Simple Summary
Reptiles are carriers of different zoonotic pathogens hazardous to other animals and humans. Salmonella enterica is one of the best adapted bacterial pathogens causing infections. The aim of this study was to investigate the prevalence of Salmonella in different reptile species and to evaluate their serological variety and patterns of antimicrobial resistance. In total, 97 samples from 25 wild and domesticated reptile species were investigated in Lithuania for the presence of Salmonella. Fifty isolates of Salmonella were obtained from the ninety-seven tested samples. Results demonstrated that lizards and snakes are frequent carriers of a large variety of Salmonella serovars. Sixty-eight per cent of Salmonella were resistant to at least one antimicrobial. The most frequent resistance of the isolates was to streptomycin (26%), cefoxitin, gentamicin, tetracycline and chloramphenicol (16%). Genes encoding resistance to different antimicrobial classes were detected. The data obtained provided knowledge on Salmonella prevalence in reptiles. Healthy individuals, irrespective of their origin, often carry Salmonella, including multi-resistant strains. Due to its large serological diversity, zoonotic potential and antimicrobial resistance, Salmonella in reptiles poses a risk to other animals and humans.
Abstract
Salmonella enterica is one of the best adapted bacterial pathogens causing infections in a wide variety of vertebrate species. The aim of this study was to investigate the prevalence of Salmonella in different reptile species and to evaluate their serological variety and patterns of antimicrobial resistance. In total, 97 samples from 25 wild and domesticated reptile species were investigated in Lithuania. Serological variety, as well as phenotypical and genotypical resistance to antimicrobials, were investigated. Fifty isolates of Salmonella were obtained from the ninety-seven tested samples (51.5%; 95% CI 41.2–61.2). A significantly higher prevalence of Salmonella was detected in domesticated individuals (61.3%; 95% CI 50.0–71.5) compared with wild ones (18.2%; 95% CI 7.3–38.5). All isolates belonged to a single species, Salmonella enterica. Results demonstrated that reptiles carry a large variety of Salmonella serovars. Thirty-four isolates (68%) of Salmonella were resistant to at least one antimicrobial drug. The most frequent resistance of the isolates was to streptomycin (26%), cefoxitin, gentamicin, tetracycline and chloramphenicol (16%). Genes encoding resistance to tetracyclines, aminoglycosides, sulphonamides and trimethoprim were detected. No integrons that are associated with horizontal gene transfer were found. Data obtained provided knowledge about the adaptation of Salmonella in reptiles. Healthy individuals, irrespective of their origin, often carry Salmonella, including multi-resistant strains. Due to its large serological diversity, zoonotic potential and antimicrobial resistance, Salmonella in reptiles poses a risk to other animals and humans.
Keywords:
antimicrobial susceptibility; epidemiology; lizards; reptiles; snakes; Salmonella enterica 1. Introduction
Salmonella is a well-known pathogen that is prevalent in multiple species of vertebrates. Although the carriage of Salmonella species (Salmonella bongori and Salmonella enterica) in the intestinal tract of reptiles usually does not cause illness to themselves, it can cause serious infections in people, in particular young children, elderly people or immunocompromised individuals. Salmonella is a zoonotic bacterium and was isolated from multiple vertebrate species, including both warm- and cold-blooded animals [,].
Salmonella is divided into 60 serogroups and more than 2300 serovars []. Except for characterizing clinical aspects of a few serovars, such as Salmonella enterica (S. enterica) serovar Typhi, serogrouping and serotyping are mainly used as public health tools to recognize outbreaks and identify and control sources of infection [,]. Salmonellaenterica spp. enterica serovars are considered zoonotic or potentially zoonotic. The most common serovars infecting humans worldwide are S. serovar (ser.) Typhimurium and S. ser. Enteritidis [].
Humans may become infected through direct contact with reptiles or indirectly by manipulating objects, home stuff or contaminated food [,,,,,]. Globally, it is estimated that there are 93.8 million cases of salmonellosis per year caused by different reasons [,]. It is estimated that over 70,000 people get salmonellosis from reptiles each year in the United States, while 160,649 human cases of salmonellosis were reported in 2006 in 25 European countries, including Bulgaria, Romania, Iceland, Liechtenstein, Latvia, Germany, France and Norway [,].
Humans and animals share bacterial species including resistant ones. Antibiotic resistance of bacteria to antimicrobials is currently a primary concern in both human and veterinary medicine. For this reason, epidemiological studies in domestic and wild animals should be performed on a regular basis [,]. Resistant pathogens, including Salmonella enterica, should be of particular attention as these bacteria are very well adapted to different hosts, carry different genes encoding for both virulence and antimicrobial resistance and are currently among the most common infectious agents isolated from humans with food-borne infections. The aim of this study was to investigate the prevalence of Salmonella in different reptile species and to evaluate their serological variety and patterns of antimicrobial resistance.
2. Materials and Methods
2.1. Samples and Place
In 2020–2021, samples (n = 97) of domesticated reptile faeces and cloacal swabs of wild reptiles were collected using sterile cotton swabs with a transport medium (Transwab® Amies, Corsham, UK). Domesticated reptiles such as pet animals were sampled all over Lithuania from private keepers as well as in the Lithuanian Zoo. All animals were clinically healthy and underwent physical examination by a veterinarian before sampling. No treatments with antibiotics were performed for at least 6 months before sampling. Wild reptiles were caught and samples were collected from three main locations in Lithuania: Raguva (55.56472 24.61574); (55.564476 24.617858); the Rumšiškės forest (54.881027 24.178046); (54.881832 24.178994) and Čepkeliai—Dzūkija National Park (54.0214 24.4289), (54.0225 24.4831), (54.0562 24.4241), (54.0606 24.4302). Ethical approval for this study was given by the Lithuanian Environmental Protection Agency (permissions numbers AS-4800 and AS-4884).
Samples were delivered to the laboratory within 24 h of collection, kept in containers with transport media on ice for 1–2 h then followed by refrigeration at +2–4 °C. In total, 97 samples were collected from 25 different species of reptiles (Table 1).

Table 1.
Species and number of tested reptiles.
2.2. Isolation and Identification of Salmonella
Isolation of Salmonella was performed according to the EN ISO 6579-1 (ISO, 2017) [] procedure for Salmonella detection. Xylose lysine deoxycholate (XLD) agar and Salmonella Shigella (SS) agar (Oxoid, Basingstoke, UK) were used as plating media after the enrichment procedure. The randomly selected separate colonies (one colony per sample) were identified using the “Microgen Gram-Negative Plus” biochemical identification system (Microgen, Camberley, UK).
Salmonella serotyping was carried out by standard slide agglutination test (CEN ISO/TR 6579-3:2014) [] with polyvalent and monovalent somatic (O) and flagella (H) antisera (Statens Serum Institute Denmark and Sifin, Berlin, Germany). Firstly, suspected colonies were picked up and tested with somatic O polyvalent and O polyvalent group antisera. In the case of a positive reaction, testing according to the Kaufman–White scheme was applied. If the suspect colony did not show any reaction with O polyvalent antisera, Salmonella species and subspecies were identified by biochemical properties. Serotyping results were evaluated according to the Kaufmann–White Salmonella serotyping scheme.
2.3. Susceptibility Testing
Antimicrobial susceptibility testing was performed using the disk diffusion method according to Kirby-Bauer. Antimicrobials of different classes were selected with the aim of addressing the risk of salmonellosis to public health. The following disks were used: ampicillin (10), cefoxitin (30), gentamicin (10), chloramphenicol (30), sulfamethoxazole-trimethoprim (25), cefpodoxime (10), ciprofloxacin (5), tetracycline (30), ofloxacin (5), streptomycin (10) and doxycycline (30). The results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing licensed by EUCAST 2022 clinical breakpoints [] whenever possible. For tetracycline, doxycycline, cefpodoxime and streptomycin the interpretation of the results was performed using Clinical and Laboratory Standards Institute (CLSI) guidelines []. In the case of resistance to at least three or more antimicrobial classes, the isolates were treated as multi-resistant isolates.
2.4. Molecular Testing
The resistant Salmonella isolates were tested by polymerase chain reaction (PCR) for detection of the genes encoding resistance. DNA material for molecular testing was obtained after bacterial lysis was performed as described previously []. PCR included 30 cycles of denaturation (94 °C, 30 s), annealing (30 s) and extension (94 °C, 90 s). Annealing temperatures and oligonucleotides used are presented in Table 2. As a negative control, DNA/RNA-free water was used instead of the antigen whereas, for the positive control strains, Enterobacteriaceae from the culture collection of the Microbiology and Virology Institute at the Lithuanian University of Health Sciences were used.

Table 2.
Antimicrobial resistance genes tested and oligonucleotide primers used in the study.
2.5. Data Analysis
Statistical analysis was performed using the IBM SPSS Statistics package, version 27 (SPSS Inc., Chicago, IL, USA). For percentage estimates, Wilson (score) 95% confidence intervals (CI 95%) and their ranges for true population proportions were calculated. Comparison between categorical variables was calculated using a chi-squared test or Fisher’s exact test for small counts. Results were considered statistically significant if p < 0.05. The number of genes encoding resistance to separate antimicrobials was expressed in % from the number of resistant isolates tested.
3. Results
3.1. Salmonella Prevalence in Reptiles
In total, 97 reptile samples were tested, of which 22 samples came from wild reptiles and 75 samples from domesticated animals. Fifty animals were positive for Salmonella (51.5%; 95% CI 41.2–61.2), as determined by isolation of the cultures with further biochemical identification. All of the isolates belonged to a single species, Salmonella enterica. The results demonstrated that the frequency of Salmonella prevalence was significantly higher in domesticated reptiles than in wild ones (p < 0.0475). Forty-six isolates (61.3% 95% CI 50.0–71.5) were obtained from domesticated reptiles and four (18.2%; 95% CI 7.3–38.5) were obtained from wild individuals. Overall, Salmonella was isolated from 17 out of the 25 reptile species (68%) included in this study. The prevalence of Salmonella in different reptile species is presented in Table 3.

Table 3.
Species of reptiles carrying Salmonella isolates.
3.2. Serological Variety of the Salmonella Isolates
In total, 34 Salmonella isolates showing antimicrobial resistance were serotyped. Twenty-seven out of thirty-four (79.4%) isolates had a positive reaction only with O (somatic) antisera, whereas only three strains had a positive reaction with H grouping antisera. Different serogroups and serovars were identified including IIIa, enterica arizonae/IIIb, enterica diarizonae, Sherbrooke, Maiduguri, Waycross, Macallen, and others. Most of the isolates belonged to O:4, O:8 and O:18 serogroups; some serogroups, including O:41, O:30 and O:3.10 were only detected in up to three isolates, and some were detected just by single isolates. Characteristics of Salmonella isolated from domesticated and wild reptiles according to their serological patterns are presented in Table 4 and Table 5.

Table 4.
Characteristics of Salmonella isolated from domesticated reptiles.

Table 5.
Characteristics of Salmonella isolated from wild reptiles.
3.3. Antimicrobial Resistance
Of 50 Salmonella isolates, 34 (68%) were resistant to at least one tested antimicrobial. The resistant isolates recovered from domesticated and wild reptiles are presented in Table 6.

Table 6.
Species of reptiles carrying antimicrobial resistant Salmonella isolates.
In total, 24 of 50 isolates were resistant (48%) to a single or two antimicrobial agents, whereas 10 (20%) of the isolates were multi-resistant, i.e., resistant to three or more antimicrobial classes. Multi-resistant isolates were obtained from grass snakes (Natrix natrix) (n = 3), boa constrictors (Boa constrictor) (n = 2), a Chinese water dragon (Physignathus cocincinus) (n = 1), a California kingsnake (Lampropeltis californiae) (n = 1), a corn snake (Pantherophis guttatus) (n = 1), a milk snake (Lampropeltis triangulum) (n = 1) and a king ratsnake (Elaphe carinata carinata) (n = 1). The phenotypical resistance of the isolates is presented in Figure 1.
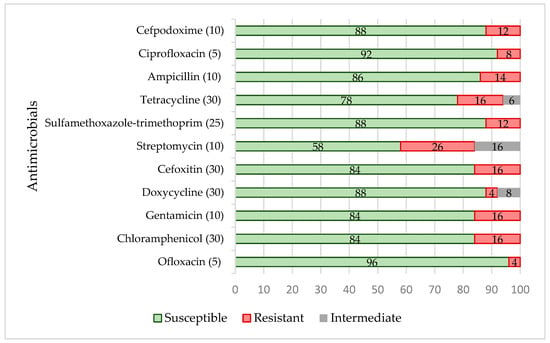
Figure 1.
Phenotypical antimicrobial resistance (%) patterns of the Salmonella isolates from reptiles (n = 50). Intermediate describes the zone of inhibition in between “susceptible” and “resistant”. The numbers in brackets near the antimicrobial agent represent the antimicrobial concentrations (µg) of the discs.
The data demonstrated that the frequency of the resistance to different antimicrobials was not high; however, the spectrum of resistance was wide, i.e., there was no antimicrobial substance tested that was effective against all Salmonella isolates. The most frequent resistance prevalence of the isolates was against streptomycin (26%; chi-squared test, p < 0.00073), cefoxitin, gentamicin, tetracycline and chloramphenicol (16%; chi-squared test, p < 0.001).
The genes encoding antimicrobial resistance to different antibiotics were as follows: aadA (37.5%) and armA (37.5%) encoding resistance to aminoglycosides, sul2 (50%) encoding resistance to sulphonamides, dfr1 (50%) and dfr7 (16.6%) encoding resistance to trimethoprim and tetA (61.5%) and tetB (53.8%) encoding resistance to tetracyclines. No genes were detected for encoding the resistance to β-lactams, fluoroquinolones, and amphenicols. No integrons associated with horizontal gene transfer were detected. The data of susceptibility profiles as well as the genes encoding resistance are presented in Table 4 and Table 5.
4. Discussion
Salmonella is a gram-negative pathogen that causes various host-specific diseases. It is one of the most widespread agents causing human gastrointestinal infections, as well as infections in pigs, poultry and calves. Although the clinical significance of Salmonella infections in wild and captive reptiles is poorly understood, it is thought that the majority of infections lead to an asymptomatic carrier state and do not result in disease []. In this study, all tested reptiles were clinically healthy, therefore our data support this opinion. From the reptiles sampled in this study, Salmonella was found in 61% and 18% of domesticated and wild individuals, respectively, across Lithuania. In other studies, the data were quite similar. For example, in central Europe (Poland, Germany and Austria), the prevalence of Salmonella in domesticated snakes and lizards ranged from 33% to 54.1% []. In Norwegian zoos, Salmonella was recovered from 62% of snakes and 67% of lizards []. Although there is a lack of data about Salmonella prevalence in wild reptiles, a study performed by Scheelings et al. demonstrated a higher prevalence of this bacterium in reptiles held in captivity (47%) compared to wild reptiles (14%) []. Such data are very similar to the data obtained in our study; however, the number of wild animals used in our study was low. More wild animals should be investigated in order to answer whether domesticated animals more often carry Salmonella than wild individuals. The origin of Salmonella in both captive and wild reptiles is also unclear. As some of the wild species, such as Coronella austriaca, dwell far away from the urban areas, it may be assumed that the carriage of Salmonella in reptiles is not necessarily associated with human activity, but this microorganism can be a part of the natural microbiota of reptiles. On the other hand, a higher prevalence of Salmonella in domesticated reptiles rather than in wild individuals, as detected in this study, may be explained by the mixing of different reptile species in a single premise, restricted area, and carriage by humans. Feed intended for reptiles can also be a reason for Salmonella spread because either raw feed (such as live or frozen rodents) or concentrated feed may be contaminated by Salmonella.
In this study, the most frequent carriage of Salmonella was detected in snakes, especially in Lampropeltis californiae, Pantherophis guttatus, Lampropeltis triangulum and Elaphe taeniura friesei and less frequently in lizards. This may be associated with a smaller number of investigated lizards, as in other studies, Salmonella was more frequently isolated from lizards rather than from snakes [,]. This may also depend on investigated species of lizards, as different species of lizards both in the wild and in captivity have different feed diets; some lizards eat arthropods and other invertebrates while larger species include small vertebrates in their diet []. As rodents are known as reservoirs of different pathogens including Salmonella, this fact of Salmonella epidemiology in reptiles could be considered very important and should be further studied.
The exact serotyping of Salmonella in reptile isolates is not always successful because of a wide variety of serovars and that the most well-known serovars with epidemiological importance for humans and domestic animals are less frequently presented in cold-blooded animals. Different serovars of Salmonella were detected in this study, including Salmonella Florida, Salmonella Sherbrooke, Salmonella Maiduguri, Salmonella Waycross and others, whereas the most widespread serovars in humans and farm animals according to responsible institutions and previous data in Lithuania were Salmonella Enteritidis, Salmonella Typhimurium, Salmonella Choleraesuis, Salmonella Infantis, Salmonella Derby and Salmonella Dublin []. Although studies about Salmonella serotypes in reptiles are scarce, some data from other countries exist. For instance, in Norway, 26 different Salmonella serovars in captive reptiles were detected, including those with high zoonotic potential, such as Salmonella Paratyphi B, subsp. arizonae, and those with low or moderate zoonotic potential, such as serovar Salmonella Lome, subsp. salamae, diarizonae and others []. In neighbouring Poland, 209 serovars of Salmonella were detected in reptiles, from which the most prevalent were Salmonella Oranienburg, Salmonella Tennessee, Salmonella Agona, Salmonella Fluntern and Salmonella Muenchen []. In French Guiana, 14 different Salmonella serovars were detected among wild reptiles. Interestingly, nearly two-thirds of the Salmonella serovars isolated from reptiles were also isolated from patients in this country []. The high prevalence of Salmonella in humans and overlapping serotypes was explained by the handling and consumption of reptiles by humans. Such data support the fact that Salmonella, regardless of the serovars, is pathogenic and can easily be transmitted from reptiles to humans.
The data on antimicrobial susceptibility of the isolates revealed a wide spectrum of resistance, as there were no antimicrobials tested that would be effective for all Salmonella isolates. This can be explained by the large diversity of Salmonella among reptiles. In a recent study performed in Poland, Salmonella isolates from reptiles were most frequently resistant to streptomycin [], which is in accordance with the results obtained in our study. In Taiwan, the most frequent resistances were to streptomycin and tetracycline []. In Spain, the most frequent resistances among Salmonella from reptiles were to gentamicin, colistin, and ampicillin []. In this study, 20% of all Salmonella isolates were multi-resistant. Such strains usually pose a high risk not only for the treatment of infections but also for the transfer of resistance genes to other microbiota. Horizontal gene transfer is very common among Enterobacteriaceae including Salmonella; however, we did not detect integrons that are associated with horizontal gene transfer. Nevertheless, as Salmonella can be easily transferred to humans or other animals and are pathogenic, multi-resistant strains are of great concern. Different studies demonstrate the unequal frequency of multi-resistant Salmonella isolated from reptiles. For example, in Poland, only single multi-resistant strains were isolated [], whereas, in Spain, 72% of the isolates were multi-resistant []. Such data also prove the large diversity of Salmonella among reptiles. Although we have detected some genes encoding antimicrobial resistance, their variety was not high, especially when compared with isolates from farm animals or humans. Genes encoding resistance to tetracyclines, aminoglycosides, sulphonamides and trimethoprim were detected. Although the same genes were recently detected in Enterobacteriaceae from domestic (unpublished data) and wild animals in Lithuania [], it is difficult to make any conclusion about their origin in Salmonella isolates from reptiles. Further studies are needed to analyse possible relations of microorganism transfer between reptiles and other hosts.
5. Conclusions
Reptiles are carriers of a wide variety of serovars and multi-resistant strains of Salmonella. Although the prevalence of Salmonella was higher in domesticated reptiles than in wild individuals, further studies are needed to support this theory, as the number of tested wild animals was much lower than in domesticated ones. Reptiles can be a reservoir of Salmonella, therefore hygienic measures should be kept when maintaining and carrying them, as well as when keeping reptiles in close contact with other animals.
Author Contributions
Conceptualization, L.M. and G.P.; methodology, R.Š. and M.R.; formal analysis, M.V. and Č.B.-A.; investigation, G.P., A.P., J.D., R.Š., M.V., Č.B.-A. and L.M.; data curation, A.D.; writing—original draft preparation, L.M. and M.R.; writing—review and editing, M.R. and L.M.; supervision, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for this study was given by the Lithuanian Environmental Protection Agency (permissions numbers AS-4800, AS-4884).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bjelland, A.M.; Sandvik, L.M.; Skarstein, M.M.; Svendal, L.; Debenham, J.J. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet. Scand. 2020, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Scheelings, T.F.; Lightfoot, D.; Holz, P. Prevelence of Salmonella in Australian reptiles. J. Wildl. Dis. 2011, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Keusch, G.T. Salmonellosis. In Harrison’s Principles of Internal Medicine; Isselbacher, K.J., Ed.; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Mermin, J.; Hutwagner, L.; Vugia, D.; Shallow, S.; Daily, P.; Bender, J.; Koehler, J.; Marcus, R.; Angulo, R.J. Reptiles, amphibians, and human Salmonella infection: A population-based, case-control study. Clin. Infect. Dis. 2004, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Geue, L.; Loschner, U. Salmonella enterica in reptiles of German and Austrian origin. Vet. Microbiol. 2002, 84, 79–91. [Google Scholar] [CrossRef]
- Corrente, M.; Madio, A.; Friedrich, K.G.; Greco, G.; Desario, C.; Tagliabue, S.; D’Incau, M.; Campolo, M.; Buonavoglia, C. Isolation of Salmonella strains from reptile faeces and comparison of different culture media. J. Appl. Microbiol. 2004, 96, 709–715. [Google Scholar] [CrossRef]
- Ebani, V.V.; Cerri, D.; Fratini, F.; Meille, N.; Valentini, P.; Andreani, E. Salmonella enterica isolates from faeces of domestic reptiles and a study of their antimicrobial in vitro sensitivity. Res. Vet. Sci. 2005, 78, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, W.C.; Chin, S.C.; Lai, Y.H.; Tung, K.C.; Chiou, C.S.; Hsu, Y.M.; Chang, C.C. Prevalence and antimicrobial susceptibility of salmonellae isolates from reptiles in Taiwan. J. Vet. Diagn. Investig. 2010, 22, 44–50. [Google Scholar] [CrossRef]
- Hydeskov, H.B.; Guardabassi, L.; Aalbaek, B.; Olsen, K.E.; Nielsen, S.S.; Bertelsen, M.F. Salmonella prevalence among reptiles in a zoo education setting. Zoonoses Public Health 2013, 60, 291–295. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Whiley, H.; Gardner, M.G.; Ross, K. A review of Salmonella and Squamates (Lizards, Snakes and Amphisbians): Implications for public health. Pathogens 2017, 6, 38. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevelation. CFSPH Technical Fact Sheets. Salmonella (Non-Typhoidal). CDC Website. Salmonellosis at Reptiles and Amphibians. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/ (accessed on 1 May 2022).
- Bertrand, S.; Rimhanen-Finne, R.; Weill, F.X.; Rabsch, W.; Thornton, L.; Perevoscikovs, J.; van Pelt, W.; Heck, M. Salmonella infections associated with reptiles: The current situation in Europe. Eur. Commun. Dis. Bull. 2008, 13, 18902. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- CEN ISO/TR 6579-3:2014; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 3: Guidelines for Serotyping of Salmonella spp. ISO: Geneva, Switzerland, 2014.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 1 May 2022).
- CLSI (2019); Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI Supplement M100. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Ružauskas, M.; Šiugždinienė, R.; Klimienė, I.; Virgailis, M.; Mockeliūnas, R.; Vaškevičiūtė, L.; Zienius, D. Prevalence of methicillin-resistant Staphylococcus haemolyticus in companion animals: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.; Chin, J.; Chapman, T.; Tran, L.T.; Coloe, P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008, 3, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ojdana, D.; Sacha, P.B.; Wieczorek, P.; Czaban, B.; Michalska, A.; Jaworowska, J.; Jurczak, A.; Poniatowski, B.; Tryniszewska, E. The occurrence of blaCTX-M, blaSHV, and blaTEM genes in extended-spectrum 𝛽-lactamase-positive strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. Int. J. Antibiot. 2014, 2014, 935842. [Google Scholar] [CrossRef]
- Bert, F.; Branger, C.; Lambert-Zechovsky, N. Identification of PSE and OXA β-lactamase genes in Pseudomonas aeruginosa using PCR–restriction fragment length polymorphism. J. Antimicrob. Chemother. 2002, 50, 11–18. [Google Scholar] [CrossRef]
- Pagani, L.; Dell’Amico, E.; Migliavacca, R.; D’Andrea, M.M.; Giacobone, E.; Amicosante, G.E.R.; Rossolini, G.M. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 2003, 41, 4264–4269. [Google Scholar] [CrossRef]
- Hasman, H.; Mevius, D.; Veldman, K.; Olesen, I.; Aarestrup, F.M. β-Lactamases among extended-spectrum β-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005, 56, 115–121. [Google Scholar] [CrossRef]
- Celenza, G.; Pellegrini, C.; Caccamo, M.; Segatore, B.; Amicosante, G.; Perilli, M. Spread of blaCTX-M-type and blaPER-2 β-lactamase genes in clinical isolates from Bolivian hospitals. J. Antimicrob. Chemother. 2006, 57, 975–978. [Google Scholar] [CrossRef]
- Maynard, C.; Fairbrother, J.M.; Bekal, S.; Sanschagrin, F.; Levesque, R.C.; Brousseau, R.; Masson, L.; Larivière, S.; Harel, J. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 2003, 47, 3214–3221. [Google Scholar] [CrossRef]
- Asadollahi, P.; Akbari, M.; Soroush, S.; Taherikalani, M.; Asadollahi, K.; Sayehmiri, K. Antimicrobial resistance patterns and their encoding genes among Acinetobacter baumannii strains isolated from burned patients. Clin. Infect. Dis. 2012, 38, 1198–1203. [Google Scholar] [CrossRef]
- Yan, J.J.; Wu, J.J.; Ko, W.C.; Tsai, S.H.; Chuang, C.L.; Wu, H.M.; Lu, Y.J.; Li, J.D. Plasmid-mediated 16S rRNA methylases conferring high-level aminoglycoside resistance in Escherichia coli and Klebsiella pneumoniae isolates from two Taiwanese hospitals. J. Antimicrob. Chemother. 2004, 54, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Galimand, M.; Courvalin, P.; Lambert, T. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 2003, 47, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Frana, T.S.; Carlson, S.A.; Griffith, R.W. Relative distribution and conserovation of genes encoding aminoglycoside-modifying enzymes in Salmonnella enterica serotype Typhimurium phage type DT104. Appl. Environ. Microbiol. 2001, 67, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Odumosu, B.T.; Akintimehin, A.R. Occurrence of extended-spectrum beta-lactamase producing Enterobacteriaceae isolates in communal water sources in Ogun State, Nigeria. Afr. J. Clin. Exp. Microbiol. 2015, 16, 28–32. [Google Scholar] [CrossRef][Green Version]
- Sandvang, D.; Aarestrupp, F.M. Characterization of aminoglycoside resistance genes and class 1 integrons in porcine and bovine gentamicin-resistant Escherichia coli. Microb. Drug Resist. 2009, 6, 19–27. [Google Scholar] [CrossRef]
- Lanz, R.; Kuhnert, P.; Boerlin, P. Antimicrobial resistance and resistance genes determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 2003, 91, 73–84. [Google Scholar] [CrossRef]
- Vassort-Bruneau, C.; Lesage-Descauses, M.; Martel, J.L.; Lafont, J.P.; Chaslus-Dancla, E. CAT III chloramphenicol resistance in Pasteurella haemolytica and Pasteurella multocida isolated from calves. J. Antimicrob. Chemother. 1996, 38, 205–213. [Google Scholar] [CrossRef]
- Keyes, K.; Hudson, C.; Maurer, J.J.; Thayer, S.; White, D.G.; Lee, M.D. Detection of florfenicol resistance genes in Escherichia coli isolated from sick chickens. Antimicrob. Agents Chemother. 2000, 44, 421–424. [Google Scholar] [CrossRef]
- Christabel, M.; Budambula, N.; Kiiru, J.; Kariuki, S. Characterization of antibiotic resistance in environmental enteric pathogens from Kibera slum in Nairobi-Kenya. J. Bacteriol. Res. 2012, 4, 46–54. [Google Scholar] [CrossRef]
- Perreten, V.; Boerlin, P.A. New sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 2003, 44, 1169–1172. [Google Scholar] [CrossRef]
- Gibreel, A.; Sköld, O. High-level resistance to trimethoprim in clinical isolates of Campylobacter jejuni by acquisition of foreign genes (dfr1 and dfr9) expressing drug-insensitive dihydrofolate reductases. Antimicrob. Agents Chemother. 1998, 42, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Šeputienė, V.; Povilonis, J.; Ružauskas, M.; Pavilonis, A.; Sužiedėlienė, E. Prevalence of trimethoprim resistance genes in Escherichia coli isolates of human and animal origin in Lithuania. J. Med. Microbiol. 2010, 59, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Navia, M.M.; Ruiz, J.; Cespedes, S.J.; Vila, J. Detection of dihydrofolate reductase genes by PCR and RFLP. Diagn. Microbiol. Infect. Dis. 2003, 46, 295–298. [Google Scholar] [CrossRef]
- Robicsek, A.; Strahilevitz, J.; Sahm, D.; Jacoby, G.A.; Hooper, D.C. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 2006, 50, 2872–2874. [Google Scholar] [CrossRef]
- Liu, J.H.; Deng, Y.T.; Zeng, Z.L.; Gao, J.H.; Chen, L.; Arakawa, Y.; Chen, Z.L. Coprevalence of plasmid-mediated quinolone resistance determinants QepA, Qnr, and AAC(6′)-Ib-cr among 16S rRNA methylase RmtB-producing Escherichia coli isolates from pigs. Antimicrob. Agents Chemother. 2008, 52, 2992–2993. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, C.C. Risk factor analysis for extended-spectrum β-lactamase-producing Enterobacter cloacae bloodstream infections in central Taiwan. BMC Infect. Dis. 2013, 13, 417. [Google Scholar] [CrossRef]
- Goldstein, C.; Lee, M.D.; Sanchez, S.; Hudson, C.; Phillips, B.; Register, B.; Grady, M.; Liebert, C.; Summers, A.O.; White, D.G.; et al. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob. Agents Chemother. 2001, 45, 723–726. [Google Scholar] [CrossRef]
- Piasecki, T.; Chrząstek, K.; Wieliczko, A. Salmonella serovar spectrum associated with reptiles in Poland. Acta Vet. Brno 2014, 83, 287–294. [Google Scholar] [CrossRef]
- Losos, J.B.; Grrene, H.W. Ecological and evolutionary implications of diet in monitor lizards. Biol. J. Linn. Soc. 1998, 35, 379–407. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Virgailis, M.; Špakauskas, V. Serological diversity and antimicrobial resistance of Salmonella isolated from different sources in Lithuania. Vet. Arh. 2005, 75, 211–221. [Google Scholar]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in captive reptiles and their environment—Can We Tame the Dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.; Le Hello, S.; Weill, F.; de Thoisy, B.; Berger, F. Salmonella serotypes in reptiles and humans, French Guiana. Vet. Microbiol. 2014, 170, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Lorenzo-Rebenaque, L.; Laso, O.; Villora-Gonzalez, J.; Vega, S. Pet Reptiles: A potential source of transmission of multidrug-resistant Salmonella. Front. Vet. Sci. 2021, 7, 613718. [Google Scholar] [CrossRef] [PubMed]
- Merkevičienė, L.; Klimienė, I.; Šiugždinienė, R.; Virgailis, M.; Mockeliūnas, R.; Ružauskas, M. Prevalence and molecular characteristics of multiresistant Escherichia coli in wild birds. Acta Vet. Brno 2018, 87, 9–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).