Resistance of Graphene/Epoxy Resin—Based Composite Materials to γ Radiation Damage and Their Mechanical Properties
Abstract
:1. Introduction
2. Experimental Materials and Methods
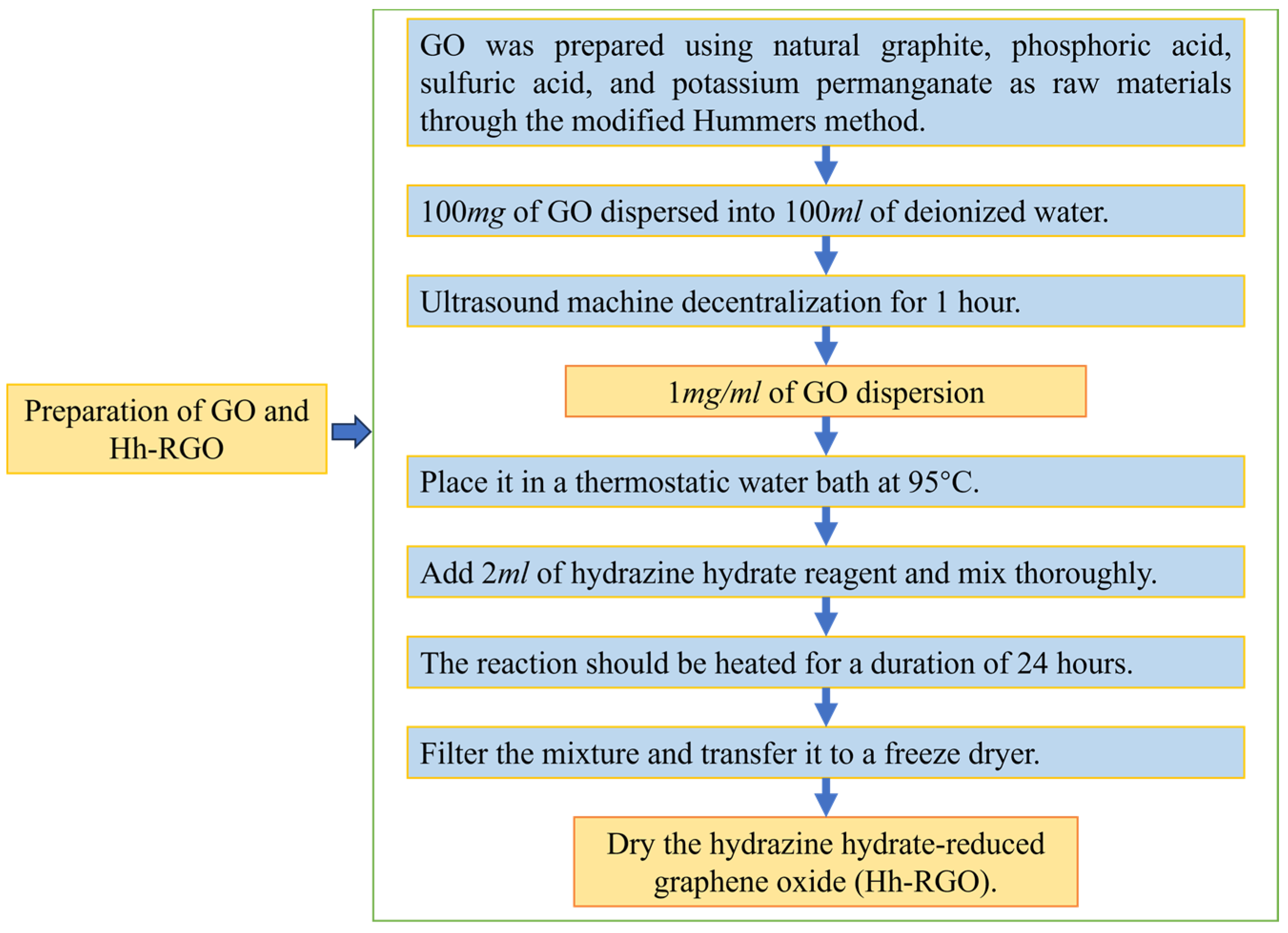
2.1. Preparation of GO and Hh-RGO
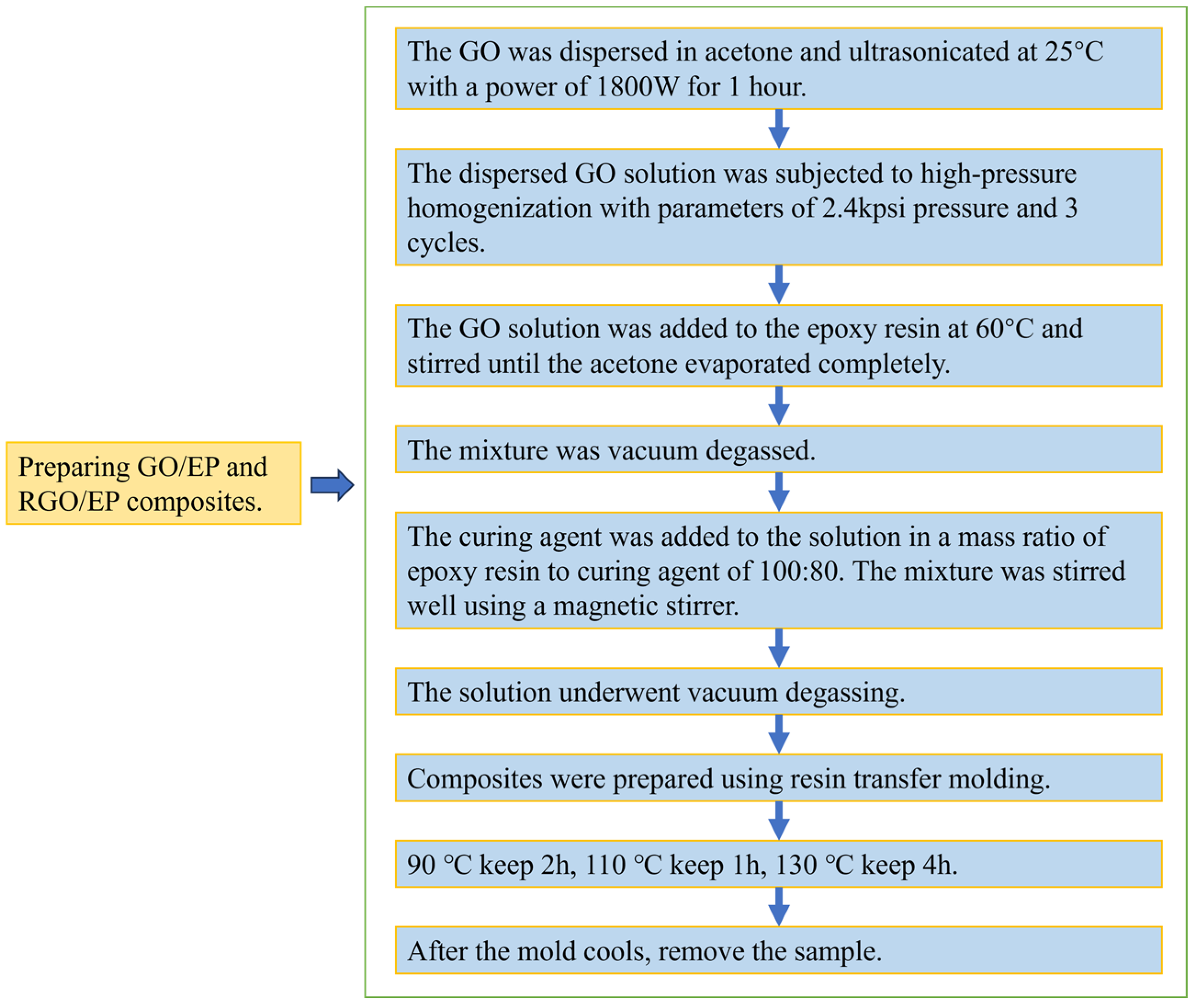
2.2. Preparation of GO/EP and Hh-RGO/EP Composite Materials
2.3. Experimental Procedure and Characterization
3. Results and Discussion
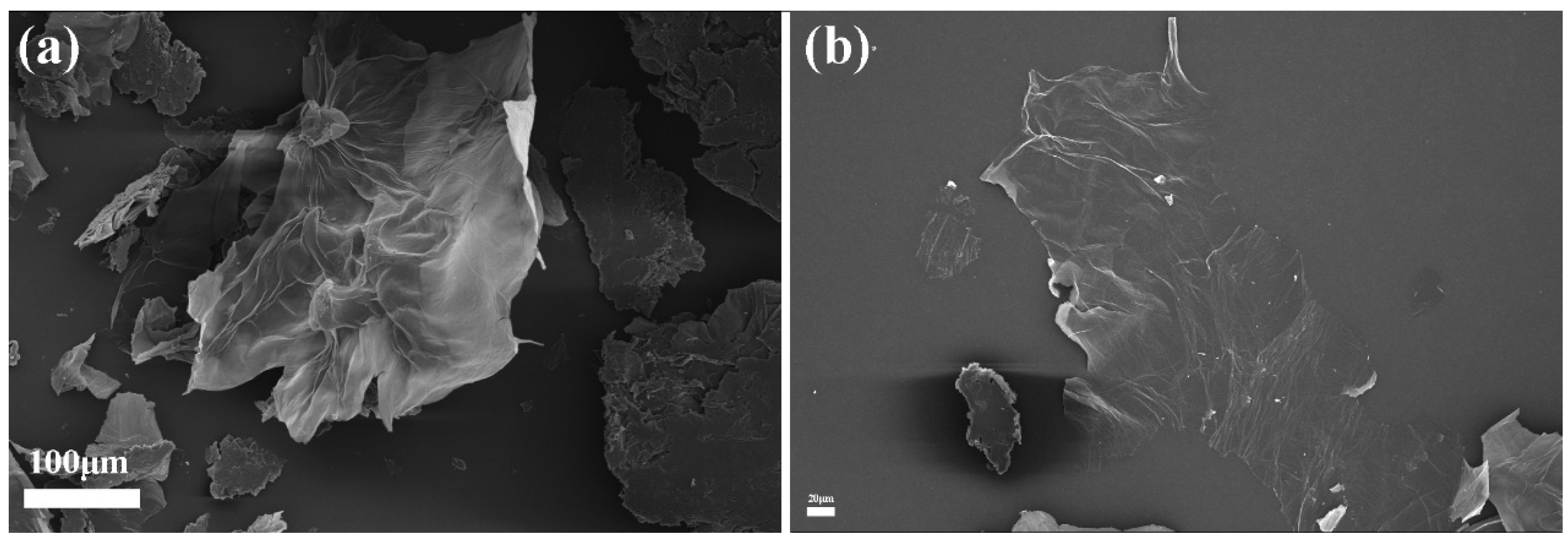
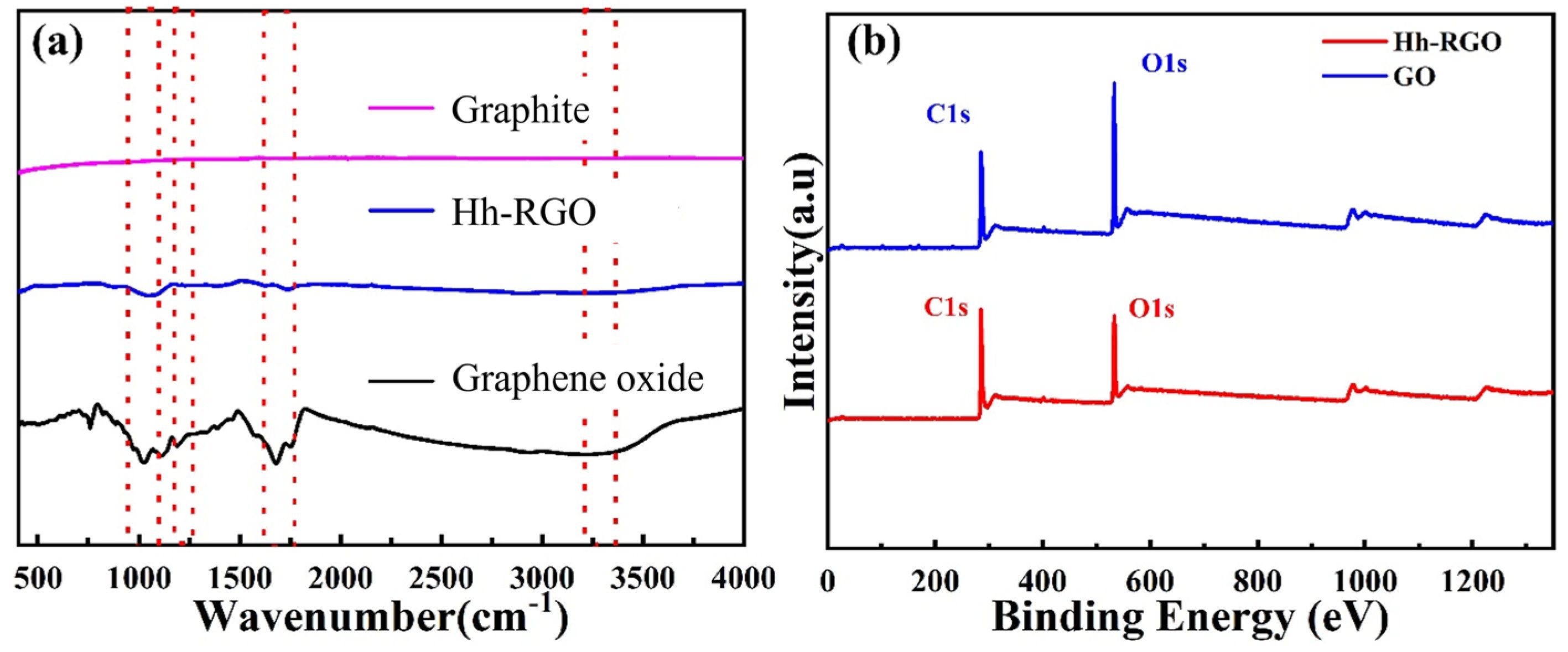
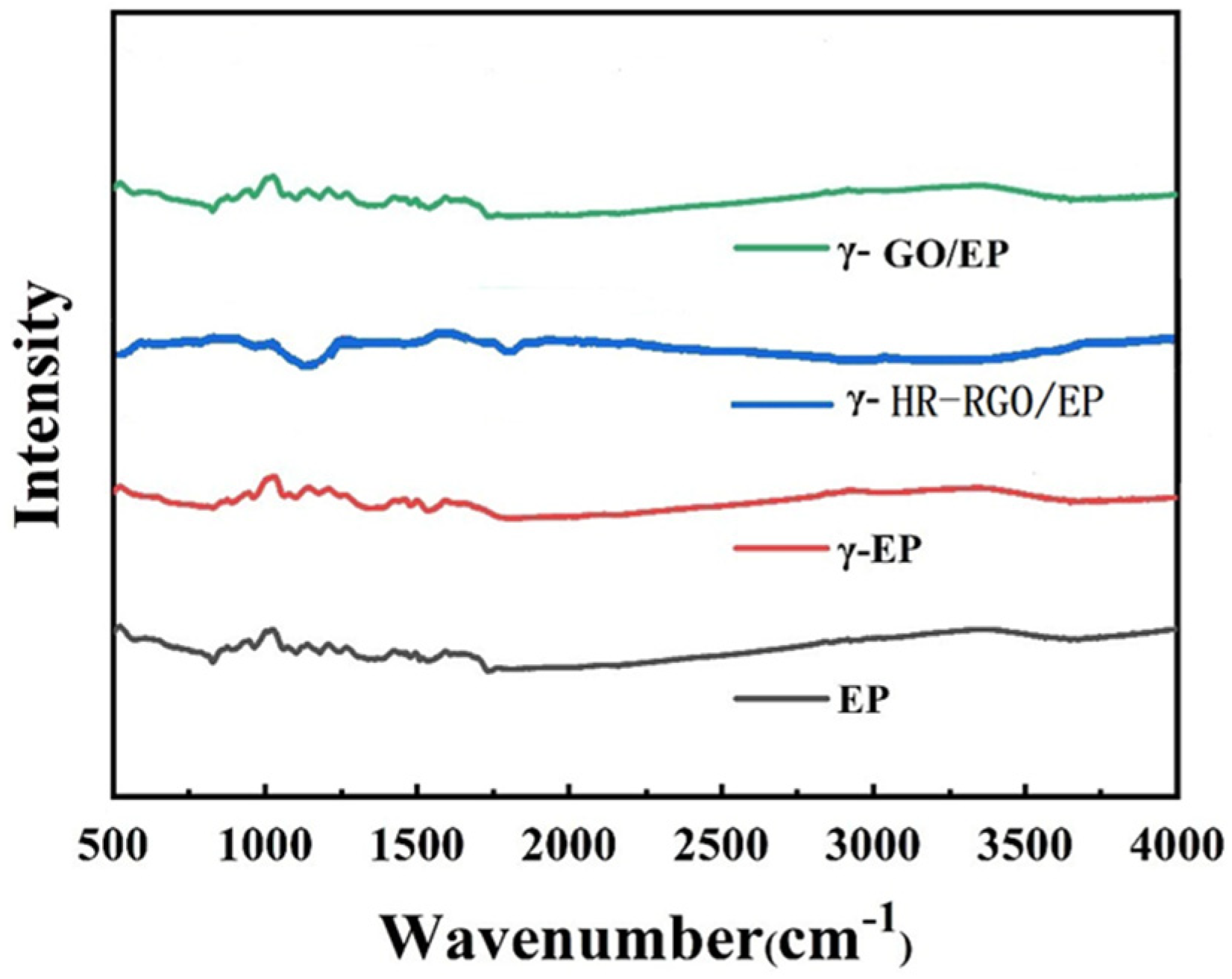
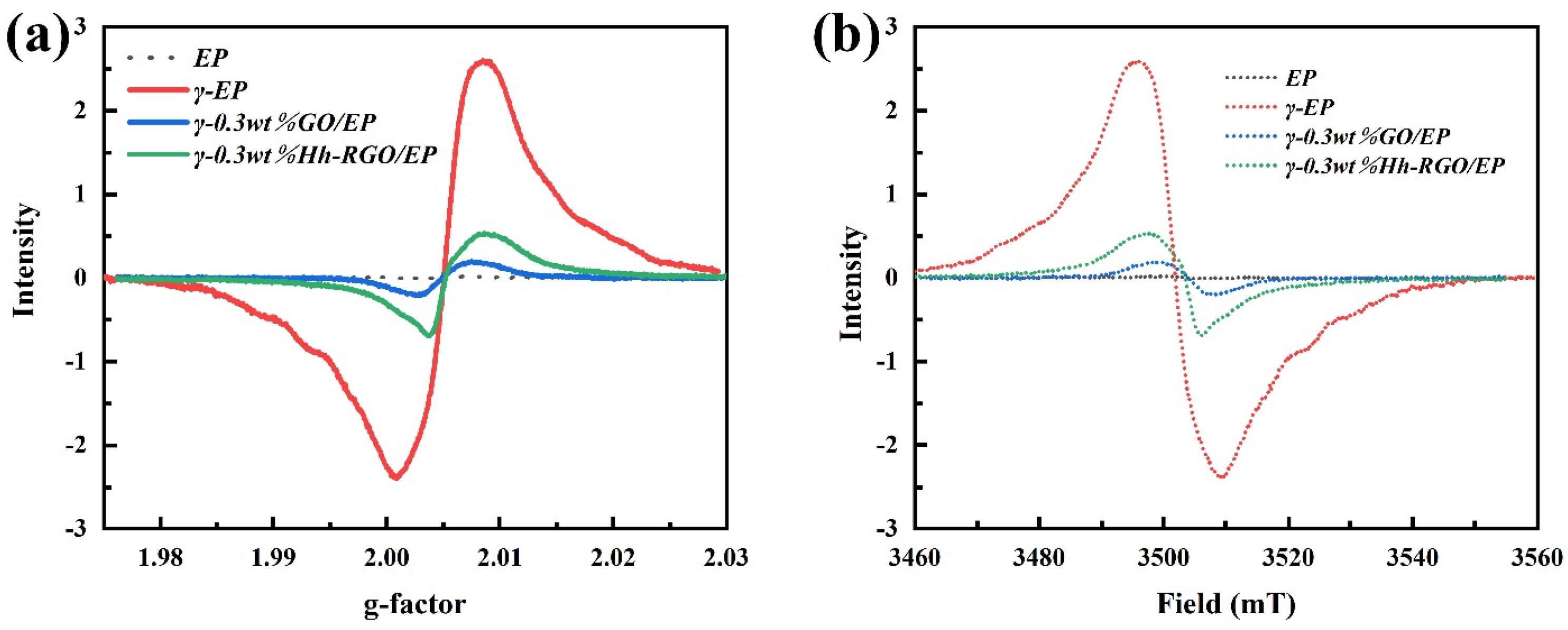
3.1. Microstructure
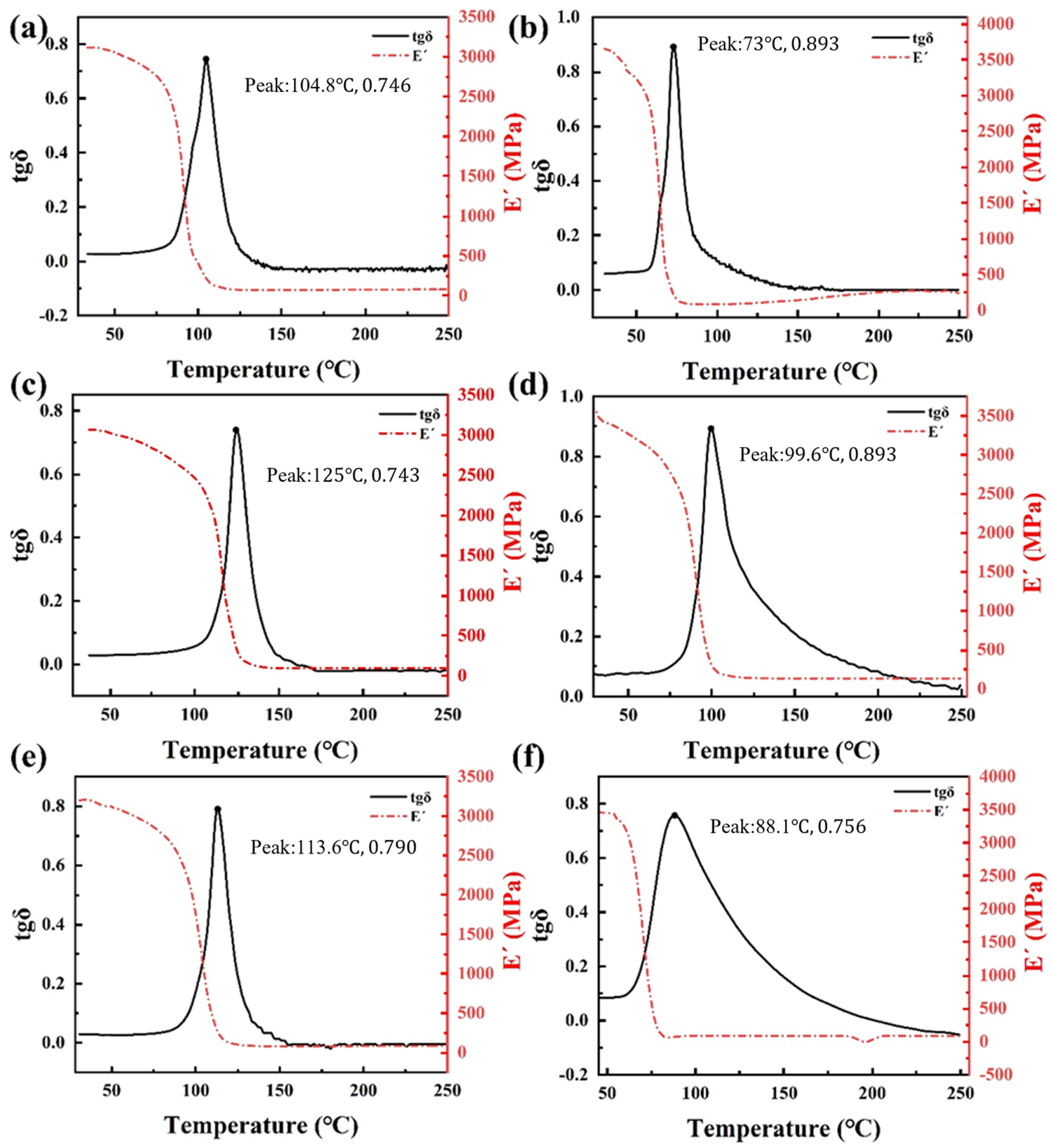
3.2. Thermal Stability
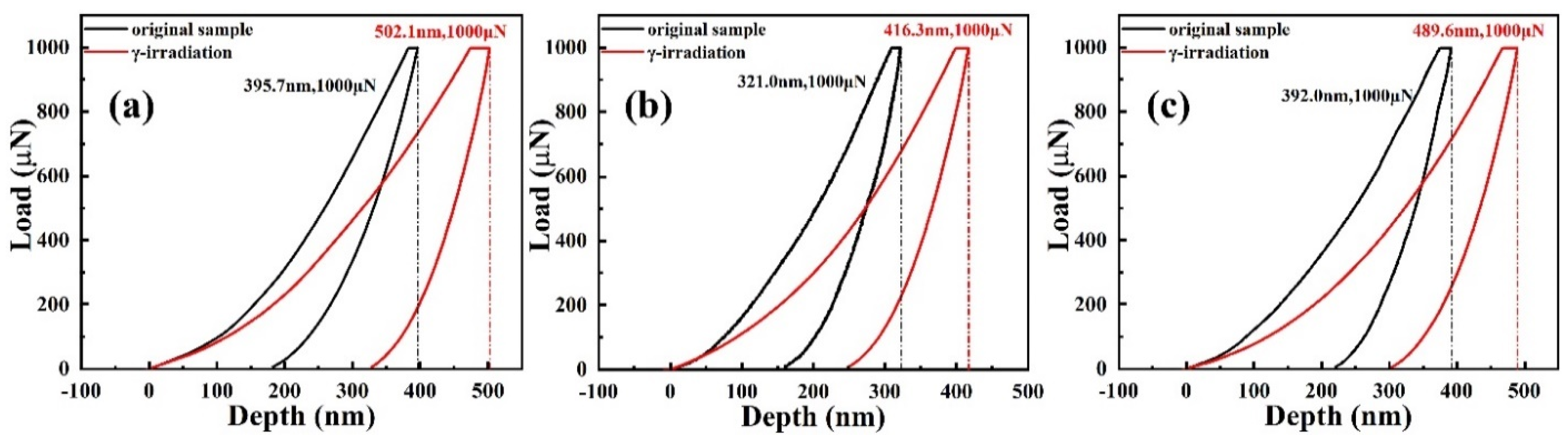
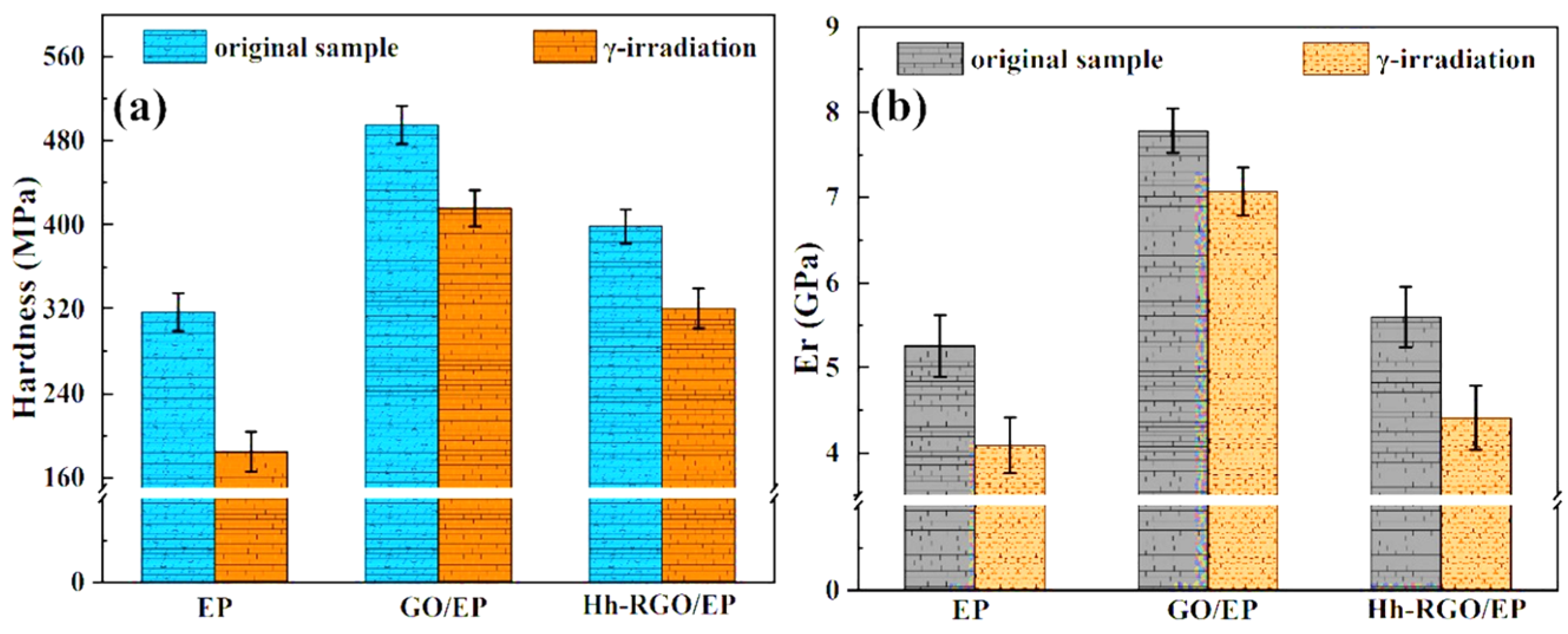
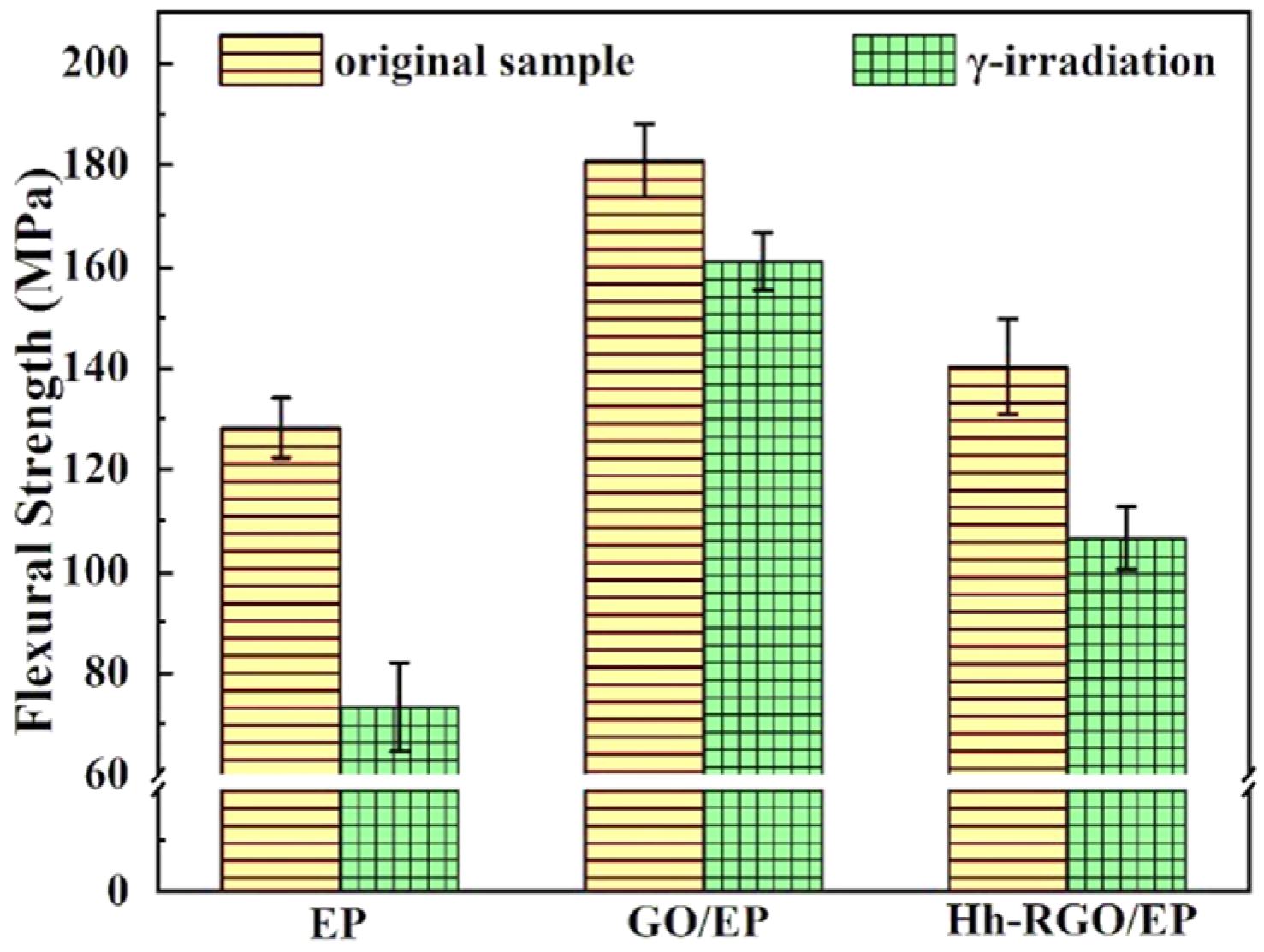
3.3. Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clare, D.C.; Marissa, A.R.; Erica, M.R.; Avi, G.B.; Khalid, H.; Amanda, S.P.; LaRico, J.F. Fabrication, thermal analysis, and heavy ion irradiation resistance of epoxy matrix nanocomposites loaded with silane-functionalized ceria nanoparticles. Phys. Chem. Chem. Phys. 2022, 24, 6552–6569. [Google Scholar] [CrossRef]
- Liu, L.; Feng, L.; Ma, T.; Xu, Z.; Pei, X.; Liu, Y.; Shi, H.; Tang, Y.; Liu, L.; Deng, H.; et al. Mechanical properties, thermal stability and microstructure evolution of carbon fiber-reinforced epoxy composites exposed to high-dose γ-rays. Radiat. Phys. Chem. 2022, 194, 110056. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Zhang, D.; Chen, C.; Wu, G. Radiation resistance of poly(methyl methacrylate)/reduced graphene oxide nanocomposites fabricated through latex mixing and in situ reduction. Chem. Eng. J. 2017, 315, 516–526. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Erol, B.; Baskan, T.; Mutlu, S.; Savaskan Yilmaz, S.; Yilmaz, A.H. Gamma Irradiation and the Radiation Shielding Characteristics: For the Lead Oxide Doped the Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers and the Polystyrene-b-Polyethyleneglycol-Boron Nitride Nanocomposites. Polymers 2021, 13, 3246. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Chen, L.; Wu, C.Y.; Chan, H.K.; Freeman, T. The Effects of Relative Humidity on the Flowability and Dispersion Performance of Lactose Mixtures. Materials 2017, 10, 592. [Google Scholar] [CrossRef]
- Xu, H.; Liu, D.; Sun, W.-Q.; Wu, R.-J.; Liao, W.; Li, X.-L.; Hu, G.; Hu, H.-S. Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials. Materials 2022, 15, 2978. [Google Scholar] [CrossRef]
- Acevedo-Del-Castillo, A.; Águila-Toledo, E.; Maldonado-Magnere, S.; Aguilar-Bolados, H. A Brief Review on the High-Energy Electromagnetic Radiation-Shielding Materials Based on Polymer Nanocomposites. Int. J. Mol. Sci. 2021, 22, 9079. [Google Scholar] [CrossRef]
- Zaharescu, T.; Mariş, M. Irradiation Effects in Polymer Composites for Their Conversion into Hybrids. J. Compos. Sci. 2022, 6, 109. [Google Scholar] [CrossRef]
- Manzhi, S.; Hong, Z.; Chunyang, L.; Xu, Y.; Zhidong, H.; Zhenguo, Y. Formulation and properties of UV crosslinked low voltage ethylene propylene diene monomer cable insulation material. Acta Mater. Compos. Sin. 2022, 39, 5922–5933. [Google Scholar] [CrossRef]
- Julmi, S.; Abel, A.; Gerdes, N.; Hoff, C.; Hermsdorf, J.; Overmeyer, L.; Klose, C.; Maier, H.J. Development of a Laser Powder Bed Fusion Process Tailored for the Additive Manufacturing of High-Quality Components Made of the Commercial Magnesium Alloy WE43. Materials 2021, 14, 887. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, B.; Han, T.; Wang, X. Gamma-ray irradiation induced variation in charge transport behavior of polyethylene based boron nitride/silica micro/nanocomposites. Materials 2020, 13, 2180. [Google Scholar] [CrossRef]
- Babu, M.; Myneni, R.B.; Sarathi, R.; Vasa, N.; Imai, T. Investigation on space charge and charge trap characteristics of gamma irradiated epoxy micro-nano composites. High Volt. 2020, 5, 191–201. [Google Scholar]
- Lv, J.; Wang, H.; Liu, Y.; Chen, J.; Chen, H.; Xu, J.; Sun, J.; Zhao, H.; Zhu, C. Nanocomposite enhanced radiation resistant effects in polyurethane elastomer with low fraction of polydoapmine nanoparticles. Compos. Sci. Technol. 2020, 186, 107908. [Google Scholar] [CrossRef]
- Dai, S.W.; Zhou, Y.J.; Li, W.D.; Bai, W.; Zhao, H.; Bai, H.; Hu, X. Interlaminar toughening of carbon fiber/epoxy composites with graphene oxide-carbon nanotube composite film. Acta Mater. Compos. Sin. 2023, 40, 3862–3873. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, P.; Wang, F. Graphene distribution and structural integrity dependent irradiation resistance of graphene/tungsten composites. Mater. Today Commun. 2022, 31, 103365. [Google Scholar] [CrossRef]
- Laskar, B.I.; Shukla, P.K. Adsorption of HOOO radical on pristine and doped graphene—A first-principles study. Struct. Chem. 2021, 32, 1171–1179. [Google Scholar] [CrossRef]
- Scalia, T.; Bonventre, L.; Terranova, M.L. From Protosolar Space to Space Exploration: The Role of Graphene in Space Technology and Economy. Nanomaterials 2023, 13, 680. [Google Scholar] [CrossRef]
- Guo, M.; Hei, Y.; Li, B.; Xing, L. Structure, mechanical property, electrical conductivity and lightning strike damage behavior of graphene/carbon nanotube co-modified CFRPs. Acta Mater. Compos. Sin. 2022, 39, 4354–4365. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Shen, Q.Q.; Wang, Y.; Li, T.; Ding, D.Q. Experimental study on mechanisms of reactions of radicals with graphene oxide particles in wastewater. J. Mol. Liq. 2023, 373, 121231. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Yao, W.J.; Fan, L.; Liu, G.Y. Advance in Properties of Graphene and Graphene/Metal Layered Composite After Irradiation Damage. Rare Met. Mater. Eng. 2019, 48, 3130–3135. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000494079600009(overlay:export/ris) (accessed on 1 June 2020).
- Hu, C.; Zhang, H.; Neate, N.; Fay, M.; Hou, X.; Grant, D.; Xu, F. Highly Aligned Ni-Decorated GO–CNT Nanostructures in Epoxy with Enhanced Thermal and Electrical Properties. Polymers 2022, 14, 2583. [Google Scholar] [CrossRef] [PubMed]
- Mostovoy, A.S.; Yakovlev, A.V.; Lopukhova, M.I. Directional control of physico-chemical and mechanical properties of epoxide composites by the addition of graphite-graphene structures. Polym. Plast. Technol. Mater. 2020, 59, 874–883. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Ladani, R.B.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C. A novel route for tether-ing graphene with iron oxide and its magnetic field alignment in polymer nanocomposites. Polymer 2016, 97, 273–284. [Google Scholar] [CrossRef]
- Wei, X.; Tao, W.; Li, S.; Hao, G.; Hu, G.; Xiaoli, F.; Bin, G.; Jun, Z.; Jianping, H. Graphene/Epoxy Composite Coating Damage under γ-ray Irradiation and Corrosion Protection. J. Inorg. Mater. 2018, 33, 35–40. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Alvarez, P.J.J.; Chen, W. Reduced graphene oxide enhances horseradish peroxidase stability by serving as radical scavenger and redox mediator. Carbon 2015, 94, 531–538. [Google Scholar] [CrossRef]
- Martínez-Morlanes, M.J.; Castell, P.; Alonso, P.J.; Martinez, M.T.; Puértolas, J.A. Multiwalled carbon nanotubes acting as free radical scavengers in gamma-irradiated ultrahigh molecular weight polyethylene composites. Carbon 2012, 50, 2442–2452. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Zhang, D.; Wu, G. Radiation resistance of polypropylene composites by incorporating reduced graphene oxide and antioxidant: A comparison study. Compos. Sci. Technol. 2017, 146, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, Y.M.; Wang, J.; Liu, H.; Liu, X.Q.; Wang, L.F.; Liu, X.G.; Xue, W.D.; Ma, N. Electrochemical synthesis of phosphorus-doped graphene quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000401022300066(overlay:export/exp) (accessed on 1 January 2020). [CrossRef]
- Ansón-Casaos, A.; Puértolas, J.A.; Pascual, F.J.; Hernández-Ferrer, J.; Castell, P.; Benito, A.M.; Maser, W.K.; Martínez, M.T. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef]
- ASTM D790-03; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org/d0790-03.html (accessed on 30 July 2023).
- Diao, F.; Zhang, Y.; Liu, Y.; Fang, J.; Luan, W. γ-Ray irradiation stability and damage mechanism of glycidyl amine epoxy resin. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 383, 227–233. [Google Scholar] [CrossRef]
- Xia, W.; Li, J.; Zhang, S.; Jiang, C.; Feng, Y.; Zhao, J.; Lin, Z.; Huang, X.; Wang, T.; He, J. In Situ Synthesis of Graphene-Phenol Formaldehyde Composites and Their Highly-Efficient Radical Scavenging Effects under the γ Irradiation. Corros. Sci. 2019, 159, 108139. [Google Scholar] [CrossRef]
- Marrale, M.; Longo, A.; Panzeca, S.; Gallo, S.; Principato, F.; Tomarchio, E.; Parlato, A.; Buttafava, A.; Dondi, D.; Zeffiro, A. ESR response of phenol compounds for dosimetry of gamma photon beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 339, 15–19. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Y.; Liu, Y.J.; Fang, J.; Luan, W.J. Study on the damage mechanism for epoxy resin under gamma irradiation. Thermosetting Resin 2016, 31, 10–14. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201702249560588765 (accessed on 1 January 2020).
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Laricheva, V.P. Radiation resistance of epoxy oligomers and prospects of creating new epoxy-oligomer based materials for space structures. In Proceedings of the 11th International Symposium on Materials in a Space Environment, Aix-en-Provence, France, 15–18 September 2009; Available online: http://esmat.esa.int/Materials_News/ISME09/pdf/4-New/Poster%20New%20Materials%20and%20Technologies%20-%20Laricheva.pdf (accessed on 1 June 2020).
- Yan, M.; Liu, L.; Chen, L.; Li, N.; Jiang, Y.; Xu, Z.; Jing, M.; Hu, Y.; Liu, L.; Zhang, X. Radiation Resistance of Carbon Fiber-Reinforced Epoxy Composites Optimized Synergistically by Carbon Nanotubes in Interface Area/Matrix. Compos. Part B Eng. 2019, 172, 447–457. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Pei, X.; Shi, H.; Li, D.; Xu, Z.; Li, S.; Xue, Y.; Song, L. Free radical scavenging behavior of multidimensional nanomaterials in γ-irradiated epoxy resin and mechanical and thermal performance of γ-irradiated composites. Compos. Part C 2021, 4, 100095. [Google Scholar] [CrossRef]
| Binding Energy (eV) | C-C (284.4–284.7) | C-O (286.1–286.4) | C=O (288.4–288.9) |
|---|---|---|---|
| EP (%) | 54.69 | 41.89 | 3.41 |
| γ-EP (%) | 75.92 | 14.72 | 9.36 |
| GO/EP (%) | 83.90 | 10.36 | 5.75 |
| γ-GO/EP (%) | 60.12 | 32.47 | 7.41 |
| Hh-RGO/EP (%) | 77.00 | 20.64 | 2.36 |
| γ-Hh-RGO/EP (%) | 63.74 | 27.54 | 8.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Pei, X.; Shao, R.; Liu, S.; Wang, W.; Zhao, C.; Xu, Z. Resistance of Graphene/Epoxy Resin—Based Composite Materials to γ Radiation Damage and Their Mechanical Properties. Coatings 2023, 13, 1536. https://doi.org/10.3390/coatings13091536
Wang H, Pei X, Shao R, Liu S, Wang W, Zhao C, Xu Z. Resistance of Graphene/Epoxy Resin—Based Composite Materials to γ Radiation Damage and Their Mechanical Properties. Coatings. 2023; 13(9):1536. https://doi.org/10.3390/coatings13091536
Chicago/Turabian StyleWang, Hongxia, Xiaoyuan Pei, Ruiqi Shao, Shengkai Liu, Wei Wang, Cun Zhao, and Zhiwei Xu. 2023. "Resistance of Graphene/Epoxy Resin—Based Composite Materials to γ Radiation Damage and Their Mechanical Properties" Coatings 13, no. 9: 1536. https://doi.org/10.3390/coatings13091536
APA StyleWang, H., Pei, X., Shao, R., Liu, S., Wang, W., Zhao, C., & Xu, Z. (2023). Resistance of Graphene/Epoxy Resin—Based Composite Materials to γ Radiation Damage and Their Mechanical Properties. Coatings, 13(9), 1536. https://doi.org/10.3390/coatings13091536






