Relationship between Oxygen Defects and Properties of Scandium Oxide Films Prepared by Ion-Beam Sputtering
Abstract
1. Introduction
2. Experimental Details
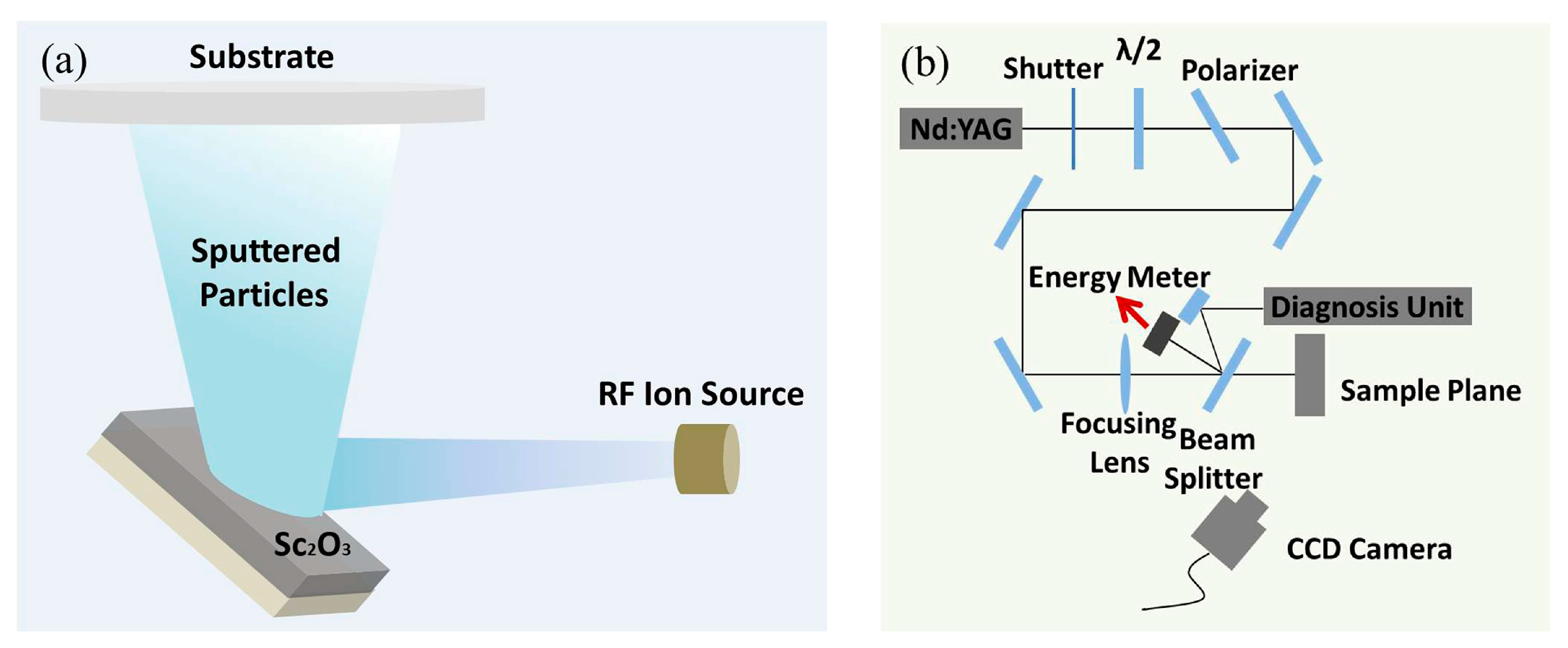
2.1. Sample Preparation
2.2. Film Characterization
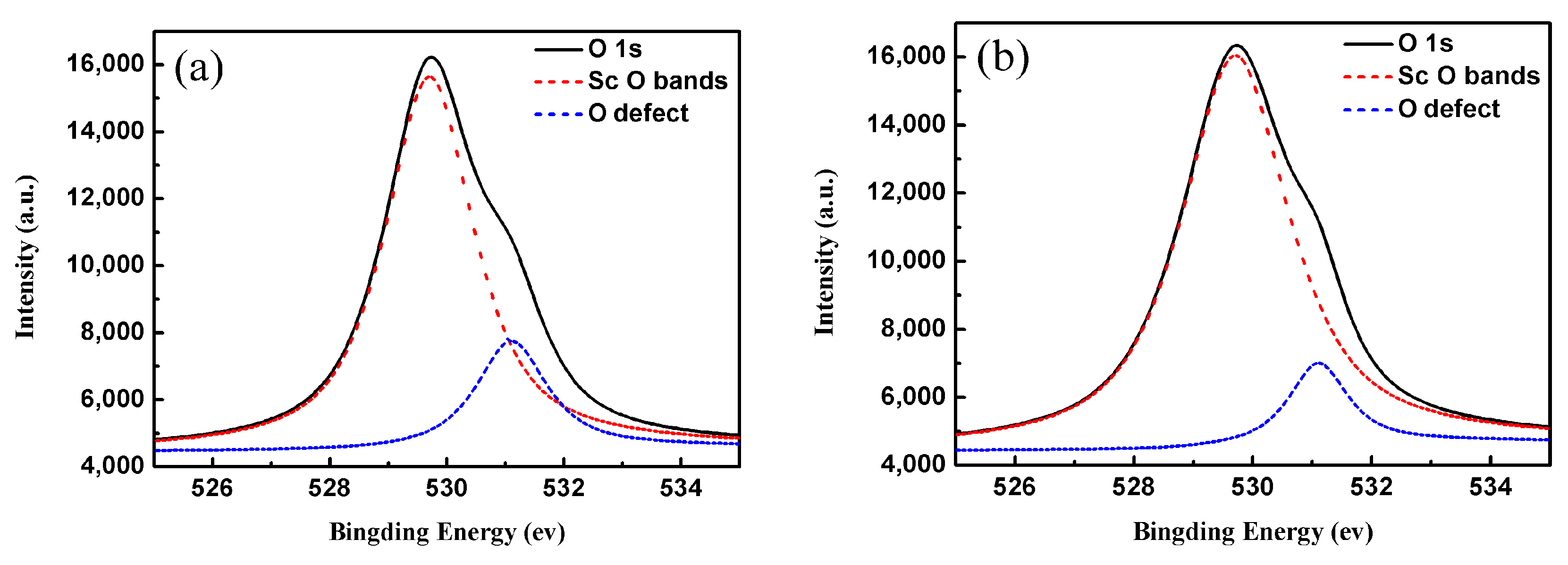
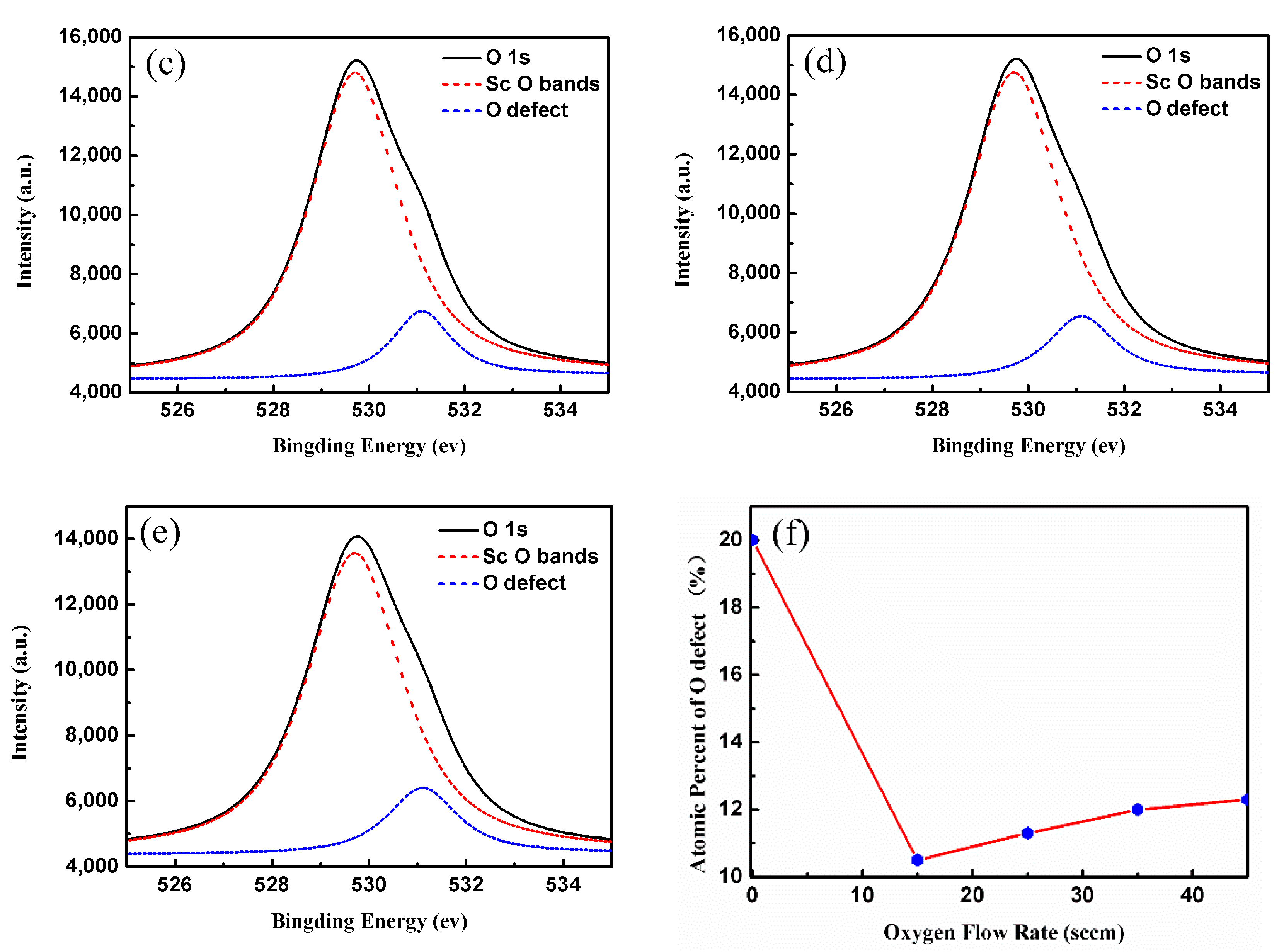
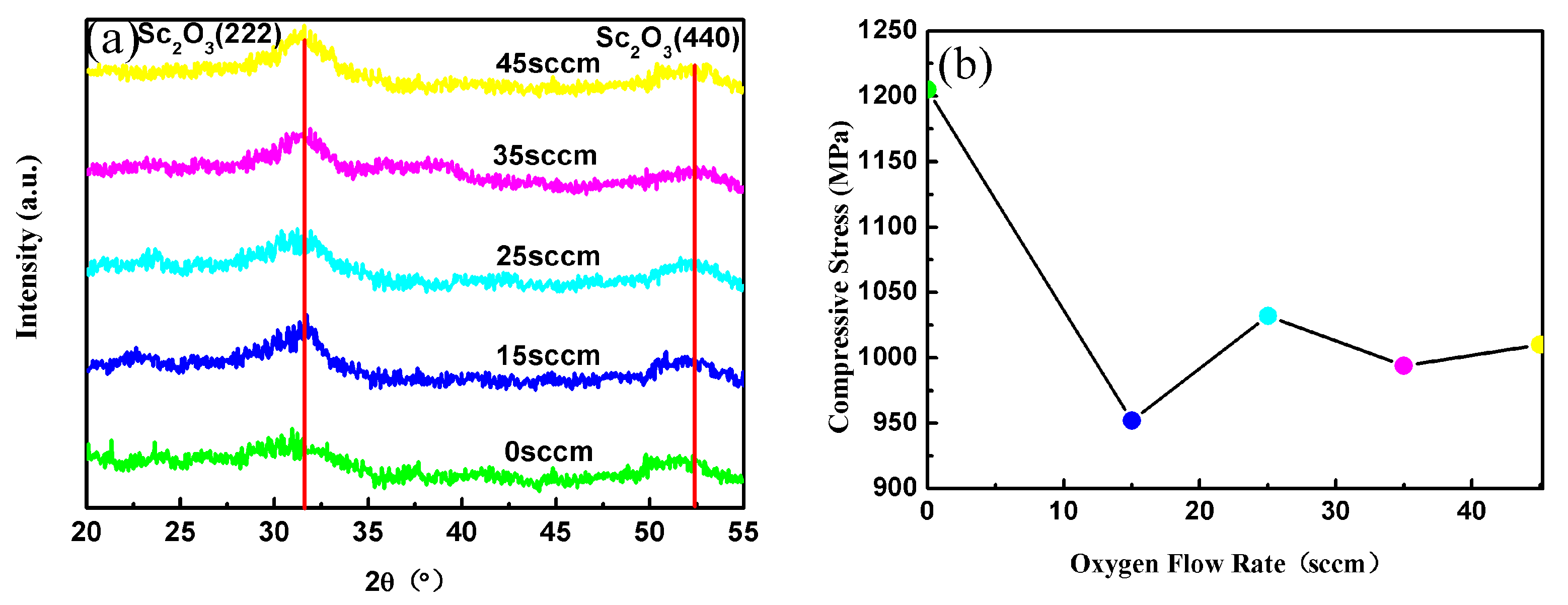
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gschneidner, K.A.; Melson, G.A.; Youngblood, D.H.; Schock, H.H. Scandium its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology; Horovitz, C.T., Ed.; Academic Press: London, UK, 1975. [Google Scholar]
- Rainer, F.; Lowdermilk, W.H.; Milam, D.; Hart, T.T.; Lichtenstein, T.L.; Carniglia, C.K. Scandium oxide coatings for high-power UV laser applications. Appl. Opt. 1982, 21, 3685–3688. [Google Scholar] [CrossRef] [PubMed]
- Langston, P.F.; Krous, E.; Schiltz, D.; Patel, D.; Emmert, L.; Markosyan, A.; Reagan, B.; Wernsing, K.; Xu, Y.; Sun, Z. Point defects in Sc2O3 thin films by ion beam sputtering. Appl. Opt. 2014, 53, A276–A280. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Abdulameer, M.R.; Emmert, L.A.; Day, T.; Patel, D.; Menoni, C.S.; Rudolph, W. Comparison of defects responsible for nanosecond laser-induced damage and ablation in common high index optical coatings. Opt. Eng. 2016, 56, 011019. [Google Scholar] [CrossRef]
- Fu, X.H.; Commandre, M.; Gallais, L.; Mende, M.; Ehlers, H.; Ristau, D. Laser-induced damage in scandium, hafnium, aluminum oxides composites with silica in the infrared. In Proceedings of the Optical Interference Coatings 2013, Whistler, BC, Canada, 16–21 June 2013. [Google Scholar]
- Fu, X.H.; Melninkaitis, A.; Gallais, L.; Kicas, S.; Drazdys, R.; Sirutkaitis, V.; Commandre, M. Measured nanosecond laser damage probabilities of niobia-silica and zirconia-silica mixtures coatings. In Laser-Induced Damage in Optical Materials, Proceedings of SPIE Laser Damage, Boulder, CO, USA, 23–26 September 2012; Exarhos, G.J., Gruzdev, V.E., Menapace, J.A., Ristau, D., Soileau, M.J., Eds.; SPIE: Bellingham, WA, USA, 2012. [Google Scholar]
- Tamura, S.; Kimura, S.; Sato, Y.; Yoshida, H.; Yoshida, K. Laser-damage threshold of Sc2O3/SiO2 high reflector coatings for a laser wavelength of 355 nm. Thin Solid Films 1993, 228, 222–224. [Google Scholar] [CrossRef]
- Hollenbeck, J.L.; Buchanan, R.C. Oxide thin films for nanometer scale electron beam lithography. J. Mater. Res. 1990, 5, 1058–1072. [Google Scholar] [CrossRef]
- Pike, G.E. AC conductivity of scandium oxide and a new hopping model for conductivity. Phys. Rev. B 1973, 6, 1572–1580. [Google Scholar] [CrossRef]
- Heitmann, W. Reactively evaporated films of Scandia and yttria. Appl. Opt. 1973, 12, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, Y.; He, H.; Fan, Z. Effect of substrate temperatures on the optical properties of evaporated Sc2O3 thin films. Thin Solid Films 2010, 518, 2920–2923. [Google Scholar] [CrossRef]
- Adelmann, C.; Lehnen, P.; Elshocht, S.; Zhao, C.; Brijs, B.; Franquet, A.; Conard, T.; Roeckerath, M.; Schubert, J.; Boissière, O. Growth of dysprosium, scandium, and hafnium-based third generation high-k dielectrics by atomic vapor deposition. Chem. Vap. Depos. 2010, 13, 567–573. [Google Scholar] [CrossRef]
- Putkonen, M.; Nieminen, M.; Niinistö, J.; Niinistö, L.; Sajavaara, T. Surface-controlled deposition of Sc2O3 thin films by atomic layer epitaxy using-diketonate and organometallic precursors. Chem. Mater. 2001, 13, 4701–4707. [Google Scholar] [CrossRef]
- Klenov, D.O.; Edge, L.F.; Schlom, D.G.; Stemmer, S. Extended defects in epitaxial Sc2O3 films grown on (111) Si. Appl. Phys. Lett. 2005, 86, 051901. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Daga, A.; Chen, H. Microstructure and optical properties of scandium oxide thin films prepared by metalorganic chemical-vapor deposition. Appl. Phys. Lett. 2001, 79, 3782–3784. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Ivanova, E.V.; Zamoryanskaya, M.V.; Smirnova, T.P.; Yakovkina, L.V.; Gritsenko, V.A. XPS and cathodoluminescence studies of HfO2/Sc2O3 and (HfO2)1−x(Sc2O3)x films. Eur. Phys. J. Appl. Phys. 2013, 64, 10302. [Google Scholar] [CrossRef]
- Liu, C.; Chor, E.F.; Tan, L.S.; Du, A. Epitaxial growth of Sc2O3 films on GaN (0001) by pulsed laser deposition. J. Vac. Sci. Technol. B 2007, 25, 754–759. [Google Scholar] [CrossRef]
- Feijoo, P.C.; Pampillón, M.A.; Andrés, E.; Lucía, M.L. Optimization of scandium oxide growth by high pressure sputtering on silicon. Thin Solid Films 2012, 526, 81–86. [Google Scholar] [CrossRef]
- Hays, D.C.; Gila, B.P.; Pearton, S.J.; Kim, B.J.; Ren, F.; Jang, T. Band offsets in Sc2O3/ZnO heterostructures deposited by RF magnetron sputtering. J. Vac. Sci. Technol. B 2015, 33, 051218. [Google Scholar] [CrossRef]
- Alexandr, B.; Kęstutis, J.; Lukas, C.; Romanas, S.; Sandra, S.; Vitalija, J.; Simonas, K. Correlation between stoichiometry and properties of scandium oxide films prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2018, 427, 312–318. [Google Scholar]
- Wang, S.; Ma, P.; Pu, Y.; Qiao, Z.; Zhang, M.; Lu, Z.; Peng, D. Effect of oxygen flow on the structure and optical properties of the Gd2O3 optical films. In 8th International Symposium on Advanced Optical Manufacturing and Testing Technologies: Advanced Optical Manufacturing Technologies, Proceedings of Eighth International Symposium on Advanced Optical Manufacturing and Testing Technology (AOMATT2016), 26–29 April 2016, Suzhou, China; Jiang, W., Yang, L., Riemer, O., Li, S., Wan, Y., Eds.; SPIE: Bellingham, WA, USA, 2016. [Google Scholar]
- Krous, E.; Patel, D.; Langston, P.; Menoni, C.; Markosyan, A.; Route, R.; Fejer, M.; Nguyen, D.; Emmert, L.; Rudolph, W. Scandium oxide thin films deposited by dual ion beam sputtering for high-power laser applications. In Proceedings of the Optical Interference Coatings 2010, Tucson, AZ, USA, 6–11 June 2010. [Google Scholar]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. The study of polycrystalline nickel metal oxidation by water vapour. J. Electron. Spectrosc. Relat. Phenom. 2009, 175, 55–65. [Google Scholar] [CrossRef]
- Belosludtsev, A.; Houska, J.; Vlcek, J.; Haviar, S.; Cerstvy, R.; Rezek, J.; Kettner, M. Structure and properties of Hf–O–N films prepared by high-rate reactive HiPIMS with smoothly controlled composition. Ceram. Int. 2017, 43, 5661–5667. [Google Scholar] [CrossRef]
- Musil, J.; Zenkin, S.; Kos, S.; Cerstvy, R.; Haviar, S. Flexible hydrophobic ZrN nitride films. Vacuum 2016, 131, 34–38. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlcek, J.; Nam, K.H.; Han, J.G. Reactive magnetron sputtering of thin films: Present status and trends. Thin Solid Films 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Klein, C. How accurate are Stoney equation and recent modifications. J. Appl. Phys. 2000, 88, 5487–5489. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts. J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef]
- Osuwa, J.C.; Oriakuand, C.I.; Kalu, I.A. Variation of optical band gap with post deposition annealing in CdS/PVA thin film. Chalcogenide Lett. 2009, 6, 433–439. [Google Scholar]
- Adam, A.; Lilov, E.; Ibrahim, E.; Petkov, P.; Panina, L.; Darwish, M. Correlation of structural and optical properties in as-prepared and annealed Bi2Se3 thin films. J. Mater. Process. Technol. 2019, 264, 76–83. [Google Scholar] [CrossRef]
- Fursenko, O.; Bauer, J.; Lupina, G.; Dudek, P.; Lukosius, M.; Wenger, C.; Zaumseil, P. Optical properties and band gap characterization of high dielectric constant oxides. Thin Solid Films 2012, 520, 4532–4535. [Google Scholar] [CrossRef]
- Hamberg, I.; Granqvist, C.G.; Berggren, K.F.; Sernelius, B.E.; Engstrom, L. Band gap widening in heavily Sn-doped In2O3. Phys. Rev. B 1984, 30, 3240–3249. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Lattice distortion and corresponding changes in optical properties of CeO2 nanoparticles on Nd doping. Curr. Appl. Phys. 2013, 13, 217–223. [Google Scholar] [CrossRef]
- Eskandari, F.; Kameli, P.; Salamati, H. Effect of laser pulse repetition rate on morphology and magnetic properties of cobalt ferrite films grown by pulsed laser deposition. Appl. Surf. Sci. 2019, 466, 215–223. [Google Scholar] [CrossRef]
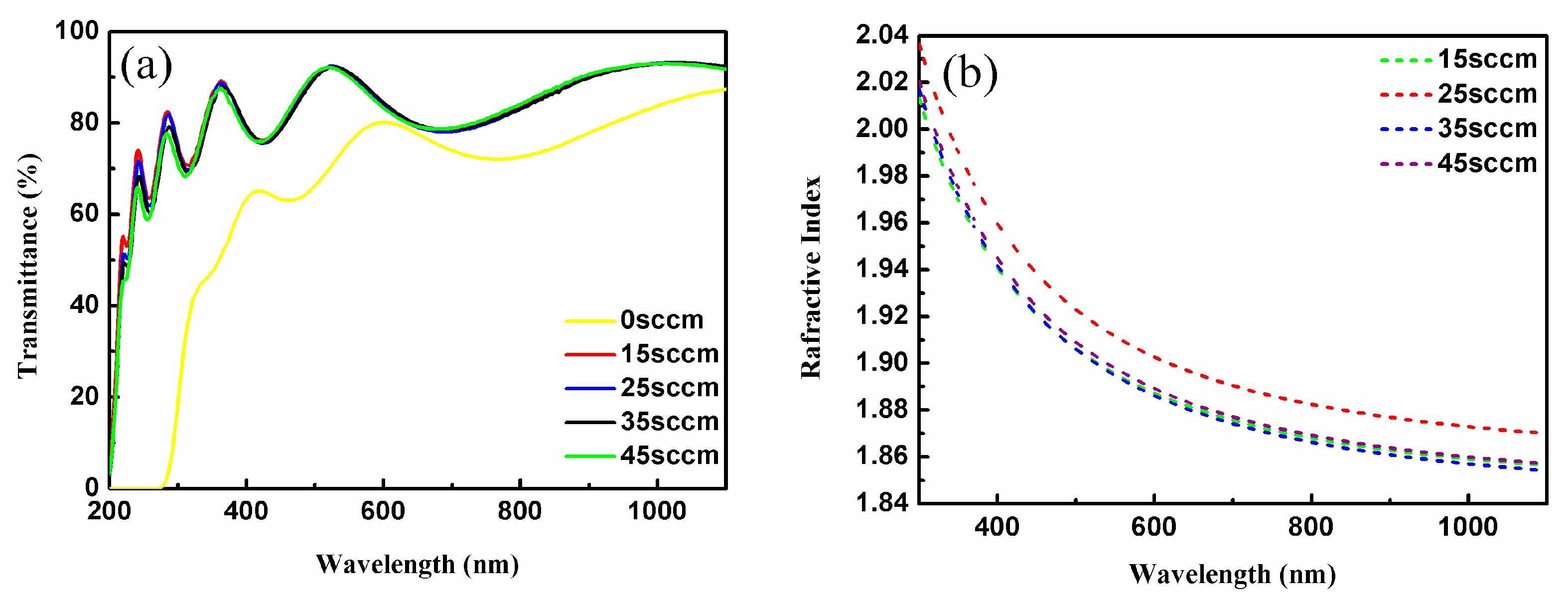
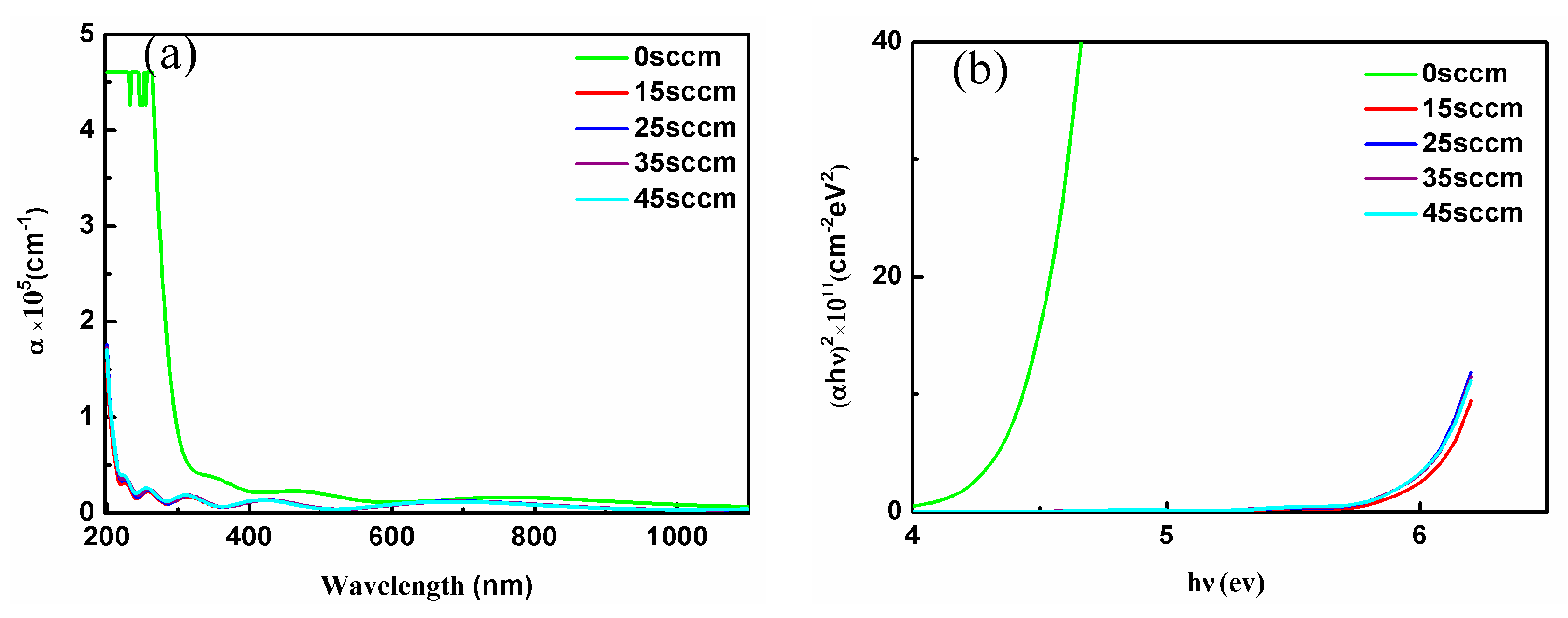
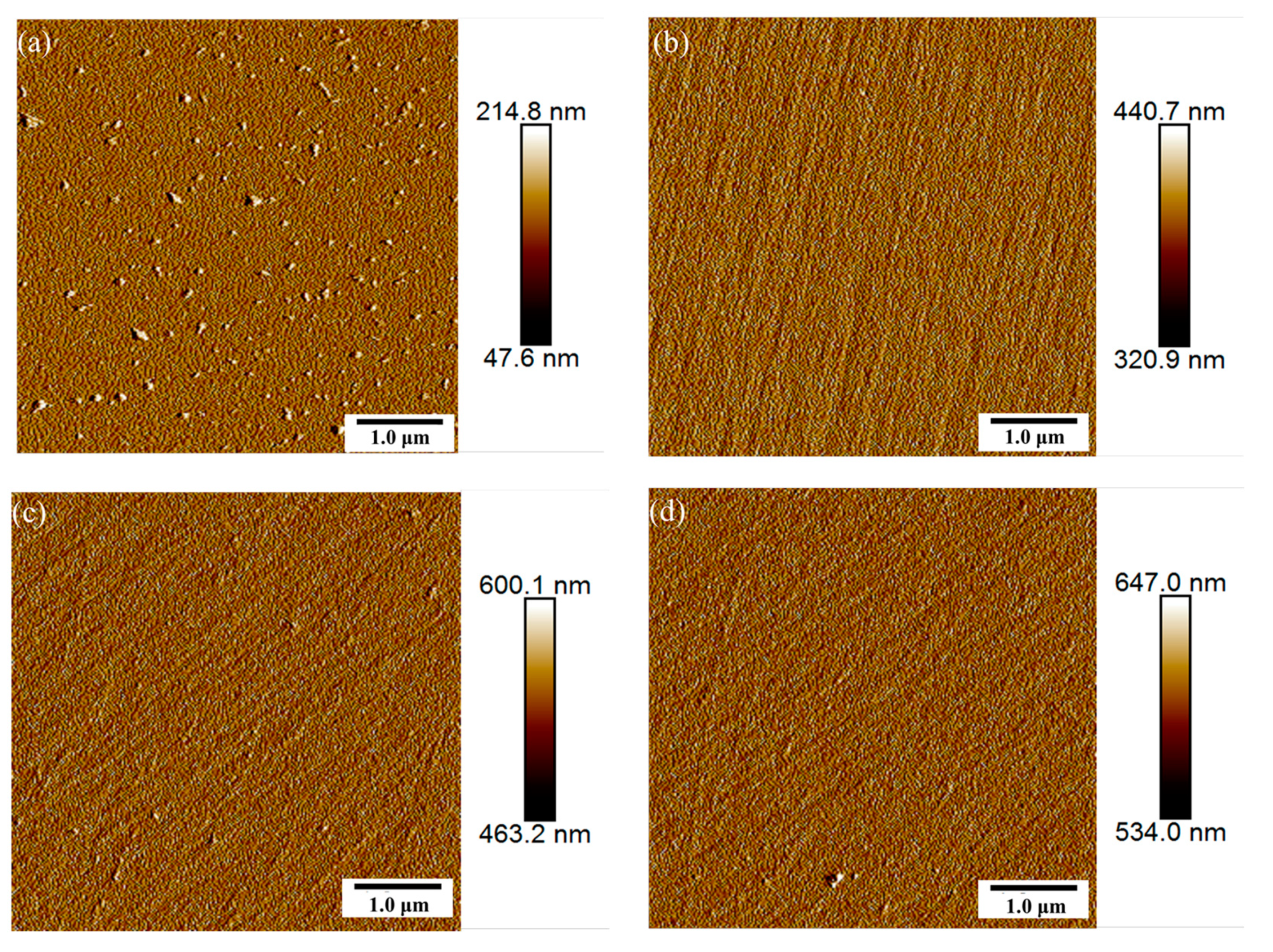
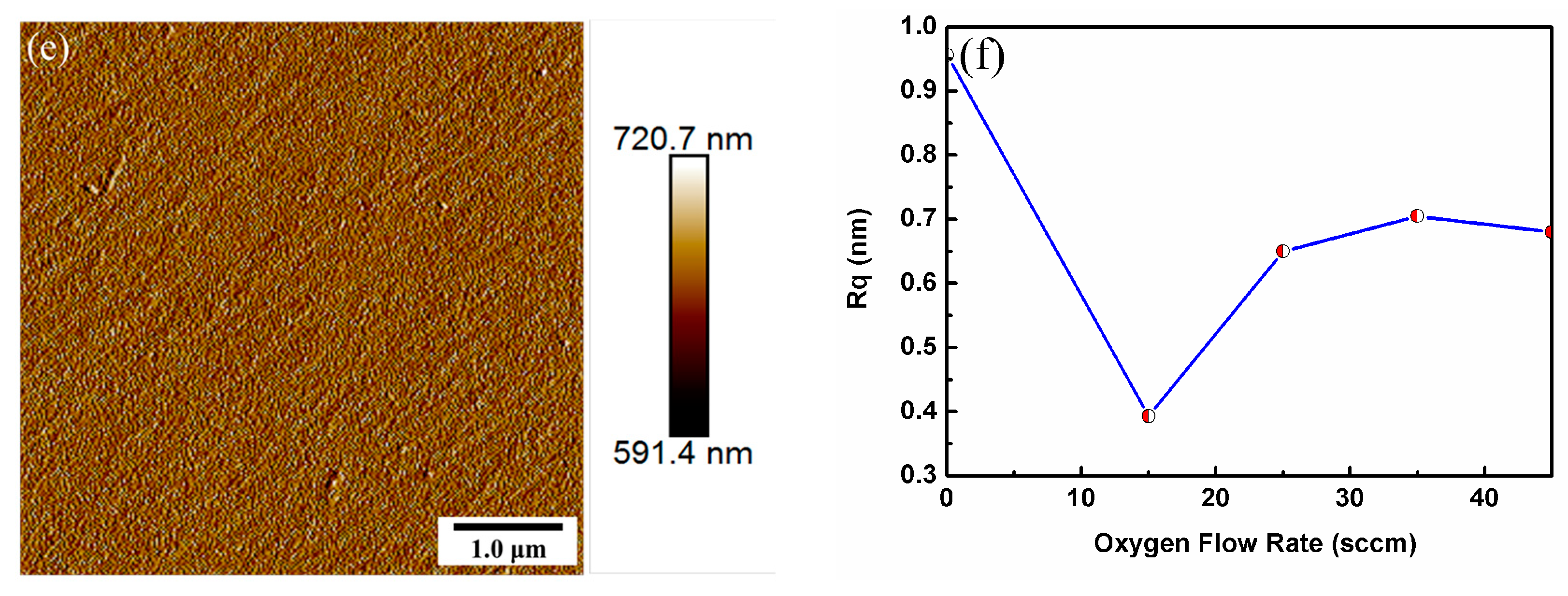
| Oxygen Flow Rates (sccm) | LIDT (J/cm2) |
|---|---|
| 0 | 0.8 |
| 15 | 7.2 |
| 25 | 5.6 |
| 35 | 5.3 |
| 45 | 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, P.; Pu, Y.; Ma, P.; Zhu, J. Relationship between Oxygen Defects and Properties of Scandium Oxide Films Prepared by Ion-Beam Sputtering. Coatings 2019, 9, 517. https://doi.org/10.3390/coatings9080517
Kong P, Pu Y, Ma P, Zhu J. Relationship between Oxygen Defects and Properties of Scandium Oxide Films Prepared by Ion-Beam Sputtering. Coatings. 2019; 9(8):517. https://doi.org/10.3390/coatings9080517
Chicago/Turabian StyleKong, Pengfei, Yunti Pu, Ping Ma, and Jiliang Zhu. 2019. "Relationship between Oxygen Defects and Properties of Scandium Oxide Films Prepared by Ion-Beam Sputtering" Coatings 9, no. 8: 517. https://doi.org/10.3390/coatings9080517
APA StyleKong, P., Pu, Y., Ma, P., & Zhu, J. (2019). Relationship between Oxygen Defects and Properties of Scandium Oxide Films Prepared by Ion-Beam Sputtering. Coatings, 9(8), 517. https://doi.org/10.3390/coatings9080517




