Abstract
Construction in cold regions faces significant challenges due to delayed cement hydration and frost damage caused by sub-zero temperatures. This study investigated the effects of three antifreeze agents on the performance of composite cement under sub-zero temperatures. The setting time, compressive strength, freezing point, and hydration mechanisms were evaluated. The results revealed that CaCl2 optimally accelerated hydration, while achieving the continuous development of compressive strengths through freezing-point depression and dense microstructure formation. NaNO2 exhibited the lowest freezing point but delayed setting at high dosages, while Li2CO3 showed limited impact due to insufficient freezing point depression. Li2CO3 showed limited efficacy under continuous low temperatures but enabled strength recovery after the temperature transition from sub-zero to ambient conditions. This research provides a basis for the application of antifreeze agents in the composite cement system for construction in cold environments.
1. Introduction
Cement technologies for cold regions have garnered global attention, as construction under such conditions is inherently more challenging than in temperate environments due to the detrimental impacts of low ambient temperatures on both project timelines and structural quality [1,2,3]. During winter construction, there are two primary risks: first, extremely cold conditions delay cement hydration, leading to suppressed early compressive strength development; second, when the internal temperature of concrete falls below −5 °C, approximately 92% of capillary water freezes, damaging the microstructure, while only minimal liquid water remains for hydration [4].
To address frost heave damage, enhancing early-age cement properties, accelerating hydration, and minimizing freezable water content prior to freezing are essential [4,5,6]. Conventional approaches, including heating water and aggregates, employing thermal storage curing, and covering poured concrete with insulating materials, are widely implemented [7,8]. While these measures can effectively mitigate risks, the long-term investment of manpower and energy leads to excessively high construction costs and significantly increases CO2 emissions, contributing to environmental pollution [7,8]. Therefore, there is an urgent need for a low-cost, environmentally friendly material that can be used at sub-zero temperatures, addressing the challenges posed by excessively cold weather conditions.
Portland cement, as the most widely used cement in engineering, offered advantages including substantial late-age strength growth, low price, and simple production processes. However, sub-zero temperatures during winter construction delay the setting and hardening of Portland cement and increase CO2 emissions [9,10,11]. Sulfoaluminate cement, known for its low carbon footprint, rapid hydration, and high early strength, is typically employed in cold-region projects and rapid repairs [12]. However, its high production costs and limited raw material availability restrict widespread adoption [13,14]. Studies demonstrated that blending sulfoaluminate cement with Portland cement create a composite system that combines rapid early hydration, shortening setting time, and boosting early strength at sub-zero temperatures with Portland cement’s economic benefits [15,16]. Nevertheless, when the temperature decreases below −5 °C, the frost resistance of the composite cement is significantly compromised due to the freezing of 92% capillary water [6]. To address this limitation, antifreeze agents have been proposed as a viable strategy to reduce frost-induced damage and expand the applicability of cement [17,18,19]. Therefore, identifying appropriate antifreeze agents compatible with composite systems is of paramount importance.
Inorganic antifreeze agents have been widely utilized in winter construction due to their cost-effectiveness, accessibility, and operational simplicity. Their mechanisms of action primarily involve lowering the freezing point of pore solutions to delay ice formation and accelerating cement hydration through ionic nucleation effects, thereby enhancing early strength development [20,21,22]. Among these agents, chloride salts (e.g., CaCl2, NaCl), nitrites (e.g., NaNO2, Ca(NO2)2), and lithium carbonate (Li2CO3) are commonly employed, with their functional advantages and limitations varying significantly based on composition and application scenarios.
Calcium chloride (CaCl2) is recognized as a highly effective antifreeze agent in cold climates. Studies demonstrate that Ca2+ ions improve particle dispersion and accelerate C3S hydration, while interactions with C3A generate insoluble chloroaluminates (e.g., Friedel’s salt), promoting aluminate phase reactions [22,23]. For instance, Wang et al. [23] reported that 3%–4% CaCl2 reduced the initial setting time of Portland cement by 60% and increased its 28 d compressive strength by 25% under 3 °C curing. However, the corrosion risk of Cl− on steel reinforcement restricts its application to non-reinforced concrete structures [24,25]. Sodium nitrite (NaNO2) has emerged as a prominent non-chloride antifreeze agent, which is particularly valued for its dual functionality in corrosion inhibition and hydration acceleration. Studies have demonstrated that NaNO2 effectively prevents steel reinforcement corrosion by forming a protective oxide layer on the steel surface, thereby neutralizing chloride-induced electrochemical reactions [26]. In Portland cement systems, NO2− ions preferentially react with C3A to form nitrite-modified AFt (NO2-AFt), which suppresses the conversion of AFt to SO42−-AFm phases, thereby increasing ettringite content and early strength [11]. Compared to chloride-based agents, NaNO2 exhibits superior compatibility with reinforced concrete, achieving a freezing point reduction to −15 °C at an 8% dosage without corrosion risks [11]. Lithium carbonate (Li2CO3) exhibits exceptional performance in sulfoaluminate cement systems. The small ionic radius and high polarization capability of Li+ shortens the hydration induction period and promotes AFt nucleation, significantly improving early strength [27,28]. Shen et al. [28] observed that 1% Li2CO3 reduces the initial and final setting times of sulfoaluminate cement from 37 and 64 min to 8 and 13 min, respectively. Although the aforementioned antifreeze agents have demonstrated significant efficacy in individual cement systems, their effects in composite systems remain unclear.
In this study, three antifreeze agents (CaCl2, NaNO2, Li2CO3) are selected to evaluate their effects on the setting time, compressive strength, and freezing point of a Portland/sulfoaluminate composite cement under −10 °C curing. X-ray diffraction (XRD) and scanning electron microscopy (SEM) are employed to analyze hydration products and microstructural evolution, elucidating the mechanisms by which these agents influence composite cement performance.
2. Materials and Methods
2.1. Materials
The chemical compositions of Portland cement (P.O 42.5) and sulfoaluminate cement (52.5) are shown in Table 1 and Table 2. Silica sand, with a fineness modulus of 2.3–3.0, was employed as the fine aggregate. Analytical-grade CaCl2, NaNO2, and Li2CO3 were used as antifreeze agents, with purities exceeding 96.0%, 99.0%, and 99.0%, respectively.

Table 1.
Chemical compositions of Portland cement (wt. %).

Table 2.
Chemical compositions of sulfoaluminate cement (wt. %).
2.2. Methods
2.2.1. Setting Time
The setting time test was conducted using cement paste with a Portland/sulfoaluminate cement ratio of 9:1 and a w/c ratio of 0.3. The 9:1 ratio was selected based on prior studies, which demonstrated that exceeding a 10% sulfoaluminate cement content in the composite system significantly altered hydration mechanisms, leading to impractical setting time reductions for field applications [6]. The w/c ratio of 0.3 was optimized to balance two critical factors: freezable water mitigation and workability-structure trade-off. Antifreeze agent dosages included CaCl2 (0%, 0.5%, 1%, 1.5%, 2%), NaNO2 (0%, 1%, 2%, 3%, 4%), and Li2CO3 (0%, 0.1%, 0.2%, 0.4%, 0.6%). The cement paste was prepared according to the mix proportions. Prior to mixing, the antifreeze admixture was dissolved in water to form a homogeneous solution, which was subsequently combined with cement. The time of complete cement/water mixing was recorded as the starting time (t1). For the initial setting test, the test needle was vertically lowered onto the paste surface, tightened for 1 to 2 s, and released. The initial setting was defined when the needle penetrated to a depth of 4 ± 1 mm from the base plate, with the corresponding time recorded as t2. The initial setting time (Δt1) was calculated as t2 − t1. The final setting time (Δt2 = t3 − t1) was determined when the annular attachment of the final setting needle failed to leave an indentation on the specimen surface, recorded as t3.
2.2.2. Compressive Strength
The compressive strength test was performed on mortar specimens with a Portland/sulfoaluminate cement ratio of 9:1, a w/c ratio of 0.3, and a cement/sand ratio of 1:1. The mortar preparation process, including specimen dimensions and testing procedures, was based on the Chinese standard GB/T 17671-2021 “Method for Testing the Strength of Cement Mortar” [29], as follows: the composite cement was dry-mixed for 120 s; the antifreeze solution was added and mixed for 30 s; sand was incorporated with slow mixing for 30 s, followed by fast mixing for 30 s and an additional 60 s. Mortar was cast into steel molds (40 mm × 40 mm × 160 mm), vibrated 60 times, and immediately sealed with a polyethylene film. The specimens were immediately transferred to a −10 °C curing chamber for low-temperature hydration. Two curing regimes were applied: (1) continuous −10 °C curing; (2) −10 °C curing for 7 d followed by 21 d at 20 ± 2 °C and 95 ± 5% relative humidity. Compressive strength was measured at four designated ages, with six specimens tested per age to ensure statistical reliability.
2.2.3. Freezing Point
The temperature history curve test was conducted using a single mortar specimen (100 mm × 100 mm × 100 mm). A T-type thermocouple embedded in the specimen center was connected to a temperature recorder, which logged data to a computer system (Figure 1) [6]. The specimen was then transferred to a −10 °C curing chamber for monitoring. Upon test completion, the temperature history curve was plotted, and the freezing point was identified using the methodology depicted in Figure 2 [6].
Figure 1.
The schematic of the test of temperature history of cement [6].
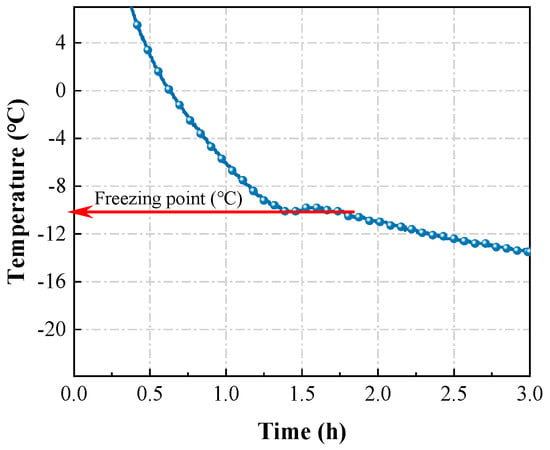
Figure 2.
The schematic of selection of the freezing point [6].
2.2.4. X-Ray Diffraction
The hydration products of the composite system were analyzed using X-ray diffraction (XRD). At designated ages, core samples were extracted, ground into powder, and immersed in isopropanol to terminate hydration. The powder was then pressed into pellets and loaded into a Bruker AXS D8 X-ray diffractometer (Billerica, MA, USA) with CuKα radiation (40 kV, 40 mA). Scans were performed over 2θ angles from 5° to 90°at a rate of 10°/min.
2.2.5. Scanning Electron Microscopy
The microstructure of the composite system was analyzed using scanning electron microscopy (SEM). At designated ages, a core region was extracted from each specimen and processed into a 5 mm × 5 mm × 5 mm cube. Then, it was immersed in isopropanol for 48 h to terminate hydration, and dried for 72 h. Prior to imaging, a fresh, smooth cross-section was exposed by fracturing the cube. The sample was sputter-coated with gold prior to imaging. SEM observations were conducted using an FEI Quanta 250 instrument (Hillsboro, OR, USA).
3. Results
3.1. Setting Time
The setting time of the composite system with varying CaCl2 dosages is shown in Figure 3a. Under a negative temperature, the hydration rate of the cement slows down, resulting in prolonged initial and final setting times (136 min and 188 min, respectively, for the 0% dosage). The addition of CaCl2 significantly mitigated this issue, with higher dosages yielding shorter setting times. As seen in Figure 3a, adding CaCl2 notably shortened both the initial and final setting time of the composite system. Additionally, a higher dosage resulted in shorter setting times. At 2.0% CaCl2, initial and final setting times were reduced by 77.9% and 69.7%, respectively, compared to the control. This acceleration was attributed to CaCl2 reacting with C3A to form hydrated chloroaluminates [30]. Additionally, CaCl2 reacted with CH, reducing the CH content in the system by forming calcium chlorate, which further accelerated the hydration of C3S and C2S [17,31,32].
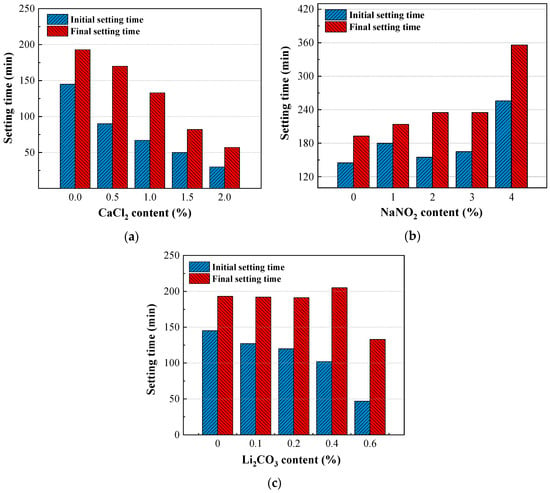
Figure 3.
Effects (a) CaCl2, (b) NaNO2, and (c) Li2CO3 on the setting time of composite cement.
In contrast, NaNO2 exhibited opposing effects, as shown in Figure 3b. While the control system (0% NaNO2) showed initial and final setting times of 136 min and 188 min, NaNO2 incorporation progressively delayed both. Moreover, as the NaNO2 dosage increased, the setting time increased slowly at first and then rapidly when the dosage exceeded 3%. At a 4% dosage, initial and final setting times increased by 88.2% (256 min) and 89.4% (356 min), respectively, compared to the control. Thus, excessive NaNO2 may delay the hydration of the composite system [33].
From Figure 3c, Li2CO3, similar to CaCl2, reduced setting times but with lesser efficacy. At a 0.6% dosage, initial and final setting times decreased by 65.4% and 29.3%, respectively, compared to the control. The reduction was mainly due to the fact that Li+ ions shorten the induction period, accelerating sulfoaluminate cement hydration [27]. However, its impact was weaker than CaCl2, likely due to limited freezing-point depression and preferential interaction with sulfoaluminate phases rather than silicates.
3.2. Compressive Strength
When subjected to continuous negative temperature curing, as shown in Figure 4a, the incorporation of CaCl2 significantly enhanced the compressive strength of the composite system at various ages. When the CaCl2 content was 0%, the compressive strength of the composite system at all ages had been suppressed by the negative temperature. The compressive strength of the composite system at −3 d, −7 d, and −28 d was only 3.1 MPa, 6.2 MPa, and 4.9 MPa, respectively. As the CaCl2 content increased, the compressive strength of the composite system also increased accordingly. When the CaCl2 content reached 2%, the compressive strength of the composite system reached the maximum value, i.e., 22.0 MPa, 41.2 MPa, and 43.7 MPa at −3 d, −7 d, and −28 d, respectively, which had been five to eight times higher than that of the blank. CaCl2 promoted the hydration of C3S and C3A in the composite system and accelerated the formation of hydration products. During the initial stage of continuous negative temperature curing, it had still rapidly constructed a microscopic skeleton with certain strength, thereby improving the compressive strength of the composite system.
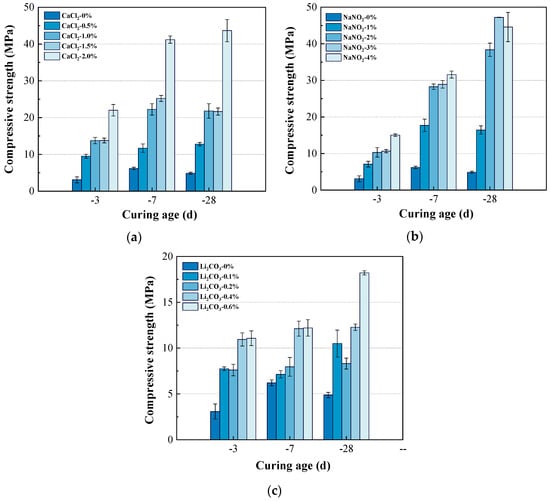
Figure 4.
Effects (a) CaCl2, (b) NaNO2, and (c) Li2CO3 on the compressive strength of composite cement at continues curing at −10 °C.
From Figure 4b, it can be observed that the incorporation of NaNO2 also improved the compressive strength of the composite system at various ages. During the early stages of negative temperature curing at −3 d and −7 d, as the NaNO2 content increased from 1.0% to 4.0%, the compressive strength of the composite system at −3 d and −7 d had continued to increase. When the NaNO2 content reached 4%, the compressive strength reached the maximum. During the later stage of −28 d, the compressive strength of the composite system reached the maximum when the NaNO2 content was 3%, which was 47.2 MPa, more than eight times higher than that of the blank. The incorporation of NaNO2 lowered the freezing point of the liquid phase, thereby reducing the content of freezable water. Under −10 °C curing, it provided a large amount of unfrozen water in the early stage to ensure the continuous hydration of the composite system.
As shown in Figure 4c, Li2CO3 improved the compressive strength of the composite system to a certain extent. However, as the age increased, the growth in compressive strength was not significant. The incorporation of Li2CO3 promoted the hydration of C3A and C4A3 in the paste, thereby accelerating the formation of the hydration product AFt. This rapidly constructed a relatively dense microscopic network structure in the early stage, resisting the freezing damage caused by negative temperature curing conditions to a certain extent. However, its effect on lowering the freezing point was limited. Under continuous negative temperature curing, the free water in the system froze, making it difficult for the composite system to undergo continuous hydration.
From Figure 5a, it can be seen that when the curing condition shifted from a negative temperature to positive temperature, the compressive strength of the composite system significantly increased at −7 + 21 d compared to continuous negative temperature curing for −28 d. After the curing temperature shifted from negative to positive, some amount of frozen water melted and was reintroduced into the hydration process [19]. Under this curing condition, CaCl2 also enhanced the compressive strength of the composite system. When the amount of CaCl2 was 0.5%, the compressive strength at −7 + 21 d reached a maximum of 89.8 MPa, representing a 68.3% increase compared to the blank. When shifting the temperature from −10 °C to 20 °C, the previously frozen free water thawed and again became available for cement hydration. In the composite system, sulfoaluminate cement initiated rapid hydration, generating AFt to enhance strength. Concurrently, CaCl2 accelerated the hydration of silicate phases, facilitating the formation of a denser microstructure, thereby achieving enhanced mechanical performance.
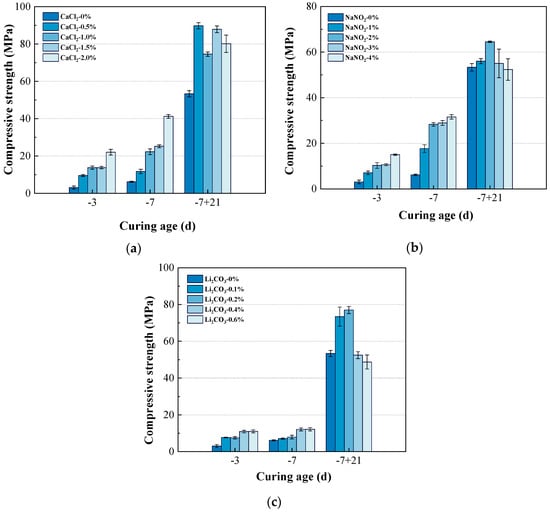
Figure 5.
Effects (a) CaCl2, (b) NaNO2, and (c) Li2CO3 on the compressive strength of composite cement at curing condition shifted from negative temperature to positive temperature.
As shown in Figure 5b, with increasing amounts of NaNO2, the compressive strength of the composite system at −7 + 21 d exhibited a trend of first increasing and then decreasing. When the amount of NaNO2 was 2%, the compressive strength at −7 + 21 d reached a maximum of 64.5 MPa. However, when the amount of NaNO2 was as high as 4%, the compressive strength of the composite system was only 52.4 MPa. This indicated that excessive NaNO2 was detrimental to the hydration of the composite system at room temperature.
From Figure 5c, it can be observed that an appropriate amount of Li2CO3 significantly improved the compressive strength of the composite system compared to continuous negative temperature curing. When the amount of Li2CO3 was 0.2%, the compressive strength at −7 + 21 d reached a maximum of 77.0 MPa, representing a 44.4% increase compared to the control group. However, excessive Li2CO3 was detrimental to the development of compressive strength. When the amount of Li2CO3 was 0.6%, the compressive strength of the composite system was only 48.7 MPa. Compared to NaNO2, Li2CO3 exhibited a more significant strength enhancement after the temperature transition to room temperature conditions. This contrast revealed that the freezing of free water substantially impaired Li2CO3’s ability to promote hydration reactions.
3.3. Freezing Point
Table 3 shows the internal liquid phase freezing point of the composite system. When without an antifreeze agent, the freezing point was −0.1 °C, indicating that the composite system was susceptible to frost damage at low temperatures of −10 °C. After adding CaCl2, the freezing point of the composite system decreased significantly. As the amount of CaCl2 increased, the freezing point of the composite system continued to drop. When the CaCl2 content was 2%, the freezing point of the composite system decreased to −3.2 °C. As an inorganic salt, CaCl2 not only lowered the freezing point of the liquid phase of the composite system but also accelerated the dissolution of C3S and C3A in the composite system, promoting the formation of hydration products and enabling the system to rapidly establish a microscopic network structure in the early stages [31]. As the pores inside the slurry were gradually filled with hydration products, the pore diameters decreased, and the freezing point of the composite system was reduced [32].

Table 3.
Effects of antifreeze agent on the freezing point of composite cement.
The effect of NaNO2 in lowering the freezing point of the composite system was more significant. With increasing the NaNO2 dosage, the freezing point of the composite system continued to decrease. When the NaNO2 dosage was 1%, 2%, 3%, and 4%, the freezing point of the composite system decreased to −2.3 °C, −3.1 °C, −4.1 °C, and −4.9 °C, respectively. According to Raoult’s Law, the addition of NaNO2 effectively increased the liquid phase concentration in the composite system, and the freezing point of the liquid phase decreased as the vapor pressure inside the pores decreased [33]. Therefore, the content of unfrozen water in the composite system increased, ensuring the adequate progression of early hydration reactions. The formed hydration products filled the pores in the composite system, reducing pore diameters and further lowering the liquid phase freezing point [6]. Thus, under continuous negative temperature curing conditions, the composite system had sufficient unfrozen water available for hydration reactions, enabling continuous strength growth.
The effect of incorporating Li2CO3 in lowering the freezing point of the composite system was relatively insignificant. When the Li2CO3 dosage ranged from 0.1% to 0.6%, the freezing point of the composite system was between −1.1 °C and −2.3 °C. CaCl2 and NaNO2 maintained high solubility at low temperatures, and their dissociation generated a greater number of ions, enabling effective freezing point depression. In contrast, Li2CO3 exhibited extremely low solubility, further reduced under sub-zero conditions, with insufficient total particle concentration post-dissociation to significantly alter the freezing point. Therefore, under continuous negative temperature curing conditions, a large amount of free water froze, and Li2CO3 had a non-significant promoting effect on the hydration of the composite system, with minimal strength growth observed. However, when the temperature transitioned to positive, a large amount of water was released. At this point, Li2CO3 effectively promoted the hydration of the composite system, leading to rapid strength growth.
3.4. XRD Analysis
The effect of CaCl2 on the hydration products of the composite system is shown in Figure 6a, and the diffraction peak intensities of the phases were quantitatively extracted and plotted in Figure 7a for comparative analysis. Compared to the raw materials and the blank system, the addition of CaCl2 resulted in a significant decrease in the diffraction peak intensity of the clinkers C3S and C2S in the composite system, while the diffraction peak intensity of the hydration product AFt and CH was notably enhanced. This indicates that the incorporation of CaCl2 promoted the hydration of the silicate phases in the system, thereby accelerating the formation of CH crystals. With the increase in CaCl2 content, the diffraction peak intensity of gypsum in the composite system significantly decreased, while the diffraction peak intensity of the hydration product AFt further increased. Studies [11] have demonstrated that Ca2+ ions enhance particle dispersion through electrical double-layer effects, while their increased concentration in pore solution elevate ionic activity coefficients and reaction kinetics, thereby accelerating C3S hydration. Concurrently, Cl− ions chemically interact with C3A phases to form insoluble chloroaluminates, which preferentially drive aluminate reaction pathways.
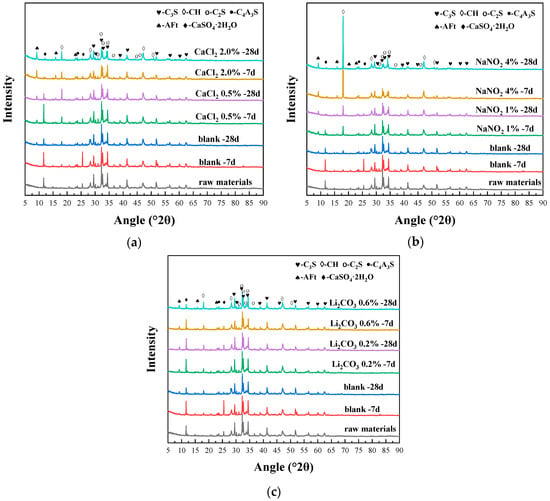
Figure 6.
XRD patterns showing the effects of (a) CaCl2, (b) NaNO2, and (c) Li2CO3 on hydration products of composite cement.
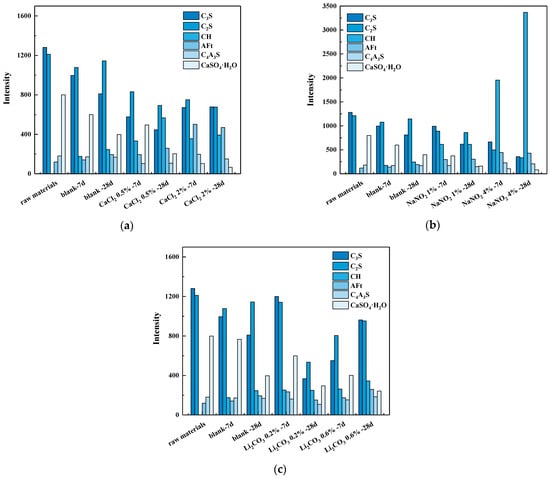
Figure 7.
Effects of (a) CaCl2, (b) NaNO2, and (c) Li2CO3 on intensity of hydration products.
The effect of NaNO2 on the hydration products of the composite system is shown in Figure 6b, and the diffraction peak intensities of the phases were quantitatively extracted and plotted in Figure 7b for comparative analysis. Compared to the blank system, the incorporation of NaNO2 decreased the diffraction peak intensity of C3S and C2S, while substantially increasing those of hydration products AFt and CH. When 1% NaNO2 was added, the gypsum diffraction peak remained detectable after −7 d of curing but nearly disappeared by −28 d, indicating the progressive consumption of gypsum during hydration. At a higher NaNO2 dosage (4%), gypsum was almost entirely consumed within −7 d of sub-zero temperature curing. The experimental results of the freezing point demonstrated that NaNO2 significantly reduced the freezing point of cement paste. This phenomenon was attributed to the colligative properties of solutions, where the extent of freezing point depression correlated with the total dissolved particle concentration. Owing to its high solubility, NaNO2 dissociated into abundant Na+ and NO2− ions in the pore solution, resulting in an effective freezing point depression through ionic interference with ice nucleation thermodynamics. In addition, Liu et al. [11] have indicated that NaNO2 promotes the rapid nucleation and growth of hydration products by providing additional nucleation sites, which also accelerate early hydration reactions.
The effect of Li2CO3 on the hydration products of the composite system is illustrated in Figure 6c, and the diffraction peak intensities of the phases were quantitatively extracted and plotted in Figure 7c for comparative analysis. Compared to the blank system, the incorporation of Li2CO3 resulted in a reduction in the peak intensities of C3S and C2S in the composite system, while the diffraction peak of hydration products (AFt and CH crystals) gradually intensified with the increasing Li2CO3 content. When the Li2CO3 dosage increased from 0.2% to 0.6%, the diffraction peak intensity of gypsum decreased under continuous sub-zero temperature curing, whereas the peak intensity of CH crystals was progressively strengthened. Zhang et al. [27] have demonstrated that Li2CO3 reacts with AH3 to produce LiOH, which increases the system’s pH and enhances the dissolution of calcium sulfoaluminate phases. This process releases additional Al3+ and SO42− ions, accelerating AFt formation. Simultaneously, Li+ ions coordinate with hydroxyl groups to stabilize [Al(OH)6]3− clusters, reducing the energy barrier for AFt nucleation and promoting rapid crystallization. Therefore, the hydration of sulfoaluminate cement in the composite cement is accelerated. However, due to its limited ability to depress the freezing point, Li2CO3 exhibits inferior effectiveness in enhancing the strength of the composite system under sub-zero temperatures compared to CaCl2 and NaNO2.
3.5. SEM Analysis
The morphological effects of different admixtures on the hydration products of the composite cement at −7 d are illustrated in Figure 8. In the blank system, limited hydration products were observed on the surface of cement particles. Combined with XRD results, the hydration reaction was significantly inhibited by the sub-zero curing temperature, leaving a substantial amount of unhydrated cement particles at −7 d. With the addition of 2% CaCl2, abundant gel-like hydration products covered the cement particle surfaces, accompanied by hexagonal plate-like CH crystals. Compared to the blank system, CaCl2 accelerated the hydration reaction under sub-zero conditions, forming a denser microstructural network. For the system incorporating 4% NaNO2, the microstructure of hydration products exhibited a high compactness, with extensive amorphous gel-like phases covering the surfaces. The addition of NaNO2 depressed the freezing point of the pore solution, reduced the content of freezable water, effectively sustained hydration at sub-zero temperatures, and mitigated frost heave-induced damage. In the case of 0.6% Li2CO3, needle-shaped AFt crystals and amorphous gel-like hydration products were observed on cement particle surfaces. However, numerous pores were evident. Although Li2CO3 promoted the hydration of sulfoaluminate cement and AFt formation, its limited freezing point depression not only hindered hydration but also increased microporosity due to frost heave-induced structural damage.

Figure 8.
Effects (a) without addition, (b) CaCl2, (c) NaNO2, and (d) Li2CO3 on the morphology of composite cement.
4. Conclusions
This study investigated the effects of three antifreeze agents (CaCl2, NaNO2, and Li2CO3) on the performance of composite cement under sub-zero temperatures (−10 °C). The main conclusions are as follows:
(1) Three antifreeze agents enhanced the strength of the composite system under sub-zero conditions to different extents. Both CaCl2 and NaNO2 demonstrated sustained strength development under continuous sub-zero temperature curing (−10 °C), with additional strength gains observed after transitioning to room temperature. In contrast, Li2CO3 showed a limited early-stage improvement under persistent sub-zero conditions but achieved remarkable strength recovery following the temperature shift to room temperature.
(2) The freezing point depression efficacy in the composite system followed the order NaNO2 > CaCl2 > Li2CO3. Both CaCl2 and NaNO2 maintained substantial unfrozen water contents, enabling continuous hydration under continuous sub-zero temperature curing. In contrast, Li2CO3 permitted the amount of water frozen, fundamentally restricting its strength-enhancing effectiveness under sustained sub-zero conditions.
(3) The three antifreeze agents acted in the composite system through different pathways. CaCl2 predominantly enhanced strength through accelerated silicate phase hydration, forming dense hydration products. NaNO2 mainly provided unfrozen water to ensure the hydration of cement by lowering the freezing point, but excessive NaNO2 inhibited hydration processes. Li2CO3 promoted the strength increase mainly by affecting the hydration rate of the sulfoaluminate phase. This only worked if unfrozen water was present, while it did not have the effect of lowering the freezing point.
(4) Practical implementation requires scenario-specific strategies: For sustained sub-zero conditions, 1.5%–2.0% CaCl2 is recommended in composite cement due to its superior freezing point depression and hydration acceleration, though corrosion inhibitors must be incorporated for steel-reinforced applications. Under intermittent low-to-ambient temperature cycles, 0.2%–0.4% Li2CO3 offers advantages by enabling rapid strength recovery in chloride-sensitive environments. For cost-driven projects, 2%–3% NaNO2 provides balanced ice suppression and strength enhancement without chloride-related durability risks. Future research should prioritize investigating the synergistic effects of hybrid antifreeze agents to advance sustainable cold-region construction practices.
Author Contributions
Conceptualization, B.Z.; methodology, B.Z.; validation, B.Z.; formal analysis, Y.D.; investigation, Y.H.; resources, D.L.; data curation, D.L.; writing—original draft preparation, D.L.; writing—review and editing, B.Z.; supervision, B.Z.; project administration, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
If needed, the corresponding author can be contacted to obtain the original data.
Acknowledgments
The financial support from Shaanxi Province No. S202410703094 for the current research is gratefully acknowledged.
Conflicts of Interest
Authors Bitao Zhang and Yongkang Du were employed by Shaanxi Building Materials Technology Group Co., Ltd. Author Dong Liu was employed by Huangling Ecological Cement Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Choi, H.; Zhang, W.; Hama, Y. Method for determining early-age frost damage of concrete by using air-permeability index and influence of early-age frost damage on concrete durability. Constr. Build. Mater. 2017, 153, 630–639. [Google Scholar]
- Elshazly, M.A.; Elakhras, A.A.; Elshami, A.A.; Ahmad, S.S.E.; Elmahdy, M.A.R. Investigating the effectiveness of a bacterial self-healing mechanism for repairing cracks in sustainable cement mortar at low temperatures. Results Eng. 2025, 25, 103907. [Google Scholar]
- Kothari, A.; Hedlund, H.; Illikainen, M.; Cwirzen, A. Effects of sodium nitrate and OPC-GGBS concrete mix composition on phase transition of pore water at subzero temperatures. Constr. Build. Mater. 2022, 327, 126901. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Isteri, V.; Hanein, T.; Geddes, D.; Poikelispää, M.; Illikainen, M. Blending eco-efficient calcium sulfoaluminate belite ferrite cement to enhance the physico–mechanical properties of Portland cement paste cured in refrigerated and natural winter conditions. Cem. Concr. Compos. 2022, 129, 104469. [Google Scholar]
- Liu, Y.; Sun, F.; Yu, K.; Yang, Y. Experimental and numerical research on development of synthetic heat storage form incorporating phase change materials to protect concrete in cold weather. Renew. Energy 2020, 149, 1424–1433. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, S.; Lei, Y.; Shi, C.; Li, G.; Hanif, A. Optimizing anti-freezing agent on the properties of Portland cement-calcium sulphoaluminate cement system based on Taguchi-GRA method. Case Stud. Constr. Mater. 2024, 20, e02998. [Google Scholar]
- Nishat, F.M.; Chakraborty, R.; Oh, H.-J.; Yoo, D.-Y.; Mohapoo, R.; Yeon, J.H. Design and performance evaluation of an electrically heated concrete panel for sustainable winter maintenance. Dev. Built Environ. 2023, 16, 100259. [Google Scholar] [CrossRef]
- Wang, T.; Liao, C.; Qi, X.; Zhang, Y. Predicting the effect of promoting ultra-low energy buildings in hot summer and warm winter regions on CO2 emission. Energy Sustain. Dev. 2025, 85, 101646. [Google Scholar]
- Zhang, G.; Yang, H.; Ju, C.; Yang, Y. Novel selection of environment-friendly cementitious materials for winter construction: Alkali-activated slag/Portland cement. J. Clean. Prod. 2020, 258, 120592. [Google Scholar]
- Li, L.; Wang, C.; Zhao, Z.; Dang, L.; He, R. Hydration behavior and micro-pore structural of Portland cement composites with crystalline nano-SiO2 at low temperature. J. Build. Eng. 2024, 98, 111276. [Google Scholar]
- Liu, Z.; Lou, B.; Barbieri, D.M.; Sha, A.; Ye, T.; Li, Y. Effects of pre-curing treatment and chemical accelerators on Portland cement mortars at low temperature (5 °C). Constr. Build. Mater. 2020, 240, 117893. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Kletti, H.; Rößler, C.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Ferritic calcium sulfoaluminate belite cement from metallurgical industry residues and phosphogypsum: Clinker production, scale-up, and microstructural characterisation. Cem. Concr. Res. 2022, 154, 106715. [Google Scholar] [CrossRef]
- Kim, N.; Seo, J.; Lee, H.K. Enhancement in clinker hydration degrees and later stage-ettringite stability of calcium sulfoaluminate cements by the incorporation of dolomite. Cem. Concr. Compos. 2025, 155, 105815. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, C.; Tan, H.; Liu, X. Potential application of Portland cement-sulfoaluminate cement system in precast concrete cured under ambient temperature. Constr. Build. Mater. 2020, 251, 118869. [Google Scholar] [CrossRef]
- Chaunsali, P.; Mondal, P. Physico-chemical interaction between mineral admixtures and OPC–calcium sulfoaluminate (CSA) cements and its influence on early-age expansion. Cem. Concr. Res. 2016, 80, 10–20. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Langås, I.; Arntsen, B.; Poikelispää, M.; Illikainen, M. Low-temperature (−10 °C) curing of Portland cement paste—Synergetic effects of chloride-free antifreeze admixture, C-S-H seeds, and room-temperature pre-curing. Cem. Concr. Compos. 2022, 125, 104319. [Google Scholar] [CrossRef]
- Khan, J.; Kumar, S.G. Influence of binary antifreeze admixtures on strength performance of concrete under cold weather conditions. J. Build. Eng. 2021, 34, 102055. [Google Scholar] [CrossRef]
- Karagol, F.; Demirboga, R.; Khushefati, W.H. Behavior of fresh and hardened concretes with antifreeze admixtures in deep-freeze low temperatures and exterior winter conditions. Constr. Build. Mater. 2015, 76, 388–395. [Google Scholar] [CrossRef]
- Polat, R. The effect of antifreeze additives on fresh concrete subjected to freezing and thawing cycles. Cold Reg. Sci. Technol. 2016, 127, 10–17. [Google Scholar] [CrossRef]
- Demirboğa, R.; Karagöl, F.; Polat, R.; Kaygusuz, M.A. The effects of urea on strength development of fresh concrete under cold weather conditions. Constr. Build. Mater. 2014, 64, 114–120. [Google Scholar] [CrossRef]
- Poirier, M.; Blotevogel, S.; Noiriel, C.; Bonnin, A.; Kaknics, J.; Olbinado, M.; Steger, L.; Patapy, C.; Cyr, M. Synchrotron X-ray micro-tomography investigation of the early hydration of blended cements: A case study on CaCl2-accelerated slag-based blended cements. Constr. Build. Mater. 2022, 321, 126412. [Google Scholar]
- Wang, H.; Tong, M. Properties and field application of the grouting material for water blocking during thawing of frozen wall of deep sand layer. Arab. J. Geosci. 2021, 14, 1–12. [Google Scholar]
- Gómez-Luna, G.F.; Lopez-Calvo, H.Z.; Bremner, T.W.; Castro-Borges, P.; Montes-García, P. Performance of fly ash and CNI as corrosion prevention methods for steel reinforcement embedded in cracked HPC concrete exposed to a natural marine environment. Constr. Build. Mater. 2024, 428, 136260. [Google Scholar]
- Vu, T.H.; Dang, L.C.; Kang, G.; Sirivivatnanon, V. Chloride-induced corrosion of steel reinforcement in alkali-activated slag concretes: A critical review. Case Stud. Constr. Mater. 2022, 16, e01112. [Google Scholar]
- Jiang, M.; Liu, X.; Hang, M.; Xu, Y.; Lai, G.; Li, S. Performance and deterioration mechanism of concrete incorporated with corrosion-inhibiting admixtures under the coupling effect of composite salt and freeze-thaw cycles. J. Build. Eng. 2023, 69, 106329. [Google Scholar]
- Zhang, Y.; Wang, Y.; Li, T.; Xiong, Z.; Sun, Y. Effects of lithium carbonate on performances of sulphoaluminate cement-based dual liquid high water material and its mechanisms. Constr. Build. Mater. 2018, 161, 374–380. [Google Scholar]
- Shen, Y.; Zhang, W.; Wang, P.; Chen, X.; Zhu, H. Influence of lithium salt on the performance of calcium sulfoaluminate cement. J. Therm. Anal. Calorim. 2022, 147, 1–9. [Google Scholar]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021.
- Ming, X.; Li, Y.; Liu, Q.; Wang, M.; Cai, Y.; Chen, B.; Li, Z. Chloride binding behaviors and early age hydration of tricalcium aluminate in chloride-containing solutions. Cem. Concr. Compos. 2023, 137, 104928. [Google Scholar]
- Karagöl, F.; Demirboğa, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polat, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar]
- Thomas, J.J.; Allen, A.J.; Jennings, H.M. Hydration kinetics and microstructure development of normal and CaCl2-accelerated tricalcium silicate pastes. J. Phys. Chem. C 2009, 113, 19836–19844. [Google Scholar]
- Saha, O.; Boulfiza, M.; Wegner, L.D. Tracking the hydration of antifreeze treated cement paste at subfreezing temperatures using the TDR technique. Constr. Build. Mater. 2020, 262, 119915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).