clear reporting guidelines for the assay. To better understand optimal reporting standards for an OKR assay in zebrafish, we performed a systematic literature review of 109 research papers exploring the animal, environmental, and technical factors that should be considered. In this article, we highlight multiple crucial factors, such as larval characteristics, sample size, fixing method, assay set-up, detailed stimulus parameters, eye recording, and eye movement analysis, necessary for preforming the assay. We have created the zebrafish optokinetic (ZOK) reflex minimal reporting guideline that will allow researchers to avoid future errors and create more reliable and transparent research.
Abstract
Optokinetic reflex (OKR) assays in zebrafish models are a valuable tool for studying a diverse range of ophthalmological and neurological conditions. Despite its increasing popularity in recent years, there are no clear reporting guidelines for the assay. Following reporting guidelines in research enhances reproducibility, reduces bias, and mitigates underreporting and poor methodologies in published works. To better understand optimal reporting standards for an OKR assay in zebrafish, we performed a systematic literature review exploring the animal, environmental, and technical factors that should be considered. Using search criteria from three online databases, a total of 109 research papers were selected for review. Multiple crucial factors were identified, including larval characteristics, sample size, fixing method, OKR set-up, distance of stimulus, detailed stimulus parameters, eye recording, and eye movement analysis. The outcome of the literature analysis highlighted the insufficient information provided in past research papers and the lack of a systematic way to present the parameters related to each of the experimental factors. To circumvent any future errors and champion robust transparent research, we have created the zebrafish optokinetic (ZOK) reflex minimal reporting guideline.
1. Introduction
The optokinetic reflex (OKR) is a visual reflex that develops within the first six months in healthy children. Optokinetic nystagmus (OKN) is induced in response to a moving image, such as rotating black and white gratings, travelling across much of the visual field. Vertically oriented gratings would elicit a horizontal OKR whilst horizontally oriented gratings would elicit vertical OKR. OKR typically has two phases of eye movements. The “slow phase” equates to the movement of the eyes in the same direction as the moving stimulus to cancel out the retinal slip velocity. To reset the eyes, the slow tracking movement is routinely interrupted by a “fast phase” or “saccadic movement” in the opposite direction [1].
Abnormalities in OKN are a clinical finding in a variety of ophthalmic, vestibular, and neurological disorders. Examples of visual disorders associated with abnormal OKN include photoreceptor dystrophies, albinism, and FRMD7-related infantile nystagmus [2]. Neurological eye-tracking disorders can be divided into pursuit system disorders, such as floccular lesions, and quick phase disorders, such as progressive supranuclear palsy [3,4].
Common visual behavioural assays used in larval zebrafish include the optomotor response (OMR) assay and the OKR assay. In an OMR, fish reflexively swim in the direction of a moving stimulus. Due to the tracking of multiple fish simultaneously, an OMR is more advantageous for high-throughput screens [5]. In comparison, an OKR has the advantages of being more robust, reliable, and able to identify underlying diseases linked to the visual system and brain development. Reductions in visual acuity can be quantified using the OKR assay by altering spatial frequencies until no OKR is elicited [5]. Similarly, nystagmus has been reported in mutant zebrafish making it suitable for precisely measuring eye movements [6]. Due to their different merits, these assays are often used in tandem.
In addition to zebrafish, OKR has been successfully elicited in other animal research models, including rodents, rabbits, and cats [7]. Ethically, animal research must strive to follow the “three Rs” of animal research ethics: refine, reduce and replace [8]. Using zebrafish as an exemplar model aims to uphold these principles.
Firstly, zebrafish form ideal models for investigating OKN disorders, sharing an 87% disease gene homology with humans [9]. By 5 days post-fertilization (dpf), they already exhibit a fully functioning OKR, at which stage they are still legally classed as “unprotected organisms” in some countries, allowing for greater ease of research. Achieving successful OKR assays in zebrafish allows researchers to “replace” more sentient animals such as mice [8].
The establishment of clear reporting guidance aids in research design, the reproducibility of results, minimizing bias, and facilitating systematic review studies [10]. The National Institute of Health (NIH) propose that researchers have a scientific as well as a social responsibility to produce rigorous and transparent research through adherence to guidelines [10]. This allows each subsequent generation of researchers to verify a study’s validity, expand upon the current body of research, and further “refine” their method. Attaining high-quality results in animal models also provides valuable time-saving and economic benefits as well as more reliable results, which may translate to “reduced” numbers of animals per experiment [11].
The ARRIVE guidance, updated in 2020, is a well-known pre-clinical guidance for animal researchers which aims to standardise reporting practices across all published works [12]. It broadly outlines ten essential items including study design, sample size, outcome measures, and a description of experimental animals to provide a basic skeleton applicable to most animal research projects. Similarly, another paper called “the gold-standard checklist” approaches this from an animal ethics perspective and follows the ‘three Rs’ [11].
Though these guides provide a strong foundation for research design, neither are specific to a particular species. Guidance tailored to fish research does exist, including specific environmental and feeding steps that differ from other animals [13]. However, this guidance is dated and too generalizable, making it insufficient for reporting on complex research procedures such as OKR assays.
More recent studies optimise experiments in fish models by summarising the literature and forming a standardised protocol [14]. Implementing this specific guidance on research methodology can further improve research rigour and quality and enhance the reproducibility of these procedures. Whilst in areas such as animal husbandry standardised protocols have been quick to emerge, only one exists for behavioural assays in larval zebrafish, which is the DAZL 2023 guidelines are for locomotion (OMR) assays for developmental neurotoxicity screening [15].
Recent reporting guidance has emerged on eye-tracking research methodology in humans but no such guidance currently exists for performing OKR behavioural assays in larval zebrafish [16].
In this paper, we aim to carry out a systematic review of different methodologies used to elicit, measure, and analyse the OKR in zebrafish. Based on the review, we developed the zebrafish optokinetic reflex minimum reporting guideline, identifying various factors in methodology that should be reported to ensure reproducibility and the correct interpretation of OKR results.
2. Materials and Methods
To perform a comprehensive literature review of current OKR reporting practices, we followed the PRISMA 2020 statement [17]. The search was conducted in May 2023 across three peer-reviewed literature platforms as follows: PubMed, Medline, and Science Direct.
For the Medline and PubMed searches, we utilised the criteria as follows: “optokinetic” or “opto kinetic” or “OKR” and “zebrafish” or “danio rerio”. When conducting the search on the Science Direct database, we also included “zebrafish” or “danio rerio” in the keywords search bar.
Inclusion and Exclusion Criteria
Papers were filtered for those in English and Italian to match the researchers’ language proficiency.
Papers were only included if an OKR was performed as part of the study on larval zebrafish. Studies that used juvenile or adult zebrafish were excluded. Many studies failed to note the larval developmental stage in the abstract, hence the exclusion of “larvae” from our search criteria. Studies solely using OKR for neuro-imaging studies and not visual behaviour were excluded.
3. Systematic Review of Optokinetic Reflex Studies in Larval Zebrafish for the Basis for a Reporting Guideline
3.1. Search Results and PRISMA 2
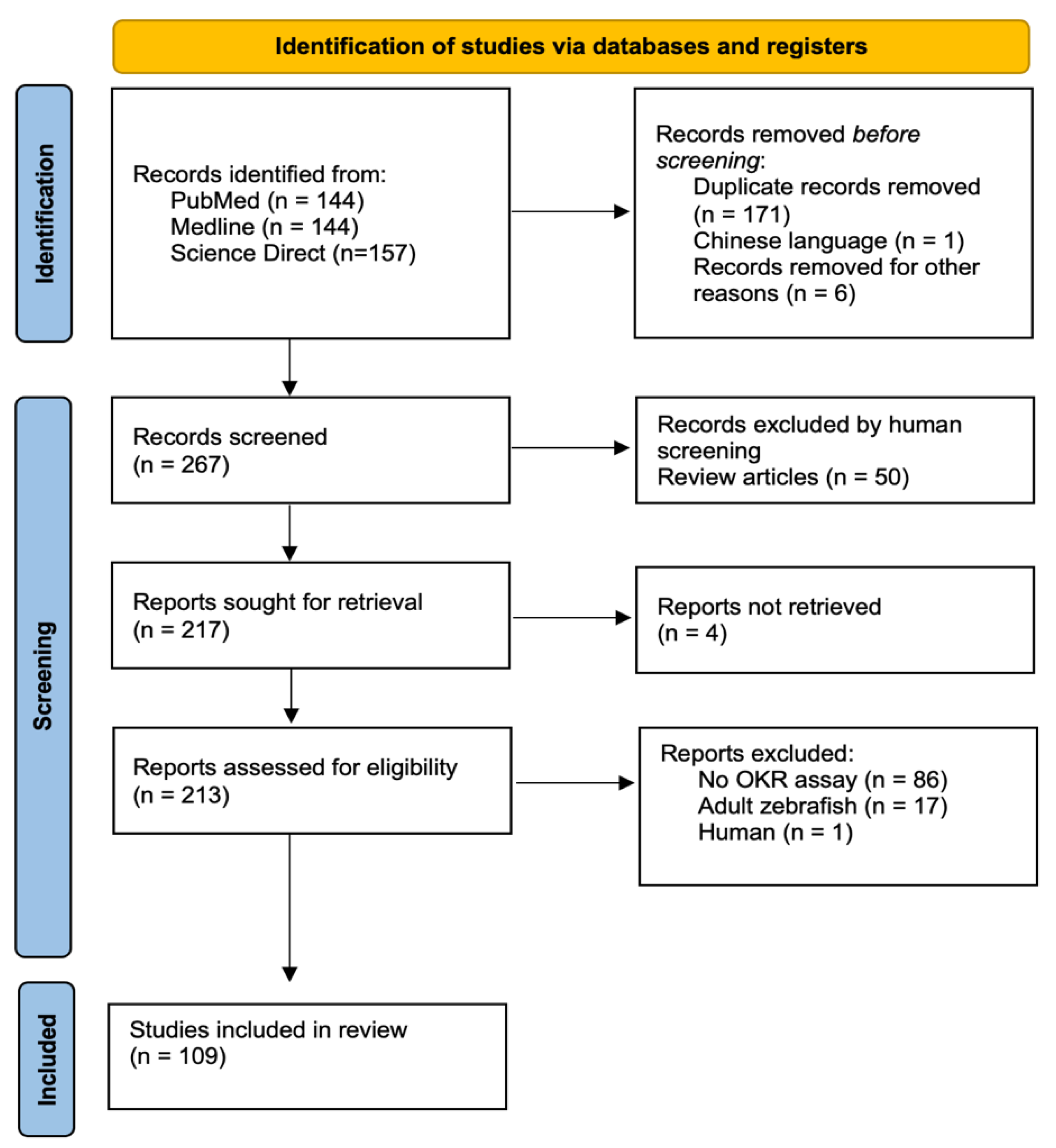
A total of 445 papers were identified across the three databases. Once duplicates and records were removed pre-screening, we had a total of 267 papers for screening. Finally, 109 relevant manuscripts were identified, with fifty of these being review papers (Figure 1).
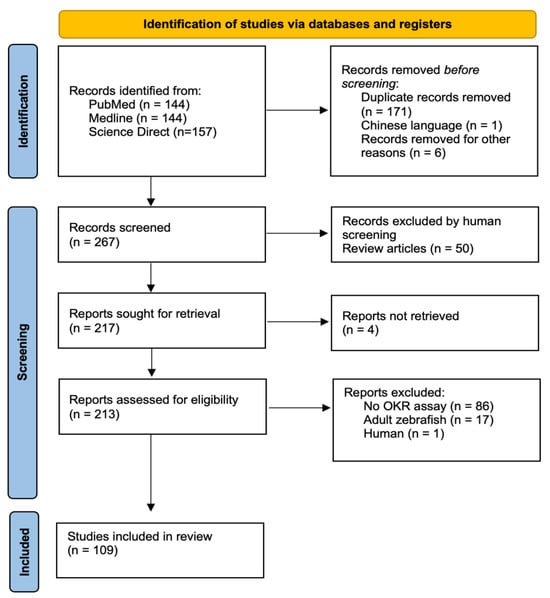
Figure 1.
The PRISMA flowchart schematic detailing the review search and selection process adapted using PRISMA guidelines [17].
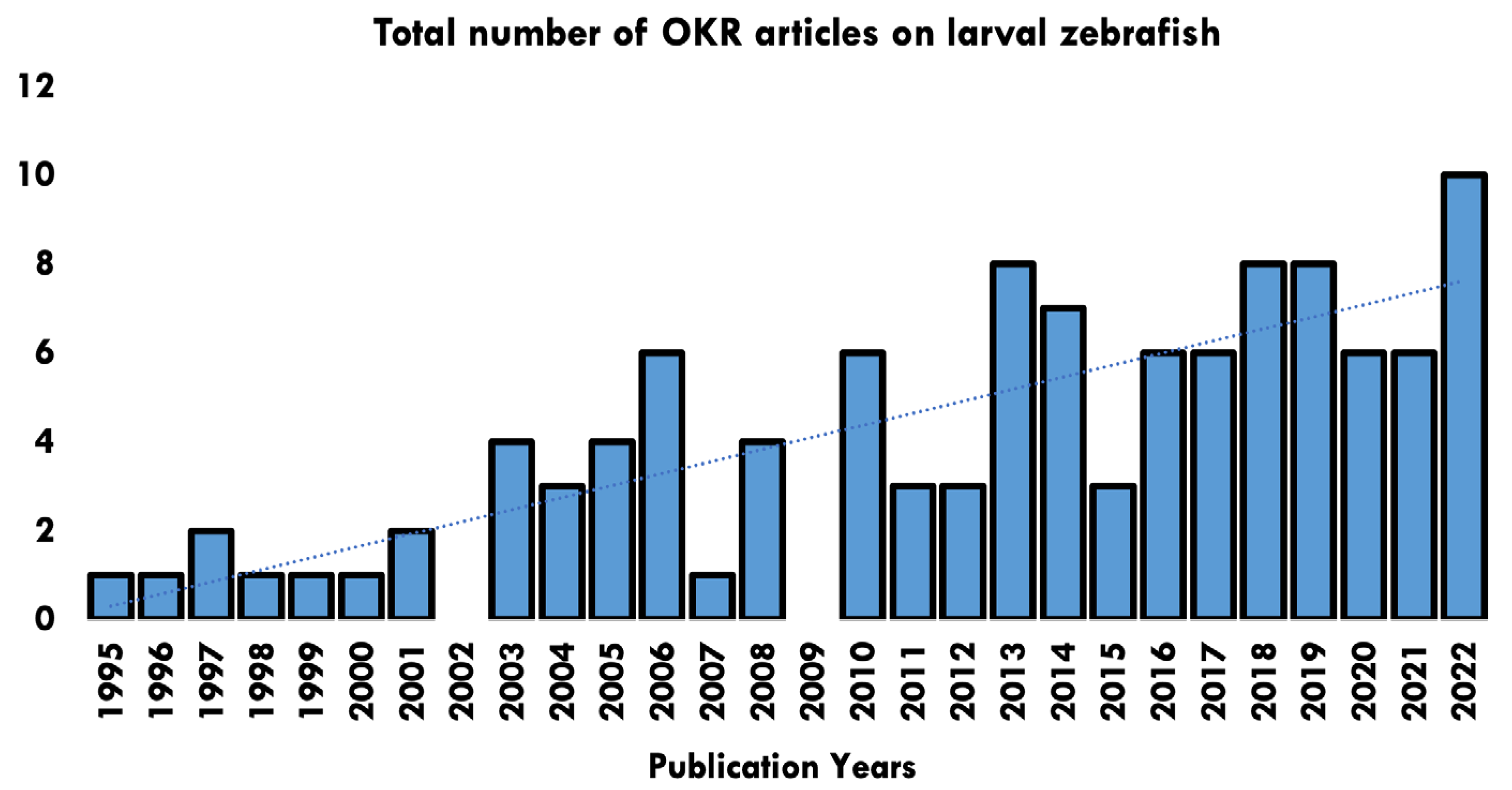
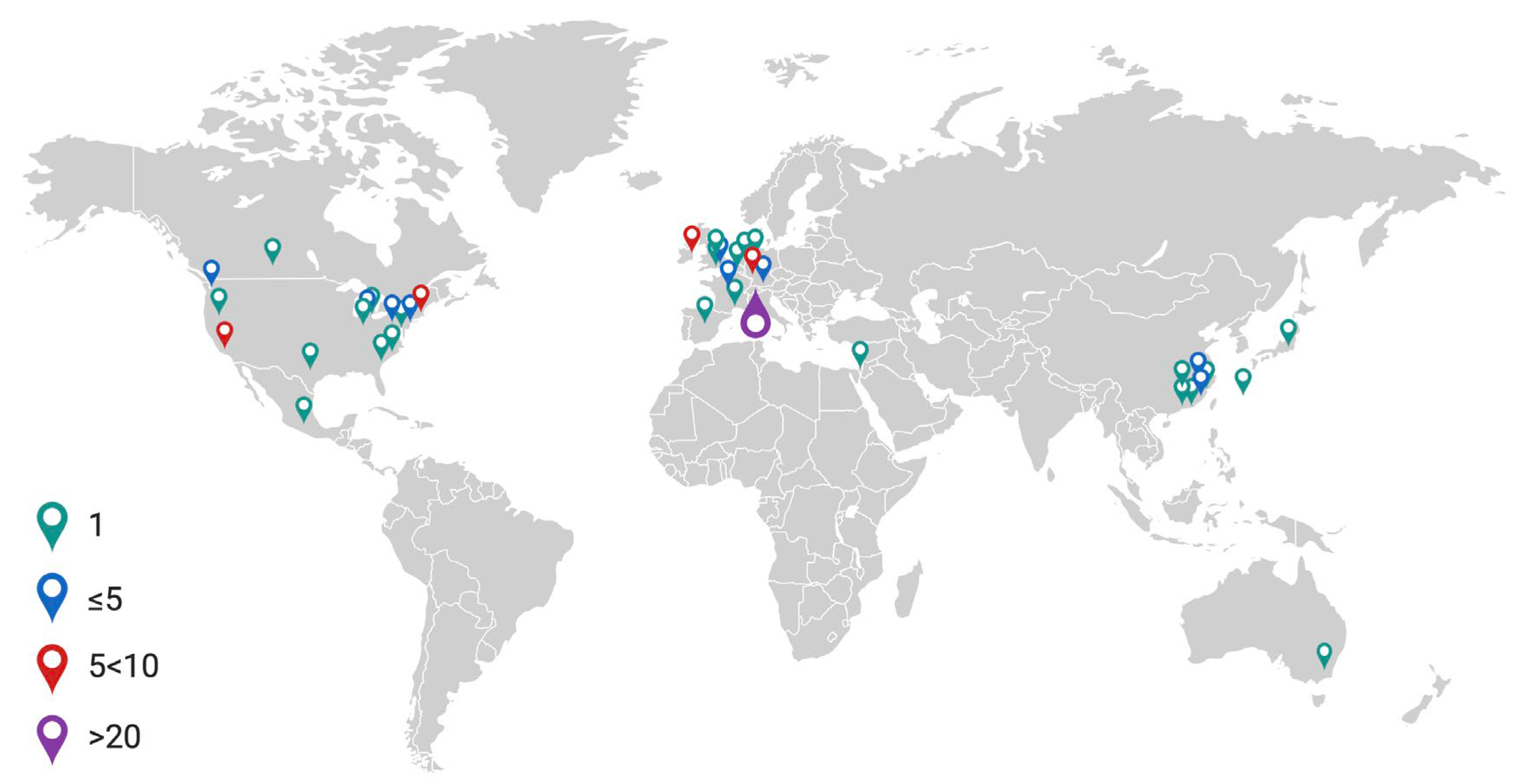
Papers ranged in date from 1995–2022, with a rising trend in the number of papers published each year (Figure 2). Studies originated across 40 research establishments worldwide (Figure 3).
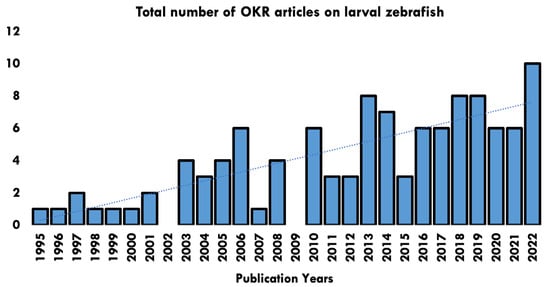
Figure 2.
Publication years of papers identified in the OKR systematic review and number of articles published per year.
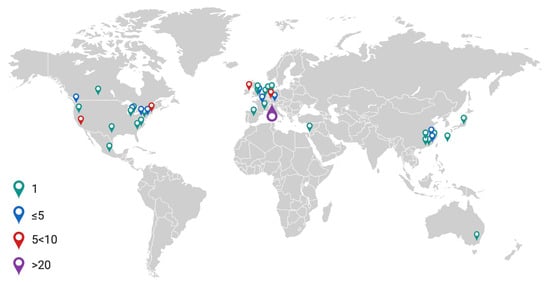
Figure 3.
World map illustrating frequency of published articles in the literature search. Created using Biorender.com.
In this review, we have organised our results into two broad sections. The first section identifies the required parameters for designing an OKR assay and the second section covers current practices regarding the assay.
3.2. OKR Assay Parameters
3.2.1. Larval Characteristics
Species of Fish
This review centres on zebrafish; however, it is important to note OKR assays used in other species such as goldfish and medaka.
Larval Developmental Stage
Days post-fertilisation (dpf) is a standard for reporting larval development. Zebrafish begin to develop an OKR at 3 dpf [18,19]. However, the developmental stage at which a mature OKR reflex can be elicited is debated, with some suggesting it is fully developed at 4 dpf [18] while others propose 5 dpf [20]. Physiologically, major ocular developmental milestones are met at 3 dpf, such as that of the retinal laminar structure, as well as the maturation of extraocular muscle function [21]. The focus in this review is on the larval stages; however, previous data suggest that visual acuity increases throughout the first year of development and then tails off at around 15 months of age [22].
3.2.2. Immobilisation
To elicit an OKR, larvae must be immobilised. This is to prevent compensatory body movements from interfering with eye movement, allowing for clear visualisation under a dissection microscope [23]. For larvae at an early developmental stage, the chorion sac itself may be sufficient for immobilisation [19].
Three principal methods identified for immobilisation are using methylcellulose, agarose, and pins/needles.
Methylcellulose
Methylcellulose is a non-toxic, gelatinous, water-based compound which can preserve larval respiratory function. Larvae are embedded inside a Petri-dish and subsequently positioned using a dissecting needle or equivalent [24]. It is suggested to take ~1 min per larvae with some experience [25]. A concentration of 3% is of adequate viscosity to impede movement [26]. The higher the concentration of methylcellulose, the greater the risk of interference from eye movement [23].
Agarose
Agarose, a polysaccharide derived from seaweed agar, can be cooled to form a block. Larvae were embedded into an agarose block with eye and head cut-outs to allow for a full range of movement [24]. This procedure is considered more time-consuming compared with methylcellulose embedding; however, it removes all barriers between the stimulus and the zebrafish’s eyes [23].
Pins/Needles
In adult zebrafish behavioural assays, the fish is too large to remain immobile in methylcellulose. It can be mounted onto a silicon base surrounded by pins/needles to restrict movement. Zebrafish are still classed as larvae at ~20 dpf, at which point they are nearing their adult size and therefore could be pinned in a similar fashion [27,28].
3.2.3. OKR Assay Set-Up
There are several different methods that have been developed for performing an OKR assay. This varies from a striped paper drum rotating around a larva to a stationary drum with projected stimuli and more modern LED arenas.
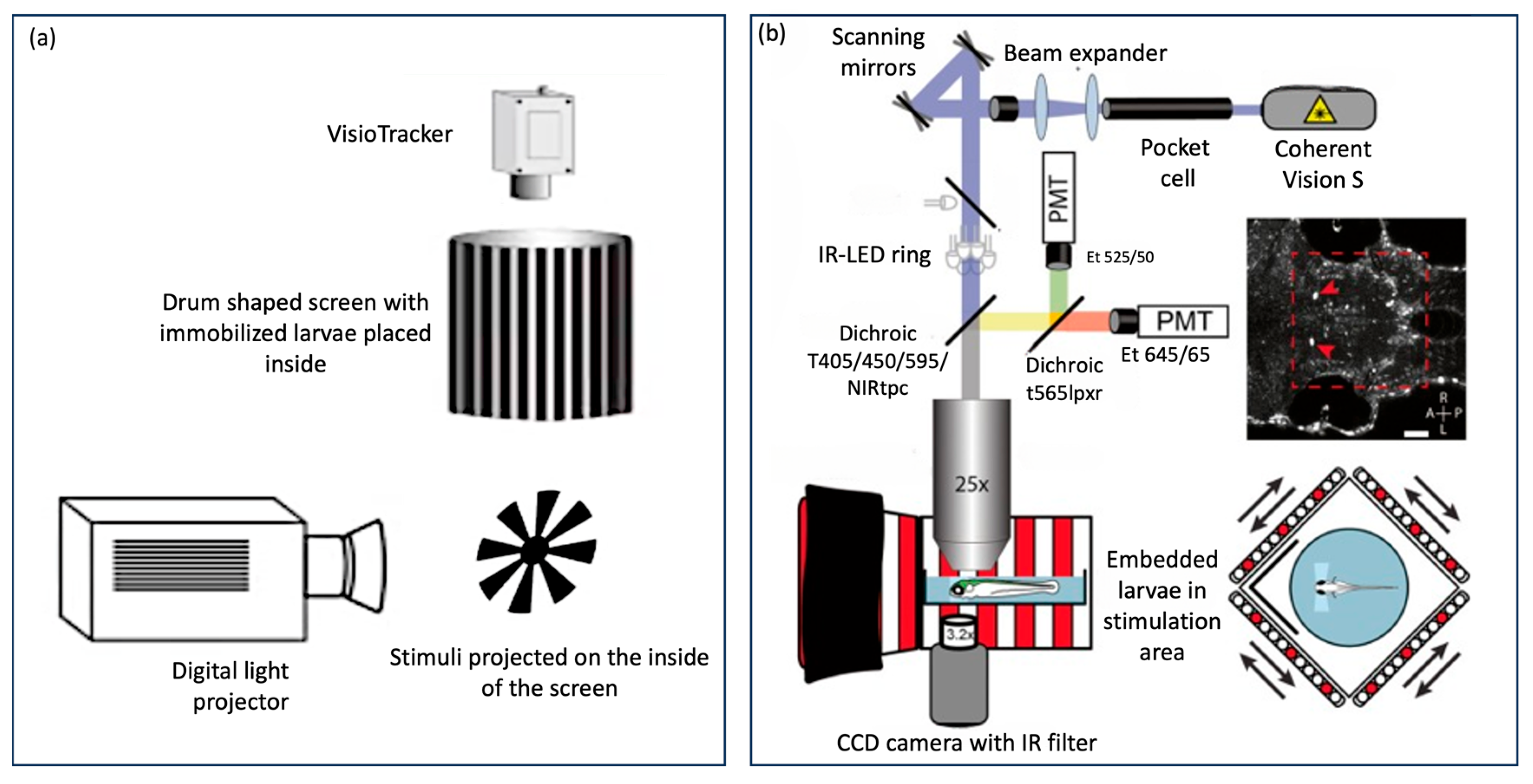
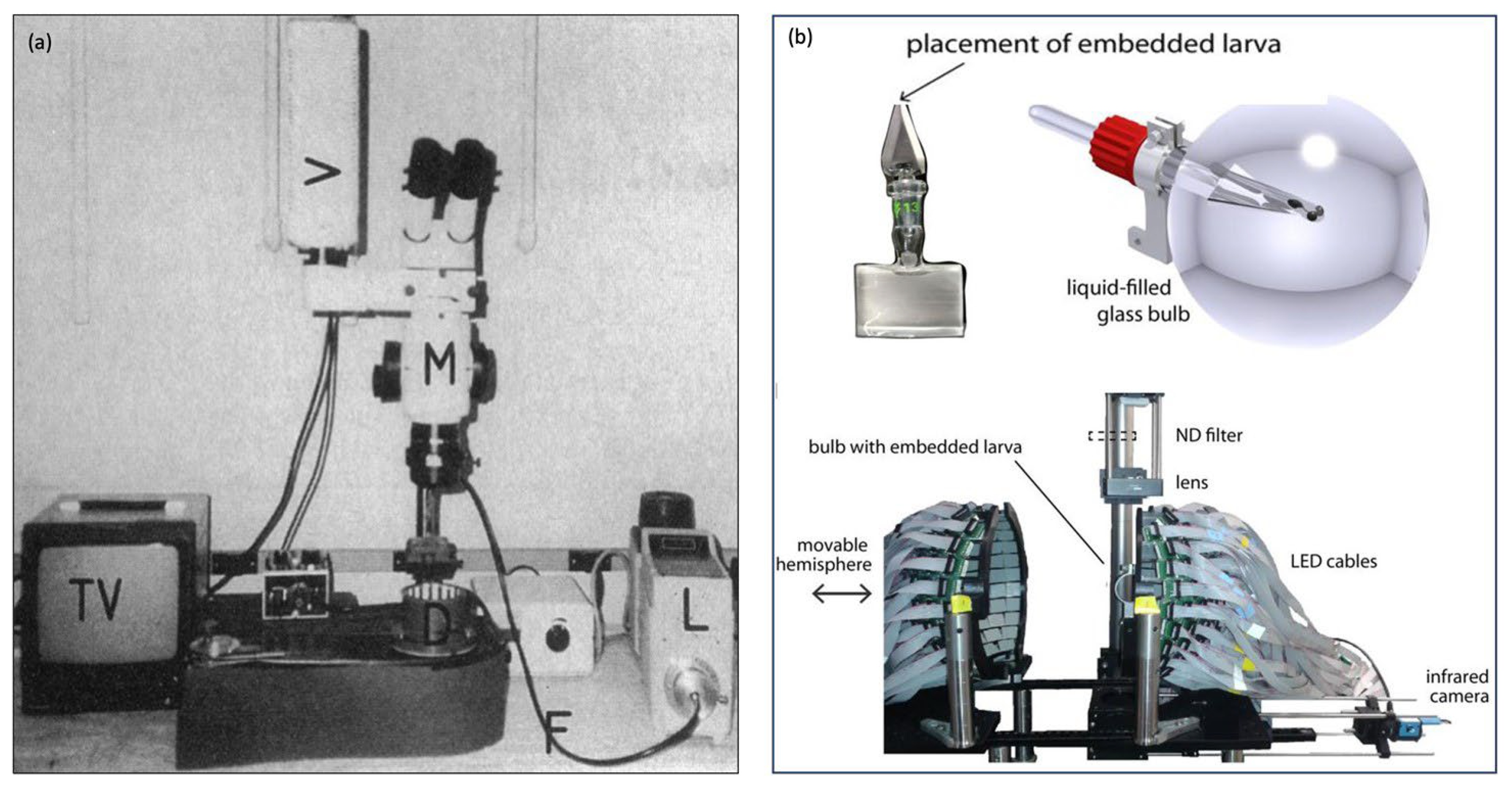
There are also automated “all-in-one” OKR assay technologies that have been used as a standard across many laboratories such as Visiotracker and ZebEyeTrack (Figure 4). These offer a combined tool for stimulus generation, eye movement recording, tracking, and analysis. Visiotracker involves the projection of stimuli onto a still paper drum [29]. ZebEyeTrack is a square LED arena [24].
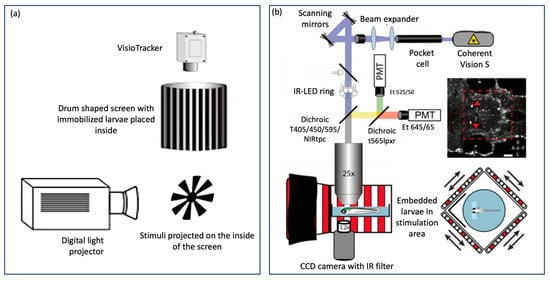
Figure 4.
Automated “all in one” assays (a) VisioTracker schematic showing stimulus projector, rotating drum, and camera adapted from Video abstract in Mueller et al. 2011 [29] and (b) ZebEyeTrack inspired set-up adapted from Brysch et al. 2019 [30].
Rotating Drum
This original technique for eliciting OKR utilised a striped paper drum which was rotated around larva by means of a belt and electrical motor (Figure 5a). These methods were pioneered in papers by Brockerhoff and Zou [20,31]. This technique is less common as more sophisticated methods of projecting stimuli have been developed.
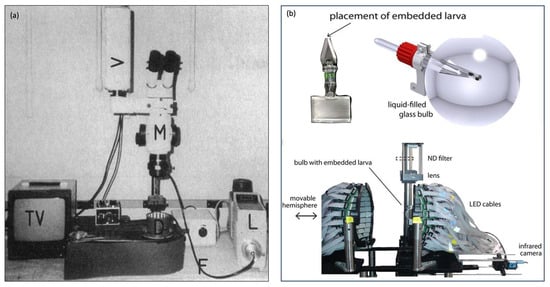
Figure 5.
Different OKR set-ups (a) stimulus on rotating striped drum, figure adapted from Brockerhoff 1995 [20] and (b) Novel circular LED arena, figure adapted from Dehmelt 2021 [32].
Projected Stimulus
This method involved the projection of a moving computer-generated OKR stimulus. This allowed for more flexibility of the stimulation but has the increased complexity of projecting flat images onto a 3D drum or screen [29]. Distortions could be prevented through the use of a mirror or through software-based techniques [33].
The stimulus was most commonly projected onto a paper drum surrounding the larva [34] (Figure 4a) or using flat 2D screen made from diffusion film [35].
LED/LCD Arenas
This involves multiple screens arranged to form an arena (Figure 5b). The original arena that was used was square-shaped [24]. Physiologically, the larval visual field is 180 degrees. It is well understood that exposing larval zebrafish to full visual field stimuli will elicit a more robust OKR, hence novel spherical or cylindrical arenas have been constructed with larvae positioned centrally inside the tip of a narrow triangular glass stage [32,36].
3.2.4. Optokinetic Stimulus
There is significant variation in the literature on reported stimulus characteristics and parameters. Computerised stimuli allow for greater flexibility in the range of stimuli provided [29].
Pattern
Vertical black and white square-wave gratings (alternating black and white bars/stripes) have been successfully used to elicit horizontal OKR in many experiments. Sinusoidal gratings have been used to evoke the natural environment which may allow for the smoother eye-tracking of stimulus [37]. Less commonly, a “random dot” pattern was used. This involves animated dots moving sinusoidally in opposite vertical directions generating a sensation of horizontal motion [38].
Stimulus Generation and Control
Traditionally, changing the stimulus on a rotating drum involves manually changing the gratings. Projection or LED assays allow for computer-generation and control of stimuli parameters, such as contrast and speed, during experiments in real-time [29]. Many different types of software can be used.
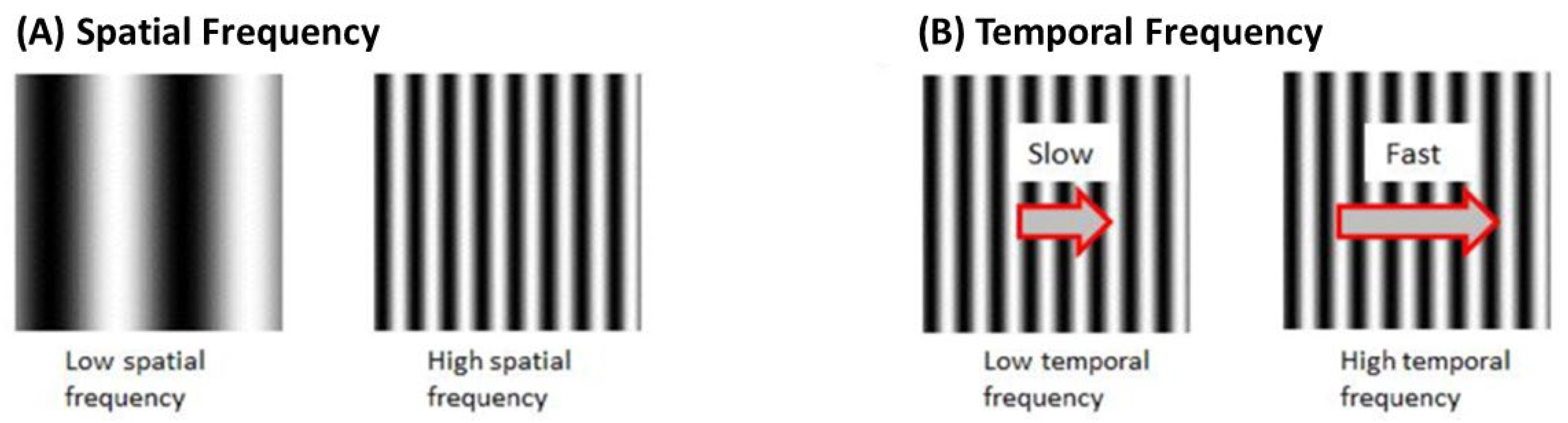
Frequency and Velocity
Each set of black and white gratings is known as one cycle. Spatial Frequency (SF) is the number of cycles per unit distance. A higher SF indicates a higher number of gratings with a smaller width. Velocity is the rate of stimulus rotation. Temporal frequency (TF) is the number of cycles per unit time. TF is the product of SF and velocity (Figure 6).
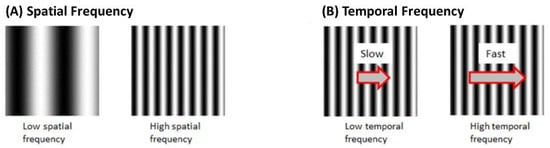
Figure 6.
(A) Spatial frequency of OKR stimulus is determined based on the number of black and white gratings (B)Temporal frequency is based on the number of cycles per unit time.
OKR gain is dependent on SF and velocity. Research shows that the stimulus is no longer detected through raising SF and velocity to the limit of larval vision, thus gain decreases [33]. The highest spatial frequency that an eye can still resolve has been described as the “optical cut-off frequency” [39].
On traditional drums, SF could be substituted for manual measurements of the drum. Computer-generated stimuli allow for accurate calculation of spatial frequencies.
Contrast
The stimulus contrast is the light to dark ratio of stimulus gratings. Research on contrast sensitivity found gain increased by 0.77 per log unit contrast [33]. A high contrast is thus recommended to evoke strong OKR in zebrafish.
Distance of Stimulus from Larval Eye
The distance from stimulus to larva impacts all the above parameters. Optimisation experiments can be performed by varying the stimulus distance while maintaining the same spatial frequency [39]. The methodology used to measure the distance of stimuli varied. Some calculated the radius of the rotating drum, assuming the larva was centrally located. Others took more precise measurements of the distance between the larval eye and stimuli [40].
Direction of Rotation
Altering the direction of rotation heightens the robustness of the OKR [41]. Frequent rapid changes in direction have been used to reduce the number of quick phases and make it easier to calculate slow phase velocity [42].
The direction of optokinetic stimulus rotation affects the intensity of the OKR elicited on monocular stimulation of lateral-eyed vertebrates, including zebrafish. A naso-temporal stimulus will elicit a greater response than a temporo-nasal stimulus [43]. This is due to the characteristic asymmetry of the OKR in temporal-to-nasal (T-N) direction under monocular stimulation.
Monocular vs. Binocular Stimulation
Traditionally, larvae were placed inside a rotating drum and binocularly stimulated. However, the OKR in the eye of interest can be affected by contralateral eye stimulation [32]. There are various methods for achieving monocular stimulation, such as positioning the larva to be perpendicular to the stimulus or isolating half the visual field with a barrier. Stationary gratings were said to be more effective than black, white, or aluminium shields at isolating one eye from receiving any stimulation [32].
Light Intensity
Initial development of zebrafish retinomotor movements at 5 dpf allows for functional light adaptation [6,44].
When considering “light intensity”, this often refers to the light emanating from the assay itself, although impact of the lighting in the surrounding environment should not be neglected.
There are many different units for light intensity. Lumen (lm) is the total quantity of visible light emitted from a source in any direction. Illumination ‘Lux’ (lx) is the measure of light intensity received on a surface that is being lit. Candela (cd) is the light intensity emitted from a source in a certain direction. Luminance (Nit, cd/m2 or Ft/L) is the light intensity emitted from a certain surface area (for example a computer screen) in a certain direction. It has been reported that when varying light intensity above 3.2 cd/m2, OKR gain was not impacted. However, gain dropped significantly below 0.36 cd/m2, indicating that zebrafish struggle to see stimuli with very low illumination [33]. Illumination at the position of the larval eye has been measured using a photometer positioned at the centre point of the assay [37,45].
3.2.5. Experiment
Throughput and Duration
The experimental throughput is the number of larvae one can measure in a given time which will determine the duration of the experimental phase of research. High throughput has been achieved through simplifying the OKR set-up, the automation of OKR analysis, or the use of commercially available systems like Visiotracker and ZebEye track [24,29]. When manually recording OKR, the throughput of an OKR assay was limited to one. When measuring one larva at a time, a throughput of ~600 larvae in a single day (10 h) was reported [25]. On a grander scale, others have managed a throughput of ~77,000 larvae over three years [34].
Eye movements of only one larva can be viewed at a time. Modern techniques combining video recordings with new eye-tracking software enable multiple simultaneous measurements of OKR [24].
Sample Size
Zebrafish studies vary immensely in the total sample size of larvae utilised for an experiment. Genetic screens can generate large sample sizes [34]. However studies trialling new OKR methods have comparatively smaller sample size [29]. In the context of a forward genetic screen for recessive mutations, it is often advocated that screening 12 fish from a single clutch can significantly increase the likelihood of detecting a genetic mutant [26]. Additionally, assessing whether fish exhibit no OKR responses can be particularly insightful for genetic screening or toxicology assays. However, it is important to note that such assessments should be tailored to the specific objectives of the experiment, taking into account the desired outcome measures and the necessary sample size calculations [26].
Time of Day
Zebrafish, like humans, possess a natural circadian rhythm which influences their behaviour. Thus, performing behavioural assays at different times of the day may skew results [25]. Overnight, we know zebrafish photoreceptors are non-active due to the dismantling of ribbon synapses, meaning they do not naturally respond to any visual stimuli [46]. More importantly, throughout the day their biological rhythms have been found to cause nuanced fluctuations in visual sensitivity [47]. There is evidence that circadian regulation weakly affects the OKR by influencing the visual transduction cascade [48].
Furthermore, when dealing with larvae 2–5 days post fertilisation, recordings ±8 h apart will affect the maturity of the OKR, as mentioned in the section of larval characteristics of OKR assay parameters.
3.2.6. Recording of Eye Motion
Manual
Traditionally, papers recorded changes in eye motion manually through a dissection microscope [20].
3.2.7. Cameras
Using cameras to record eye movements provides more flexibility. Recordings can provide rapid automated eye movement analysis in real-time and an accurate calculation of gain by measuring eye velocity [24]. Alternatively, videos can be used for manual analysis [49]. The industry standard camera is the CCD (charge-coupled device) which transmits data through analogue signals. A CMOS (complementary metal-oxide semiconductor) camera is a more modern cost-effective device which transmits data through digital signals. The quality of new-generation CMOS cameras is comparable to a CCD.
3.2.8. Analysis of Eye Motion
Manual vs. Software
Historically, manual screening has been favoured in OKR assays, particularly for its perceived ability to reduce false positives [25]. However, an often overlooked aspect in these studies is the implementation of double-blind methodologies. Such approaches, while instrumental in reducing observer bias, especially in manual evaluations, are not commonly reported. Furthermore, their applicability can be limited in contexts where fish display distinct phenotypes, such as hypopigmentation, which can inadvertently influence the observer’s assessment. Despite the potential benefits, it is important to note that manual methods also pose a risk of increased bias and human error [33].
In contrast, the emergence of eye-tracking software has provided an alternative that offers both rapidity and accuracy. Some of these software solutions utilise proprietary codes not openly shared with the public, while others are based on open-source environments like Python or MATLAB, offering broader accessibility and customisation options [1]. Advanced all-in-one assay models, such as Visiotracker and ZebEyeTrack, integrate eye-movement analysis software, further streamlining the OKR assessment process (Figure 4).
The choice between manual and software-based analysis in OKR assays should consider these factors, with an emphasis on minimizing bias and enhancing the reliability of results. Future studies employing manual screening are encouraged to explore and report the use of double-blind procedures where applicable to enhance the objectivity of their findings.
Quantifying the OKR Assay
Generally, a “positive OKR” outcome is defined as the presence of at least one slow phase or quick phase [18]. Some require both to be present for a positive response [50]. OKR outcomes can also be quantified. Quick phase/saccades can be counted or provided as a frequency [51,52].
Using software for eye-tracking, slow-phase velocity can be used to determine gain, a well-known indicator of visual impairment [53]. Gain is calculated as the ratio of eye velocity to stimulus velocity. Alternatively, an index for rating OKR outcomes on a custom-made scale can be devised [34,54,55].
4. Overview of Parameters Utilised in Designing the OKR Assay
The literature review highlights various factors reported when planning and conducting an OKR assay in zebrafish. We have explored how these factors (developmental stage of larvae, immobilisation method, assay set-up, pattern, stimulus parameters and presentation of stimulus) could influence the OKR assay results and its interpretation.
Key methodological considerations reported to elicit a robust OKR response involve selecting larvae of 5 dpf or above and using an assay set-up with full-field monocular stimulation. Strong evidence indicates selection of the stimulus spatial frequency and velocity is important for OKR outcome, with attention required to not surpass the “optical cut-off frequency” which results in poor OKR responses due to the inability of the fish to resolve the stimulus. A higher stimulus contrast will also produce a better response. Once robust OKR responses have been elicited, the final data quality is further dependent on eye movement recording and analysis. Utilising appropriate software-based approaches instead of relying on manual methods can greatly enhance the automation of eye movement measurements and effectively mitigate observer bias.
4.1. Existing Reporting Practices
The above-mentioned variables directly or indirectly impact on OKR experimental design, analysis, and interpretation. From the 109 relevant studies identified, we calculate the percentage of papers which contain the appropriate details for each variable. We will go on to discuss the minimal reporting guidance considering the literature.
4.1.1. Larval Characteristics
Species of Fish
A total of 100% of papers referenced the species either as ‘zebrafish’ or ‘Danio rerio’.
Larval Developmental Stage
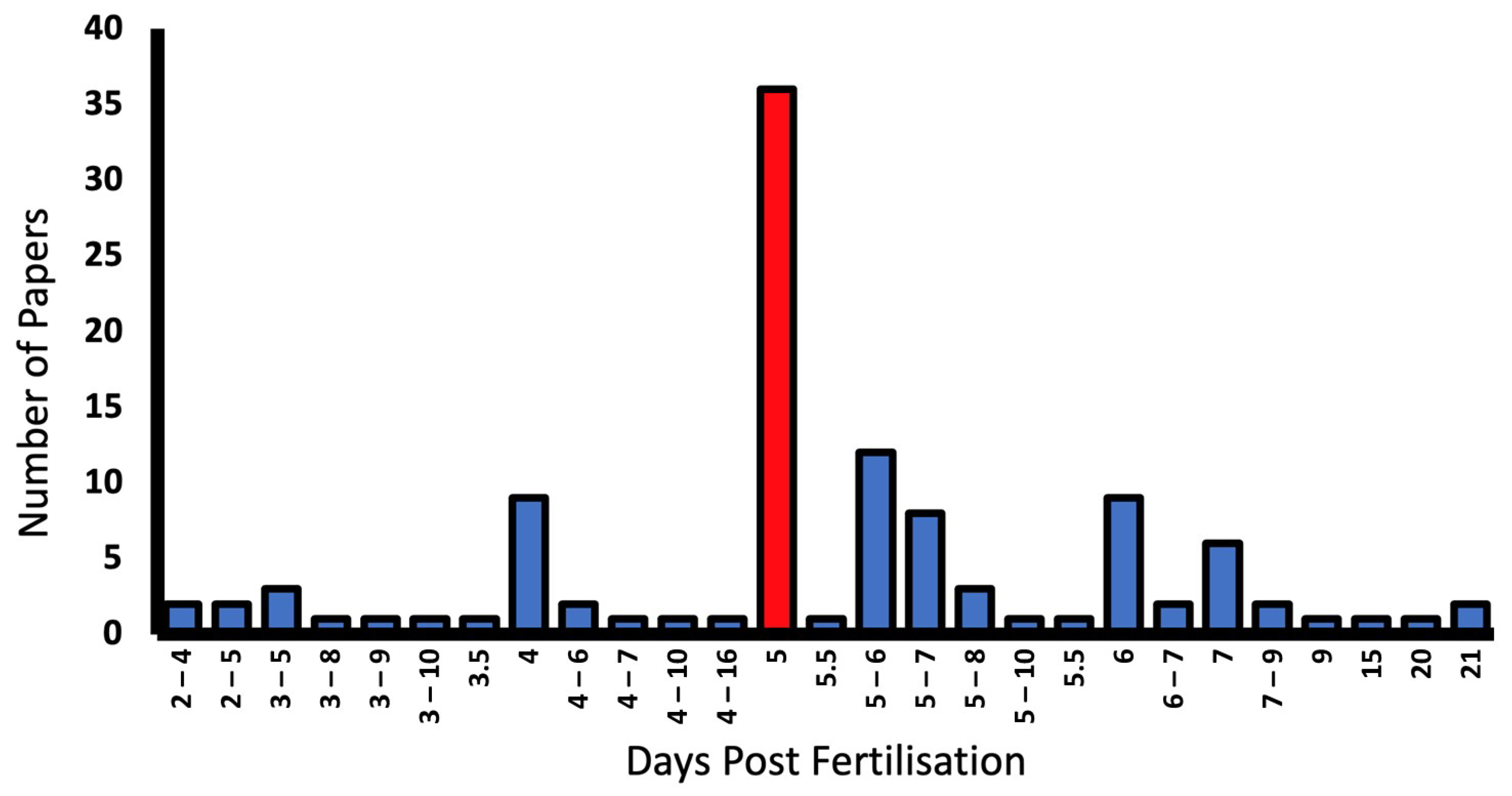
Only 7.3% of studies failed to report the developmental stage of larvae [34,35,56,57,58,59,60]. Larvae from 2–21 dpf were used, with 5 dpf being most commonly reported in 35.6% of papers. Some report dpf as a range as outlined in the graph (Figure 7).
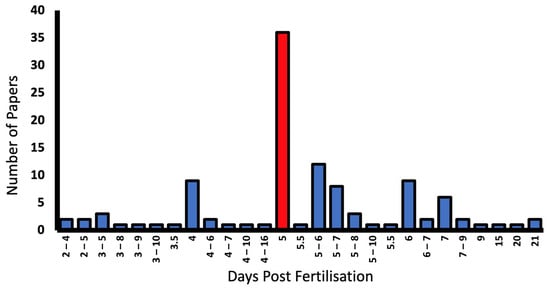
Figure 7.
Larval developmental stage (days post fertilisation (dpf)), and number of reporting papers in the literature. The most common larval stage used in OKR experiments was 5 dpf (red bar).
4.1.2. Immobilisation
The immobilisation method was detailed in 88% of studies, with a further 6.4% referencing past methodology [57,61,62].
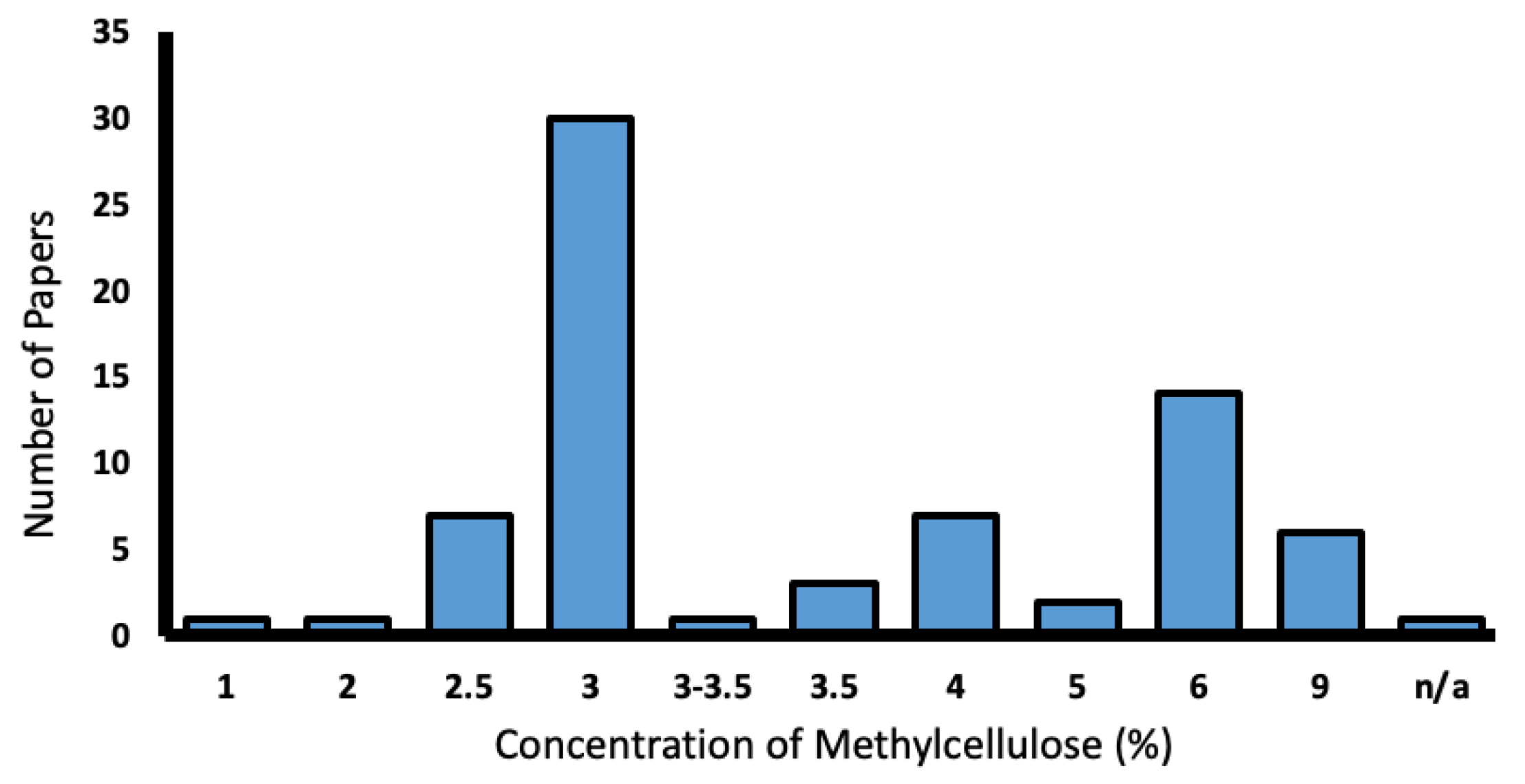
From this total of 103 papers, methylcellulose was the most popular mounting medium and was utilised in 70.9% of papers. Concentrations of methylcellulose varied from 2.5–9%, with 3% being the most common (Figure 8).
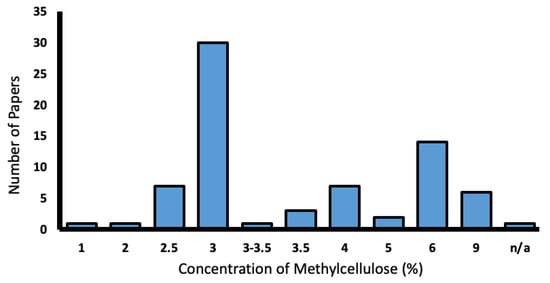
Figure 8.
Range of methylcellulose concentrations used in the literature (%).
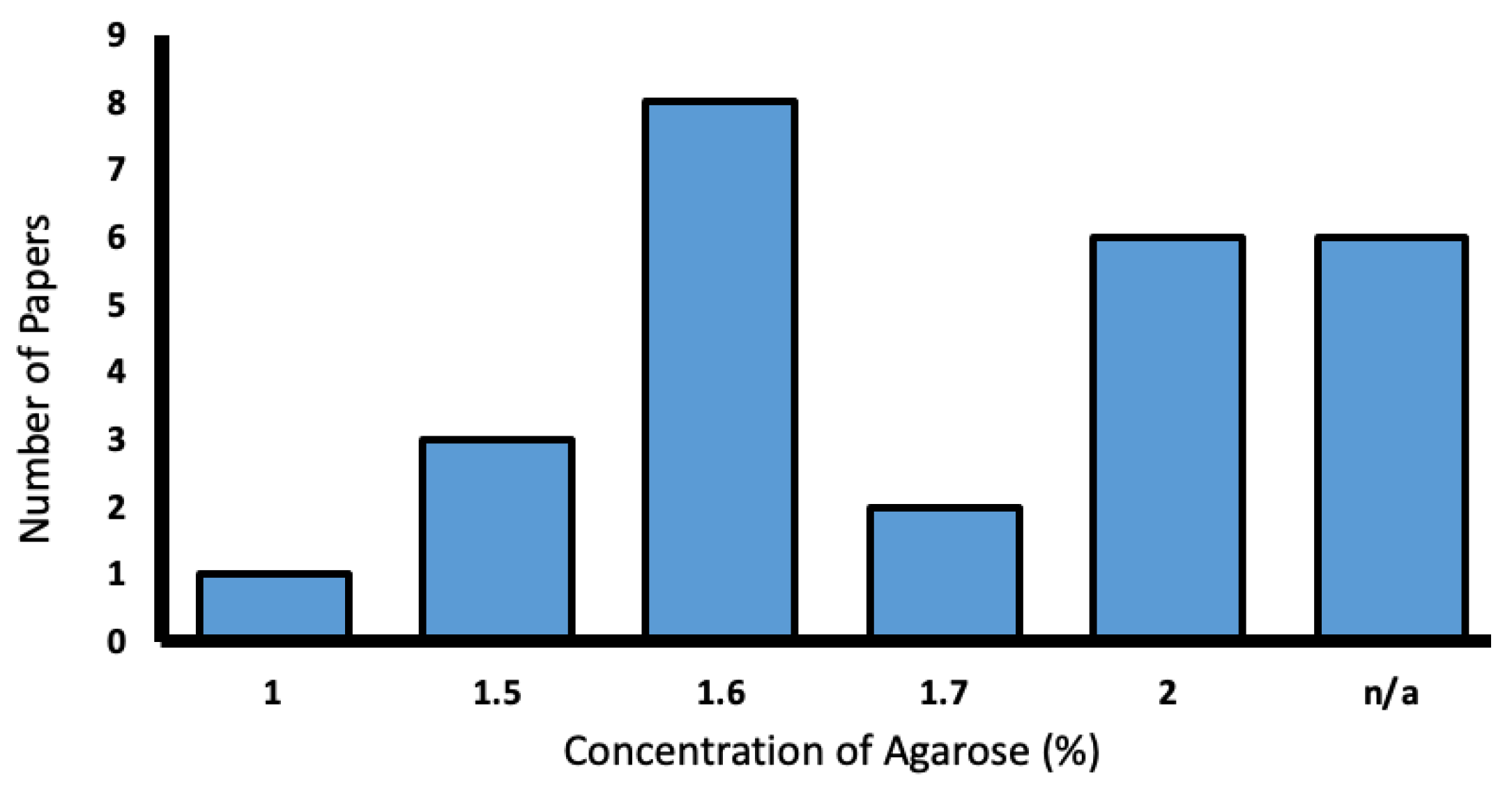
Agarose was used in 25.2% of the reporting studies. Concentrations varied from 1–2%, with most favoring 1.6% (Figure 9).
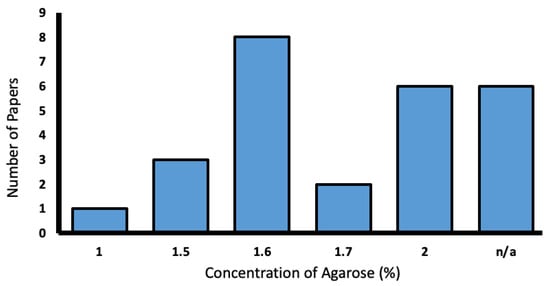
Figure 9.
Range of low-melting agarose concentrations used in the literature (%).
Pins/needles were used in 1.9% papers, where the larvae exceeded 20 dpf [63]. A further 1.9% reported no immobilisation method due to larvae being inside the chorion.
4.1.3. OKR Assay Set-Up
The OKR assay set-up was detailed in 89.9% of studies, with a further 5.5% referencing another paper’s methodology. A total of 3.7% do not mention OKR methodology [58,64,65,66].
Popularity of each method:
- A.
- In 39.4% of papers, the OKR assay used a standard rotating drum.
- B.
- The OKR stimulus was projected onto a stationary surface in 43.1% of papers.
- C.
- The LED/LCD arena method was used in 13.8% of papers.
4.1.4. Optokinetic Stimulus
Patterns
In 86.2% of papers the pattern of the stimulus was mentioned. A total of 55% of manuscripts utilised a standard stimulus of black and white stripes/gratings. A total of 4.7% of papers did not report their stimulus, but gratings were present in images of the OKR set-up. In 23.8% of papers the stimulus was sinusoidal and 0.03% used random dot patterns [38,67]. Seven papers with no pattern used Visiotracker software and thus likely used their standard gratings.
Stimulus Generation and Control
From 60 manuscripts with projected or LED-based stimuli, 66.7% referenced software for stimulus generation, with 22.5% of these opting for LabVIEW (National Instruments) [68,69,70], whilst 12.5% used MATLAB [1,32]. The rest used other software: Image-J, Simple DirectMedia Layer, PsychoPy, NIH Object Image and Python Library Vision (Table 1). This is excluding papers known to use Visiotracker or Zebeyetrack software.

Table 1.
Number of papers that reference each software for stimulus control.
Stimulus Parameters: Frequency and Velocity
A total of 81.7% of papers included a value for SF or a corresponding parameter. A total of 84.4% of papers provided a value for the stimulus velocity/temporal frequency. They have been divided based on the OKR method (Table 2).

Table 2.
Frequency of various stimulus rotation parameters reported in the literature.
For papers using the rotating drum method, 90.7% reported stimulus parameters. Of these, 84.6% reported the SF as degrees of a cycle (ranging from 9–40 degrees), with 18 degrees as the most popular. The TF was provided as the number of rotations per minute in 69.2% of papers (ranging from 3–20 rpm), with 6–8 rpm being the most common.
From all methods using a projected stimulus, 91.5% provided some stimulus parameters. Of these, 55.8% recorded SF in cycles per degree (cpd) (from 0.01–0.6 cpd). The velocity in degrees per second (from 2.7–30 deg/s) was noted in 81.4%. Median values of 0.06 cpd and 7.5 deg/s were most common [33].
For LED assays, 73.3% of papers reported stimulus parameters. Notably, all of these papers also reported velocity in degrees per second (from 5–48 deg/s). SF was mostly reported in cycles per degree (from 0.033–0.066 cpd), with one paper using a random pattern omitting a SF [81]. The most commonly used settings were 0.033 cpd with 10–15 deg/s [36].
Stimulus Duration
The time of exposure to a stimulus was given or referenced in 67% of papers. This varied greatly from just 3 s [33] to as long as 20 min [81]. Over a third of these papers used a timing of 1 min [41,51,54,84,85]. A duration of 2 min was also common [52,86,87].
Direction of Rotation
72.5% of manuscripts highlighted the direction of stimulus rotation. A bidirectional stimulus was used or referenced in 65.1% of papers [79,88].
4.1.5. Lighting
Distance of Stimulus from Larval Eye
Authors provided the distance between stimulus and larva in 26.6% of papers. Of these, 51.7% provided a drum diameter and 34.5% noted the specific measurement from the larval eye to the stimulus, from 6 cm to 22 mm (Table 3).

Table 3.
Distances measured from larval eye to stimulus (cm).
A further 13.8% of the papers provided a complex 3D location with more modern circular LED arenas.
Monocular vs. Binocular Stimulation
20.2% of manuscripts stimulated larvae monocularly [38,81,88,93]. Most papers, 48.6%, did not specify whether the stimulus was monocular or binocular.
Contrast
Contrast was mentioned in 31.1% of studies. One third of these studies, measuring contrast sensitivity, provided a broad range of contrast levels tested from ~1–5% to 100% contrast [6,44,79], 50–100% [55], and another from 20–99% [71]. The rest of these studies used a fixed high constant contrast of 85–100% [88,93,94].
In studies that did not mention contrast, light parameters in the forms of intensity or luminance were stated in 14.7% of papers. Only 11% mentioned both contrast and lighting parameters (Table 4).

Table 4.
Number of papers that report light parameters in the literature.
4.1.6. Experiment
Throughput
Only one trial using ZebEyeTrack reported on the assay throughput accuracy. A high OKR accuracy was found when measuring six zebrafish larvae simultaneously, comparable to individual stimulation [24].
Sample Size
A total of 84.4% of publications mentioned the sample size of larvae. One paper measured OKR from over 70,000 larvae over a three-year time period [34]. Most studies reported an average sample of 6–15 per larval group.
OKR Time of Day
Only 14.7% of manuscripts include time parameters for performing an OKR. Just over half of these had broad parameters spanning morning and afternoon, between 8 am and 7 pm. The most common time of day was between 12 and 6 pm, with 37.5% of these papers performing an OKR exclusively in the afternoon [53,101,102].
Recording of Eye Motion
64.2% of researchers used cameras for recording eye movement. Of these, 55.7% used CCD cameras [103], with 8.6% utilising newer CMOS technology [67]. A total of 44.2% were stated to be infrared sensitive.
Analysis of Eye Motion
Analysis software was used in 54.1% of papers (Table 5). A total of 35.5% of these used LabView.
Eye movement analysis tools were not used in 45.9% of publications. Only 16.6% of these papers explicitly stated the analysis was done manually [104].

Table 5.
Number of papers that reference eye movement analysis software.
Table 5.
Number of papers that reference eye movement analysis software.
| Analysis Software | Number of Papers | Paper Reference |
|---|---|---|
| LabView | 16 | [6,33,39,42,44,55,68,69,71,75,82,83,90,91,103,105] |
| LabView/Matlab | 4 | [73,93,106,107] |
| Labview/Tracker 4.0 | 1 | [72] |
| LabView/ImageJ | 1 | [88] |
| Cell F | 1 | [88,108] |
| Viewpoint OKR | 2 | [61,84] |
| Matlab | 9 | [1,40,56,65,70,78,86,89,109] |
| ZebEyeTrack (Matlab) | 7 | [24,30,32,65,110,111,112] |
| AmScope/Matlab | 1 | [113] |
| Python/Matlab | 1 | [67] |
| Bonzai/Matlab | 1 | [94] |
| Proprietary | 1 | [29] |
| ScanImage | 1 | [77] |
| Media Cybernetics IPP6 | 1 | [114] |
| Image J | 4 | [34,38,59,100] |
| Object Image2 | 1 | [99,108] |
Movement analysis software.
Quantifying OKR Assay
Authors reported on how they quantified the OKR in 89.9% of publications. From these, the most common outcomes measured were eye velocity/gain and saccade rate in 51% and 28.6% of papers, respectively. The number of positive OKR responses or ‘saccades’ was reported in 33.7% of papers (Table 6).

Table 6.
Number of papers outcomes for reporting the OKR.
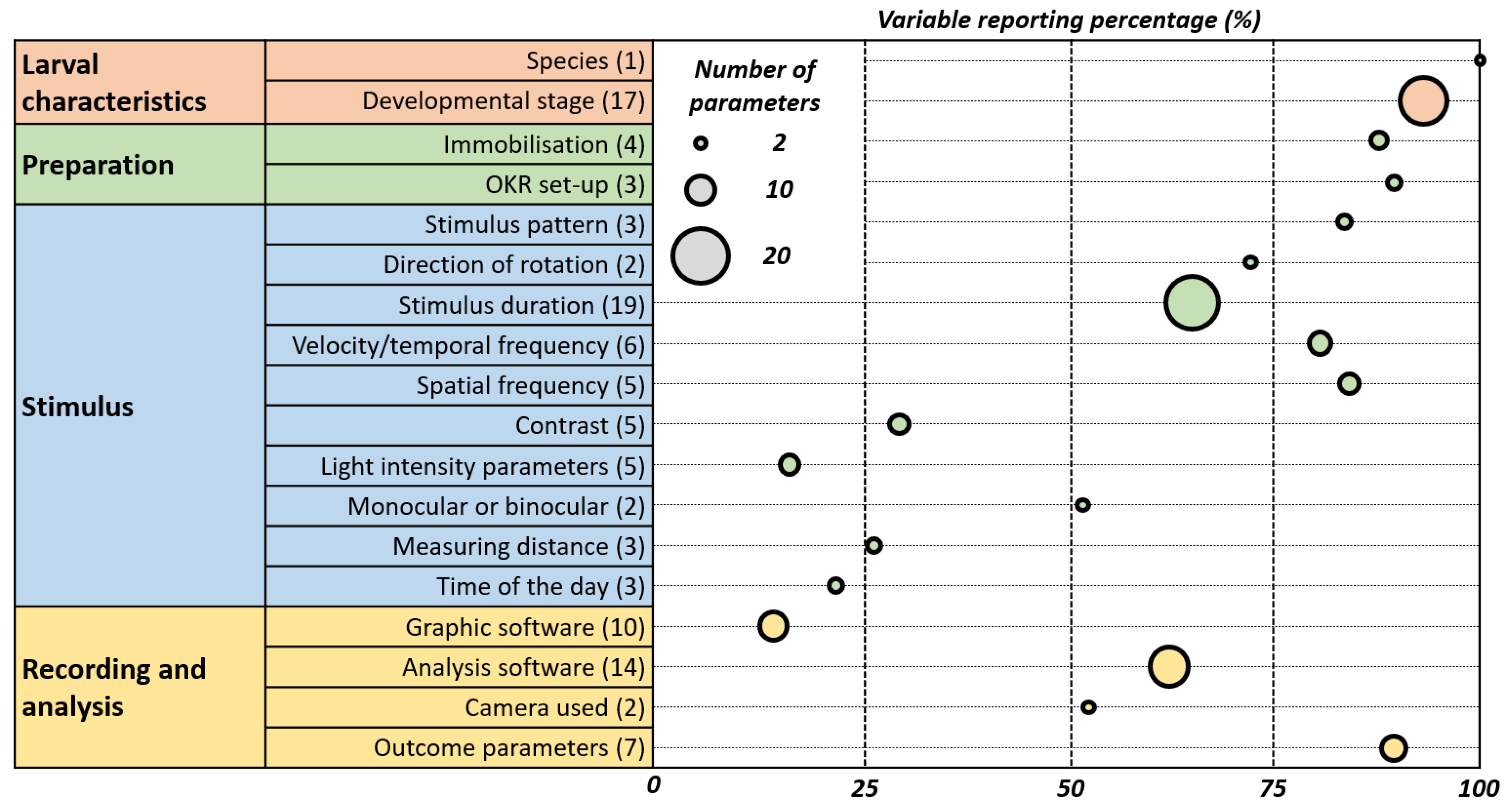
The inconsistencies in OKR assay reporting are shown in Figure 10 which emphasizes the need for its standardization.
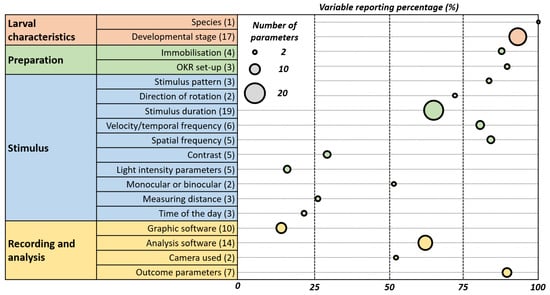
Figure 10.
Weighted Bubble Chart Illustrating Reporting Variability and Prevalence in OKR Studies. This chart depicts the reporting practices in optokinetic reflex (OKR) studies. Each bubble represents an OKR parameter, with its position on the x-axis indicating the percentage of papers reporting it. The size of the bubble reflects the variability in how the parameter is reported across studies—larger bubbles denote greater variability. This visualisation highlights the prevalence and consistency of reporting for each parameter, underscoring areas where standardised practices may be needed.
5. ZOK Reporting Guideline for Optokinetic Reflex Studies in Larval Zebrafish
Based on our systematic literature review, there is significant variability in the methodologies employed and the level of detail reported for OKR assays in larval zebrafish. New researchers require more support to fully take advantage of the OKR assay. Hence, the following minimal reporting guideline has been devised to aid future researchers (Table 7). As a primary step, the provided guidance table offers a structured outline of the necessary procedures. This entails the researcher to make the choice of an appropriate OKR assay method from (a) a rotating drum, (b) a projection, or (c) an LED arena. When uncertainty arises in the selection of parameter values for each method, we suggest referring to the section of existing reporting practices of this review.

Table 7.
The ZOK recommended minimal reporting guidelines.
6. Discussion
After reviewing the existing literature, it is clear that several factors impact measurements reported from OKR assays, including larval characteristics, immobilization methods, assay set-ups, stimulus parameters, and analysis techniques. Our systematic review of 109 papers highlights the prevalent issue of insufficiently reported variables, possibly due to lab-specific approaches and a lack of standardized reporting guidelines. We propose using a simple minimal reporting guidance table (Table 7) which serves as both a checklist for researchers establishing an OKR assay and a tool for peer reviewers assessing a study. Whilst different laboratories may utilise various ranges of parameters, the accuracy of OKR assays can still be achieved. The proposed reporting guidelines are designed to foster reproducibility and transparency rather than dictate a uniform experimental approach. This flexibility is particularly crucial when working with a spectrum of phenotypes in behavioural research. Our goal is to support a broad application of these guidelines which are adaptable to diverse research settings and methodologies.
With the advent of CRISPR-Cas9 mutagenesis techniques, the ease of modeling human mutations in zebrafish has significantly increased [116,117]. Through integrating these advances into genetic engineering with state-of-the-art visual behavioural assays, such as the OKR, researchers are not only able to model diseases linked to retinal development [118,119,120,121,122,123] but also explore cis-regulatory variants affecting neurological development or retinal function [124,125]. This approach has shown effectiveness in modeling infantile nystagmus [6], examining its impact on OKR development, and evaluating therapeutic responses analogous to those used in human treatments [75]. Such studies underscore the potential of using zebrafish as a versatile model for understanding and treating human visual disorders and, importantly, the role of well-designed OKR assays to objectively determine disease status and therapeutic responses.
Certain challenges were encountered during the execution of this review. Determining the influence of variables on the zebrafish OKR proved challenging due to limited existing research in the field. A potential weakness was that nine assay methodology papers were included in the review which conducted no OKR investigations other than displaying the set-up of their equipment. This could have slightly negatively affected the percentages concerning reporting practices. However, these papers were included in the review because assays were carried out on wildtype zebrafish. Employing better reporting in methodology papers can also be advantageous in proving that a proposed method is effective.
It was also difficult to identify the optimal parameter for a variable where values differed greatly across the studies. The modal “ideal” value we calculated may be subject to sampling bias as larger laboratories publishing multiple papers use the same protocol, potentially influencing these results. However, it is noteworthy that these high frequency publications prove their OKR assay protocol to be highly effective and perhaps it is a good opportunity for others to adopt these practices. This review provides great transparency for researchers through its systematic tables with references for various parameters. Additionally, legal restrictions in some countries probably influenced the popularity of using 5 dpf larvae. It is important for new researchers to consider that older larvae might exhibit a stronger OKR response. While our paper focused on larval OKR studies, our examination of studies involving adult zebrafish indicates that, despite marked differences in experimental procedures such as age and immobilisation methods, the fundamental reporting parameters are similar. Consequently, we propose that our reporting guidelines can also be relevant and beneficial for studies involving adult zebrafish.
Finally, the OKR assay has been found to be a popular and accurate tool for visual system analyses in larval zebrafish. Our goal is to establish ZOK as a baseline standard that enhances research transparency while remaining adaptable to individual research needs. Notably, this guidance is flexible and accommodates various assay configurations. We anticipate the use of the ZOK reporting guideline can lead to higher quality research being performed on zebrafish models.
Author Contributions
Conceptualization, M.G.T. and V.R.; methodology, M.G.T. and V.R.; validation, M.G.T. and V.R.; formal analysis, V.R.; investigation, V.R.; resources, M.G.T. and W.H.J.N.; writing—original draft preparation, V.R.; writing—review and editing, M.G.T., H.J.K., S.C.F.N., W.H.J.N., M.P. and V.R; visualization, V.R.; supervision, M.G.T. and W.H.J.N.; project administration, V.R. and M.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Wellcome Trust (RM38J0003M20), the Academy of Medical Sciences (SGL021\1066), BBSRC, Ulverscroft Foundation and Fight for Sight (SGANYN2210).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented is available within the manuscript and references.
Acknowledgments
We would like to acknowledge the support shown by the University of Leicester and Leicester Royal Infirmary library services for subscription access to necessary databases. We thank the staff at the Pre-clinical Research Facility (PRF) at the University of Leicester for their helpful discussions on zebrafish visual behaviour assays.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scheetz, S.D.; Shao, E.; Zhou, Y.; Cario, C.L.; Bai, Q.; Burton, E.A. An open-source method to analyze optokinetic reflex responses in larval zebrafish. J. Neurosci. Methods 2018, 293, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Proudlock, F.A.; Sarvananthan, N.; Roberts, E.O.; Awan, M.; McLean, R.; Surendran, M.; Kumar, A.S.A.; Farooq, S.J.; Degg, C.; et al. Phenotypical characteristics of idiopathic infantile nystagmus with and without mutations in FRMD7. Brain 2008, 131, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.D.; Baloh, R.W.; Honrubia, V. Eye movement abnormalities in rod monochromacy. Ophthalmology 1981, 88, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.; Crosier, M.; Lindsay, S.; Kumar, A.; Thomas, S.; Araki, M.; Talbot, C.J.; McLean, R.J.; Surendran, M.; Taylor, K.; et al. The clinical and molecular genetic features of idiopathic infantile periodic alternating nystagmus. Brain 2011, 134, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, S.E.; Dowling, J.E.; Hurley, J.B. Zebrafish retinal mutants. Vision Res. 1998, 38, 1335–1339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.-Y.; Rinner, O.; Hedinger, P.; Liu, S.-C.; Neuhauss, S.C.F. Oculomotor instabilities in zebrafish mutant belladonna: A behavioral model for congenital nystagmus caused by axonal misrouting. J. Neurosci. 2006, 26, 9873–9880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Damé, M.C.F.; Xavier, G.M.; Oliveira-Filho, J.P.; Borges, A.S.; Oliveira, H.N.; Riet-Correa, F.; Schild, A.L. A nonsense mutation in the tyrosinase gene causes albinism in water buffalo. BMC Genet. 2012, 13, 62. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Koroshetz, W.J.; Behrman, S.; Brame, C.J.; Branchaw, J.L.; Brown, E.N.; Clark, E.A.; Dockterman, D.; Elm, J.J.; Gay, P.L.; Green, K.M.; et al. Framework for advancing rigorous research. eLife 2020, 9, e55915. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Leenaars, M.; Ritskes-Hoitinga, M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 2010, 38, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Brattelid, T.; Smith, A.J. Guidelines for reporting the results of experiments on fish. Lab. Anim. 2000, 34, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ohnesorge, N.; Heinl, C.; Lewejohann, L. Current Methods to Investigate Nociception and Pain in Zebrafish. Front. Neurosci. 2021, 15, 632634. [Google Scholar] [CrossRef]
- Hill, B.N.; Britton, K.N.; Hunter, D.L.; Olin, J.K.; Lowery, M.; Hedge, J.M.; Knapp, B.R.; Jarema, K.A.; Rowson, Z.; Padilla, S. Inconsistencies in variable reporting and methods in larval zebrafish behavioral assays. Neurotoxicol. Teratol. 2023, 96, 107163. [Google Scholar] [CrossRef]
- Holmqvist, K.; Örbom, S.L.; Hooge, I.T.C.; Niehorster, D.C.; Alexander, R.G.; Andersson, R.; Benjamins, J.S.; Blignaut, P.; Brouwer, A.; Chuang, L.L.; et al. Eye tracking: Empirical foundations for a minimal reporting guideline. Behav. Res. Methods 2023, 55, 364–416. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Easter, S.S.J.; Nicola, G.N. The development of eye movements in the zebrafish (Danio rerio). Dev. Psychobiol. 1997, 31, 267–276. [Google Scholar] [CrossRef]
- Easter, S.S.J.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef]
- Brockerhoff, S.E.; Hurley, J.B.; Janssen-Bienhold, U.; Neuhauss, S.C.; Driever, W.; Dowling, J.E. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA 1995, 92, 10545–10549. [Google Scholar] [CrossRef]
- Portugues, R.; Engert, F. The neural basis of visual behaviors in the larval zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.; Rassamdana, F.; Tam, P.; Dang, K.; Yanez, C.; Ghaemmaghami, S.; Dehkordi, M.I. The Optokinetic Response as a Quantitative Measure of Visual Acuity in Zebrafish. JoVE 2013, 80, e50832. [Google Scholar] [CrossRef]
- Huang, Y.; Neuhauss, S.C.F. The optokinetic response in zebrafish and its applications. Front. Biosci. 2008, 13, 1899–1916. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, F.A.; von Daranyi, A.; Leyden, C.; Arrenberg, A.B. Evoking and tracking zebrafish eye movement in multiple larvae with ZebEyeTrack. Nat. Protoc. 2018, 13, 1539–1568. [Google Scholar] [CrossRef]
- Brockerhoff, S.E. Measuring the optokinetic response of zebrafish larvae. Nat. Protoc. 2006, 1, 2448–2451. [Google Scholar] [CrossRef]
- Orger, M.B.; Gahtan, E.; Muto, A.; Page-McCaw, P.; Smear, M.C.; Baier, H. Behavioral screening assays in zebrafish. Methods Cell Biol. 2004, 77, 53–68. [Google Scholar] [CrossRef]
- Vogalis, F.; Shiraki, T.; Kojima, D.; Wada, Y.; Nishiwaki, Y.; Jarvinen, J.L.P.; Sugiyama, J.; Kawakami, K.; Masai, I.; Kawamura, S.; et al. Ectopic expression of cone-specific G-protein-coupled receptor kinase GRK7 in zebrafish rods leads to lower photosensitivity and altered responses. J. Physiol. 2011, 589, 2321–2348. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Komori, A.; Sagara, H.; Suzuki, E.; Manabe, T.; Hosoya, T.; Nojima, Y.; Wada, H.; Tanaka, H.; Okamoto, H.; et al. Mutation of cGMP phosphodiesterase 6α′-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech. Dev. 2008, 125, 932–946. [Google Scholar] [CrossRef]
- Mueller, K.P.; Schnaedelbach, O.D.R.; Russig, H.D.; Neuhauss, S.C.F. VisioTracker, an innovative automated approach to oculomotor analysis. J. Vis. Exp. 2011, 56, e3556. [Google Scholar] [CrossRef]
- Brysch, C.; Leyden, C.; Arrenberg, A.B. Functional architecture underlying binocular coordination of eye position and velocity in the larval zebrafish hindbrain. BMC Biol. 2019, 17, 110. [Google Scholar] [CrossRef]
- Zou, S.; Yin, W.; Zhang, M.; Hu, C.; Huang, Y.; Hu, B. Using the optokinetic response to study visual function of zebrafish. J. Vis. Exp. 2010, 36, e1742. [Google Scholar] [CrossRef]
- Dehmelt, F.A.; Meier, R.; Hinz, J.; Yoshimatsu, T.; Simacek, C.A.; Huang, R.; Wang, K.; Baden, T.; Arrenberg, A.B. Spherical arena reveals optokinetic response tuning to stimulus location, size, and frequency across entire visual field of larval zebrafish. eLife 2021, 10, e63355. [Google Scholar] [CrossRef]
- Rinner, O.; Rick, J.M.; Neuhauss, S.C.F. Contrast sensitivity, spatial and temporal tuning of the larval zebrafish optokinetic response. Investig. Ophthalmol. Vis. Sci. 2005, 46, 137–142. [Google Scholar] [CrossRef]
- Muto, A.; Orger, M.B.; Wehman, A.M.; Smear, M.C.; Kay, J.N.; Page-McCaw, P.S.; Gahtan, E.; Xiao, T.; Nevin, L.M.; Gosse, N.J.; et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005, 1, e66. [Google Scholar] [CrossRef]
- Daie, K.; Goldman, M.S.; Aksay, E.R.F. Spatial patterns of persistent neural activity vary with the behavioral context of short-term memory. Neuron 2015, 85, 847–860. [Google Scholar] [CrossRef]
- Wang, K.; Hinz, J.; Haikala, V.; Reiff, D.F.; Arrenberg, A.B. Selective processing of all rotational and translational optic flow directions in the zebrafish pretectum and tectum. BMC Biol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Neuhauss, S.C.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar] [CrossRef]
- Roeser, T.; Baier, H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J. Neurosci. 2003, 23, 3726–3734. [Google Scholar] [CrossRef]
- Haug, M.F.; Biehlmaier, O.; Mueller, K.P.; Neuhauss, S.C. Visual acuity in larval zebrafish: Behavior and histology. Front. Zool. 2010, 7, 8. [Google Scholar] [CrossRef]
- Sylvester, S.J.G.; Lee, M.M.; Ramirez, A.D.; Lim, S.; Goldman, M.S.; Aksay, E.R.F. Population-scale organization of cerebellar granule neuron signaling during a visuomotor behavior. Sci. Rep. 2017, 7, 16240. [Google Scholar] [CrossRef]
- Kainz, P.M.; Adolph, A.R.; Wong, K.Y.; Dowling, J.E. Lazy eyes zebrafish mutation affects Müller glial cells, compromising photoreceptor function and causing partial blindness. J. Comp. Neurol. 2003, 463, 265–280. [Google Scholar] [CrossRef]
- Bögli, S.Y.; Afthinos, M.; Bertolini, G.; Straumann, D.; Huang, M.Y. Unravelling Stimulus Direction Dependency of Visual Acuity in Larval Zebrafish by Consistent Eye Displacements Upon Optokinetic Stimulation. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1721–1727. [Google Scholar] [CrossRef][Green Version]
- Qian, H.; Zhu, Y.; Ramsey, D.J.; Chappell, R.L.; Dowling, J.E.; Ripps, H. Directional asymmetries in the optokinetic response of larval zebrafish (Danio rerio). Zebrafish 2005, 2, 189–196. [Google Scholar] [CrossRef]
- Hodel, C.; Neuhauss, S.C.F. Computer-Based Analysis of the Optokinetic Response in Zebrafish Larvae. Cold Spring Harb. Protoc. 2008, 2008, 4961. [Google Scholar] [CrossRef][Green Version]
- Grønskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43. [Google Scholar] [CrossRef]
- Emran, F.; Rihel, J.; Adolph, A.R.; Dowling, J.E. Zebrafish larvae lose vision at night. Proc. Natl. Acad. Sci. USA 2010, 107, 6034–6039. [Google Scholar] [CrossRef]
- Li, L.; Dowling, J.E. Zebrafish visual sensitivity is regulated by a circadian clock. Vis. Neurosci. 1998, 15, 851–857. [Google Scholar] [CrossRef]
- Zang, J.; Gesemann, M.; Keim, J.; Samardzija, M.; Grimm, C.; Neuhauss, S.C. Circadian regulation of vertebrate cone photoreceptor function. eLife 2021, 10, e68903. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Song, P.; Nivar, G.C.; Piccillo, E.M.; Fogerty, J.; Rozic, R.; Perkins, B.D. Ciliary genes arl13b, ahi1 and cc2d2a differentially modify expression of visual acuity phenotypes but do not enhance retinal degeneration due to mutation of cep290 in zebrafish. PLoS ONE 2019, 14, e0213960. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, S.E.; Hurley, J.B.; Niemi, G.A.; Dowling, J.E. A new form of inherited red-blindness identified in zebrafish. J. Neurosci. 1997, 17, 4236–4242. [Google Scholar] [CrossRef]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Richards, F.M.; Alderton, W.K.; Kimber, G.M.; Liu, Z.; Strang, I.; Redfern, W.S.; Valentin, J.; Winter, M.J.; Hutchinson, T.H. Validation of the use of zebrafish larvae in visual safety assessment. J. Pharmacol. Toxicol. Methods 2008, 58, 50–58. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Fogerty, J.; Gaivin, R.J.; Song, P.; Perkins, B.D. The Ciliopathy Gene ahi1 Is Required for Zebrafish Cone Photoreceptor Outer Segment Morphogenesis and Survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 448–460. [Google Scholar] [CrossRef][Green Version]
- Roosing, S.; Lamers, I.J.C.; de Vrieze, E.; van den Born, L.I.; Lambertus, S.; Arts, H.H.; POC1B Study Group; Peters, T.A.; Hoyng, C.B.; Kremer, H.; et al. Disruption of the basal body protein POC1B results in autosomal-recessive cone-rod dystrophy. Am. J. Hum. Genet. 2014, 95, 131–142. [Google Scholar] [CrossRef]
- Maurer, C.M.; Schönthaler, H.B.; Mueller, K.P.; Neuhauss, S.C.F. Distinct retinal deficits in a zebrafish pyruvate dehydrogenase-deficient mutant. J. Neurosci. 2010, 30, 11962–11972. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Lin, Z.; Liao, X.; Zou, T.; Qi, Z.; Cai, Z. Toxic effects of triclocarban on larval zebrafish: A focus on visual dysfunction. Aquat. Toxicol. 2021, 241, 106013. [Google Scholar] [CrossRef]
- Ben Fredj, N.; Hammond, S.; Otsuna, H.; Chien, C.; Burrone, J.; Meyer, M.P. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J. Neurosci. 2010, 30, 10939–10951. [Google Scholar] [CrossRef]
- Rick, J.M.; Horschke, I.; Neuhauss, S.C. Optokinetic behavior is reversed in achiasmatic mutant zebrafish larvae. Curr. Biol. 2000, 10, 595–598. [Google Scholar] [CrossRef]
- Chen, F.; Chen, S.; Liu, S.; Zhang, C.; Peng, G. Effects of lorazepam and WAY-200070 in larval zebrafish light/dark choice test. Neuropharmacology 2015, 95, 226–233. [Google Scholar] [CrossRef]
- Kay, J.N.; Finger-Baier, K.C.; Roeser, T.; Staub, W.; Baier, H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron 2001, 30, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiang, L.; Cheng, W.; Cheng, F.; He, K.; Zhang, B.; Zheng, S.; Han, R.; Zheng, Y.; Xu, X.; et al. Mutation of IPO13 causes recessive ocular coloboma, microphthalmia, and cataract. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Taylor, M.R.; Hurley, J.B.; Van Epps, H.A.; Brockerhoff, S.E. A zebrafish model for pyruvate dehydrogenase deficiency: Rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc. Natl. Acad. Sci. USA 2004, 101, 4584–4589. [Google Scholar] [CrossRef]
- Maldonado, E.; Hernandez, F.; Lozano, C.; Castro, M.E.; Navarro, R.E. The zebrafish mutant vps18 as a model for vesicle-traffic related hypopigmentation diseases. Pigment Cell Res. 2006, 19, 315–326. [Google Scholar] [CrossRef]
- Matsui, H.; Dorigo, A.; Buchberger, A.; Hocking, J.C.; Distel, M.; Köster, R.W. Zebrafish jam-b2 Gal4-enhancer trap line recapitulates endogenous jam-b2 expression in extraocular muscles. Dev. Dyn. 2015, 244, 1574–1580. [Google Scholar] [CrossRef]
- Hernández-Bejarano, M.; Gestri, G.; Monfries, C.; Tucker, L.; Dragomir, E.I.; Bianco, I.H.; Bovolenta, P.; Wilson, S.W.; Cavodeassi, F. Foxd1-dependent induction of a temporal retinal character is required for visual function. Development 2022, 149, dev200938. [Google Scholar] [CrossRef]
- Reynolds, A.L.; Alvarez, Y.; Sasore, T.; Waghorne, N.; Butler, C.T.; Kilty, C.; Smith, A.J.; McVicar, C.; Wong, V.H.Y.; Galvin, O.; et al. Phenotype-based Discovery of 2-[(E)-2-(Quinolin-2-yl)vinyl]phenol as a Novel Regulator of Ocular Angiogenesis. J. Biol. Chem. 2016, 291, 7242–7255. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Nörenberg, W.; Arrenberg, A.B. A robust receptive field code for optic flow detection and decomposition during self-motion. Curr. Biol. 2022, 32, 2505–2516.e8. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Z.; Chen, L.; Fu, J.; Han, J.; Hu, B.; Zhou, B. Optical toxicity of triphenyl phosphate in zebrafish larvae. Aquat. Toxicol. 2019, 210, 139–147. [Google Scholar] [CrossRef]
- Huang, D.; Wang, M.; Yin, W.; Ma, Y.; Wang, H.; Xue, T.; Ren, D.; Hu, B. Zebrafish Lacking Circadian Gene per2 Exhibit Visual Function Deficiency. Front. Behav. Neurosci. 2018, 12, 53. [Google Scholar] [CrossRef]
- Lin, T.; Mohammadi, M.; Fathalla, A.M.; Pul, D.; Lüthi, D.; Romano, F.; Straumann, D.; Cullen, K.E.; Chacron, M.J.; Huang, M.Y. Negative optokinetic afternystagmus in larval zebrafish demonstrates set-point adaptation. Sci. Rep. 2019, 9, 19039. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Wang, K.; Huang, D.; Huang, Y.; Yin, W.; Ren, D.; Wang, H.; Hu, B. Effects of circadian clock protein Per1b on zebrafish visual functions. Chronobiol. Int. 2018, 35, 160–168. [Google Scholar] [CrossRef]
- Le, H.T.; Dowling, J.E.; Cameron, D.J. Early retinoic acid deprivation in developing zebrafish results in microphthalmia. Vis. Neurosci. 2012, 29, 219–228. [Google Scholar] [CrossRef]
- Portugues, R.; Feierstein, C.E.; Engert, F.; Orger, M.B. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron 2014, 81, 1328–1343. [Google Scholar] [CrossRef]
- Bahadori, R.; Biehlmaier, O.; Zeitz, C.; Labhart, T.; Makhankov, Y.V.; Forster, U.; Gesemann, M.; Berger, W.; Neuhauss, S.C.F. Nyctalopin is essential for synaptic transmission in the cone dominated zebrafish retina. Eur. J. Neurosci. 2006, 24, 1664–1674. [Google Scholar] [CrossRef]
- Bögli, S.Y.; Afthinos, M.; Huang, M.Y. Effect of Gabapentin/Memantine on the Infantile Nystagmus Syndrome in the Zebrafish Model: Implications for the Therapy of Ocular Motor Diseases. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3149–3157. [Google Scholar] [CrossRef]
- Deveau, C.; Jiao, X.; Suzuki, S.C.; Krishnakumar, A.; Yoshimatsu, T.; Hejtmancik, J.F.; Nelson, R.F. Thyroid hormone receptor beta mutations alter photoreceptor development and function in Danio rerio (zebrafish). PLoS Genet. 2020, 16, e1008869. [Google Scholar] [CrossRef]
- Kramer, A.; Wu, Y.; Baier, H.; Kubo, F. Neuronal Architecture of a Visual Center that Processes Optic Flow. Neuron 2019, 103, 118–132.e7. [Google Scholar] [CrossRef]
- Shao, E.; Scheetz, S.D.; Xie, W.; Burton, E.A. Modulation of the zebrafish optokinetic reflex by pharmacologic agents targeting GABA(A) receptors. Neurosci. Lett. 2018, 671, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, P.J.; Arrenberg, A.B.; Del Bene, F.; Baier, H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J. Neurosci. 2010, 30, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Schuster, V.; Kulkarni, A.; Nouvian, M.; Romano, S.A.; Lygdas, K.; Jouary, A.; Dipoppa, M.; Pietri, T.; Haudrechy, M.; Candat, V.; et al. Sustained Rhythmic Brain Activity Underlies Visual Motion Perception in Zebrafish. Cell Rep. 2016, 17, 3089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arrenberg, A.B. High throughput, rapid receptive field estimation for global motion sensitive neurons using a contiguous motion noise stimulus. J. Neurosci. Methods 2019, 326, 108366. [Google Scholar] [CrossRef] [PubMed]
- Huber-Reggi, S.P.; Mueller, K.P.; Neuhauss, S.C.F. Analysis of optokinetic response in zebrafish by computer-based eye tracking. Methods Mol. Biol. 2013, 935, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Franz-Odendaal, T.A.; Hodel, C.; Gehring, I.; Geisler, R.; Schwarz, H.; Neuhauss, S.C.F.; Dahm, R. The zebrafish mutant bumper shows a hyperproliferation of lens epithelial cells and fibre cell degeneration leading to functional blindness. Mech. Dev. 2010, 127, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xiang, L.; Wen, X.; Shen, R.; Zhao, N.; Zheng, S.; Han, R.; Qu, J.; Lu, F.; Jin, Z. Slc7a14 Is Indispensable in Zebrafish Retinas. Front. Cell Dev. Biol. 2019, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Raine, J.C.; Lallemand, L.; Pettem, C.M.; Janz, D.M. Effects of Chronic Dietary Selenomethionine Exposure on the Visual System of Adult and F1 Generation Zebrafish (Danio rerio). Bull. Environ. Contam. Toxicol. 2016, 97, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kubo, F.; Hablitzel, B.; Dal Maschio, M.; Driever, W.; Baier, H.; Arrenberg, A.B. Functional architecture of an optic flow-responsive area that drives horizontal eye movements in zebrafish. Neuron 2014, 81, 1344–1359. [Google Scholar] [CrossRef]
- Crouzier, L.; Richard, E.M.; Diez, C.; Denus, M.; Peyrel, A.; Alzaeem, H.; Cubedo, N.; Delaunay, T.; Maurice, T.; Delprat, B. NCS1 overexpression restored mitochondrial activity and behavioral alterations in a zebrafish model of Wolfram syndrome. Mol. Ther. Methods Clin. Dev. 2022, 27, 295–308. [Google Scholar] [CrossRef]
- Huber-Reggi, S.P.; Mueller, K.P.; Straumann, D.; Huang, M.Y.; Neuhauss, S.C.F. Individual larvae of the zebrafish mutant belladonna display multiple infantile nystagmus-like waveforms that are influenced by viewing conditions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3971–3978. [Google Scholar] [CrossRef][Green Version]
- Tuschl, K.; White, R.J.; Trivedi, C.; Valdivia, L.E.; Niklaus, S.; Bianco, I.H.; Dadswell, C.; González-Méndez, R.; Sealy, I.M.; Neuhauss, S.C.F.; et al. Loss of slc39a14 causes simultaneous manganese hypersensitivity and deficiency in zebrafish. Dis. Model. Mech. 2022, 15, dmm044594. [Google Scholar] [CrossRef]
- Biehlmaier, O.; Makhankov, Y.; Neuhauss, S.C.F. Impaired retinal differentiation and maintenance in zebrafish laminin mutants. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2887–2894. [Google Scholar] [CrossRef][Green Version]
- Bahadori, R.; Rinner, O.; Schonthaler, H.B.; Biehlmaier, O.; Makhankov, Y.V.; Rao, P.; Jagadeeswaran, P.; Neuhauss, S.C.F. The Zebrafish fade out mutant: A novel genetic model for Hermansky-Pudlak syndrome. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4523–4531. [Google Scholar] [CrossRef] [PubMed]
- Rinner, O.; Makhankov, Y.V.; Biehlmaier, O.; Neuhauss, S.C.F. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron 2005, 47, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Huber-Reggi, S.P.; Chen, C.; Grimm, L.; Straumann, D.; Neuhauss, S.C.F.; Huang, M.Y. Severity of infantile nystagmus syndrome-like ocular motor phenotype is linked to the extent of the underlying optic nerve projection defect in zebrafish belladonna mutant. J. Neurosci. 2012, 32, 18079–18086. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.; Privat, M.; Sumbre, G. Fourier Motion Processing in the Optic Tectum and Pretectum of the Zebrafish Larva. Front. Neural Circuits 2022, 15, 814128. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, P.; Mills-Henry, I.; Padmanabhan, K.R.; Pascuzzi, P.; Hassan, M.; Zhang, J.; Zhang, X.; Ma, P.; Pang, C.P.; Dowling, J.E.; et al. Rods Contribute to Visual Behavior in Larval Zebrafish. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Matsui, J.I.; Egana, A.L.; Sponholtz, T.R.; Adolph, A.R.; Dowling, J.E. Effects of ethanol on photoreceptors and visual function in developing zebrafish. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4589–4597. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Matsui, J.I.; Miller, J.; Dowling, J.E.; Perkins, B.D. Myosin 7aa(−/−) Mutant Zebrafish show Mild Photoreceptor Degeneration and Reduced Electroretinographic Responses. Exp. Eye Res. 2014, 122, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Allwardt, B.A.; Lall, A.B.; Brockerhoff, S.E.; Dowling, J.E. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J. Neurosci. 2001, 21, 2330–2342. [Google Scholar] [CrossRef]
- Page-McCaw, P.S.; Chung, S.C.; Muto, A.; Roeser, T.; Staub, W.; Finger-Baier, K.C.; Korenbrot, J.I.; Baier, H. Retinal network adaptation to bright light requires tyrosinase. Nat. Neurosci. 2004, 7, 1329–1336. [Google Scholar] [CrossRef]
- Muto, A.; Taylor, M.R.; Suzawa, M.; Korenbrot, J.I.; Baier, H. Glucocorticoid receptor activity regulates light adaptation in the zebrafish retina. Front. Neural Circuits 2013, 7, 145. [Google Scholar] [CrossRef]
- Hertle, R.W. Albinism: Particular attention to the ocular motor system. Middle East. Afr. J. Ophthalmol. 2013, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ranspach, L.E.; Luo, X.; Cianciolo, L.T.; Fogerty, J.; Perkins, B.D.; Thummel, R. Vision and sensorimotor defects associated with loss of Vps11 function in a zebrafish model of genetic leukoencephalopathy. Sci. Rep. 2022, 12, 3511. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, R.; Huber, M.; Rinner, O.; Seeliger, M.W.; Geiger-Rudolph, S.; Geisler, R.; Neuhauss, S.C.F. Retinal function and morphology in two zebrafish models of oculo-renal syndromes. Eur. J. Neurosci. 2003, 18, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.L.; O’Connor, J.J.; Dillon, E.T.; Duffy, D.J.; Kolch, W.; Cagney, G.; et al. A Brain-Derived Neurotrophic Factor Mimetic Is Sufficient to Restore Cone Photoreceptor Visual Function in an Inherited Blindness Model. Sci. Rep. 2017, 7, 11320–11325. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dal Maschio, M.; Kubo, F.; Baier, H. An Optical Illusion Pinpoints an Essential Circuit Node for Global Motion Processing. Neuron 2020, 108, 722–734.e5. [Google Scholar] [CrossRef]
- Carretero-Rodriguez, L.; Guðjónsdóttir, R.; Poparic, I.; Reilly, M.L.; Chol, M.; Bianco, I.H.; Chiapello, M.; Feret, R.; Deery, M.J.; Guthrie, S. The Rac-GAP alpha2-Chimaerin Signals via CRMP2 and Stathmins in the Development of the Ocular Motor System. J. Neurosci. 2021, 41, 6652–6672. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Mohammadi, M.; Cullen, K.E.; Chacron, M.J.; Huang, M.Y. Optokinetic set-point adaptation functions as an internal dynamic calibration mechanism for oculomotor disequilibrium. iScience 2022, 25, 105335. [Google Scholar] [CrossRef]
- Collery, R.; McLoughlin, S.; Vendrell, V.; Finnegan, J.; Crabb, J.W.; Saari, J.C.; Kennedy, B.N. Duplication and divergence of zebrafish CRALBP genes uncovers novel role for RPE- and Muller-CRALBP in cone vision. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3812–3820. [Google Scholar] [CrossRef]
- Beck, J.C.; Gilland, E.; Tank, D.W.; Baker, R. Quantifying the Ontogeny of Optokinetic and Vestibuloocular Behaviors in Zebrafish, Medaka, and Goldfish. J. Neurophysiol. 2004, 92, 3546–3561. [Google Scholar] [CrossRef]
- Leyden, C.; Brysch, C.; Arrenberg, A.B. A distributed saccade-associated network encodes high velocity conjugate and monocular eye movements in the zebrafish hindbrain. Sci. Rep. 2021, 11, 12644. [Google Scholar] [CrossRef]
- Leyden, C.; Brüggemann, T.; Debinski, F.; Simacek, C.A.; Dehmelt, F.A.; Arrenberg, A.B. Efficacy of Tricaine (MS-222) and Hypothermia as Anesthetic Agents for Blocking Sensorimotor Responses in Larval Zebrafish. Front. Vet. Sci. 2022, 9, 864573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, Z.; Lu, S.; Chen, X.; Liao, X.; Qi, Z.; Cai, Z. Azole-Induced Color Vision Deficiency Associated with Thyroid Hormone Signaling: An Integrated In Vivo, In Vitro, and In Silico Study. Environ. Sci. Technol. 2022, 56, 13264–13273. [Google Scholar] [CrossRef] [PubMed]
- Rozenblat, R.; Tovin, A.; Zada, D.; Lebenthal-Loinger, I.; Lerer-Goldshtein, T.; Appelbaum, L. Genetic and Neurological Deficiencies in the Visual System of mct8 Mutant Zebrafish. Int. J. Mol. Sci. 2022, 23, 2464. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Hu, B. Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish. Exp. Neurol. 2014, 251, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Fries, R.; Scholten, A.; Säftel, W.; Koch, K. Zebrafish guanylate cyclase type 3 signaling in cone photoreceptors. PLoS ONE 2013, 8, e69656. [Google Scholar] [CrossRef] [PubMed]
- Rosello, M.; Serafini, M.; Concordet, J.; Del Bene, F. Precise mutagenesis in zebrafish using cytosine base editors. Nat. Protoc. 2023, 18, 2794–2813. [Google Scholar] [CrossRef] [PubMed]
- Kroll, F.; Powell, G.T.; Ghosh, M.; Gestri, G.; Antinucci, P.; Hearn, T.J.; Tunbak, H.; Lim, S.; Dennis, H.W.; Fernandez, J.M.; et al. A simple and effective F0 knockout method for rapid screening of behaviour and other complex phenotypes. eLife 2021, 10, e59683. [Google Scholar] [CrossRef]
- Kuht, H.J.; Bmedsci; De Maconachie, G.; Han, J.; Kessel, L.; Van Genderen, M.M.; Mclean, R.J.; Hisaund, M.; Tu, Z.; Hertle, R.W.; et al. Genotypic and Phenotypic Spectrum of Foveal Hypoplasia: A Multi-centre Study. Ophthalmology 2022, 129, 708–718. [Google Scholar] [CrossRef]
- Kuht, H.J.; Han, J.; Maconachie, G.D.E.; Park, S.E.; Lee, S.; Mclean, R.; Sheth, V.; Hisaund, M.; Dawar, B.; Sylvius, N.; et al. SLC38A8 mutations result in arrested retinal development with loss of cone photoreceptor specialization. Hum. Mol. Genet. 2020, 29, 2989. [Google Scholar] [CrossRef]
- Thomas, M.G.; Papageorgiou, E.; Kuht, H.J.; Gottlob, I. Normal and abnormal foveal development. Br. J. Ophthalmol. 2022, 106, 593–599. [Google Scholar] [CrossRef]
- Thomas, M.G.; Crosier, M.; Lindsay, S.; Kumar, A.; Araki, M.; Leroy, B.P.; McLean, R.J.; Sheth, V.; Maconachie, G.; Thomas, S.; et al. Abnormal retinal development associated with FRMD7 mutations. Hum. Mol. Genet. 2014, 23, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.; McLean, R.J.; Kohl, S.; Sheth, V.; Gottlob, I. Early signs of longitudinal progressive cone photoreceptor degeneration in achromatopsia. Br. J. Ophthalmol. 2012, 96, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.; Kumar, A.; Mohammad, S.; Proudlock, F.A.; Engle, E.C.; Andrews, C.; Chan, W.; Thomas, S.; Gottlob, I. Structural Grading of Foveal Hypoplasia Using Spectral-Domain Optical Coherence Tomography: A Predictor of Visual Acuity? Ophthalmology 2011, 118, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Donato, L.; Alafaci, C.; Crisafulli, C.; Mucciardi, M.; Rinaldi, C.; Sidoti, A.; D’angelo, R. CCM3/SERPINI1 bidirectional promoter variants in patients with cerebral cavernous malformations: A molecular and functional study. BMC Med. Genet. 2016, 17, 74. [Google Scholar] [CrossRef]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’angelo, R.; Sidoti, A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).