Simple Summary
Our growing aging population and increased prevalence of obesity have become an emerging health problem as these conditions lead to the development of related diseases. Specifically, obesity and aging are associated with cardiovascular disease and a decline in cognitive function underlying the inflammatory mechanisms. However, the literature regarding how both interact and impact these complex physiological processes across the lifespan remains to be elucidated. As such, this review discusses how obesity in aging adults mediates inflammatory, cardiovascular, and neurobiological effects of exercise in this population.
Abstract
Obesity with advancing age leads to increased health complications that are involved in various complex physiological processes. For example, inflammation is a critical cardiovascular disease risk factor that plays a role in the stages of atherosclerosis in both aging and obesity. Obesity can also induce profound changes to the neural circuitry that regulates food intake and energy homeostasis with advancing age. Here we discuss how obesity in older adults impacts inflammatory, cardiovascular, and neurobiological functions with an emphasis on how exercise mediates each topic. Although obesity is a reversible disorder through lifestyle changes, it is important to note that early interventions are crucial to prevent pathological changes seen in the aging obese population. Lifestyle modifications such as physical activity (including aerobic and resistance training) should be considered as a main intervention to minimize the synergistic effect of obesity on age-related conditions, such as cerebrovascular disease.
Keywords:
obesity; aging; inflammation; vascular function; neurobiology; aerobic exercise; resistance exercise 1. Introduction
Biological aging is associated with increased visceral adipose tissue [1] and a shift in energy homeostasis that often leads to sarcopenic obesity [2] and subclinical chronic pro-inflammation (e.g., inflammaging) [1]. These chronic obese and age-related inflammation are precursors to severe health conditions such as hypertension, dyslipidemia, type 2 diabetes, stroke, coronary artery disease, and various forms of cancer [3]. Many of these complications manifest with normal aging but are more prevalent with age-related obesity as more than 65% of adults in the United States are overweight or obese, substantially increasing the risk of morbidity and mortality [4]. With relative risk of mortality increasing between 27–93% depending on severity of obesity [5]. Particularly, inflammation is a critical cardiovascular disease risk factor that plays a role in the process of atherosclerosis in both aging and obesity. This chronic inflammatory disease is involved with endothelial cell permeability, accumulation of low-density lipoproteins as a major source of atherosclerotic lipid storage, and leucocyte trafficking, eventually leading to endothelial/vascular dysfunction [6]. Additionally, the literature has demonstrated that obesity can induce profound changes to the neural circuitry that regulates food intake and energy homeostasis [7], thereby affecting cognitive development [8]. In this regard, research has previously found a negative relationship between body mass index (BMI) and cognitive test performance, even after controlling for age [9]. Lifestyle modifications including diet and exercise are proposed as a first-line defense to counteract obesity. Thus, while exercise has been extensively shown to effectively provide many physiological health benefits relative to other therapeutic treatments, this review provides an overview of how aerobic exercise, resistance exercise, or in combination would modulate inflammatory, cardiovascular, and neurobiological responses in older adults with obesity based on the latest available data. We anticipated that exercise interventions would prove effective at improving inflammatory, cardiovascular, and neurobiological responses in older adults with obesity.
2. The Effect of Exercise on Inflammation in Obese Older Adults
Obese individuals have immune dysfunction leading to higher infection rates and impaired wound healing [10]. Excess body fat increases leukocyte count (neutrophils, monocytes) but lowers T- and B-cell mitogen-induced proliferation [10]. Obesity-related inflammation is mediated by excessive adipose tissue storage and subsequent apoptotic-related macrophage infiltration, as well as neutrophil, CD4+ and CD8+ T cell recruitment that consequently leads to insulin resistance within the adipose tissue [11,12]. With macrophage infiltration and the associated metabolic shift from glycolytic to oxidative, reactive oxygen species (ROS) are produced due to the electron transport chain and respiration being attenuated, which allows for the upshot in ROS production, signaling the production of pro-inflammatory cytokines [13]. This overproduction of ROS has been linked to oxidative stress and inflammation [14]. Pro-inflammatory cytokines that are associated with obesity and aging include tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and C-reactive protein (CRP), which cause peripheral blood mononuclear cells to be in a pro-inflammatory state [15]. This pro-inflammatory profile changes the macrophage polarization state from the M2 (anti-inflammatory) to the M1 (pro-inflammatory) phenotype [16]. Additionally, the ratio of CD8+ to CD4+ T-cells have been shown to increase with obesity, limiting the secretion of anti-inflammatory cytokines that inhibit macrophage migration from CD4+ regulatory T-cells [17,18]. In fact, obesity can lead to accelerated aging, and older adults that are obese have impaired immune responses, for example in response to vaccination [19]. Obesity and age-related increases in inflammatory cytokines (e.g. TNF-α, IL-6, and CRP) cause exacerbated danger-associated molecular patterns and immunosenescence, thereby increasing morbidity and mortality [20].
Adipocytes release signaling molecules (adipokines), including leptin and adiponectin, that have immunomodulatory actions [21]. While leptin has effects on the central nervous system to stimulate satiety and energy expenditure [22], an increase in the circulating levels with obesity can lead to leptin resistance, resulting in the activation of immune cells [23]. Specifically, leptin stimulates monocyte proliferation and differentiation into macrophages, modulating the activation of natural killer cells (NK cells) and stimulating the release of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12 [10,24]. In contrast, adiponectin is an anti-inflammatory and insulin-sensitizing hormone that is inversely related to body weight and has opposite immunomodulatory actions from that of leptin [25]. In macrophages, adiponectin inhibits phagocytosis and decreases the production of TNF-α [23]. Adiponectin prevents the differentiation of monocytes and lowers the production of endothelial cell adhesion molecules [26]. Adiponectin also induces the production of the anti-inflammatory cytokines IL-10 and IL-1 [24].
Importantly, exercise is a main lifestyle modification that is considered a first-line defense to combat age-related obesity by counteracting positive energy balance and also modulate immune cells, adipokines, and inflammatory cytokines [27]. Our previous work showed that young adults with obesity exhibited a comparable concentrations of IL-6 to acute aerobic exercise when compared to normal-weight counterparts [28]. Additionally, these young adults with obesity elicited greater microtubule-associated protein 1A/1B-light chain 2 (LC3-II) to microtubule-associated protein 1A/1B-light chain 1 (LC3-I) ratio and LC3-II/LC3-I AUCi (potentially elevating autophagic activity) when compared to normal-weight subjects in response to maximal aerobic exercise [29], along with a positive relationship BMI, waist/hip ratio, and fasting insulin levels [29]. However, evaluation of autophagic modulators need to be utilized to fully assess autophagic flux [30]. More recently, we demonstrated young adults with obesity had similar responses to both acute traditional continuous moderate intensity exercise and acute high-intensity interval exercise in adipokines (e.g., C1q-TNF related protein 9 [CTRP9]) compared to normal-weight controls, which has anti-inflammatory effects and improves endothelial function [31]. Additionally, we also show that young adults with obesity have an attenuated response to pentraxin 3 (PTX3; an anti-inflammatory mediator released by neutrophils into circulation) compared to normal weight individuals in response to acute high-intensity interval exercise [32]. However, it is known that exercise training can improve overall health in obese older adults by lower body fat and systemic inflammation [33], which is partially supported by our recent results with an improvement of body weight and BMI following 12 weeks of both swimming and cycling exercise training, with no change in appetite stimulating hormones (e.g. leptin) [34]. A meta-analysis demonstrated that increased physical activity improves body composition and insulin-like growth factor 1 (IGF-1) in older adults with sarcopenic obesity [35]. These increased levels of IGF-1 also inhibit astrocyte response to inflammatory insult, thereby modulating neuroinflammation [36]. Exercise training attenuates obesity-associated meta-inflammation [37] and modifies metabolic hormones that may counteract chronic inflammation and obesity-related conditions [38]. With just 16 weeks of aerobic exercise training, CRP was significantly decreased in obese young women [39]. A similar study showed that 10 months of aerobic exercise training significantly decreased CRP, IL-6, and IL-18 in overweight older adults [40]. In older adults with obesity and diabetes, 6 months of aerobic exercise training decreased CRP and IL-18 with an increase in the anti-inflammatory cytokine IL-10 [41]. A recent meta-analysis demonstrated that exercise interventions also attenuate inflammaging (IL-6, CRP, and TNF-α) in middle-aged and older adults with type 2 diabetes [42]. Studies utilizing animal models provide organ-specific mechanistic insight (e.g., liver, lung, stomach) for improvements in inflammation, oxidative damage, cellular senescence, and hepatic function after 3 months of aerobic exercise training via changes in sirtuin activity, nuclear factor kappa B (NF-κB), and peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) [43]. Collectively, these findings demonstrate that long-term aerobic exercise interventions are an effective method to reduce systemic inflammation in obese older adults.
Moreover, resistance exercise can also be used to combat obesity and age-related chronic inflammation. One year of resistance exercise training has shown to significantly decease CRP levels and increase adiponectin concentrations in young overweight women [44]. In fact, a study with just 10 weeks of resistance training demonstrated a decrease in CRP with overweight and obese individuals [45]. Prior evidence found decreases in CRP and IL-6 after resistance training for 18 months in obese older adults but not with aerobic training [33]. Furthermore, a meta-analysis showed resistance training decreasing CRP and tended to lower IL-6 levels in older adults [46]. As such, overweight older adults with higher levels of physical activity express lower concentrations of IL-6 independent of weight loss [47]. These studies support the utilization of resistance exercise to improve systemic inflammation in older adults with obesity, with the added benefit of attenuating sarcopenia commonly seen with this population.
Indeed, a combination of diet, aerobic, and resistance exercise training is likely to be the most effective lifestyle modification aimed at improving visceral adipose tissue, systemic inflammation, blood pressure (BP), lipid profile, and insulin sensitivity in obese older adults [48]. For example, a 1 year diet and exercise intervention improved insulin sensitivity more than diet or exercise alone in obese older adults [49]. This study also demonstrated decreases in CRP, TNF-α, and increases in adiponectin concentrations after the combined diet and exercise intervention [49]. Reductions in visceral adipose tissue that are hormonally active in obese adults are mediated by IL-6 released from skeletal muscles with exercise, which also acts in an anti-inflammatory manner to reduce chronic systemic inflammation [50]. Another study using combined aerobic and resistance exercise training exhibited reduced immune cells and inflammation (TNF-α and IL-8) in subcutaneous adipose tissue with decreased levels of hypoxia-inducible factor 1-α and superoxide dismutase, a biomarker of oxidative stress, in overweight females [51]. Similarly, a combination of aerobic and resistance exercise training for 12 months in obese diabetic older adults decreased CRP and risk factors for cardiovascular disease (CVD) [52]. Additionally, 15 weeks of caloric restriction and aerobic exercise training significantly reduced systemic inflammation (with decreases in CRP, IL-6, TNF-α, IL-8, monocyte chemoattractant protein-1 [MCP-1]), subcutaneous adipose tissue inflammation, increased adiponectin and insulin sensitivity in severely obese participants [53]. A recent systemic review highlighted improvements in inflammatory biomarkers in adults with obesity using aerobic exercise training (decreased CRP, IL-6, and TNF-α), resistance training (decreased TNF-α), and concurrent training including both aerobic and resistance training (decreased TNF-α) [54]. In overweight and obese middle-aged and older adults, 12 weeks of aerobic training alone, resistance training alone, or in combination decreased TNF-α [55]. Collectively, these studies demonstrate that lifestyle interventions including diet, aerobic and resistance exercise training are very effective at reducing body fat and improving systemic inflammation in older males and females (Figure 1).
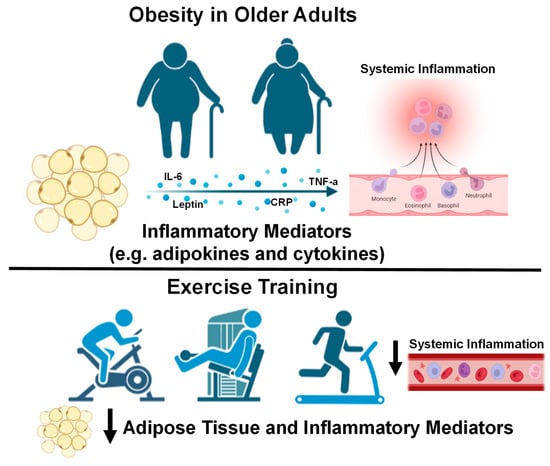
Figure 1.
Older adults with obesity and the effect of exercise interventions on systemic inflammation. Down arrows indicate decreases in systemic inflammation with exercise training. Images created with Biorender.com accessed on 13 March 2023.
3. Aging: Cardiovascular Responses to Exercise in Obesity
Excess adipose tissue increases cardiovascular (CV) strain which, over time, places profound stress on the CV system. CV late effects, such as stroke, left ventricular hypertrophy, heart failure (HF), and various forms of cardiomyopathies, are highly prevalent with age-related obesity [56,57,58,59]. In fact, more than 80% of patients with HF with preserved ejection fraction (HFpEF), a cardiac consequence of chronic hypertension, are overweight or obese [60]. There is also a reduction in the elastic properties of the arteries leading to stiffening, termed arterial stiffness, which is associated with abdominal obesity [61] and is independently associated with CVD and mortality [62]. Additionally, obesity-related hypercholesterolemia increases plaque buildup within the myocardial vasculature leading to dysrhythmias, coronary artery disease, myocardial ischemia and infarction, and premature death [63,64]. Properly functioning endothelial cells that line the lumen of arteries, arterioles and capillaries are crucial for controlling systemic blood flow, BP, and prevents the development of atherosclerosis via nitric oxide production, a potent vasodilator, anticoagulant, and antithrombotic biomolecule [65,66,67,68]. Endothelial dysfunction is a modifiable CV risk factor and a hallmark characteristic of accelerated vascular aging that is associated with obesity-related hypertension and CV disease (CVD) [69,70,71]. Endothelial dysfunction is also associated with sarcopenia, the age-related loss in muscle mass, strength and/or function, where there is less nutritive blood flow to skeletal muscles from reduced capillarization [72]. Sarcopenia is highly related to the development of hypertension [73], arterial stiffness [74,75], endothelial dysfunction [76], and CVD via physical inactivity and inflammation [77,78]. Sarcopenic obesity is more catastrophic than sarcopenia and obesity alone [79] and is becoming increasingly prevalent in the sedentary aging population, where there is a large imbalance of adipose-to-muscle tissue ratio and is associated with disability, CVD and mortality [80,81]. Low muscle strength (dynapenia) occurs independently of the loss in muscle mass [82] and is highly associated with poor physical function [83], quality of life [84], mortality [85], and fall risk [86,87] in obese middle-aged and older adults. Older adults diagnosed with CVD have a high prevalence of dynapenia which is associated with increased mortality rate, highlighting the prognostic utility of dynapenia in patients with CVD [88]. Dynapenia is also highly correlated with endothelial dysfunction in elderly women [89], demonstrating early-onset CV consequences related to low muscle strength. Unfortunately, obesity-related CVD prevalence is still on the uprise [90] and is attributed to factors like physical inactivity and diets high in saturated fats and refined carbohydrates [91,92]. Moreover, endothelial dysfunction is the earliest vascular maladaptation with obesity [93], which progressively worsens with age [94]. Similarly, obesity across the lifespan leads to increased arterial stiffness [61,95,96]. However, age- and obesity-associated disorders may be improved with lifestyle interventions that promote weight loss, such as physical activity and exercise. To combat the severe CV consequences of obesity in older adults, the United States Department of Health and Human Services recommends that adults ≥18 years old participate in ≥150 min/week of moderate or ≥75 min/week of vigorous aerobic exercise to reduce all-cause mortality risk [97].
Exercise capacity and tolerance in obese adults are less than their lean counterparts. In a cohort of obese women, acute submaximal capacity, peak aerobic capacity and recovery rate were reduced compared to lean controls [98], demonstrating the acute obesity-related reduction in CV responses to physical activity. An explanation for this may be chronotropic incompetence, which can be defined as the inability to increase heart rate to meet blood flow demands of the working muscles during exercise and is an independent predictor of major CV outcomes and mortality in obese adults [99,100,101]. Exaggerated sympathetic nerve activity at rest and during exercise may provide further explanation for the abnormal increases in heart rate (HR) and BP at rest, as well as the delay in returning to basal levels post-exercise [102,103,104,105]. Furthermore, impaired endothelial function has been seen in sedentary obese women after a bout of strenuous weightlifting compared to lean controls [106], suggesting that obesity compromises the functionality of the peripheral vasculature and promotes possible vascular risk when high-intensity exercise is performed. One benefit after acute aerobic [107] and resistance exercise [108] is a sustained reduction in BP for up to 24 h, termed postexercise hypotension, although these reductions in BP are short-lived. Complications hinder obese older adults from exercising for long durations, including discomfort, reduced motivation for exercise, and decreased adherence to exercise training [109,110]. Since chronic exercise is the most effective route to reduce excess adiposity and improve overall CV health, the adverse CV responses to acute exercise are important to monitor as obese adults begin exercise training.
Repeated bouts of aerobic and resistance exercise, or a combination of both, are crucial to improve exercise tolerance and overall CV health in obese populations. Participating in regular exercise training can enhance functional characteristics of the CV system and begin to reverse CV risk associated with obesity. Chronic exercise training improves vascular function such as endothelial function [111,112], exercise performance [113,114], overall CV health, and modulates cardiac autonomic function in obese adolescents [115,116,117,118]. Existing literature also show the beneficial CV adaptations to exercise training in the adult obese population. Regarding aerobic exercise training, Amano and colleagues conducted a 12-week moderate-intensity aerobic exercise training intervention in middle-aged obese adults and found reductions in body fat, preserved muscle mass, increased aerobic capacity, and improved parasympathetic activity via increased HR variability [119]. These results suggest a “reset” mechanism of the autonomic nervous system (such as improved baroreflex sensitivity) and significant improvements in CV health after only 12 weeks of aerobic exercise training in obese adults. In another intervention-based exercise training study, high-intensity aerobic exercise training elicited the largest reduction in systolic BP during exercise after 6 months versus low and moderate intensities in obese postmenopausal women [120]. Six months of aerobic exercise training also improved endothelial function in hypertensive obese postmenopausal women regardless of intensity [121]. Resistance exercise training alone in obese adults have also yielded improvements in CV health. Figueroa and colleagues tested the effects of a 12-week whole-body vibration training program, a form of resistance exercise, in hypertensive obese postmenopausal women and found decreases in leg arterial stiffness which were associated with reduced ankle and aortic systolic BP [122]. Twelve weeks of low-intensity resistance exercise training also reduced mean arterial pressure in obese postmenopausal women, along with reductions in systemic arterial stiffness when exercise was combined with a hypocaloric diet [123]. These findings are important to consider, since hypertension is a hallmark risk factor of obesity, nearly 40% of postmenopausal women are obese [124], and more than 75% of women ≥60 years are hypertensive [125,126,127]. Although it can be speculated that hypertension may be due to reduced estrogen, hormone replacement therapy shows no effect and possibly an increase in BP [128,129], demonstrating no cardioprotective traits compared to women not on hormone therapy [130,131,132]. These findings suggest that obesity, and not age-related reductions in sex hormones, is associated with hypertension in postmenopausal women. A combination of moderate-intensity aerobic and resistance exercise training for 12 weeks has shown to improve cardiorespiratory fitness in middle-aged obese adults [133]. Park and colleagues also demonstrated that combined aerobic and resistance exercise at moderate intensity for 12 weeks reduced systolic and diastolic BP, mean arterial pressure, pulse pressure, systemic arterial stiffness, low-density lipoprotein cholesterol, and increased aerobic capacity in obese older men [134]. Data from the Maastricht study also found that 12 weeks of combined aerobic and resistance exercise improved oxygen uptake, left ventricular ejection fraction and cardiac lipid profile in overweight/obese middle-aged males [135]. Results from these studies suggest that a combination of aerobic and resistance exercise training for at least 12 weeks may yield more robust improvements in CV function in obese middle-aged and older adults compared to performing aerobic or resistance exercise alone.
Lifestyle changes (diet and exercise training) are suggested to be the most optimal strategy to improve physical limitations [136] and systemic inflammation [137] in sarcopenic/dynapenic obese adults. It is recommended that combined aerobic and resistance exercise training should be individually, progressively prescribed for sarcopenic obese adults to improve muscle mass and function [138]. However, to date, only one study in obese sarcopenic rats has examined the effects of exercise training (aerobic, 20 weeks) on CV health [139]. A study performed by Sénéchal and colleagues found that 12 weeks of a combined hypocaloric diet and resistance exercise training intervention improved body weight (due to reductions in fat mass), waist circumference, blood lipid profile, and systolic BP along with enhanced physical capacity in 38 dynapenic obese postmenopausal women [140]. Although it is known that sarcopenic obesity could contribute to exercise intolerance and reduced cardiorespiratory fitness in HF patients [141,142], identifying the benefits of exercise training (aerobic, resistance, and combined) on CV health in sarcopenic/dynapenic obese adults are of critical need. Mechanistically, oxidative stess seem to play a critical role in exacerbating the negative effects of sarcopenic obesity [143], which is associated with CVD risk [80,144,145]). Resistance exercise training has shown to decrease oxidative damage and stress in older adults [146,147]. Recently, it has been demonstrated that 8 weeks of low-intensity resistance exercise training with slow movement combined with L-citrulline supplementation improved leg lean mass and leg curl strength, and L-citrulline alone improved leg endothelial function in hypertensive postmenopausal women. Although women in this study were not diagnosed with sarcopenia, they were physically inactive [148]. L-citrulline is a key promoter of endogenous nitric oxide production, a potent antioxidant [149], provides vascular and muscular benefits to exercise training in older adults [150], and is suggested to be combined with exercise to manage sarcopenia [151]. Given these findings, L-citrulline supplementation paired with aerobic, resistance or combined exercise training may yield improvements in muscle mass, function, and CV health in sarcopenic and/or dynapenic obese adults.
Epidemiological data show marked reductions in 10-year CVD risk in overweight and obese middle-aged adults who perform ≥150 min/week of moderate-to-vigorous exercise [152]. This is in line with the US Physical Activity Guidelines, which established that ≥150 min/week of moderate- or ≥75 min/week of vigorous-intensity aerobic physical activity reduces CVD morbidity and mortality, BP and incidence of hypertension, weight loss and prevention of weight loss, type 2 diabetes prevalence, and an adverse blood lipid profile [153]. When examining obesity-related CVD, evidence from the Cochrane database show reductions in CVD-related mortality and decreased risk of myocardial infarction from exercise-based cardiac rehabilitation programs ranging from 6 months to >3 years [154]. Further, 6 months to 1 year of community-based cardiac rehabilitation has shown to improve left ventricular function, exercise time, and mobility in patients who experienced prior acute myocardial infarction [155]. Further, 20 weeks of aerobic exercise training has shown to improve peak aerobic capacity in obese older patients with HFpEF [156]. These results are important to consider, since obesity is associated with blunted skeletal muscle blood flow during low-intensity exercise in middle-aged and older HFpEF patients, which may contribute to exercise intolerance in this population [157]. Since a high percentage of HFpEF patients are obese [60], exercise training is suggested to improve exercise tolerance in this patient population, and lower risk of all-cause mortality in this patient population [158], but more research is needed to elucidate the CV mechanisms behind the beneficial effects seen in the literature thus far. Altogether, these studies show substantial improvements in exercise tolerance, aerobic capacity, CV function and, importantly, reductions in CVD and risk factors in obese adults after long-term exercise training as illustrated in Figure 2. Evidence from this section suggests that although acute exercise unveils physiological abnormalities, chronic exercise training, preferably combined aerobic and resistance, can improve obesity-related CVD in young and old obese populations.
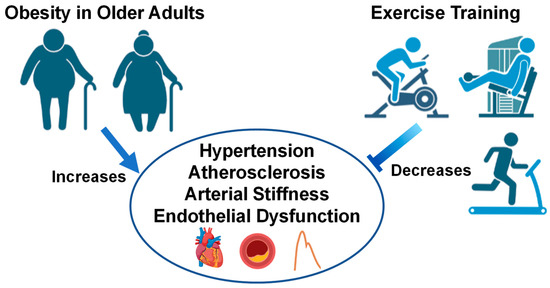
Figure 2.
Older adults with obesity and the effect of exercise interventions on cardiovascular health. Images created with Biorender.com accessed on 13 March 2023.
4. Neurobiological Responses in Older Adults with Obesity and Effects of Exercise
Within the central nervous system, eating behavior is thought to be largely regulated by an intricate interaction between the circuitry that regulates energy intake requirement and reward pathways [159]. In this context, the hypothalamus is seen as a key hub in the regulation of hunger and satiety cues [160], while the mesolimbic and mesocortical dopaminergic pathways (highly activated by palatable food) are crucial for the positive behavioral response to food consumption (e.g., food-associated reward) [159,161]. Found in the forebrain, the hypothalamus is one of the smallest structures in the human brain, integral for key physiological processes such as thermoregulation, neuroendocrine integration, energy metabolism, and energy expenditure [162]. Traditionally, the hypothalamus has been functionally divided into thirds, and components of the tuberal hypothalamus are credited with the regulation of hunger and satiety [159,162]. Since the tuberal hypothalamus contains the arcuate nucleus, the venteromedial, and the lateral areas, it contains neurons that control hunger and food intake including proopiomelanocortin (POMC) and agouti-related peptide and neuropeptide Y (AgRP/NPY) neurons [159,160]. POMC neurons are stimulated by the secretion of peripheral hormones such as adipocyte-derived leptin and pancreatic-derived insulin, and function to regulate satiety following food consumption [163]. On the other hand, as their name implies, AgRP/NPY neurons are associated with the generation of orexigenic peptides AgRP and NPY that stimulate appetite during states of energy deficits such as fasting [164,165]. Importantly, inhibition of POMC neurons, via knockout or ablation, leads to uncontrolled food consumption and weight gain [166,167], while ablation of AgRP/NPY neurons leads to anorexia and starvation in mice [168]. From an aging standpoint, aged rats were previously reported to have significantly lower POMC neuronal activity within the hypothalamus and higher weight gain [169] while increasing POMC tone in aged rats was associated with weight loss, lower visceral adipose deposits, and improved fat metabolism [170]. Intriguingly, aged rats have also been reported to have lower protein expression of NPY protein in the hypothalamus [171] and reduced AgRP gene expression in response to fasting [172]. However, it is important to note that although fasting can lower hypothalamic gene expression of AgRP in old rats, intracerebroventricular injection of AgRP in old rats led to similar levels of food intake as those seen in younger rats exposed to intracerebroventricular injection of AgRP [172]. As such, chronic obesity may first lead to early adaptations that hinder the neurobiological mechanisms behind food satiety that, along with the process of biological aging, may lead to accrued energetic imbalances that favor the development of sarcopenic obesity and obesity-related metabolic syndromes [169]. Although not fully understood, research has found that obese individuals have elevated circulating insulin and leptin while decreased circulating ghrelin [160]. These changes in circulating hormones may, in turn, lead to insulin and leptin resistance within the tubular hypothalamus causing reduced POMC neuronal activity and undisrupted AgRP/NPY neuronal activity, possibly resulting in sustained hunger [160,165]. This is substantiated by data showing that obese older adults display hypothalamic insulin and leptin resistance [173,174], which can, in turn, facilitate hypothalamic neuroinflammation and neuronal stress [175]. In fact, it is well established that microglial cells express leptin receptors, and leptin resistance can induce the release of inflammatory mediators such as NF-kB, IL-1β, and TNF-α by microglial cells [176]. Moreover, because plasma leptin has been shown to positively associate with the number of activated microglia in the hypothalamus of mice fed with a high fat diet, it is possible that in obesity, insulin resistance can lead to not only dysregulated peripheral immune responses, but also maladaptive neuroinflammatory states that ultimately contribute to energy imbalance and diet-induced obesity [177]. However, it must be kept in mind that that advanced chemogenetic and optogenetic approaches suggest the roles of AgRP/NPY and POMC neurons are not fully antagonistic, and their activity and behavioral consequences (eating behaviors) may be modulated by stimuli such as sustained environmental stress [178] and thus, other external stimuli may also lead to neurobiological adaptations in obesity that disrupt the neuroendocrine and neuroimmune regulation of food intake. Importantly, it must also be noted here that hypothalamic POMC and AgRP/NPY circuits may also fluctuate during biological aging and facilitate imbalances in energy metabolism [169]. Indeed, hypothalamic POMC neuronal activity is significantly lower in aged rodents when compared to younger counterparts [169] and AgRP/NPY receptor levels and signaling have been observed to fluctuate in aged rats based on feeding status [179]. Unsurprisingly, imbalances in energy metabolism are commonly found during biological aging, which can, in turn, lead to changes in body composition and give rise to conditions such as sarcopenic obesity [2]. Defined, sarcopenic obesity is an age-related chronic condition characterized by decreases in skeletal muscle mass and function concomitant to increases in high body fat and loss of appetite [2]. Although not exclusively, the age-related neurobiological changes in POMC and AgRP/NPY hypothalamic circuitry have been postulated to partially contribute to the energy imbalance and loss of appetite seen during sarcopenic obesity [2]. However, to which degree remains poorly understood, and should be studied further. Lastly, emerging lines of evidence suggest there may be differences in the neurobiological adaptations during obesity based on biological sex [180]. For example, recent work by Freire-Regatillo and colleagues found that POMC and AgRP/NPY adaptations to high fat diet differed based on sex in middle-aged TgAPP mice [180]. Specifically, following a high fat diet, female mice had significantly greater increases in body weight, visceral and subcutaneous adipose tissue, as well as higher expression of POMC mRNA when compared to males, and males displayed lower AgRP/NPY mRNA than females [180]. As such, the neurobiological systems that regulate energy homeostasis and appetitive behaviors are not only dysregulated during obesity but can also be influenced by biological aging, sex, and even environmental stress. Thus, future research should strive to better characterize how these neurobiological systems are influenced from an integrative point of view.
Meanwhile, the mesolimbic and mesocortical pathways are both dopaminergic components of the brain’s reward circuitry that are heavily involved in addictive behavior such as substance abuse [181,182]. In fact, because these two pathways are activated by highly palatable foods and have been implicated in behavioral overeating [159,183], the concept of food addiction continues to be heavily scrutinized as another possible neurobiological adaptation in chronic obesity [184]. Although relatively novel and somewhat controversial, food addiction is an important concept to recognize given that obese individuals display behavioral constructs associated with addiction such as impulsivity, increased sensitivity to reward, and heightened need to consume for pleasure (e.g., food cravings and hedonic eating) [185,186]. From a neuroanatomical point of view, the homeostatic circuitry of food intake (hypothalamus) and the reward pathways display a large degree of interconnectivity [159] and data from animal studies indicate structures within the mesolimbic pathway such as the nucleus accumbens can lead to increased appetitive behavior [187]. Thus, it is possible that the brain’s reward circuitry may influence food intake, give rise to compulsive food consumption, and increase the risk of developing obesity [186,188]. This notion is supported by human and animal studies showing dopaminergic release becoming altered in the presence of food cues and is associated with the motivation of wanting to seek food consumption [189,190,191]. There is also data to suggest that obesity leads maladaptive changes in reward circuitry similar to those found in substance abuse, such as decreases in dopamine receptors, that may trigger compulsive food intake [192]. Moreover, human studies have found that when compared to lean counterparts, obese individuals display lower activation of reward circuitry during food consumption, higher somatosensory activation in the presence of food cues, and greater anticipation of food consumption [193]. Together, these lines of evidence showcase similarities with models of substance abuse whereby a mismatch in the expected reward of food consumption leads to compulsive eating in an attempt to seek reward [194], possibly explaining why food addiction is gaining traction as a likely etiological component of obesity.
The benefits of aerobic exercise towards brain health and synaptic plasticity are well established in the literature [195,196]. Unsurprisingly, exercise is seen as a powerful tool to combat the prevalence of obesity [195] given that in obese older adults, chronic exercise training can improve insulin sensitivity, reduce inflammation, and improve body composition [197,198]. Indeed, chronic exercise training is postulated to improve energy balance through modulation of neuroplasticity in POMC and AgRP/NPY neurons [199,200]. A recent study in mice provides compelling evidence showing aerobic exercise can increase POMC neuronal excitability, with leptin-dense POMC neurons showing the most robust response to exercise [201]. Intriguingly, in this same study, exercise was reported to inhibit NPY neuronal activity, suggesting that exercise can exert rapid reorganization in multiple neuronal populations within the tuberal hypothalamus [201]. Further, there is limited evidence to suggest POMC mRNA expression is differentially altered following acute and chronic aerobic exercise in not only the hypothalamus, but also the frontal cortex and hippocampus [200]. Together, these lines of evidence suggest aerobic exercise can facilitate neuroplasticity within POMC neurons throughout the brain, and ultimately benefit the efficacy of satiety cues. Moreover, although not specific to the hypothalamus, human data also suggests exercise-related reductions in leptin and high-density lipoprotein, as well as increases in serum brain derived neurotrophic factor (BDNF) which are all related to improved gray and white matter integrity within the brain, suggesting improved exercise-modulated neuroplasticity in obese humans [202]. Moreover, it is also worth noting several independent research groups have found, in animal models, that aerobic exercise can be neuroprotective to obesity-related neuroinflammation within the hypothalamus [203,204], which may ultimately further facilitate the reestablishment of satiety signaling within the brain [205]. Intriguingly, there is evidence to suggest the effects of exercise may differ between sexes. In a recent study, Wilson et. al., (2020) found that in obese mice, only female mice downregulate mRNA expression NPY within the hypothalamus following 12 weeks of aerobic exercise and intermittent fasting [206]. However, it is worth noting that the ways by which exercise may differentially influence POMC and AgRP/NPY systems based on sex is a relatively novel concept that needs to be studied extensively. Lastly, there is limited data to suggest that in obese adults, chronic resistance exercise training may improve circulating biomarkers associated with appetitive behavior such as glucose and NPY, as well as reduce circulating pro-inflammatory markers such as TNF-α and IFN-γ [207,208]. Moreover, while randomized controlled exercise trials in humans are limited in individuals with sarcopenic obesity, a combination of aerobic exercise and resistance training may be the most beneficial [209]. For example, Villareal and colleagues found that in dieting obese older adults at risk of developing sarcopenia, performance in the physical performance test improved more robustly in older adults that performed concurrent exercise training than in aerobic or resistance training alone [210]. Meanwhile, in a smaller study including individuals diagnosed with sarcopenic obesity, concurrent exercise training was found to induce similar improvements in body weight and fat mass, while resistance training alone yielded greater improvements in muscular strength than aerobic or concurrent training [211]. Together, these studies suggest that exercise training routines that incorporate resistance training or a combination of resistance training plus aerobic exercise will improve the clinical outcomes in obese older adults and adults with sarcopenic obesity. However, it is important to highlight here that whether resistance exercise can more directly benefit the neurocircuitry affected in obesity remains understudied in the literature. Indeed, future studies should aim to bridge this knowledge gap from an acute and chronic point of view with an emphasis on neuro-centric biomarkers to better understand whether resistance exercise can serve as an ancillary tool against obesity. Nevertheless, although not fully elucidated, it is possible that exercise may aid in re-establishing a balance in the brain circuitry that regulates hunger and food intake as illustrated in Figure 3. There is ample evidence demonstrating that aerobic and resistance exercise training can be beneficial in older adults, adults with obesity, and older adults with sarcopenic obesity. However, it remains challenging to address in humans the neurobiological mechanisms by which exercise can influence the neurocircuitry behind energy homeostasis and appetitive behavior. Nevertheless, animal studies continue to be crucial in bridging this gap in the literature and provide compelling evidence that exercise can act directly on POMC and AgRP/NPY neurons, as well as by influencing neuroinflammatory mediators such as pro-inflammatory cytokines. Moreover, emerging frameworks suggest there may be sex differences in some of the mechanisms discussed above, yet to what extent remains to be fully elucidated. Future studies would benefit from addressing some of the gaps in the literature highlighted in this section to better characterize the neurobiological responses to obesity, sarcopenic obesity, possible sex differences, and how exercise influences each.
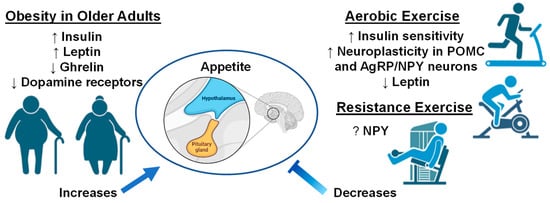
Figure 3.
Older adults with obesity and the effect of exercise interventions on appetite. Up arrows indicate increases, down arrows indicate decreases, and question mark indicates unknown if changes occur with exercise interventions. Images created with Biorender.com accessed on 13 March 2023.
5. Conclusions
Obesity and aging are involved in a decline in cognitive function underlying the inflammatory mechanisms; however, the literature regarding how both interact and impact this complex process of cognitive impartment across the lifespan still remains elucidated. Although obesity is a reversible disorder through lifestyle changes, it is important to note that early interventions are crucial to prevent accelerated CV aging and reduce the risk of severe CV late effects seen in the aging obese population. Lifestyle modifications such as physical activity should be considered as a main intervention to minimize the synergistic effect of obesity on age-related conditions as summarized in Table 1, such as cerebrovascular disease. Of particular note, obesity has been recently shown to be a primary predictor for a long-term risk of cerebrovascular mortality in black vs. white individuals [212]. Thus, future investigation should also explicate the influence of racial/ethnic disparities in the development of related diseases to better understand how obesity accelerates biological changes in brain health across the life course.

Table 1.
Exercise Interventions in Obese Middle-Aged and Older Adults.
Author Contributions
Conceptualization, B.G.F., A.M., G.S.P. and C.-J.H.; writing—review and editing, B.G.F., A.M., G.S.P. and C.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D. Several areas of overlap between obesity and aging indicate obesity as a biomarker of accelerated aging of human B cell function and antibody responses. Immun. Ageing 2022, 19, 48. [Google Scholar] [CrossRef]
- Xu, H.; Cupples, L.A.; Stokes, A.; Liu, C.-T. Association of Obesity with Mortality Over 24 Years of Weight History. JAMA Netw. Open 2018, 1, e184587. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef]
- Bischof, G.N.; Park, D.C. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef]
- Hassing, L.B.; Dahl, A.K.; Pedersen, N.L.; Johansson, B. Overweight in Midlife Is Related to Lower Cognitive Function 30 Years Later: A Prospective Study with Longitudinal Assessments. Dement. Geriatr. Cogn. Disord. 2010, 29, 543–552. [Google Scholar] [CrossRef]
- Marti, A.; Marcos, A.; Martinez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Bouloumie, A.; Casteilla, L.; Lafontan, M. Adipose tissue lymphocytes and macrophages in obesity and insulin resistance. Arter. Thromb. Vasc. Biol. 2008, 28, 1211–12132008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Aljada, A.; Hofmeyer, D.; Syed, T.; Mohanty, P.; Dandona, P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004, 110, 1564–1571. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Thomas, A.L.; Alarcon, P.C.; Divanovic, S.; Chougnet, C.A.; Hildeman, D.A.; Moreno-Fernandez, M.E. Implications of inflammatory states on dysfunctional immune responses in aging and obesity. Front. Aging 2021, 2, 732414. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Koerner, A.; Kratzsch, J.; Kiess, W. Adipocytokines: Leptin—The classical, resistin—The controversical, adiponectin—The promising, and more to come. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 525–546. [Google Scholar] [CrossRef]
- Park, Y.; Myers, M.; Vieira-Potter, V. Adipose tissue inflammation and metabolic dysfunction: Role of exercise. Mo. Med. 2014, 111, 65–72. [Google Scholar]
- Ferrandi, P.J.; Fico, B.G.; Whitehurst, M.; Zourdos, M.C.; Bao, F.; Dodge, K.M.; Rodriguez, A.L.; Pena, G.; Huang, C.-J. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci. 2018, 202, 161–166. [Google Scholar] [CrossRef]
- Huang, C.-J.; Rodriguez, A.L.; Visavadiya, N.P.; Fico, B.G.; Slusher, A.L.; Ferrandi, P.J.; Whitehurst, M. An exploratory investigation of apoptotic and autophagic responses in peripheral blood mononuclear cells following maximal aerobic exercise in obese individuals. Arch. Physiol. Biochem. 2022, 128, 209–216. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Fico, B.G.; Garten, R.S.; Zourdos, M.C.; Whitehurst, M.; Ferrandi, P.J.; Dodge, K.M.; Pena, G.S.; Rodriguez, A.A.; Huang, C.-J. The Impact of Obesity on C1q/TNF-Related Protein-9 Expression and Endothelial Function following Acute High-Intensity Interval Exercise vs. Continuous Moderate-Intensity Exercise. Biology 2022, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; Fico, B.G.; Dodge, K.M.; Garten, R.S.; Ferrandi, P.J.; Rodriguez, A.A.; Pena, G.; Huang, C.-J. Impact of acute high-intensity interval exercise on plasma pentraxin 3 and endothelial function in obese individuals—A pilot study. Eur. J. Appl. Physiol. 2021, 121, 1567–1577. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Marsh, A.P.; Fanning, J.; Ambrosius, W.T.; Walkup, M.P.; Nicklas, B.J. Dietary Weight Loss, Exercise, and Inflammation in Older Adults with Overweight or Obesity and Cardiometabolic Disease. Obesity 2019, 27, 1805–1811. [Google Scholar] [CrossRef]
- Fico, B.G.; Alkatan, M.; Tanaka, H. No Changes in Appetite-Related Hormones Following Swimming and Cycling Exercise Interventions in Adults with Obesity. Int. J. Exerc. Sci. 2020, 13, 1819–1825. [Google Scholar] [PubMed]
- Zhuang, M.; Jin, M.; Lu, T.; Lu, L.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of three modes of physical activity on physical fitness and hematological parameters in older people with sarcopenic obesity: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 917525. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-like growth factor-1 and neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Ringseis, R.; Eder, K.; Mooren, F.C.; Krüger, K. Metabolic signals and innate immune activation in obesity and exercise. Exerc. Immunol. Rev. 2015, 21, 793575724. [Google Scholar]
- McMurray, R.G.; Hackney, A.C. Interactions of metabolic hormones, adipose tissue and exercise. Sport. Med.-ADIS Int. 2005, 35, 393–412. [Google Scholar] [CrossRef]
- Arikawa, A.Y.; Thomas, W.; Schmitz, K.H.; Kurzer, M.S. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med. Sci. Sport. Exerc. 2011, 43, 1002–1009. [Google Scholar] [CrossRef]
- Kohut, M.; McCann, D.; Russell, D.; Konopka, D.; Cunnick, J.; Franke, W.; Castillo, M.; Reighard, A.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Xing, H.; Lu, J.; Yoong, S.Q.; Tan, Y.Q.; Kusuyama, J.; Wu, X.V. Effect of Aerobic and Resistant Exercise Intervention on Inflammaging of Type 2 Diabetes Mellitus in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 823–830.e13. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, L.; Hall, Z.; Lemos, H.; Vacca, M.; Paish, H.; Green, K.; Elliott, B.; Tiniakos, D.; Passos, J.F. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 2021, 206, 904–916. [Google Scholar] [CrossRef]
- Olson, T.P.; Dengel, D.; Leon, A.; Schmitz, K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int. J. Obes. 2007, 31, 996–1003. [Google Scholar] [CrossRef]
- Donges, C.E.; Duffield, R.; Drinkwater, E.J. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med. Sci. Sport. Exerc. 2010, 42, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; Tomeleri, C.M.; Cyrino, E.S.; Fernhall, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Exp. Gerontol. 2018, 111, 188–196. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Hsu, F.C.; Brinkley, T.J.; Church, T.; Goodpaster, B.H.; Kritchevsky, S.B.; Pahor, M. Exercise training and Plasma C-reactive Protein and Interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008, 56, 2045–2052. [Google Scholar] [CrossRef]
- Colleluori, G.; Napoli, N.; Phadnis, U.; Villareal, R.-A.; Villareal, D.T. Effect of Weight Loss, Exercise, or Both on Undercarboxylated Osteocalcin and Insulin Secretion in Frail, Obese Older Adults. Oxidative Med. Cell. Longev. 2017, 2017, 4807046. [Google Scholar] [CrossRef] [PubMed]
- Bouchonville, M.; Armamento-Villareal, R.; Shah, K.; Napoli, N.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: Results of a randomized controlled trial. Int. J. Obes. 2014, 38, 423–431. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.-S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef] [PubMed]
- Čížková, T.; Štěpán, M.; Daďová, K.; Ondrůjová, B.; Sontáková, L.; Krauzová, E.; Matouš, M.; Koc, M.; Gojda, J.; Kračmerová, J. Exercise training reduces inflammation of adipose tissue in the elderly: Cross-sectional and randomized interventional trial. J. Clin. Endocrinol. Metab. 2020, 105, e4510–e4526. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Cardelli, P.; Salerno, G.; Fallucca, S.; Nicolucci, A.; Pugliese, G. Supervised exercise training counterbalances the adverse effects of insulin therapy in overweight/obese subjects with type 2 diabetes. Diabetes Care 2012, 35, 39–41. [Google Scholar] [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef]
- Gonzalo-Encabo, P.; Maldonado, G.; Valadés, D.; Ferragut, C.; Pérez-López, A. The Role of Exercise Training on Low-Grade Systemic Inflammation in Adults with Overweight and Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13258. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. Effects of chronic exercise training on inflammatory markers in Australian overweight and obese individuals in a randomized controlled trial. Inflammation 2013, 36, 625–632. [Google Scholar] [CrossRef]
- Brady, T.M. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr. Hypertens. Rep. 2016, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Sala, C.; Grassi, G. Left-ventricular hypertrophy and obesity: A systematic review and meta-analysis of echocardiographic studies. J. Hypertens. 2014, 32, 16–25. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef] [PubMed]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef]
- Strasser, B.; Arvandi, M.; Pasha, E.; Haley, A.; Stanforth, P.; Tanaka, H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Association, A.H. Heart Disease and Stroke Statistics 2018–At-a-Glance; American Heart Association: Dallas, TX, USA, 2018. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, J.R. Exploring vascular benefits of endothelium-derived nitric oxide. Am. J. Hypertens. 2005, 18, 177S–183S. [Google Scholar] [CrossRef]
- Gewaltig, M.T.; Kojda, G. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef]
- Tziros, C.; Freedman, J.E. The many antithrombotic actions of nitric oxide. Curr. Drug Targets 2006, 7, 1243–1251. [Google Scholar] [CrossRef]
- Engin, A. Endothelial dysfunction in obesity. Obes. Lipotoxicity 2017, 960, 345–379. [Google Scholar]
- Perticone, F.; Ceravolo, R.; Pujia, A.; Ventura, G.; Iacopino, S.; Scozzafava, A.; Ferraro, A.; Chello, M.; Mastroroberto, P.; Verdecchia, P. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia is associated with lower skeletal muscle capillarization and exercise capacity in older adults. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Fang, F.; Li, F.; Ren, Y.; Hu, J.; Cao, J. Sarcopenia is associated with hypertension in older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Chung, G.E.; Lee, H.; Kim, M.J.; Choi, S.Y.; Lee, W.; Yoon, J.W. Significance of Low Muscle Mass on Arterial Stiffness as Measured by Cardio-Ankle Vascular Index. Front. Cardiovasc. Med. 2022, 9, 857871. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Klich-Rączka, A.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Pulse Wave Velocity and Sarcopenia in Older Persons-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6477. [Google Scholar] [CrossRef]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 2021, 50, 725–732. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship between sarcopenia and cardiovascular diseases in the elderly: An overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Sasaki, K.I.; Fukumoto, Y. Sarcopenia as a comorbidity of cardiovascular disease. J. Cardiol. 2022, 79, 596–604. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Stephen, W.C.; Janssen, I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J. Nutr. Health Aging 2009, 13, 460–466. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Endocrinol. Metab. 2013, 28, 86–89. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, D.R.; Janssen, I. Dynapenic-obesity and physical function in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 71–77. [Google Scholar] [CrossRef]
- Batsis, J.A.; Zbehlik, A.J.; Pidgeon, D.; Bartels, S.J. Dynapenic obesity and the effect on long-term physical function and quality of life: Data from the osteoarthritis initiative. BMC Geriatr 2015, 15, 118. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574. [Google Scholar] [CrossRef]
- Lv, D.; Shen, S.; Chen, X. Association Between Dynapenic Abdominal Obesity and Fall Risk in Older Adults. Clin. Interv. Aging 2022, 17, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. Prognostic utility of dynapenia in patients with cardiovascular disease. Clin. Nutr. 2021, 40, 2210–2218. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, M.J.; Na, J.B.; Chun, Y.H.; Park, Y.J.; Park, Y.; Hah, Y.S.; Ha, Y.C.; Park, K.S. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachexia Sarcopenia Muscle 2018, 9, 1034–1041. [Google Scholar] [CrossRef]
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef] [PubMed]
- Dubbert, P.M.; Carithers, T.; Hall, J.E.; Barbour, K.A.; Clark, B.L.; Sumner, A.E.; Crook, E.D. Obesity, physical inactivity, and risk for cardiovascular disease. Am. J. Med. Sci. 2002, 324, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Tataranni, P.A. Dietary fat and human obesity. J. Am. Diet. Assoc. 1997, 97, S42–S46. [Google Scholar] [CrossRef]
- Al Suwaidi, J.; Higano, S.T.; Holmes, D.R.; Lennon, R.; Lerman, A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J. Am. Coll. Cardiol. 2001, 37, 1523–1528. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Zebekakis, P.E.; Nawrot, T.; Thijs, L.; Balkestein, E.J.; Van Der Heijden-Spek, J.; Van Bortel, L.M.; Struijker-Boudier, H.A.; Safar, M.E.; Staessen, J.A. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005, 23, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased Rate of Arterial Stiffening with Obesity in Adolescents: A Five-Year Follow-Up Study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.; Muls, E. Exercise capacity in lean versus obese women. Scand. J. Med. Sci. Sport. 2001, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.S.; Francis, G.S.; Okin, P.M.; Pashkow, F.J.; Snader, C.E.; Marwick, T.H. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999, 281, 524–529. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Arbit, B.; Azarbal, B.; Hayes, S.W.; Gransar, H.; Germano, G.; Friedman, J.D.; Thomson, L.; Berman, D.S. Prognostic contribution of exercise capacity, heart rate recovery, chronotropic incompetence, and myocardial perfusion single-photon emission computerized tomography in the prediction of cardiac death and all-cause mortality. Am. J. Cardiol. 2015, 116, 1678–1684. [Google Scholar] [CrossRef]
- Dipla, K.; Nassis, G.P.; Vrabas, I.S. Blood pressure control at rest and during exercise in obese children and adults. J. Obes. 2012, 2012, 298953. [Google Scholar] [CrossRef]
- Hargens, T.A.; Guill, S.G.; Zedalis, D.; Gregg, J.M.; Nickols-Richardson, S.M.; Herbert, W.G. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep 2008, 31, 104–110. [Google Scholar] [CrossRef]
- Deniz, F.; Katircibasi, M.T.; Pamukcu, B.; Binici, S.; Sanisoglu, S.Y. Association of metabolic syndrome with impaired heart rate recovery and low exercise capacity in young male adults. Clin. Endocrinol. 2007, 66, 218–223. [Google Scholar] [CrossRef]
- Itagi, A.B.H.; Jayalakshmi, M.; Yunus, G. Effect of obesity on cardiovascular responses to submaximal treadmill exercise in adult males. J. Fam. Med. Prim. Care 2020, 9, 4673. [Google Scholar] [CrossRef]
- Franklin, N.C.; Ali, M.; Goslawski, M.; Wang, E.; Phillips, S.A. Reduced vasodilator function following acute resistance exercise in obese women. Front. Physiol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Marcal, I.R.; Goessler, K.F.; Buys, R.; Casonatto, J.; Ciolac, E.G.; Cornelissen, V.A. Post-exercise hypotension following a single bout of high intensity interval exercise vs. a single bout of moderate intensity continuous exercise in adults with or without hypertension: A systematic review and meta-analysis of randomized clinical trials. Front. Physiol. 2021, 12, 675289. [Google Scholar] [CrossRef]
- Tibana, R.; Pereira, G.; Navalta, J.; Bottaro, M.; Prestes, J. Acute effects of resistance exercise on 24-h blood pressure in middle aged overweight and obese women. Int. J. Sport. Med. 2013, 34, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Baillot, A.; Chenail, S.; Barros Polita, N.; Simoneau, M.; Libourel, M.; Nazon, E.; Riesco, E.; Bond, D.S.; Romain, A.J. Physical activity motives, barriers, and preferences in people with obesity: A systematic review. PLoS ONE 2021, 16, e0253114. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Pirozzi, T.M.; Cattaneo, G.; Solana-Sánchez, J.; Gomes-Osman, J.; Pascual-Leone, A. The importance of motivation to older adult physical and cognitive exercise program development, initiation, and adherence. Front. Aging 2022, 3, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Yang, Y.-J.; Ge, R.-K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.-L.; Dong, Y.-F. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.P.; Dengel, D.R.; Leon, A.S.; Schmitz, K.H. Moderate resistance training and vascular health in overweight women. Med. Sci. Sport. Exerc. 2006, 38, 1558–1564. [Google Scholar] [CrossRef]
- D’Agostino, E.M.; Day, S.E.; Konty, K.J.; Armstrong, S.C.; Skinner, A.C.; Neshteruk, C.D. Longitudinal Association between Weight Status, Aerobic Capacity, Muscular Strength, and Endurance among New York City Youth, 2010–2017. Child. Obes. 2022, 19, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.S.; Farnesi, B.-C.; Lafleche, A.; Legault, L.; Komorowski, J. The effects of resistance exercise training on body composition and strength in obese prepubertal children. Physician Sportsmed. 2013, 41, 103–109. [Google Scholar]
- Farah, B.; Ritti-Dias, R.; Balagopal, P.; Hill, J.; Prado, W. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr. Obes. 2014, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Burke, G.L.; Savage, P.J.; Sidney, S.; Gardin, J.M.; Oberman, A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: The CARDIA study. Am. J. Hypertens. 1994, 7, 234–241. [Google Scholar]
- Janz, K.; Dawson, J.; Mahoney, L. Increases in physical fitness during childhood improve cardiovascular health during adolescence: The Muscatine Study. Int. J. Sport. Med. 2002, 23, 15–21. [Google Scholar] [CrossRef]
- Farinatti, P.; Neto, S.R.M.; Dias, I.; Cunha, F.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Short-term resistance training attenuates cardiac autonomic dysfunction in obese adolescents. Pediatr. Exerc. Sci. 2016, 28, 374–380. [Google Scholar] [CrossRef]
- Amano, M.; KANDA, T.; UE, H.; MORITANI, T. Exercise training and autonomic nervous system activity in obese individuals. Med. Sci. Sport. Exerc. 2001, 33, 1287–1291. [Google Scholar] [CrossRef]
- Swift, D.L.; Earnest, C.P.; Katzmarzyk, P.T.; Rankinen, T.; Blair, S.N.; Church, T.S. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause 2012, 19, 503–509. [Google Scholar] [CrossRef]
- Swift, D.L.; Earnest, C.P.; Blair, S.N.; Church, T.S. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: Results from the DREW study. Br. J. Sport. Med. 2012, 46, 753–758. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Wong, A. Whole-body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause 2015, 22, 423–427. [Google Scholar] [CrossRef]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- National Center for Health Statistics (US). Health, United States: 2010, with Special Feature on Death and Dying; February Report No.: 2011-1232; National Center for Health Statistics: Washington, DC, USA, 2011. [Google Scholar]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Reckelhoff, J.F. Postmenopausal hypertension. Am. J. Hypertens. 2011, 24, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Prelevic, G.M.; Kwong, P.; Byrne, D.J.; Jagroop, I.A.; Ginsburg, J.; Mikhailidis, D.P. A cross-sectional study of the effects of hormon replacement therapy on the cardiovascular disease risk profile in healthy postmenopausal women. Fertil. Steril. 2002, 77, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Kalantaridou, S.N.; Naka, K.K.; Papanikolaou, E.; Kazakos, N.; Kravariti, M.; Calis, K.A.; Paraskevaidis, E.A.; Sideris, D.A.; Tsatsoulis, A.; Chrousos, G.P. Impaired endothelial function in young women with premature ovarian failure: Normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3907–3913. [Google Scholar] [CrossRef]
- Writing Group for The Women, S.H.I.I. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women’s Health Initiative Randomized Controlled Trial. JAMA J. Am. Med. Assoc. 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Herrington, D.M. The HERS trial results: Paradigms lost? Heart and Estrogen/progestin Replacement Study. Ann. Intern. Med. 1999, 131, 463–466. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Jung, W.-S.; Hong, K.; Kim, Y.-Y.; Kim, S.-W.; Park, H.-Y. Effects of moderate combined resistance-and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7233. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Meex, R.; Van Der Made, S.; Schär, M.; Lamb, H.; Wildberger, J.E.; Glatz, J.; Snoep, G.; Kooi, M.E. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J. Clin. Endocrinol. Metab. 2010, 95, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Konieczna, J.; Zulet, M.A.; Galmés-Panades, A.M.; Ibero-Baraibar, I.; Babio, N.; Estruch, R.; Vidal, J.; Toledo, E.; Razquin, C.; et al. Association of lifestyle factors and inflammation with sarcopenic obesity: Data from the PREDIMED-Plus trial. J. Cachexia Sarcopenia Muscle 2019, 10, 974–984. [Google Scholar] [CrossRef]
- Hershberger, D.; Bollinger, L. Sarcopenic Obesity: Background and Exercise Training Strategies. Strength Cond. J. 2015, 37, 78–83. [Google Scholar] [CrossRef]
- França, G.O.; Frantz, E.D.C.; Magliano, D.C.; Bargut, T.C.L.; Sepúlveda-Fragoso, V.; Silvares, R.R.; Daliry, A.; Nascimento, A.R.D.; Borges, J.P. Effects of short-term high-intensity interval and continuous exercise training on body composition and cardiac function in obese sarcopenic rats. Life Sci. 2020, 256, 117920. [Google Scholar] [CrossRef]
- Sénéchal, M.; Bouchard, D.R.; Dionne, I.J.; Brochu, M. The effects of lifestyle interventions in dynapenic-obese postmenopausal women. Menopause 2012, 19, 1015–1021. [Google Scholar] [CrossRef]
- Upadhya, B.; Haykowsky, M.J.; Eggebeen, J.; Kitzman, D.W. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2015, 12, 205–214. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Del Buono, M.G.; Canada, J.M.; Kim, Y.; Damonte, J.I.; Trankle, C.R.; Halasz, G.; Mihalick, V.; Vecchié, A.; Markley, R.R.; et al. Sarcopenic Obesity Is Associated with Reduced Cardiorespiratory Fitness Compared with Nonsarcopenic Obesity in Patients with Heart Failure with Reduced Ejection Fraction. Circ. Heart Fail. 2022, 15, e009518. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxidative Med. Cell. Longev. 2021, 2021, 4493817. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Parise, G.; Brose, A.N.; Tarnopolsky, M.A. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp. Gerontol. 2005, 40, 173–180. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2022, 15, 74. [Google Scholar] [CrossRef]
- Coles, K.E. Investigation into the Antioxidant Capacity of L-Arginine and L-Citrulline in Relation to Their Vascular Protective Properties. Ph.D. Thersis, Cardiff University (United Kingdom), Wales, UK, 2007. [Google Scholar]
- Figueroa, A.; Jaime, S.J.; Morita, M.; Gonzales, J.U.; Moinard, C. L-Citrulline Supports Vascular and Muscular Benefits of Exercise Training in Older Adults. Exerc. Sport Sci. Rev. 2020, 48, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cash, R.E.; Bower, J.K.; Focht, B.C.; Paskett, E.D. Physical activity and risk of cardiovascular disease by weight status among US adults. PLoS ONE 2020, 15, e0232893. [Google Scholar]
- Piercy, K.L.; Troiano, R.P. Physical activity guidelines for Americans from the US department of health and human services: Cardiovascular benefits and recommendations. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, Cd001800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Jiang, P.; Tang, H. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: A community-based study. Medicine 2018, 97, e9785. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ratchford, S.M.; Lee, J.F.; Bunsawat, K.; Alpenglow, J.K.; Zhao, J.; Ma, C.L.; Ryan, J.J.; Khor, L.L.; Wray, D.W. The impact of obesity on the regulation of muscle blood flow during exercise in patients with heart failure with a preserved ejection fraction. J. Appl. Physiol. 2022, 132, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.J.; O’Driscoll, J.M. Exercise Training in Heart failure with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Sport. Med.-Open 2022, 8, 76. [Google Scholar] [CrossRef]
- Lee, D.J.; Elias, G.J.; Lozano, A.M. Neuromodulation for the treatment of eating disorders and obesity. Ther. Adv. Psychopharmacol. 2018, 8, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Belfort-DeAguiar, R.; Seo, D. Food cues and obesity: Overpowering hormones and energy balance regulation. Curr. Obes. Rep. 2018, 7, 122–129. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s reward system in health and disease. Circadian Clock Brain Health Dis. 2021, 1344, 57–69. [Google Scholar]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef]
- Biebermann, H.; Castañeda, T.R.; van Landeghem, F.; von Deimling, A.; Escher, F.; Brabant, G.; Hebebrand, J.; Hinney, A.; Tschöp, M.H.; Grüters, A. A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006, 3, 141–146. [Google Scholar] [CrossRef]
- Kastin, A. Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Yaswen, L.; Diehl, N.; Brennan, M.B.; Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 1999, 5, 1066–1070. [Google Scholar] [CrossRef]
- Greenman, Y.; Kuperman, Y.; Drori, Y.; Asa, S.L.; Navon, I.; Forkosh, O.; Gil, S.; Stern, N.; Chen, A. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol. Endocrinol. 2013, 27, 1091–1102. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Yang, S.B.; Tien, A.C.; Boddupalli, G.; Xu, A.W.; Jan, Y.N.; Jan, L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012, 75, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Wilsey, J.; Scarpace, P. Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia 2005, 48, 2376–2385. [Google Scholar] [CrossRef]
- Kowalski, C.; Micheau, J.; Corder, R.; Gaillard, R.; Conte-Devolx, B. Age-related changes in cortico-releasing factor, somatostatin, neuropeptide Y, methionine enkephalin and β-endorphin in specific rat brain areas. Brain Res. 1992, 582, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wolden-Hanson, T.; Marck, B.T.; Matsumoto, A.M. Blunted hypothalamic neuropeptide gene expression in response to fasting, but preservation of feeding responses to AgRP in aging male Brown Norway rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R138–R146. [Google Scholar] [CrossRef]
- Carter, S.; Caron, A.; Richard, D.; Picard, F. Role of leptin resistance in the development of obesity in older patients. Clin. Interv. Aging 2013, 8, 829–844. [Google Scholar]
- Ono, H. Molecular mechanisms of hypothalamic insulin resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e183. [Google Scholar] [CrossRef]
- Henn, R.E.; Noureldein, M.H.; Elzinga, S.E.; Kim, B.; Savelieff, M.G.; Feldman, E.L. Glial-neuron crosstalk in health and disease: A focus on metabolism, obesity, and cognitive impairment. Neurobiol. Dis. 2022, 170, 105766. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. The Effects of Leptin on Glial Cells in Neurological Diseases. Front. Neurosci. 2019, 13, 828. [Google Scholar] [CrossRef]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: An integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Veyrat-Durebex, C.; Quirion, R.; Ferland, G.; Dumont, Y.; Gaudreau, P. Aging and long-term caloric restriction regulate neuropeptide Y receptor subtype densities in the rat brain. Neuropeptides 2013, 47, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Freire-Regatillo, A.; Diaz-Pacheco, S.; Frago, L.M.; Arévalo, M.; Argente, J.; Garcia-Segura, L.M.; de Ceballos, M.L.; Chowen, J.A. Sex Differences in Hypothalamic Changes and the Metabolic Response of TgAPP Mice to a High Fat Diet. Front. Neuroanat. 2022, 16, 910477. [Google Scholar] [CrossRef]
- MacNicol, B. The biology of addiction. Can. J. Anesth. J. Can. D’anesthésie 2017, 64, 141–148. [Google Scholar] [CrossRef]
- Cooper, S.; Robison, A.; Mazei-Robison, M.S. Reward circuitry in addiction. Neurotherapeutics 2017, 14, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Morris, M.J. The role of reward circuitry and food addiction in the obesity epidemic: An update. Biol. Psychol. 2018, 131, 31–42. [Google Scholar] [CrossRef]
- Lerma-Cabrera, J.M.; Carvajal, F.; Lopez-Legarrea, P. Food addiction as a new piece of the obesity framework. Nutr. J. 2015, 15, 5. [Google Scholar] [CrossRef]
- Adams, R.C.; Sedgmond, J.; Maizey, L.; Chambers, C.D.; Lawrence, N.S. Food addiction: Implications for the diagnosis and treatment of overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef]
- Lee, P.C.; Dixon, J.B. Food for thought: Reward mechanisms and hedonic overeating in obesity. Curr. Obes. Rep. 2017, 6, 353–361. [Google Scholar] [CrossRef]
- Reynolds, S.M.; Berridge, K.C. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 2002, 22, 7308–7320. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, A. It wasn’t me; it was my brain–Obesity-associated characteristics of brain circuits governing decision-making. Physiol. Behav. 2017, 176, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Temple, J.L.; Roemmich, J.N.; Bouton, M.E. Habituation as a determinant of human food intake. Psychol. Rev. 2009, 116, 384. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Jayne, M.; Franceschi, D.; Wong, C.; Gatley, S.J.; Gifford, A.N.; Ding, Y.S. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002, 44, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. ‘Liking’and ‘wanting’food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Felder, C.; Fowler, J.S.; Levy, A.V.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 2002, 13, 1151–1155. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef]
- Beavers, K.M.; Ambrosius, W.T.; Rejeski, W.J.; Burdette, J.H.; Walkup, M.P.; Sheedy, J.L.; Nesbit, B.A.; Gaukstern, J.E.; Nicklas, B.J.; Marsh, A.P. Effect of exercise type during intentional weight loss on body composition in older adults with obesity. Obesity 2017, 25, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Ibeas, K.; Herrero, L.; Mera, P.; Serra, D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochem. Pharmacol. 2021, 190, 114640. [Google Scholar] [CrossRef] [PubMed]
- Jiaxu, C.; Weiyi, Y. Influence of acute and chronic treadmill exercise on rat brain POMC gene expression. Med. Sci. Sport. Exerc. 2000, 32, 954–957. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Gao, Y.; Alhadeff, A.L.; Castorena, C.M.; Huang, Y.; Lieu, L.; Afrin, S.; Sun, J.; Betley, J.N.; Guo, H. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 2018, 18, 107–119. [Google Scholar] [CrossRef]
- Mueller, K.; Möller, H.E.; Horstmann, A.; Busse, F.; Lepsien, J.; Blüher, M.; Stumvoll, M.; Villringer, A.; Pleger, B. Physical exercise in overweight to obese individuals induces metabolic-and neurotrophic-related structural brain plasticity. Front. Hum. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef]
- Yi, C.X.; Al-Massadi, O.; Donelan, E.; Lehti, M.; Weber, J.; Ress, C.; Trivedi, C.; Müller, T.D.; Woods, S.C.; Hofmann, S.M. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol. Behav. 2012, 106, 485–490. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Muñoz, V.R.; Formigari, G.P.; Kuga, G.K.; Nakandakari, S.; Botezelli, J.D.; da Silva, A.S.R.; Cintra, D.E.; de Moura, L.P.; Ropelle, E.R.; et al. Acute physical exercise increases the adaptor protein APPL1 in the hypothalamus of obese mice. Cytokine 2018, 110, 87–93. [Google Scholar] [CrossRef]
- Rochete Ropelle, E.; Ramos da Silva, A.S.; Esper Cintra, D.; Pereira de Moura, L.; Teixeira, A.M.; Rodrigo Pauli, J. Physical Exercise: A Versatile Anti-Inflammatory Tool Involved in the Control of Hypothalamic Satiety Signaling. Exerc. Immunol. Rev. 2021, 27, 7–23. [Google Scholar]
- Wilson, R.A.; Stathis, C.G.; Hayes, A.; Cooke, M.B. Intermittent Fasting and High-Intensity Exercise Elicit Sexual-Dimorphic and Tissue-Specific Adaptations in Diet-Induced Obese Mice. Nutrients 2020, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Onambélé-Pearson, G.L.; Breen, L.; Stewart, C.E. Influence of exercise intensity in older persons with unchanged habitual nutritional intake: Skeletal muscle and endocrine adaptations. Age 2010, 32, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. A cross-sectional study evaluating the effects of resistance exercise on inflammation and neurotrophic factors in elderly women with obesity. J. Clin. Med. 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M. Baseline Obesity Increases 25-Year Risk of Mortality due to Cerebrovascular Disease: Role of Race. Int. J. Environ. Res. Public Health 2019, 16, 3705. [Google Scholar] [CrossRef]
- Marcell, T.J.; McAuley, K.A.; Traustadóttir, T.; Reaven, P.D. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism 2005, 54, 533–541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).