Dusky-like Is Critical for Morphogenesis of the Cellular Protuberances and Formation of the Cuticle in Henosepilachna vigintioctopunctata
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Insect
2.2. Molecular Cloning
2.3. Preparation of dsRNAs
2.4. Injection of dsRNA
2.5. Observation of Feeding Ability
2.6. Quantitative Real-Time PCR (qRT-PCR)
3. Results
3.1. Dyl Is Conserved in Insects
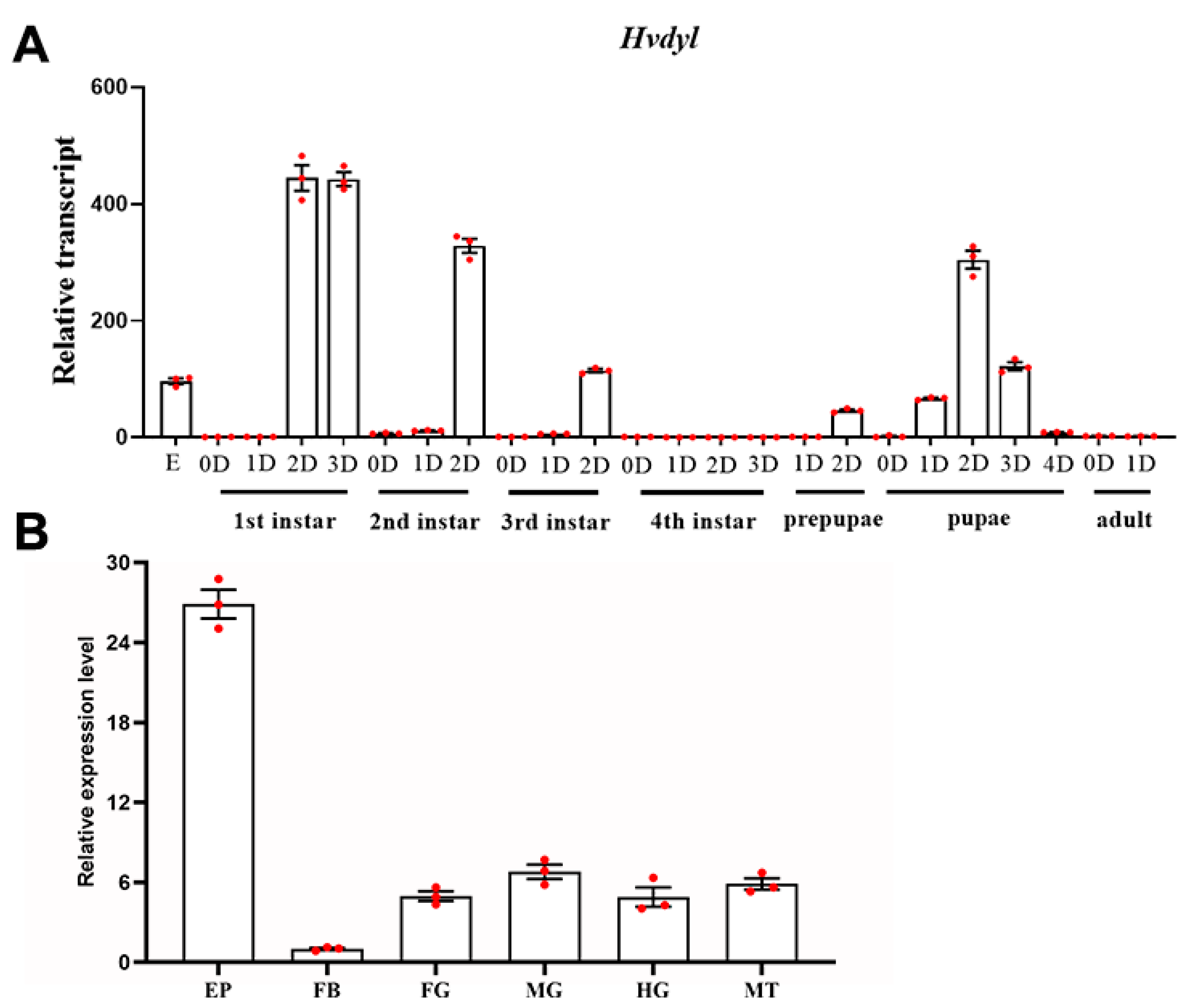
3.2. Expression Profiles of Hvdyl
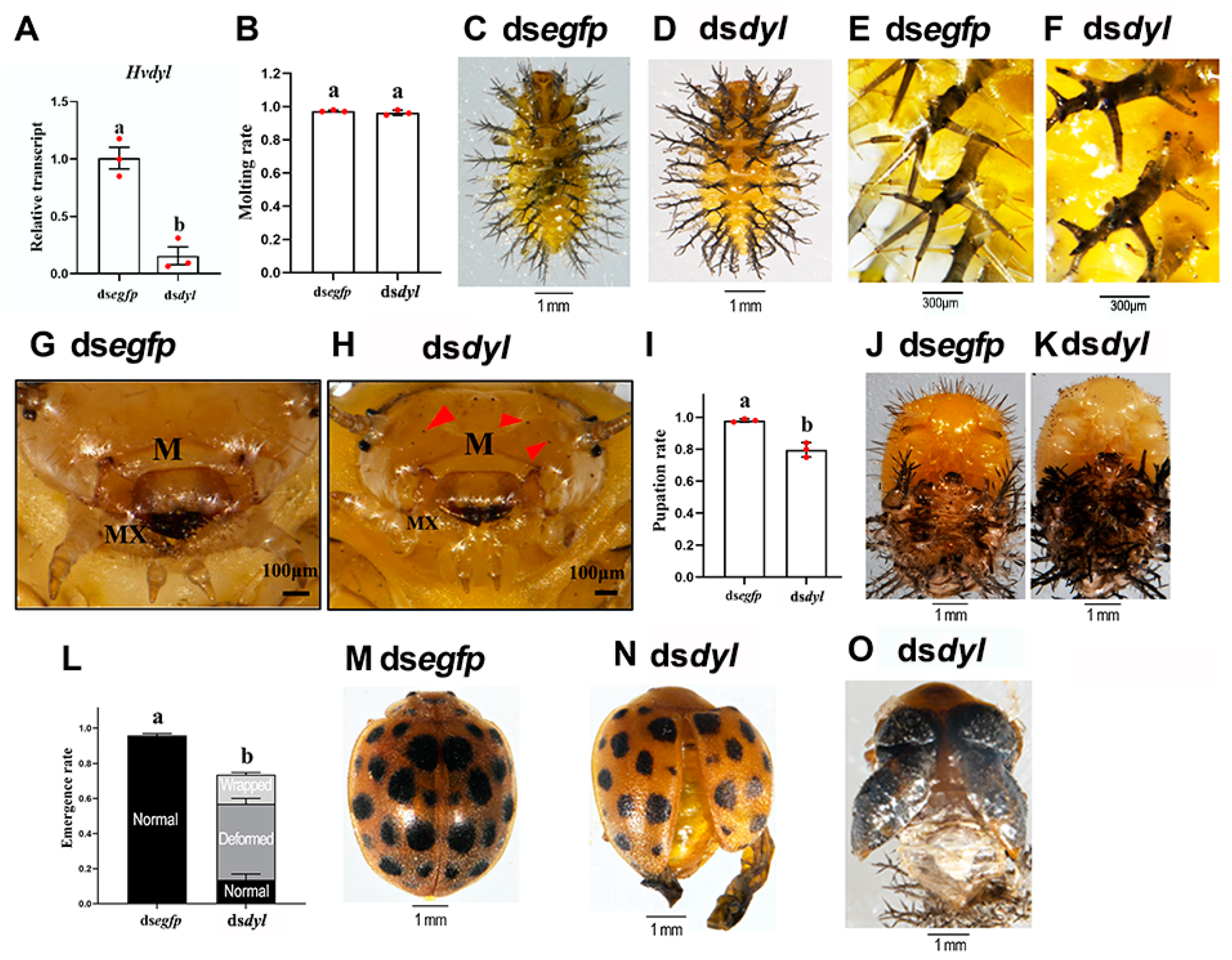
3.3. Hvdyl RNAi at the Third-Instar Stage Inhibited the Growth of Scoli and Setae
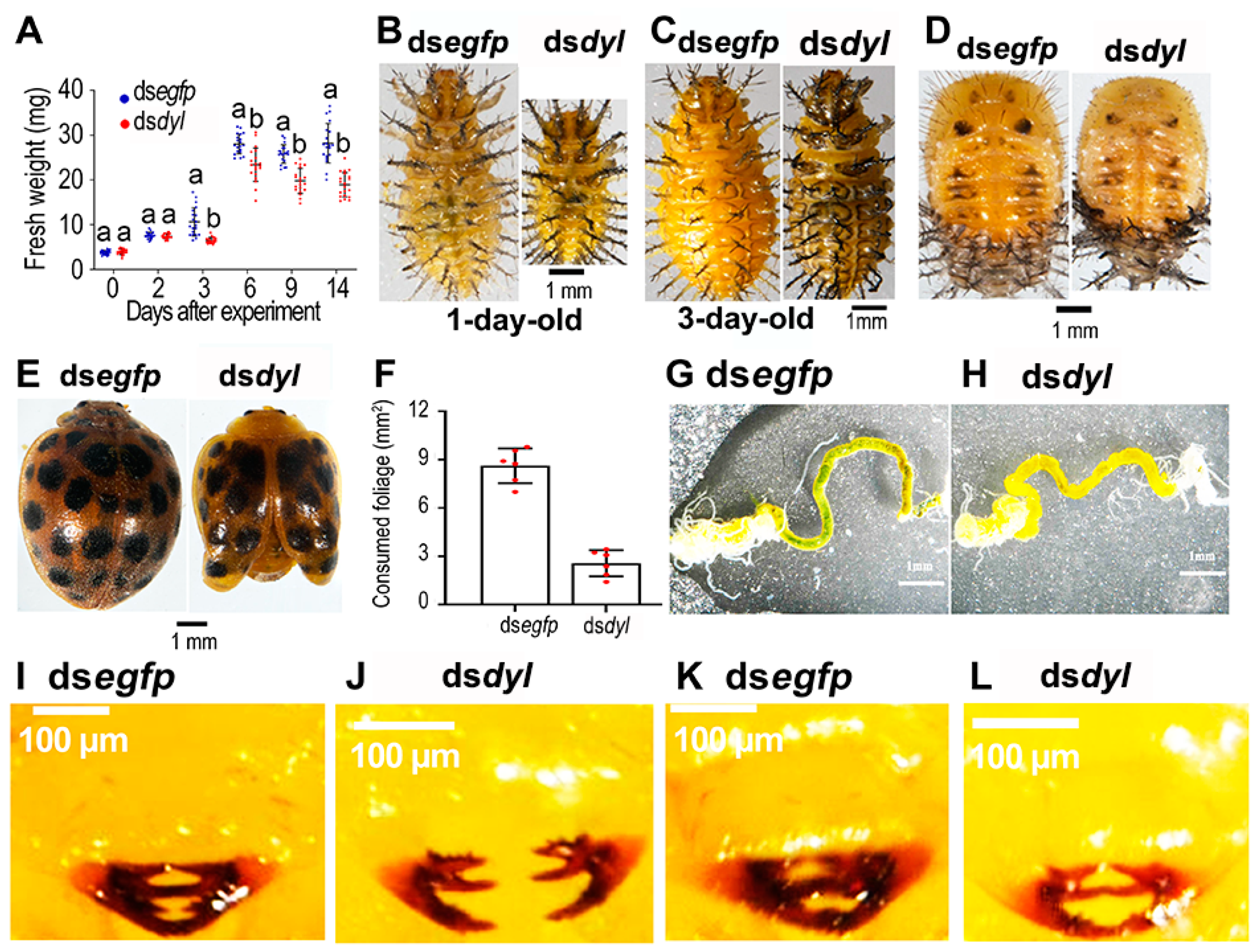
3.4. Reduced Growth and Foliage Consumption in the Hvdyl RNAi Beetles
3.5. Knockdown of Hvdyl at the Fourth-Instar Stage
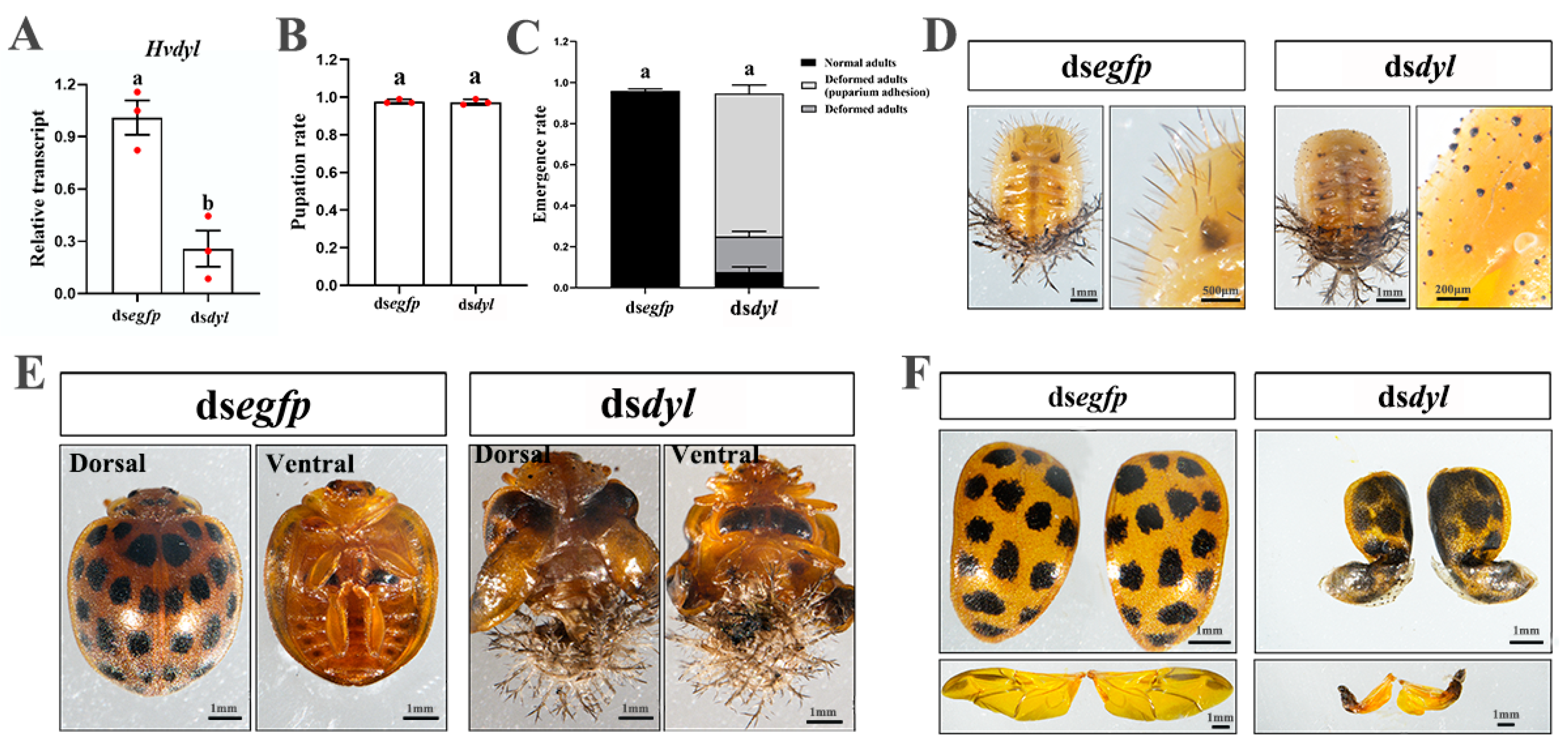
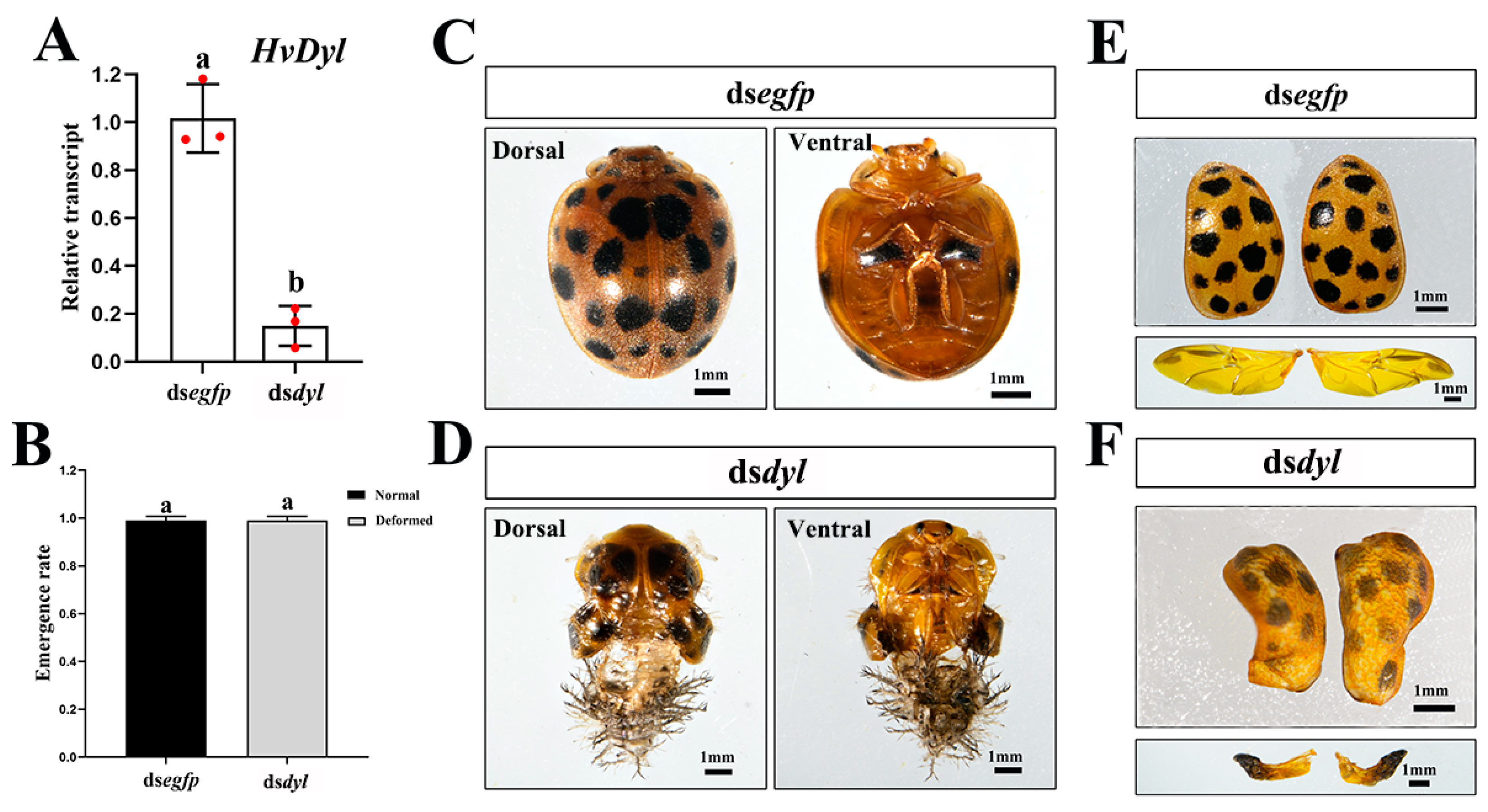
3.6. Depletion of Hvdyl at the Pupal Stage
4. Discussion
4.1. Dyl Is Critical for the Development of Epidermic Cellular Protuberances
4.2. Dyl May Regulate Chitin Deposition
4.3. Chitin Is Not the Only Target of Dyl
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bokel, C.; Prokop, A.; Brown, N.H. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J. Cell Sci. 2005, 118, 633–642. [Google Scholar] [CrossRef]
- Brodu, V.; Baffet, A.D.; Le Droguen, P.M.; Casanova, J.; Guichet, A. A Developmentally Regulated Two-Step Process Generates a Noncentrosomal Microtubule Network in Drosophila Tracheal Cells. Dev. Cell 2010, 18, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Chanut-Delalande, H.; Ferrer, P.; Latapie, Y.; Waltzer, L.; Affolter, M.; Payre, F.; Plaza, S. Zona Pellucida Domain Proteins Remodel the Apical Compartment for Localized Cell Shape Changes. Dev. Cell 2010, 18, 64–76. [Google Scholar] [CrossRef]
- Jazwinska, A.; Affolter, M. A family of genes encoding zona pellucida (ZP) domain proteins is expressed in various epithelial tissues during Drosophila embryogenesis. Gene Expr. Patterns 2004, 4, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.; Adler, P.N. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development 2012, 139, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Roch, F.; Alonso, C.R.; Akam, M. Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J. Cell Sci. 2003, 116, 1199–1207. [Google Scholar] [CrossRef]
- Plaza, S.; Chanut-Delalande, H.; Fernandes, I.; Wassarman, P.M.; Payre, F. From A to Z: Apical structures and zona pellucida-domain proteins. Trends Cell Biol. 2010, 20, 524–532. [Google Scholar] [CrossRef]
- Adler, P.N.; Sobala, L.F.; Thom, D.; Nagaraj, R. dusky-like is required to maintain the integrity and planar cell polarity of hairs during the development of the Drosophila wing. Dev. Biol. 2013, 379, 76–91. [Google Scholar] [CrossRef]
- Li, C.J.; Yun, X.P.; Li, B. Dusky-like is required for epidermal pigmentation and metamorphosis in Tribolium castaneum. Sci. Rep. 2016, 6, 20102. [Google Scholar] [CrossRef]
- Ze, L.J.; Wang, P.; Peng, Y.C.; Jin, L.; Li, G.Q. Silencing tyrosine hydroxylase or dopa decarboxylase gene disrupts cuticle tanning during larva-pupa-adult transformation in Henosepilachna vigintioctopunctata. Pest. Manag. Sci. 2022, 78, 3880–3893. [Google Scholar] [CrossRef]
- Casari, S.A.; Teixeira, E.P. Immatures of Epilachna Chevrolat (Coleoptera, Coccinellidae, Epilachninae). Rev. Bras. Entomol. 2015, 59, 113–120. [Google Scholar] [CrossRef]
- Wang, P.; Ze, L.J.; Jin, L.; Li, G.Q. Yellow-b, -c, -d, and -h are required for normal body coloration of Henosepilachna vigintioctopunctata. Arch. Insect Biochem. 2022, 109, e21856. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Cheng, M.D.; Ze, L.J.; Shen, C.H.; Jin, L.; Li, G.Q. Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval-Larval and Larval-Pupal Ecdyses in Henosepilachna vigintioctopunctata. Insects 2022, 13, 228. [Google Scholar] [CrossRef]
- Ze, L.J.; Jin, L.; Li, G.Q. Silencing of Adc and Ebony Causes Abnormal Darkening of Cuticle in Henosepilachna vigintioctopunctata. Front. Physiol. 2022, 13, 8296755. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Kang, W.N.; Jin, L.; Anjum, A.A.; Li, G.Q. Vacuolar ATPase subunit F is critical for larval survival in Henosepilachna vigintioctopunctata. Insect Mol. Biol. 2022, 31, 177–189. [Google Scholar] [CrossRef]
- Wu, J.J.; Mu, L.L.; Kang, W.N.; Ze, L.J.; Shen, C.H.; Jin, L.; Anjum, A.A.; Li, G.Q. RNA interference targeting ecdysone receptor blocks the larval-pupal transition in Henosepilachna vigintioctopunctata. Insect Sci. 2021, 28, 419–429. [Google Scholar] [CrossRef]
- Xu, P.; Ze, L.J.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Functional divergence of white genes in Henosepilachna vigintioctopunctata revealed by RNA interference. Insect Mol. Biol. 2020, 29, 466–476. [Google Scholar] [CrossRef]
- Ze, L.-J.; Xu, P.; Kang, W.-N.; Wu, J.-J.; Jin, L.; Anjum, A.A.; Li, G.-Q. Disruption of ommochrome biosynthesis affects eye coloration, phototaxis and climbing in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef]
- Lu, J.; Chen, S.M.; Guo, M.J.; Ye, C.Y.; Qiu, B.L.; Wu, J.H.; Yang, C.X.; Pan, H.P. Selection and Validation of Reference Genes for RT-qPCR Analysis of the Ladybird Beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Cant, K.; Knowles, B.A.; Mooseker, M.S.; Cooley, L. Drosophila Singed, a Fascin Homolog, Is Required for Actin Bundle Formation during Oogenesis and Bristle Extension. J. Cell Biol. 1994, 125, 369–380. [Google Scholar] [CrossRef]
- Fei, X.Y.; He, B.; Adler, P.N. The growth of Drosophila bristles and laterals is not restricted to the tip or base. J. Cell Sci. 2002, 115, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Adler, P.N. Cellular mechanisms in the development of the Drosophila arista. Mech. Dev. 2001, 104, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.S.; Lankenau, D.H.; Mitchell, H.K.; Young, P.; Corces, V.G. Forked Proteins Are Components of Fiber-Bundles Present in Developing Bristles of Drosophila melanogaster. Genetics 1994, 136, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Tilney, L.G.; Connelly, P.S.; Vranich, K.A.; Shaw, M.K.; Guild, G.M. Actin filaments and microtubules play different roles during bristle elongation in Drosophila. J. Cell Sci. 2000, 113, 1255–1265. [Google Scholar] [CrossRef]
- Turner, C.M.; Adler, P.N. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech. Dev. 1998, 70, 181–192. [Google Scholar] [CrossRef]
- Tilney, L.G.; Connelly, P.; Smith, S.; Guild, G.M. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J. Cell Biol. 1996, 135, 1291–1308. [Google Scholar] [CrossRef]
- Ren, N.; Zhu, C.M.; Lee, H.; Adler, P.N. Gene expression during Drosophila wing morphogenesis and differentiation. Genetics 2005, 171, 625–638. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. RNAi for chitin synthase 1 rather than 2 causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Pestic. Biochem. Phys. 2021, 178, 104934. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. Silencing uridine diphosphate N-acetylglucosamine pyrophosphorylase gene impairs larval development in Henosepilachna vigintioctopunctata. Pest. Manag. Sci. 2022, 78, 3894–3902. [Google Scholar] [CrossRef]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Shi, J.F.; Fu, J.; Mu, L.L.; Guo, W.C.; Li, G.Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylases are specialized for chitin synthesis in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochem. Mol. 2016, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of Tribolium castaneum. PLoS Genet. 2013, 9, e1003268. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet. 2018, 14, e1007307. [Google Scholar] [CrossRef]
- Gunsalus, K.C.; Bonaccorsi, S.; Williams, E.; Verni, F.; Gatti, M.; Goldberg, M.L. Mutations in Twinstar, a Drosophila Gene Encoding a Cofilin Adf Homolog, Result in Defects in Centrosome Migration and Cytokinesis. J. Cell Biol. 1995, 131, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Charlton, J.; Adler, P.N. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef]
- Tilney, L.G.; Tilney, M.S.; Guild, G.M. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J. Cell Biol. 1995, 130, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kiehart, D.P.; Franke, J.D.; Chee, M.K.; Montague, R.A.; Chen, T.L.; Roote, J.; Ashburner, M. Drosophila crinkled, mutations of which disrupt morphogenesis and cause lethality, encodes fly myosin VIIA. Genetics 2004, 168, 1337–1352. [Google Scholar] [CrossRef]
- Franke, J.D.; Montague, R.A.; Kiehart, D.P. Nonmuscle myosin II is required for cell proliferation, cell sheet adhesion and wing hair morphology during wing morphogenesis. Dev. Biol. 2010, 345, 117–132. [Google Scholar] [CrossRef]
- Winter, C.G.; Wang, B.; Ballew, A.; Royou, A.; Karess, R.; Axelrod, J.D.; Luo, L.Q. Drosophila Rho-associated kinase (Drok) links frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 2001, 105, 81–91. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tan, Q.; Lin, M.; Shen, C.; Jin, L.; Li, G. Dusky-like Is Critical for Morphogenesis of the Cellular Protuberances and Formation of the Cuticle in Henosepilachna vigintioctopunctata. Biology 2023, 12, 866. https://doi.org/10.3390/biology12060866
Zhang Y, Tan Q, Lin M, Shen C, Jin L, Li G. Dusky-like Is Critical for Morphogenesis of the Cellular Protuberances and Formation of the Cuticle in Henosepilachna vigintioctopunctata. Biology. 2023; 12(6):866. https://doi.org/10.3390/biology12060866
Chicago/Turabian StyleZhang, Yuxing, Qiao Tan, Mengjiao Lin, Chenhui Shen, Lin Jin, and Guoqing Li. 2023. "Dusky-like Is Critical for Morphogenesis of the Cellular Protuberances and Formation of the Cuticle in Henosepilachna vigintioctopunctata" Biology 12, no. 6: 866. https://doi.org/10.3390/biology12060866
APA StyleZhang, Y., Tan, Q., Lin, M., Shen, C., Jin, L., & Li, G. (2023). Dusky-like Is Critical for Morphogenesis of the Cellular Protuberances and Formation of the Cuticle in Henosepilachna vigintioctopunctata. Biology, 12(6), 866. https://doi.org/10.3390/biology12060866






