Simple Summary
Lingula anatina has been attracted researchers because its morphological characteristics show limited changes compared to the ancestor. Even though L. anatina is a common brachiopod in the western Pacific region, a few studies were performed to investigate genetic variations of this species. To understand the mitogenomic diversity of L. anatina in the region, the present study conducted comparative analyses of mitogenome sequences from Korea, Vietnam, and Japan. The sequencing results indicated that L. anatina mitogenomes are extraordinarily longer than the typical animal mitogenome. Besides, the gene orders of L. anatina mitogenomes are variable among localities. The calculation based on protein-coding genes revealed relatively low substitution rates among mitogenome sequences from Buan (Korea), Doson (Vietnam) and Yanagawa (Japan). The phylogenetic analyses indicated the divergence of L. anatina in the western Pacific region. This investigation provides new information on the molecular systematics of L. anatina and could be helpful in exploring the diversity and evolution of mitogenomes in brachipod.
Abstract
Lingula anatina is a brachiopod widely distributed in the western Pacific region. Even though L. anatina has been targeted for a number of biological studies, there is still limited information on intraspecific genetic variations of L. anatina. In this study, L. anatina specimens were collected from Korea and Vietnam, and complete mitochondrial genome (mitogenome) sequences were analyzed and compared with previous records. The total mitogenomes of L. anatina were 24,875 bp and 25,305 bp in size for Korean and Vietnamese specimens, respectively. Those mitogenomes are extraordinarily longer than the typical mitogenome size for an animal but shorter than the previous record from Yanagawa (Japan) for this species. The gene orders and the sizes of the protein-coding genes are also different from those for the Japanese specimen. Furthermore, the nonsynonymous (Ka) and synonymous (Ks) substitution rates in protein-coding genes (PCGs) were calculated to test the idea of evolutionary rate differences in mitochondrial genomes. The analyses showed relatively low Ka and Ks for the complete mitogenomes from Buan (Korea), Doson (Vietnam) and Yanagawa (Japan). The Ka/Ks ratio was less than 1 in comparisons of three localities, indicating the existence of purifying selection in this species. The phylogenetic analyses showed that L. anatina diverged among localities in the western Pacific region.
1. Introduction
Mitochondria are the energy production organelles of eukaryotic cells and have double-stranded circular genomes. Typically, mitochondrial genomes (mitogenome) are approximately 14 to 18 kb long in metazoans and contain 13 protein-coding genes, 22 transfer RNA (tRNA) genes, two ribosomal RNA (rRNA) genes and a control region [1]. Their categorization is based on several factors that have high variability, even at the family level, such as size, gene content, gene order, tRNA location and the replication of tRNAs [2,3]. These variable factors are phylogenetic markers [4,5,6,7]. Additionally, the stability of the mitogenome structure is variable. For instance, different from invertebrates, mitochondrial genomes in invertebrates such as Brachiopoda are more variable, even within species [8].
The phylum Brachiopoda consists of distinctive members of a marine invertebrate with approximately 404 extant species [9]. Having appeared in the early Cambrian period, this phylum has a long evolutionary history and a complete fossil record through extinction events on Earth [10]. Conservation characteristics and long geological records make brachiopods model animals for multiple studies, especially evolutionary systematics [11]. The studies on the evolutionary history of brachiopods are usually performed based on their fossil records, and a few studies are based on molecular approaches [12]. The application of molecular markers can provide new insights into the genetic diversity and historical processes of brachiopods [13]. Lingula anatina is widely found in the Indo-Western Pacific region and is one of the most studied organisms among the brachiopods [13,14]. There is a report that limited changes have occurred in L. anatina compared to its ancestor in the Cambrian period [15]. However, molecular markers, such as mitochondrial DNA and elongation factor 1α (ef1α) vary among sampling sites [13,14,16,17]. In this case, complete mitogenome analysis might provide more comprehensive information about genetic variation and the evolutionary history of L. anatina.
To date, a limited number of mitochondrial genomes of brachiopods have been sequenced and analyzed. For L. anatina, previous studies reported an unusual mitogenome of larger size, more extended repetitive sequences and more tRNA genes compared to other groups [18]. Additionally, comparisons of L. anatina mitogenome genes indicated high levels of variation among different localities [8,19]. This variation resulted in the speculation that there are cryptic species from various surveyed sites [8]. Therefore, further studies on the Lingula mitogenome will shed more light on mitogenome diversity, species identification and the evolution of this brachiopod. The present study aimed to investigate mitogenome variation and the evolutionary history of L. anatina. For this purpose, the complete mitogenomes of L. anatina from Korea and Vietnam were sequenced and compared to previous records from Korea and Japan. A phylogenetic tree was constructed based on mitogenome sequences to elucidate the relationships among L. anatina from different localities. Additionally, cox1-based phylogenetic analyses were performed to investigate the relationships of L. anatina from a broad geographical range in the western Pacific region.
2. Results and Discussion
2.1. Mitogenome Structure
The mitogenomes lengths were 24,875 and 25,305 bp, and they were submitted to GenBank with accession numbers MW528457 and MH371361 for specimens from Buan, Korea, and Doson, Vietnam, respectively (Figure S1). The Buan mitogenome was almost identical to the previously recorded specimen from Incheon, Korea (KX774482). Only two complete mitogenomes belonging to L. anatina have been previously recorded from Incheon, Korea (KX774482) and Yanagawa, Japan (AB178773) that are 24,876 and 28,818 bp in length. One more L. anatina mitogenome was acquired from Amami Island, Japan (KP881498), but it was a partial genome (17,970 bp). All the mitogenome sequences examined to date have revealed that the mitogenome of L. anatina is extraordinary long for that of a metazoan.
A typical metazoan mitogenome consists of 13 protein-coding genes (PCGs), 22 tRNA genes and two rRNA genes [1]. However, 13 PCGs, 34 tRNA genes and two rRNA genes were identified in the mitogenome of the Buan specimen (Table 1), and 13 PCGs, 31 tRNA genes and two rRNA genes were identified in the mitogenome of the Doson specimen (Table 2). The main difference between the two records was caused by the number of tRNA-Gly genes. While there were ten tRNA-Gly genes in the Buan specimen, there were eight genes in the Doson specimen. The nucleotide distribution of the L. anatina mitoge-nome was 26.3%, A, 21.3% G, 15.9 % C and 36.5% T for the Buan specimen. Meanwhile, the nucleotide distribution was 26.1% A, 21.5% G, 15.8% C and 36.6% T for the Doson specimen. These values are slightly different from those of the Yanagawa specimen, which contained 26.1% A, 21.9% G, 16.1% C and 35.9% T. The whole genes were encoded on the heavy strands of the two new mitogenomes, which is consistent with the previous records from Incheon, Korea and Yanagawa, Japan [18,19]. Although the number of coding genes was variable, relative orders of PCGs and two rRNA genes were similar in the mitogenomes of L. anatina from Buan, Incheon, Doson and Yanagawa, while the Amami Island mitogenome is different from these sequences. Overall, mitogenomes from Buan, Doson and Incheon had identical positions for the first nine genes, from cox1 gene to tRNA-Leu (Figure 1). After tRNA-Leu, Doson and Yanagawa mitogenomes shared four more tRNA genes, including two tRNA-Met genes, tRNA-Val and tRNA-Gln. Similarly, if counted from the right direction (tRNA-Cys), gene positions were identical for these sequences from tRNA-Cys to atp6. The gene rearrangements occurred in the position between tRNA-Leu and atp8 gene. A number of events such as duplication, translocation, insertion and deletion may result in this rearrangement (Figure 1).

Table 1.
Structure and orientations of Lingula anatina complete mitochondrial genome genes collected from Buan, Korea (MW528457).

Table 2.
Structure and orientations of Lingula anatina complete mitochondrial genome genes collected from Doson, Vietnam (MH371361).

Figure 1.
Gene organizations of Lingula anatina mitogenomes from Buan-Incheon (Korea), Doson (Vietnam), Yanagawa and Amami Island (Japan). Color indicators represent different groups: Yellow: Protein-coding genes, Green: tRNA genes, Orange: rRNA genes.
There were 45 intergenic sequences in the mitogenome of Buan specimen and 41 intergenic sequences in the mitogenome of the Doson specimen. The ranges of intergenic sequences varied from 1 to 1633 bp in the Buan specimen and from 1 to 1628 bp in the Doson specimen. On the other hand, there was no overlapping region identified within the two mitogenomes. The GC skewness of the mitogenomes was slightly positive for both new sequences of L. anatina, indicating a higher content of G than C; AT skewness had slightly negative values indicating a higher occurrence of T than A (Figure S2). This finding is consistent with the mitogenome sequence reported in the previous study [18,19].
2.2. Protein-Coding Genes
The PCGs of Buan and Doson L. anatina were 11,919 and 11,952 bp in length, constituting 47.9% and 47.2% of their total mitogenomes, respectively. By comparison, the relative rearrangements of PCG positions were similar in all the complete mitogenome records (MW528457, KX774482, MH371361 and AB178773,). However, although the Amami Island sequence KP881498 is incomplete, this record has 12 protein-coding genes (without atp8), and its gene order is dramatically different. The previous study already mentioned that this specimen could belong to a cryptic species [8]. The authors also suggested improving record numbers from different localities to resolve this unclear point. Our comparison between Buan, Doson and Yanagawa specimens revealed that there is no dramatic difference in PCG structures or orientations in L. anatina mitogenomes. However, the sizes of the PCGs were slightly variable among the L. anatina records (Table S1).
Most of PCGs in the mitogenomes of the Buan and Doson specimens used AT- codons for initiation. Only cox1 and cox3 in the Buan specimen and cox3 and nd4l in the Doson specimen used GTC as a start codon. TAA and TAG were only two common termination codons in the mitogenomes of L. anatina. In the Buan specimen, TAA was used by the genes atp6, atp8, cox2, cox3, nd1 and nd2, while TAG was used by the genes cox1, cytb, nd3, nd4, nd4l, nd5 and nd6. In the Doson specimen, TAA was used by the genes atp6, atp8, cox1, cox3, nd1, nd2 and nd4, while TAG was used by the genes cox2, cytb, nd4l, nd3, nd5 and nd6. The amino acids corresponding to the codons of PCGs were also analyzed (Figure 2). According to codon usage analysis, the most used amino acids were Leu and Ser, while the least-used amino acid was Gln.
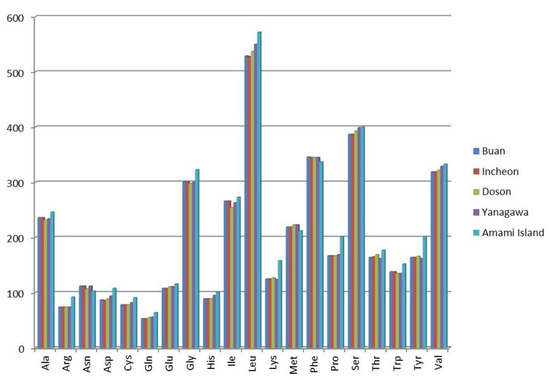
Figure 2.
Amino acids corresponding to the codons of protein-coding genes of the Lingula anatina specimens. The specimens represented with localities: Buan, Korea (MW528457), Incheon, Korea (KX774482), Doson, Vietnam (MH371361), Yanagawa, Japan (AB178773) and Amami Island, Japan (KP881498).
Furthermore, the nonsynonymous (Ka) and synonymous (Ks) substitution rates in PCGs of the specimens were calculated to test the idea of evolutionary rate differences in mitochondrial genomes since the evolutionary rates of mitochondrial genomes have proven to be correlated with speciation rates [20,21,22]. Due to Yanagawa specimen having two atp8 genes and Amami Island specimen having no atp8 gene, this gene was excluded for the Ka-Ks substitution rate analysis. Additionally, two mitogenomes of Korean specimens were almost identical, the newly sequenced mitogenome from Buan was used for the calculation. The comparison among Buan, Doson and Yanagawa specimens showed that Ka values ranged from 0 to 0.087 and Ks values ranged from 0.032 to 1.098 (Table S2). In addition, Ka values between Doson and Yanagawa specimens were the smallest for all genes, except nd4l gene. The Ka values from these comparisons were smaller than the values obtained from the comparison between specimens from Yanagawa and Amami Island, Japan [8].
Comparing the complete mitogenome sequences from Buan and Doson to the mitogenome sequence (KP881498) from Amami Island, Japan showed high values for both Ka (0.059–0.531) and Ks (0.153–4.329) (Table S2). This is consistent with the analysis in a previous study that compared two mitogenomes of L. anatina collected from Yanagawa and Amami Island [8]. Luo et al. (2015) revealed that Ka values between these two specimens varied from 0.078 (cox1) to 0.357 (nd5). Additionally, compared to other invertebrate mitogenomes, these values were comparable to interspecific variation [8]. Due to the high Ka and Ks, the authors had doubts about the species identification and suggested that the specimen collected from Amami Island may belong to a different species. Our finding here supported the finding by Luo et al. (2015) and further study should be performed to confirm the existence of cryptic L. anatina from Amami Island.
The calculation of Ka/Ks ratios among Buan, Doson and Yanagawa that all values were less than 1 for all three mitogenome comparisons (Table S2, Figure S3). The obtained results revealed purifying selection in L. anatina from these localities. Comparing Buan-Amami Island mitogenomes and Doson-Amami Island mitogenomes also showed a similar pattern, except for cox2 and nd4 genes with Ka > Ks (Table S2).
2.3. Transfer RNAs and Ribosomal RNAs
There were 34 tRNA genes in L. anatina mitogenome from Buan that were 52–72 bp in length. For the L. anatina mitogenome from Doson, there were 31 tRNA genes with the absence of tRNA-Lys. tRNAs of the Doson mitogenome also ranged from 52 to 72 bp. The number of tRNAs was variable in the L. anatina records. The number of tRNA genes was 27 for the complete mitogenome from Yanagawa, Japan and 23 for the incomplete mitogenome from Amami Island, Japan. It is worth noting that the number of tRNA genes in the Yanagawa mitogenome is possibly higher than 27. In addition to 27 tRNA genes, previous study reported tRNA-like sequences in this mitogenome [18]. The search for tRNA genes of the Yanagawa mitogenome with the prediction program ARWEN suggested a number of tRNA-like sequences could be real tRNA genes [23].
Twenty-two tRNAs with repetition of tRNA-Leu and tRNA-Ser are seen in typical metazoan mitochondrial genomes [24]. The mitogenomes of L. anatina are atypical regarding tRNAs. All four records showed some variation in the repetition of tRNAs (Figure S1). In the Buan specimen, tRNA-Gly was repeated ten times, while in the Yanagawa specimen it was seen only one time. Besides, the number of tRNA-Trp and tRNA-Met in L. anatina mitogenome were also higher than those of typical animal mitogenome. In addition, tRNA structures also showed variety for the tRNA-Ser. Typically, the dihydrouridine arm of all the tRNAs is a large loop instead of a conserved stem structure in the metazoan mitochondrial genomes, excluding tRNA-Ser. In most tRNA-Ser for AGY/N codons lack the dihydrouridine arm in the metazoan [25,26,27,28]. However, the dihydrouridine arms were 3 to 4 bp long in the L. anatina mitogenomes. In addition, the amino acid arms and the TΨC arms varied in length from 4 to 5 bp in all of the analyzed L. anatina mitogenome tRNA genes.
There were two ribosomal RNA genes observed in the mitogenome of all the L. anatina specimens. They were all encoded on the major strand. The size of 16S rRNAs varied from 1299 to 1483 bp with 56.9% to 61.7% A–T content. The shortest 16S rRNA was observed in the incomplete mitogenome from Amami Island, Japan. Apart from this incomplete sequence, comparing only complete mitogenomes indicated that 16S rRNAs were almost identical, at 1462 to 1465 bp in length. In 12S rRNA, a similar pattern also occurs. The 12S rRNA of the complete mitogenomes of L. anatina were almost identical, at 1270 to 1271 bp in length.
2.4. Non-Coding Region
Non-coding regions of the mitogenome were confirmed with primer sets listed in Table S3. Of 12 amplified sequences, nine sequences had similar sizes with NGS data, while three sequences had shorter sizes than NGS data (Table S3). The shorter sizes in obtained sequences were caused by incorrect repetitive elements in the assembly of NGS data. The final non-coding regions were assembled with sequences generated by PCR and Sanger sequencing.
The comparison of the non-coding region among localities is presented in Table S4. The size, repeat and potential open reading frame in non-coding regions were variable for the mitogenomes from Korea, Vietnam and Japan. In total, there were 45 non-coding regions in the Buan mitogenome and 41 non-coding regions in the Doson mitogenome (Tables S5 and S6). The largest non-coding region of the Buan mitogenome was 1633 bp in length and located between tRNA-Met and tRNA-Val (Table S5). The largest non-coding region of the Doson mitogenome was 1628 bp in length and located between tRNA-Gln and tRNA-Met (Table S6). Meanwhile, the largest non-coding region of the Yanagawa mitogenome was up to 4017 bp and located between tRNA-Ser and atp8 genes.
In total, six unassigned repeated sequences (URSs), were observed in the non-coding region of the Buan mitogenome with lengths from 31 to 223 bp (Table S7). Additionally, seven URSs were observed in the non-coding region of the Doson mitogenome, with lengths from 53 to 223 bp (Table S8). The numbers of URSs in the Buan and Doson mitogenomes are smaller than those in the Yanagawa mitogenome and the lengths of repeated sequences are also different (Table S4). The longest URS in the Yanagawa mitogenome was 1092 bp, whereas the longest in the Buan and Doson mitogenome was 223 bp.
Similar to the Yanagawa mitogenome, the repeated sequences in Buan and Doson mitogenomes were mainly located in Repeat region I: cox2-tRNA-Val and Repeat region II: cytb-atp6. The details of comparison between the Buan and Doson mitogenomes are presented in Figure S4. In Repeat region I, even though each mitogenome contained two URSs, their locations are different. The pseudo-gene and tandem repeat observed in the Yanagawa mitogenome was not found in the Buan and Doson mitogenomes. In addition to URS, one and two unassigned unique sequences were found in Repeat region I of the Buan and Doson mitgenomes, respectively. Compared to Repeat region I, more URSs was observed in Repeat region II for both mitgenomes. There were four URSs in the Buan mitogenome and five URSs in the Doson mitogenomes observed in this. As shown in Figure S4, URSs in both mitogenomes were mostly located in the second half of Repeat region II.
Additionally, a number of ORFs were found in the Buan and Doson mitogenomes (Tables S9 and S10). There were 15 ORFs in the Buan mitogenome that varied from 156 to 396 bp. The Doson mitogenome included 17 ORFs with the length of 153 to 492 bp. The translated amino acid sequences from ORFs are presented in Figure S5. ORFs in L. anatina may encode for amino acid sequences with unassigned function [18].
2.5. Phylogenetic Studies
Bayesian inference and maximum likelihood phylogenetic analyses were performed with five L. anatina and two Rhynchonelliformea as representatives of outgroup records (Figure 3). The results of two maximum likelihood and Bayesian inference analyses based on nucleotide datasets of 12 PCGs (atp8 excluded) are similar (Figure 3). Additionally, analyses suggested that the Vietnamese specimen has a sister relationship with the Yanagawa specimen, and the closest relatives to this clade were two Korean specimens that are almost identical. The phylogenetic tree also indicated that the Korean specimens diverged early from the Doson and Yanagawa specimens. Additionally, phylogenetic analysis showed that although the Amami Island specimen has a relationship with other L. anatina specimens, the distance is far in comparison with the relationships of the other records. These findings may suggest that there is a cryptic species among complete mitogenomes of L. anatina [8].
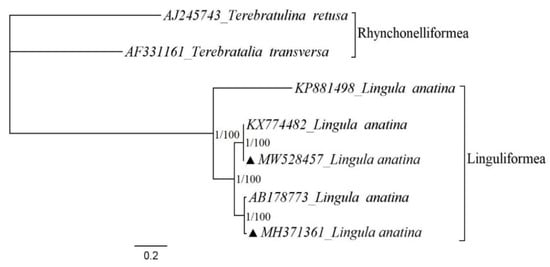
Figure 3.
Phylogenetic relationships of Lingula anatina specimens from different localities based on mitochondrial protein-coding genes. The complete mitochondrial genomes for reconstruction of phylogenetic tree retrieved from GenBank and the subphylum Rhynchonelliformea records were chosen as representatives of the outgroup. The triangle shows the records sequenced for the present study. The numbers at the internodes are bootstrap values of Maximum likelihood (right) and posterior probability values of Bayesian inference (left).
In addition, 37 partial cox1 sequences (MW519149-MW519185), including 25 specimens from Buan and 12 specimens from Incheon, were generated to investigate the relationships among L. anatina from different localities in the western Pacific region. The phylogenetic analyses suggested that the Korean specimens formed a cluster. These specimens were collected from two localities of the eastern side of the Yellow sea that were positioned close to a Chinese specimens. The phylogenetic tree also revealed that the Yanagawa specimen had a sister relationship with the Doson specimen (Figure S6). Meanwhile, Amami Island and Hong Kong specimens are distant from sequences of the remaining localities. The genetic separation of species is mainly explained by several historical and contemporary processes, such as genetic drift, natural selection, population isolation and range expansion [29,30]. In a previous study, two distinct lineages in Scomber japonicus populations representing Chinese and Japanese types were observed based on the cytb gene marker [30]. Future studies are necessary to clarify the mechanisms of genetic variations of L. anatina in the western Pacific region.
The phylogenetic trees also revealed that specimens from different localities formed different clusters. This finding suggests distinct phylogeographic differentiation, and there are different groups of L. anatina. The genetic variations of L. anatina among localities were observed in previous studies based on several markers, such as cox1, ef1α and 18S rRNA [8,17,18]. Analyses of partial cox1 and ef1α sequences demonstrated that genetic distances of L. anatina from different localities were relatively high and that there were close relationships between East and South China Sea specimens [18]. Our study supports the recent genetic studies that consider L. anatina to not be an unchanging species [13,18].
Both complete mitogenome and partial cox1 gene-based analyses showed that the specimen from Amami Island is distant from the other L. anatina specimens. The pattern of phylogenetic trees is congruent with Ka and Ks analyses, which resulted in high Ka and Ks values when comparing the Buan, Doson and Yanagawa mitogenomes with the Amami Island mitogenome. Recent investigations showed that the breeding season of L. anatina at Amami Island is also different from those of other groups in Japan [31]. The evidence supported the suggestion that the Amami Island specimen is a cryptic species. Taxonomical study in the future study is required to clarify the species complex of L. anatina.
3. Material and Methods
3.1. Sampling and DNA Sequencing
The L. anatina specimens were collected from Incheon, Korea (37°26′53″ N, 126°22′13″ E) in February 2016, Buan, Korea (35°34′55.87″ N, 126°30′52.56″ E) in July 2019) and Doson, Vietnam (20 46′16,22″ N, 106° 40′05,93″ E) in September 2016 (Figure 4). The collected specimens were deposited at the Department of Biotechnology, Sangmyung University, Korea University; 97% ethanol preservation. Procedures to obtain partial cox1 sequences were performed following a previous study [16]. One individual from each Buan (Korea) and Doson (Vietnam) specimens was chosen for mitogenome sequencing. The Buan specimen was collected approximately 200 km away from the previously recorded Incheon specimen [19]. Briefly, the mitochondrial DNA was isolated using a mitochondrial DNA isolation kit (Biovision, Milpitas, CA, USA) according to manufacturer’s recommendations. Isolated DNA was measured for purity using the DropSense96 UV/VIS droplet reader (Trinean, Gentbrugge, Ghent, Belgium). The quantification of the DNA concentration was performed using Quanti-IT PicoGreen dsDNA kit (Invitrogen, Carlsbad, CA, USA), and the integrity of extracted DNA was verified using an agarose gel. A total of 200 ng of mitochondrial DNA was sheared using S220 Ultrasonicator (Covaris, Woburn, MA, USA). Library preparation was performed with an Illumina Truseq Nano DNA sample prep kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocols. Finally, the quality and the band size of libraries were assessed using Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA, USA). Libraries were quantified by qPCR using CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). After normalization, sequencing of the prepared library was conducted on Miseq system (Illumina, San Diego, CA, USA) with 300 bp paired-end reads.
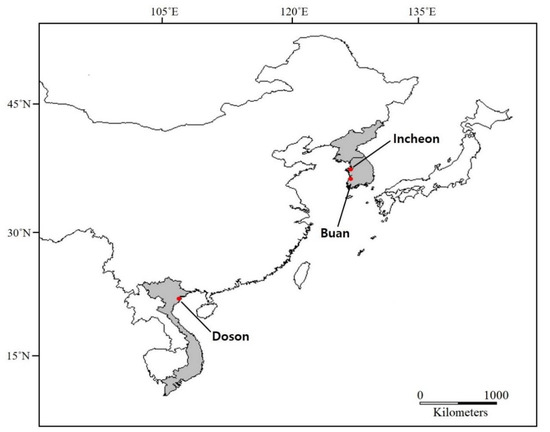
Figure 4.
Map shows sampling localities in this study: Buan and Incheon (Korea) and Doson (Vietnam).
3.2. Analysis of Mitochondrial DNA
Mitochondrial genes were assembled and annotated by MITObim software and MITOS web server [32,33]. PCGs and rRNA genes were also confirmed by BLAST searches in GenBank by alignment with homologous genes from other L. anatina species. Transfer RNA genes were identified by comparing the results predicted by tRNAscan-SE Search Server v.1.21 and ARWEN based on cloverleaf secondary structure information [23,34]. The secondary structures of ribosomal RNAs were predicted by the LocARNA webserver [35]. The annotation of mitogenome sequences was manually refined using Geneious software version 9.1.2 [36].
Due to repeated sequences in non-coding regions of L. anatina mitogenome, 12 overlapping primer sets were designed to confirm these sequences (Table S3). The PCR mixture was prepared, containing 10 μL of 2× TOPsimpleTM DyeMIX-Tenuto (Enzynomics, Daejeon, Korea), 1 μL of each primer (10 pmoles/μL), 100 ng of DNA, and distilled water to make the mixture 20 μL. The amplification procedure was performed as: 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 50 °C for 1 min, extension at 72 °C for 1 min, and final elongation at 72 °C for 5 min. The PCR products were run with electrophoresis in 1% agarose gels in 1× TAE buffer. Sequencing was conducted using an ABI 3730 DNA Analyzer (Applied Biosystems, Waltham, MA, USA).
L. anatina mitogenome records from Korea and Japan were retrieved from GenBank for further analyses. Gene orders were reoriented as starting from cox1 gene. Furthermore, non-synonymous (Ka) and synonymous (Ks) substitution rates were computed with the Ka/Ks calculator (v2.0) [37]. The repeated sequences and open reading frames were searched in Geneious software version 9.1.2 [36]. Unassigned unique sequence was searched for following previous study [18].
3.3. Phylogenetic Analyses
To better resolve the molecular phylogeny of L. anatina, the phylogenetic tree of L. anatina based on mitogenomes was reconstructed using previous records and two new sequences, including MW528457 (Buan, Korea), KX774482 (Incheon, Korea), MH371361 (Doson, Vietnam), AB178773 (Yanagawa, Japan), KP881498 (Amami Island, Japan). Among them, only KP881498 is not a complete mitochondrial genome, and these are the entire mitogenome records belonging to the subphylum Linguliformea. Additionally, two mitogenomes from rhynchonelliformean species, Terebratalia transversa (AF331161) and Terebratulina retusa (AJ245743) were selected as an outgroup. Since the atp8 gene is not stable in the records, it has been excluded, and only sequences of 12 protein-coding genes were used in phylogenetic analysis. For further investigation, partial cox1 sequences from L. anatina specimens were generated to investigate the cox1-based phylogenetic relationships. Furthermore, cox1 sequences of Lingula species were retrieved from GenBank for analysis. The best fit model for the cox1 dataset was searched with jModeltest [38]. Meanwhile, the model and partitioning of the complete mitogenome dataset were determined with Partition Finder 2 [39]. The phylogenies were reconstructed with the Bayesian inference method using Mrbayes version 3.2.7a [40] and the maximum likelihood method using RAxML version 8.0.0 [41]. For maximum likelihood method, the GTR + G model was used for each analysis and analyzed with 1000 bootstrap replicates. For the Bayesian inference method, four simultaneous Markov chains were run for ten million generations, with tree sampling occurring every 100 generations and a burn-in of 25% of the trees. The convergence of chain was implied by ESS > 200 in Tracer version 1.7 [42]. The obtained tree was visualized in FigTree v.1.4.3 [43].
4. Conclusions
In this study, the complete mitogenomes of L. anatina collected from Korea and Vietnam were sequenced to investigate mitogenome structures and genetic variations of the species in the western Pacific region. The mitogenomes of L. anatina are variable in gene order, sizes of PCGs, and the number of tRNAs. Phylogenetic trees based on PCGs of mitogenomes and partial cox1 sequences showed that L. anatina was divided into distinct clades, and the most distant clade was the incomplete mitogenome from Amami Island, Japan. This result is consistent with a previous investigation of L. anatina among various geographical localities. In future studies, broader geographical collection and additional genetic makers are recommended to provide comprehensive genetic variations of L. anatina.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10050367/s1, Figure S1: Mitochondrial genomes of Lingula anatina collected from Buan, Korea (A) and Doson, Vietnam (B), Figure S2: AT and GC skew values in the mitochondrial genome records of Lingula anatina specimens, Figure S3: Calculations of nonsynonymous and synonymous Ka/Ks ratio of protein-coding genes of Lingula anatina specimens from Buan, Doson and Yanagawa, Figure S4: Structures of Repeat regions I and II of Lingula anatiana mitogenomes from Buan and Doson, Figure S5. Amino acid sequences encoded by unassigned open reading frames in non-coding regions of the Buan mitogenome (BO1-BO15) and Doson mitogenome (DO1-DO17), Figure S6: Phylogenetic relationships of Lingula species based on partial sequences of the mitochondrial cox1 genes, Table S1: Protein-coding gene sizes in the Lingula anatina specimens, Table S2. The nonsynonymous and synonymous substitutions (Ka and Ks) estimated based on 12 protein-coding genes of Lingula anatina from Korea, Vietnam and Japan, Table S3: Primers and PCR products used for confirmation of non-coding regions of Lingula anatina from Buan, Korea, Table S4: Comparison of non-coding regions of Lingula anatina mitogenomes from different localities, Table S5: The non-coding regions of Lingula anatina from Buan, Table S6: The non-coding regions of Lingula anatina from Doson, Table S7: Unassigned repeated sequences found in non-coding regions of Lingula anatina from Buan, Korea, Table S8: Unassigned repeated sequences found in non-coding regions of Lingula anatina from Doson, Vietnam, Table S9: Unassigned open reading frames (≥50 aa) found in non-coding regions of Lingula anatina from Buan, Korea, Table S10: Unassigned open reading frames (≥50 aa) found in non-coding regions of Lingula anatina from Doson, Vietnam.
Author Contributions
Conceptualization, M.Z.K. and C.-B.K.; resources, T.D.D. and S.-G.K.; methodology, M.Z.K., T.D.D., J.-I.K., T.-J.C., S.-G.K. and C.-B.K.; software, T.D.D., J.-I.K., T.-J.C.; investigation, J.-I.K., T.-J.C. and S.-G.K.; data curation, T.D.D., J.-I.K., T.-J.C. writing—original draft preparation, M.Z.K., T.D.D. and C.-B.K.; writing—review and editing, M.Z.K. and C.-B.K.; supervision, C.-B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Marine Biotechnology Program (20170431) and a grant (Grant PM 59120) by the Ministry of Oceans and Fisheries, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in NCBI GenBank (Accession number: MW528457 and MH371361).
Acknowledgments
Authors are grateful to Duri Lee and Hyung-Eun An for their support in lab works.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, D.X.; Hewitt, G.M. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997, 25, 99–120. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Curole, J.P.; Kocher, T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999, 14, 394–398. [Google Scholar] [CrossRef]
- Lin, C.P.; Danforth, B.N. How do insect nuclear and mitochondrial gene substitution patterns differ? Insights from Bayesian analyses of combined datasets. Mol. Phylogenet. Evol. 2004, 30, 686–702. [Google Scholar] [CrossRef]
- Avise, J.C. Mitochondrial DNA polymorphism and a connection between genetics and demography of relevance to conservation. Conser. Biol. 1995, 9, 686–690. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Luo, Y.J.; Satoh, N.; Endo, K. Mitochondrial gene order variation in the brachiopod Lingula anatina and its implications for mitochondrial evolution in lophotrochozoans. Mar. Genomics 2015, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Emig, C.C.; Álvarez, F.; Bitner, M.A. Brachiopoda Database. Available online: http://paleopolis.rediris.es/brachiopoda_database/ (accessed on 1 February 2021).
- Harper, D.A.; Popov, L.E.; Holmer, L.E. Brachiopods: Origin and early history. Paleontology 2017, 60, 1–24. [Google Scholar] [CrossRef]
- Merkel, C.; Deuschle, J.; Griesshaber, E.; Enders, S.; Steinhauser, E.; Hochleitner, R.; Brand, U.; Schmahl, W.W. Mechanical properties of modern calcite-(Mergerlia truncata) and phosphate-shelled brachiopods (Discradisca stella and Lingula anatina) determined by nanoindentation. J. Struct. Biol. 2009, 168, 396–408. [Google Scholar] [CrossRef]
- Sperling, E.A.; Pisani, D.; Peterson, K.J. Molecular paleobiological insights into the origin of the Brachiopoda. Evol. Dev. 2011, 13, 290–303. [Google Scholar] [CrossRef]
- Yang, S.; Lai, X.; Sheng, G.; Wang, S. Deep genetic divergence within a “living fossil” brachiopod Lingula anatina. J. Paleontol. 2013, 87, 902–908. [Google Scholar] [CrossRef]
- Endo, K. The phylogenetic position of brachiopods inferred from mitochondrialgene orders. In Brachiopods: Past and Present. Systemic Association Special Volume; Brunton, H., Cocks, R., Long, S., Eds.; Taylor & Francis: London, UK, 2001; pp. 129–137. [Google Scholar]
- Emig, C.C. Proof that Lingula (Brachiopoda) is not a living fossil, and emended diagnoses of the family Linulidae. Carnets Geol. Noteb. Geol. 2003. [Google Scholar] [CrossRef]
- Kim, S.G.; Karagozlu, M.Z.; Kim, C.B. Phylogenetic investigations of Lingula anatina among some northwestern Pacific populations, based on mitochondrial DNA cytochrome c oxidase subunit I gene. J. Asia Pac. Biodivers 2017, 10, 162–166. [Google Scholar] [CrossRef]
- Nishizawa, A.; Sarashina, I.; Tsujimoto, Y.; Iijima, I. Artificial fertilization, early development and chromosome numbers in the brachiopod Lingula anatina. Palaeontology 2010, 84, 309–316. [Google Scholar]
- Endo, K.; Noguchi, Y.; Ueshima, R.; Jacobs, H.T. Novel repetitive structures, deviant protein-encoding sequences and unidentified ORFs in the mitochondrial genome of the brachiopod Lingula anatina. J. Mol. Evol. 2005, 61, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Karagozlu, M.Z.; Kim, S.G.; Thinh, D.D.; Kim, C.B. Complete mitochondrial genome analysis of Lingula anatina from Korea (Brachiopoda, Lingulida, Lingulidae). Mitochondrial DNA B Resour. 2017, 2, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.H.; DeWoody, J.A. Evolutionary rates of mitochondrial genomes correspond to diversification rates and to contemporary species richness in birds and reptiles. Proc. Biol. Sci. 2010, 277, 3587–3592. [Google Scholar] [CrossRef]
- Wu, X.; Xu, X.; Yu, Z.; Wei, J.; Xia, J. The relationship between the rate of molecular evolution and the rate of genome rearrangement in animal mitochondrial genomes. J. Mol. Evol. 2006, 63, 375–392. [Google Scholar]
- Sun, Y.B.; Shen, Y.Y.; Irwin, D.M.; Zhang, Y.P. Evaluating the roles of energetic functional constraints on teleost mitochondrial-encoded protein evolution. Mol. Bio. Evol. 2011, 28, 39–44. [Google Scholar] [CrossRef]
- Laslett, D.; Canback, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef]
- Yang, H.; Xia, J.; Zhang, J.E.; Yang, J.; Zhao, H.; Wang, Q.; Sun, J.; Xue, H.; Wu, Y.; Chen, J.; et al. Characterization of the complete mitochondrial genome sequences of three croakers (perciformes, sciaenidae) and novel insights into the phylogenetics. Int. J. Mol. Sci. 2018, 19, 1741. [Google Scholar] [CrossRef]
- Wolff, M.; Uribe, A.; Ortiz, A.; Duque, P. A preliminary study of forensic entomology in Medellin, Colombia. Forensic Sci. Int. 2001, 120, 53–59. [Google Scholar] [CrossRef]
- Yamauchi, M.M.; Miya, M.U.; Nishida, M. Complete mitochondrial DNA sequence of the swimming crab, Portunus trituberculatus (Crustacea: Decapoda: Brachyura). Gene 2003, 311, 129–135. [Google Scholar] [CrossRef]
- Ki, J.S.; Dahms, H.U.; Hwang, J.S.; Lee, J.S. The complete mitogenome of the hydrothermal vent crab Xenograpsus testudinatus (Decapoda, Brachyura) and comparison with brachyuran crabs. Comp. Biochem. Physiol. Part D Genom. Proteom. 2009, 4, 290–299. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173. [Google Scholar] [PubMed]
- Ye, Y.; Li, J.; Wu, C. Genetic diversity and population connectivity of the Asian green mussel Perna viridis in South China Sea, inferred from mitochondria DNA markers. Biochem. Syst. Ecol. 2015, 61, 470–476. [Google Scholar] [CrossRef]
- Yan, S.; Catanese, G.; Brown, C.L.; Wang, M.; Yang, C.; Yang, T.B. Study on the chub mackerel (Scomber japonicus) in the northwestern Pacific indicates the late Pleistocene population isolation. Marine Ecol. Evol. Perspect. 2015, 36, 753–765. [Google Scholar] [CrossRef]
- Fujii, R.; Ueno, R.; Yamamoto, T. Breeding season and life history of Lingula anatina after settlement in Amami-Oshima Island, Kagoshima, Japan. Plankton Benthos Res. 2019, 14, 45–51. [Google Scholar] [CrossRef]
- Hahn, C.; Bachmann, L.; Chevreux, B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads-abaiting and iterative mapping approach. Nucleic Acids Res. 2013, 41, e129. [Google Scholar] [CrossRef]
- Bernt, M.; Dnonath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucl. Aci. Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Raden, M.; Ali, S.M.; Alkhnbashi, O.S.; Busch, A.; Costa, F.; Davis, J.A.; Eggenhofer, F.; Gelhausen, R.; Georg, J.; Heyne, S. Freiburg RNA tools: A central online resource for RNA-focused research and teaching. Nucleic Acids Res. 2018, 46, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.S.; Yu, J. Kaks_calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinf. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.4.4. Available online: http://tree.bio.ed.ac.uk/softwar (accessed on 1 February 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).