Abstract
Multilayer cermet coatings based on a TiNbZrTaHf high-entropy alloy were produced on solid substrates by plasma-assisted vacuum-arc deposition. The assisting multicomponent metal-gas plasma was generated by evaporating TiNbZrTaHf cathodes in a gas mixture of nitrogen and argon. It was found that the coatings were nanocrystalline in structure (with nanocrystal sizes ranging from 2.5 to 4 nm). The metallic layer had a body-centered cubic lattice (a = 0.33396 nm), and the ceramic layer had a face-centered cubic lattice (a = 0.44465 nm). Transition layers formed between the substrate and the metallic layer and between the metallic and the ceramic layers were revealed. The hardness of the coatings was 36.7 GPa and their Young’s modulus was 323 GPa.
1. Introduction
High-entropy alloys (HEAs) are multielement alloys composed of at least five elements, the content of each being no more than 35%. They are equiatomic single-phase thermodynamically stable substitutional solid solutions having predominantly a body-centered cubic (bcc) or a face-centered cubic (fcc) structure. The possibility of producing HEAs was first demonstrated by [1,2]. A five-component Fe20Cr20Mn20Ni20Co20 alloy [1], later called the Cantor alloy, was produced by casting and melt spinning. This alloy was a single-phase system with an fcc lattice and a dendritic structure. This approach to designing alloys was suggested as a promising line in materials science and engineering [2]. Somewhat later, the authors of [3,4] reported on the possibility of creating ceramic materials based on HEAs. HEA-based nitride films were produced by ion-assisted DC magnetron sputtering [3]. The sputtering targets were made of Fe-Co-Ni-Cr-Cu-Al-Mn and Fe-Co-Ni-Cr-Cu-Al0.5 HEAs. It was found that the metallic films produced were close in atomic composition to the targets. They had a multiphase structure represented by grains with fcc and bcc lattices or by solid solution grains with an fcc lattice. When the sputtering targets were made of Co-Ni-Cr-Cu-Al-Mn and Fe-Co-Ni-Cr-Cu-Al0.5 alloys [4], it was observed that as the nitrogen content increased, the size of crystallites (grains) in the nitride films decreased, and the material became more amorphous. The hardness of the films reached 11 GPa.
It was found that nitride coatings based on HEAs, like nitride coatings based on transition metals, had a single-phase nanoscale structure with an fcc lattice [5,6,7,8]. The nitride films were produced by reactive RF magnetron sputtering of AlCrTaTiZr HEA targets [5,6]. It was found that they had a crystalline structure with an fcc lattice, while the AlCrTaTiZr metallic films had an amorphous structure. Nitride films with an fcc lattice, the maximum hardness of which was 30 GPa, were also produced by reactive RF magnetron sputtering of AlCrNbSiTiV HEA targets [7]. The authors of [8] report on the preparation of nitride films with an fcc lattice on (AlMoNbSiTaTiVZr)Nx films having a more complicated composition. Less often, HEA-based nitride coatings were two-phase coatings. Thus, it was shown that AlCrTiZrNbY HEA coatings produced by vacuum-arc deposition consisted of two nanocrystalline phases [9]. One phase had a bcc lattice with an average crystallite size of 15 nm and a lattice period of 0.342 nm, and the other had an fcc lattice with an average crystallite size of 7 nm and a lattice period of 0.437 nm.
Ceramic materials based on HEAs are sometimes considered a separate family of HEAs [10]. However, as noted in [11], these materials are not metal alloys but metal-like compounds in which metallic bonds between metal atoms coexist with ion-covalent bonds between metal and nonmetal atoms. It was found that HEAs and HEA-based ceramic materials have a number of remarkable properties (high corrosion resistance [12,13], excellent mechanical characteristics at high [14] and low temperatures [15,16], high wear resistance [17,18], high strength and plasticity [19,20], high hardness [5,7,8], etc.). It is expected that in the near future the following areas of application of HEAs and ceramic materials based on them may become promising [21,22,23]. Since HEAs are as light as aluminum alloys and stronger than some metallic glasses, they can be used in transport, aerospace, and power generation industries, where lightweight high-strength materials are in great demand. The HEAs containing refractory elements, such as Nb, Mo, Ta, and Hf, retain high strength at temperatures above 1450 K, outperforming traditional nickel-based superalloys such as Inconel 718 and Haynes 230. They are possible candidates for use as high-temperature materials in gas turbines, rocket nozzles, and components of nuclear reactors. In the aerospace industry, where lightweight materials resistant to high temperatures are in demand, low-density refractory HEAs can be used widely. The high cryogenic properties of HEAs make them excellent materials for cryogenic applications, such as rocket casings and pipelines for transporting and storing liquid oxygen or nitrogen. A surfacing technology holds promise in which HEAs are applied on tools and machine parts by plasma-arc or thermal spraying of HEA rods or powders. It can be expected that HEAs will be used to suppress electromagnetic interferences, especially in electronics. HEAs, as well as HEA-based nitrides, can be used as radiation-resistant coatings on the shells of fuel elements in nuclear reactors, which expands the scope of their high-tech applications.
In most cases, ceramic materials, including those based on HEAs, are used for various types of coatings. Among many types of coatings, multilayer coatings consisting of a combination of layers with different elemental compositions and, therefore, having different physical and chemical properties depending on the thickness and composition of each layer are of chief interest [24,25,26,27].
The authors of [24] investigated the mechanism of cracking in SZR-ZrN-(ZrCrAl)N, Cr-CrN-(TiCrAl)N, and Zr-ZrN-(ZrNbTiAl) multilayer composite nanostructured coatings, deposited using technologies of filtered cathodic vacuum-arc deposition, and in Ti-TiAlN-TiAlN coatings, deposited using side rotating cathodes. Typical cracking mechanisms were identified and their influence on the wear kinetics of coated hard-alloy tools was investigated. It was found that all test coatings increased the service life of carbide tools by a factor of three to four in comparison with uncoated tools. Single-layer and multilayer PVD coatings based on Cr and Ti, which are widely used in the tool industry, and coatings based on W and WN, which are not so widespread, were studied by the authors of [25]. They observed that the presence of a metallic interlayer in the test materials additionally improved the adhesion of the coatings because they gradually approached in composition to the substrates. Reviews [26,27] systematically consider the design concept and properties of various types of multilayer coatings, including transition-metal nitride coatings, DLC coatings, and other multilayer coatings. It is shown that the multilayer coatings consisting of a combination of several materials give developers an additional chance to optimize coating properties.
Multilayer coatings with alternating metallic and ceramic layers (La2O3-ZrO2-CeO2, ZrB2-ZrC-SiC, ZrB2 and HfB2, ZrB2-SiC) [28,29,30,31,32] are of much practical interest. Coatings of this type have higher rigidity compared to metals and higher fracture toughness compared to single-phase ceramics [33,34,35,36]. It was demonstrated [33,34] that the volume fraction of the metallic phase and the size of metal particles of the Al2O3-Ni/Al2O3-Cu-Ni cermet composite can be controlled by varying its hardness and fracture toughness. The authors of [36] studied laminated ZrB2-Mo5SiB2 ceramics with an intermediate Mo-Mo5SiB2 layer. They observed that the fracture toughness and bending strength of the ceramics showed a tendency first to increase and then to decrease with an increase in the ratio of layer thicknesses. High values of fracture toughness (9.89 ± 0.26 MPa m1/2) and bending strength (431.6 ± 15.1 MPa) were obtained for the layer thicknesses ratio equal to 13.
Vacuum-arc coating deposition techniques provide high productivity, and the produced coatings show high density and high adhesive strength to metal and cermet substrates [37]. Therefore, they are considered a promising way of fabricating metallic and cermet coatings.
The study presented in this paper was aimed to analyze the structure and properties of two-layer HEA-based (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coatings produced on solid substrates by plasma-assisted vacuum-arc deposition.
2. Materials and Methods
The HEA-based (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coatings used in the experiment were produced on solid substrates by plasma-assisted vacuum-arc deposition in the QUINTA facility [38]. For this purpose, a sintered multielement cathode of near-equiatomic TiNbZrTaHf composition was used. The experimental arrangement is shown schematically in Figure 1.
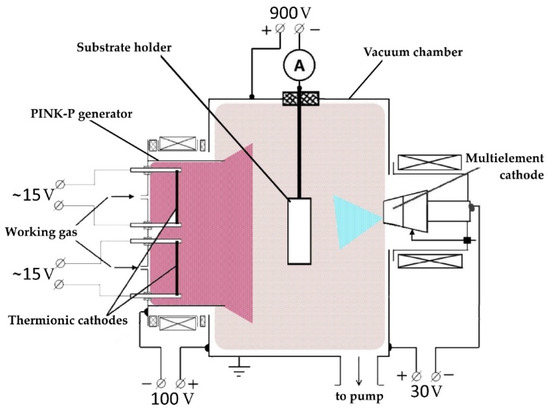
Figure 1.
Experimental arrangement for coating deposition using a multielement cathode.
The samples were cleaned with alcohol in an ultrasonic bath, fixed on the substrate holder, and placed in the vacuum chamber opposite the arc evaporator at a distance of 16 cm. With the samples, the vacuum chamber was evacuated with a turbomolecular pump to a limiting pressure of 5 × 10−3 Pa. Thereafter, argon was supplied into the chamber until its pressure reached 0.3 Pa, the PINK-P generator, having an exit aperture of 40 mm × 400 mm, was turned on, and the surfaces of the samples were cleaned with argon plasma using plasma-ion etching for 20 min. In this case, the discharge current of the PINK-P generator was 50 A and the repetitively pulsed bias voltage was –900 V with 50% pulse duty cycle. The temperature of the samples during surface cleaning reached 350 °C in 20 min. After cleaning, the arc evaporator (75 A discharge current), comprising a multielement composite cathode, was switched on, and the substrate surface was bombarded with high-energy metal ions at a constant bias voltage (−900 V) for 2 min. Thereafter, the bias voltage was reduced to −35 V, the pulse duty cycle was increased to 85%, and a HEA metal layer was deposited on the substrate. When a HEA ceramic layer was deposited, the bias voltage was increased to −150 V with the same pulse duty factor (85%). Depending on the type of supplied gas, either HEA metallic or HEA ceramic layers were produced. In the first case, only argon was supplied into the working chamber, while in the second case, nitrogen and argon in equal proportions were used. To produce a cermet coating, layer-by-layer deposition of metallic and ceramic films was performed. In our experiment, the metallic layer thickness was ≈1 µm and the ceramic layer thickness was ≈3 µm. After the deposition was completed, the samples were rest to cool in the vacuum chamber to a temperature below 100 °C and removed for examination.
To examine the elemental and phase composition of the coatings and the state of their defective substructure, a scanning electron microscope (Philips SEM-515 with an EDAX ECON IV microanalyzer) (Philips, Amsterdam, The Netherlands) and a transmission electron diffraction microscope (JEOL JEM-2100F) (Akishima, Tokyo, Japan) were used. The phase composition and structural parameters of the coatings were investigated by the method of X-ray diffraction analysis using an XRD-6000 diffractometer (Shimadzu, Kyoto, Japan) with CuKα radiation. Scanning was carried out at a voltage of 40.0 kV, current of 30.0 mA, scan range of 10.0–80.0 deg, scan speed of 2.0 deg/min, sampling pitch of 0.0200 deg, and preset time of 1.00 s. To analyze the phase composition, the PDF 4+ databases and the POWDER CELL 2.4 full-profile analysis code were invoked. The hardness of the HEA films was measured using a PMT-3 (LOMO, Saint Petersburg, Russia) instrument equipped with a Vickers indenter (the load on the indenter was 0.5 N). The physical and mechanical properties of the coatings were investigated using a TTX-NHT nanohardness tester (CSM Instruments, Peuseux, Switzerland). Indentations were made with an acquisition rate of 20.0 Hz using a linear loading to a maximum load of 30.0 mN at a loading/unloading rate of 60.00 mN/min; the time interval between successive loading/unloading cycles was 5.0 s. The indentation depth was less than 10% of the total coating thickness to rule out the substrate effect [39]. The measurement data were analyzed by the Oliver–Pharr method. Tribological investigations of the HEA films were carried out using an oscillating pin-on-disk tribometer (TRIBOtechnic, Clichy, France) with a WC–8 wt.% Co hard alloy ball 6 mm in diameter. The wear track radius was 2 mm, the indenter load was 2 N, the track length was 50 m, and the sample rotation speed was 25 mm/s. The degree of wear of the material was evaluated from the results of wear track profilometry. The wear rate (V) was calculated based on the load (F), the distance covered (L), the cross-sectional area of the wear track (A), and the wear track radius (R) using a well-known formula:
The substrate material was made of a WC–8%Co hard alloy, selected to study physical, mechanical and tribological properties, elemental and phase analysis, and AISI 304 non-magnetic stainless steel [(up to 0.08)C; (17,5–20)Cr; (up to 2)Mn; (8–11)Ni; (up to 0.045)P; (up to 0.03)S; (up to 1)Cu; (up to 0.8)Si; the rest Fe, wt.%] for transmission electron microscopy (TEM) analysis.
3. Results
X-ray microanalysis of one of the samples has shown that the cermet film had the following elemental composition (at.%), averaged over five sections of area 0.043 mm2 each: 4.8Ti-16.7Nb-16.7Zr-9.8Ta-13.9Hf-38.1N, i.e., the metal/nitrogen ratio was 62/38. Figure 2 and Table 1 show the characteristic energy spectra and the results of a quantitative analysis of the elemental composition of one of the analyzed sections of the film.
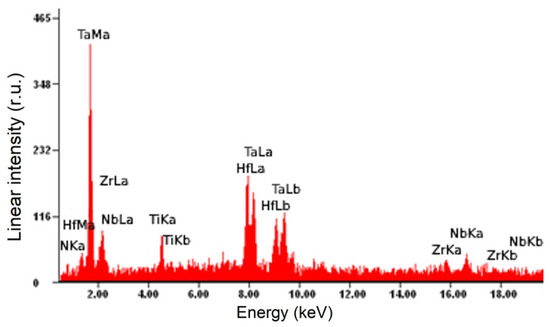
Figure 2.
Energy spectra of a section of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet sample obtained by X-ray microanalysis of its elemental composition.

Table 1.
Quantitative results of the elemental analysis of the sample.
X-ray diffraction analysis has shown that the HEA cermet film was a multiphase system, the main phases of which were (TiNbZrTaHf)N and Ta4N nitrides and a TiNbZrTaHf alloy (Figure 3). The lattice parameters and the relative content of these phases are given in Table 2.
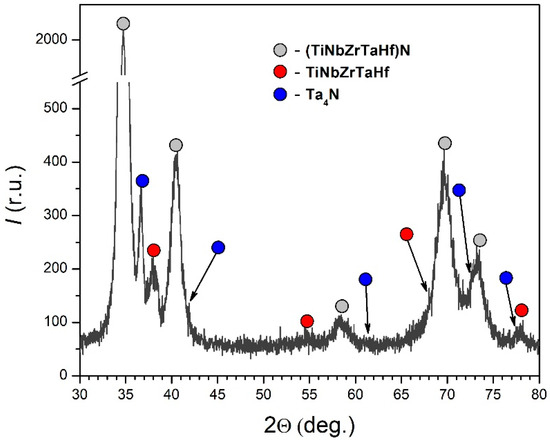
Figure 3.
Fragment of the X-ray diffraction pattern of a HEA cermet film produced by evaporating a TiNbZrTaHf cathode.

Table 2.
Results of X-ray phase analysis of a HEA cermet film produced by evaporating a TiNbZrTaHf cathode.
The data presented in Table 2 indicate that the total content ratio of the nitride phases to the metallic phase was 2.5:1, which correlates well with the thicknesses of the deposited nitride and metallic layers. The (TiNbZrTaHf)N nitride had an fcc lattice with a = 4.4465 Å (D = 22 nm; Δd/d = 7 × 10−3). Preliminary investigations have shown that the separately deposited HEA ceramic film had an fcc lattice with a = 4.5084 Å, a width of coherent scattering regions D = 75 nm, and a relative lattice distortion Δd/d = 3.6 × 10−3. Comparing these data, it can be noted that the ceramic film formed in the cermet system had a smaller (by 0.0619 Å) value of the lattice parameter and smaller (by a factor of 3.4) widths of coherent scattering regions (CSR width) at somewhat larger (by a factor of 1.9) values of the relative lattice distortions in comparison with the single-layer ceramic film. The Ta4N nitride had a tetragonal lattice with a = 6.8272 Å and c = 4.1697 Å.
The TiNbZrTaHf metallic phase had a bcc lattice with a = 3.3396 Å (D = 8 nm; Δd/d = 5 × 10−3). Preliminary investigations have shown that the sputtered TiNbZrTaHf cathode had a bcc lattice with a = 3.4278 Å (D = 85 nm; Δd/d = 5 × 10−3). Comparing these data, it can be noted that the metallic layer of the cermet coating had a smaller lattice parameter and smaller CSR widths in comparison with the cathode alloy with the same relative lattice distortion. The data obtained by X-ray diffraction analysis obviously indicate the occurrence of elastic stresses in the cermet coating, which was due to the differences in lattice types and parameters between the ceramic and the metallic film. As will be shown below, the stresses partially relaxed due to the transition sublayer formed at the interface between the metallic and the ceramic layer.
The electron microscopic images given in Figure 4a indicate that the (TiNbZrTaHf + (TiNbZrTaHf)N) cermet film was a multilayer system consisting of two main layers (layers 2 and 4) and two transition layers, significantly smaller in thickness (layers 1 and 3). Layers 2 and 4 had a columnar structure (Figure 4b). Analysis of the microelectron diffraction patterns taken from these layers (the sections from which the microelectron diffraction patterns were taken are circled) indicates that layer 2 had a bcc lattice (Figure 4c) and was obviously a metallic layer; layer 4 had an fcc lattice (Figure 4d) and was a ceramic layer. Dark-field analysis has shown that layers 2 and 4 had a nanocrystalline structure with a crystallite size varying between 2.5 and 4 nm, regardless of whether the layer was metallic or ceramic (Figure 4e,f).
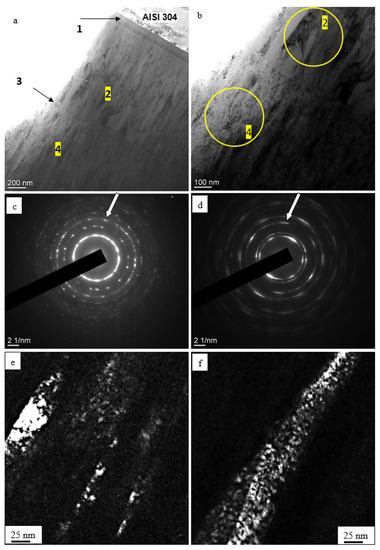
Figure 4.
Electron microscopic images showing the structure of (a) (TiNbZrTaHf + (TiNbZrTaHf)N) cermet film: (a,b)—light-field images; (c,d)—microelectron diffraction patterns for (b); (e,f)—dark-field images taken from the [112] (e) and the [022] reflex (f). The reflexes from which the dark-field images were taken are indicated by arrows in (e,f). The foil sections from which microelectron diffraction patterns (c,d) were taken are circled in (b). Substrate: AISI-304 stainless steel.
Layer 1, which separates the metallic coating from the substrate, had a thickness of 100–110 nm (Figure 5a,b) and consisted of three sublayers (Figure 5c): sublayer 1.1, adjacent to the substrate, was amorphous in structure; sublayer 1.2 consisted of rounded crystallites 2.5–5 nm in size (Figure 5e), and sublayer 1.3, adjacent to the HEA metallic film, was columnar in structure (see Figure 5e). Nanoscale size of the crystallites is evident from the ring structure of the electron diffraction patterns (Figure 5d).
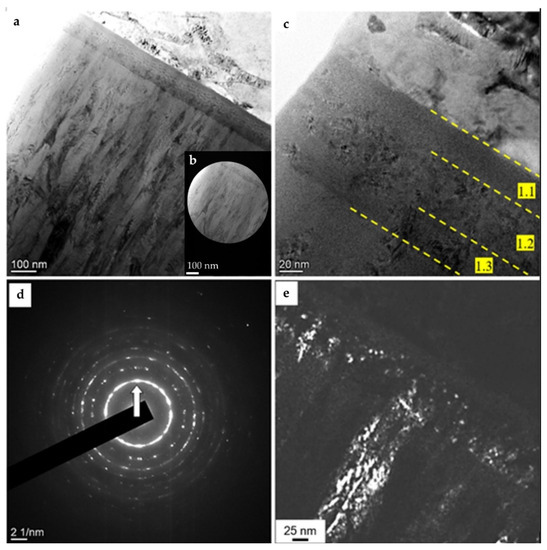
Figure 5.
Electron microscopic image of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet film, taken in the region of formation of the first transition layer: (a–c)—light-field images, (d)—microelectron diffraction pattern, (e)—dark-field image taken from the [110] reflex (indicated by the arrow in (d)). Substrate: AISI-304 stainless steel.
Figure 6a,b shows a cermet coating specimen image, taken in the region of formation of the second transition layer, obtained in the light-field mode by the TEM method. Figure 6c demonstrates a microdiffraction image of the region. Transition layer 3 of thickness 30–45 nm, separating the metallic and the ceramic layer (Figure 6a,b), consisted mainly of round-shaped crystallites 2.5–3 nm in size (Figure 6d).
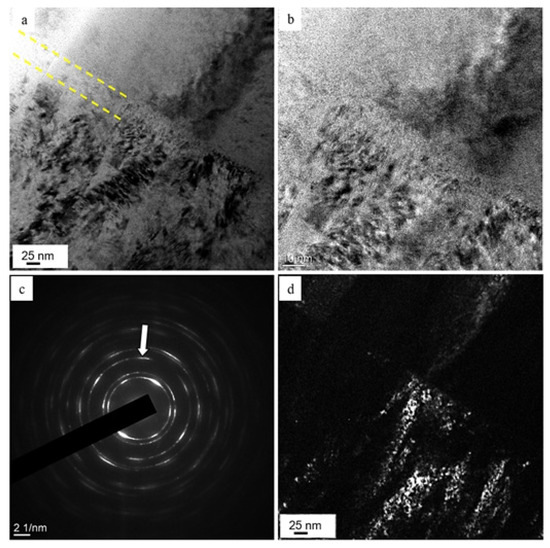
Figure 6.
Electron microscopic image of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet film, taken in the region of formation of the second transition layer: (a,b)—light-field images, (c)—microelectron diffraction pattern, (d)—dark-field image taken from the [220] reflex (indicated by the arrow in (c)). The transition layer is indicated by dashed lines in (a).
It can be supposed that one of the main reasons for the formation of transition layer 3, located along the contact boundary of the HEA metallic and ceramic layers, was, as shown above, elastic stresses that arose due to different types and parameters of the crystal lattices. The reason for the formation of transition layer 1, located at the contact boundary between the substrate and the HEA metallic layer, was the sputtering of the substrate atoms and their mixing with particles entered at the substrate, which was followed by the settlement of the mixture on the substrate at the initial stage of coating formation. In addition, this layer was most likely stressed, which was due to its complex elemental composition. It was noted [3,40] that the more chemical elements with very different atomic radii (Δ = Rmax − Rmin) make up the coating, the greater the intracrystalline deformation and the higher the probability of formation of an amorphous-like state of the alloy. It can be speculated that the activation of the substrate surface before deposition of the metallic layer was accompanied by sputtering of the substrate and formation of a transition layer containing HEA atoms and stainless-steel atoms. The atoms of the stainless-steel components have minimum radii R(Ni) = 0.124 nm and R(Cr) = 0.125 nm, the HEA atoms have a maximum radius R(Zr) = 0.160 nm, and, hence, we have Δ = Rmax − Rmin = 0.036 nm. In a study of TiAlCuZrNb HEA films (Δ = R(Zr) − R(Cu) = 0.032 nm) [41], the formation of an amorphous-crystalline state was revealed.
The physical and mechanical characteristics of the coatings were investigated using a TTX-NHT nanohardness tester at a normal load of 30 mN. Figure 7 shows a typical loading/unloading diagram obtained during the hardness test of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coating (Fn is the normal load and Pd is the penetration depth). The results of calculating the parameters of the diagram are given in Table 3.
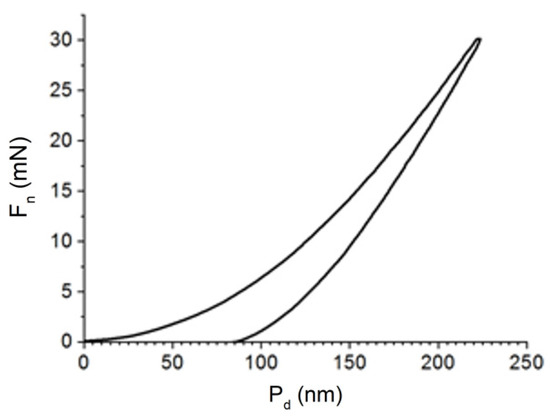
Figure 7.
Loading/unloading diagram obtained during the hardness test of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coating.

Table 3.
Nanoindentation characteristics of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coating.
Table 4 presents the average values of the film physicomechanical characteristics derived from the nanoindentation data given in Table 3. The hardness of the (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coatings produced on the WC–8 wt.% Co hard alloy was 36.7 GPa and their Young’s modulus was 323 GPa (Table 4). Additionally, the hardness of the cermet coatings measured using a PMT-3 instrument at a normal load of 0.5 N was 27.0 GPa.

Table 4.
Physicomechanical characteristics of a (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coating.
For the (TiNbZrTaHf + (TiNbZrTaHf)N) cermet films, the wear parameter k (inversely proportional to wear resistance) was 2.9 × 10−5 mm3/N·m and the friction coefficient μ was 0.71. In preliminary investigations of the TiNbZrTaHfN ceramic films produced by plasma-assisted vacuum-arc deposition, we have found that k = 3.3 × 10−5 mm3/N·m and μ = 0.89. The (TiNbZrTaHf)N ceramic coatings, produced on C45 and M2 stainless-steel substrates by dc magnetron sputtering in confocal geometry in a mixture of argon and nitrogen using an AJA ATC ORION system equipped with Ti, Zr, Nb, Hf, and Ta cathodes (each having a purity of 99.95% and diameter 0.0508 m) showed the following characteristics: HV = 30.9 GPa, k = 0.29 × 10−6 mm3/N·m, and μ = 0.17 [45].
4. Conclusions
A plasma-assisted vacuum-arc deposition method has been developed and used to produce HEA-based (TiNbZrTaHf + (TiNbZrTaHf)N) multilayer ceramic-metal (cermet) coatings. A multicomponent gas-metal plasma was generated during the evaporation of a multielement cathode made of TiNbZrTaHf alloy of near-equiatomic composition. Depending on the gas supplied into the working chamber, a HEA metallic layer (argon) or a HEA ceramic layer (nitrogen and argon in equal proportions) was formed. The thickness of the metallic layer was 1 µm, and that of the ceramic layer was 3 µm.
The (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coating was a multilayer system consisting of two main layers and two transition layers. It was found that transition layers were formed between the substrate and the metallic layer and between the metallic and the ceramic layers.
X-ray diffraction analysis and transmission electron microscopy have shown that the HEA cermet coatings was a multiphase system, the main phases of which were (TiNbZrTaHf)N and Ta4N nitrides and a TiNbZrTaHf alloy. The ceramic layer was found to be a two-phase ((TiNbZrTaHf)N + Ta4N) system. The TiNbZrTaHf metallic phase had a bcc lattice with a = 3.3396 Å (D = 8 nm; Δd/d = 5 × 10−3). The (TiNbZrTaHf)N nitride had an fcc lattice with a = 4.4465 Å (D = 22 nm; Δd/d = 7 × 10−3). The Ta4N nitride had a tetragonal lattice with a = 6.8272 Å and c = 4.1697 Å. The cermet coatings had a nanocrystalline structure with a crystallite size of 2.5–4 nm, regardless of whether the layer was metal or ceramic.
The hardness of the cermet coatings was 36.7 GPa and their Young’s modulus (at a Poisson’s ratio of 0.25) was 323 GPa. The wear parameter of the multilayer (TiNbZrTaHf + (TiNbZrTaHf)N) cermet coatings was 2.9 × 10−5 mm3/N·m and their coefficient of friction was 0.71.
Due to the high concentrations of many expensive metals, a potential application of presented HEAs-based coatings is depositing HEAs as thin coatings on low-cost substrates. The obtained results may also contribute to the further development of such multilayer coatings for specific applications.
The advantages of the plasma-assisted vacuum-arc deposition method are the ecological safety of the process and the possibility of complete automation of the cleaning of the substrate surface before coating deposition. This method makes it possible to realize a plasma-ion deposition process to produce HEA films of required elemental composition. It also enables a step-by-step formation of metallic, cermet, and ceramic films and coatings with strictly controlled thickness and elemental composition.
Author Contributions
Conceptualization, Y.F.I.; methodology Y.F.I.; validation, O.V.K.; investigation E.A.P., N.A.P. and O.S.T.; resources V.V.S.; data curation N.N.K.; writing—original draft preparation, Y.F.I. and O.V.K.; writing—review and editing, Y.F.I., N.A.P. and O.V.K.; visualization, E.A.P., N.A.P., Y.K.A. and O.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support of the Russian Federation represented by the Ministry of Science and Higher Education (project No. 075-15-2021-1348) within the framework of event No.3.1.4, 3.1.5, 3.1.12 and 3.1.13.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The measurements were carried out using the equipment of the CSU NMNT TPU, supported by the RF MES Project No. 075-15-2021-710.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Chen, T.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Chen, T.-K.; Wong, M.-S.; Shun, T.-T.; Yeh, J.-W. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2005, 200, 1361–1365. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Lai, C.-H.; Tsai, M.-H.; Lin, S.-J.; Yeh, J.-W. Influence of substrate temperature on structure and mechanical, properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2007, 201, 6993–6998. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Lai, C.-H.; Yeh, J.-W.; Gan, J.-Y. Effects of nitrogen flow ratio on the structure and properties of reactively sputtered (AlMoNbSiTaTiVZr)Nxcoatings. J. Phys. D Appl. Phys. 2008, 41, 235402. [Google Scholar] [CrossRef]
- Beresnev, V.M.; Sobol’, O.V.; Litovchenko, S.V.; Pogrebnyak, A.D.; Srebnyuk, P.A.; Novikov, V.Y.; Kolesnikov, D.A.; Meilekhov, A.A.; Postel’nik, A.A.; Nemchenko, U.S. Effect of bias voltage and nitrogen pressure on the structure and properties of vacuum-arc (Mo + Ti6%Si)N coatings. Tech. Phys. 2017, 62, 795–798. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Rogachev, A.S. Structure, Stability, and Properties of High-Entropy Alloys. Phys. Met. Metallogr. 2020, 121, 733–764. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Lee, T.-D.; Chen, S.-K.; Chang, Y.-S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Qiao, J.W.; Ma, S.; Huang, E.; Chuang, C.; Liaw, P.; Zhang, Y. Microstructural Characteristics and Mechanical Behaviors of AlCoCrFeNi High-Entropy Alloys at Ambient and Cryogenic Temperatures. Mater. Sci. Forum 2011, 688, 419–425. [Google Scholar] [CrossRef]
- Laktionova, M.A.; Tabchnikova, E.D.; Tang, Z.; Liaw, P.K. Mechanical properties of the high-entropy alloy Ag0.5CoCrCuFeNi at temperatures of 4.2–300 K. Low Temp. Phys. 2013, 39, 630–632. [Google Scholar] [CrossRef]
- Wu, J.-M.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Qiao, Y.; Chen, G. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; A Bagdasaryan, A.; Yakushchenko, I.V.; Beresnev, V.M. The structure and properties of high-entropy alloys and nitride coatings based on them. Russ. Chem. Rev. 2014, 83, 1027–1061. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Grigoriev, S.N. Study of cracking mechanisms in multi-layered composite nano-structured coatings. Wear 2017, 378–379, 43–57. [Google Scholar] [CrossRef]
- Horník, J.; Krum, S.; Tondl, D.; Puchnin, M.; Sachr, P.; Cvrček, L. Multilayer coatings Ti/Tin, Cr/Crn and W/Wn deposited by magnetron sputtering for improvement of adhesion to base materials. Acta Polytech. 2015, 55, 388–392. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Yang, H.-K.; Kim, D.-E. Tribology of multilayer coatings for wear reduction: A review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Multilayer Coatings for Tribology: A Mini Review. Nanomaterials 2022, 12, 1388. [Google Scholar] [CrossRef]
- Park, J.; Cho, S.; Kwon, H. Aluminum-ceramic composites for thermal management in energy-conversion systems. Sci. Rep. 2018, 8, 17852. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Tietz, F.; Stoever, D. New double-ceramic-layer thermal barrier coatings based on zirconia–rare earth composite oxides. J. Eur. Ceram. Soc. 2006, 26, 247–251. [Google Scholar] [CrossRef]
- Xing, Y.; Baumann, S.; Uhlenbruck, S.; Rüttinger, M.; Venskutonis, A.; Meulenberg, W.; Stöver, D. Development of a metallic/ceramic composite for the deposition of thin-film oxygen transport membrane. J. Eur. Ceram. Soc. 2013, 33, 287–296. [Google Scholar] [CrossRef]
- Guo, S.-Q.; Kagawa, Y.; Nishimura, T.; Chung, D.; Yang, J.-M. Mechanical and physical behavior of spark plasma sintered ZrC–ZrB2–SiC composites. J. Eur. Ceram. Soc. 2008, 28, 1279–1285. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Zaykoski, J.A. Oxidation-based materials selection for 2000 °C + hypersonic aerosurfaces: Theoretical considerations and historical experience. J. Mater. Sci. 2004, 39, 5887–5904. [Google Scholar] [CrossRef]
- Konopka, K.; Maj, M.; Kurzydłowski, K.J. Studies of the effect of metal particles on the fracture toughness of ceramic matrix composites. Mater. Charact. 2003, 51, 335–340. [Google Scholar] [CrossRef]
- Piotrkiewicz, P.; Zygmuntowicz, J.; Wachowski, M.; Cymerman, K.; Kaszuwara, W.; Midor, A.W. Al2O3-Cu-Ni Composites Manufactured via Uniaxial Pressing: Microstructure, Magnetic, and Mechanical Properties. Materials 2022, 15, 1848. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Fan, H.; Fang, Y.; Hu, L. Design of structure parameters and corrugated interfaces for optimal mechanical properties in alumina/graphite laminated nanocomposites. Mater. Des. (1980–2015) 2015, 65, 1205–1213. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wang, H.; Shao, G.; Zhu, J.; Liu, W.; Wang, H.; Fan, B.; Xu, H.; Lu, H.; et al. The Fabrication and Mechanical Properties of Laminated ZrB2-Mo5SiB2 Ceramics with an Mo-Mo5SiB2 Interlayer. Metals 2021, 11, 2018. [Google Scholar] [CrossRef]
- Andreev, A.A.; Sablev, L.P.; Grigoriev, S.N. Vacuum-Arc Coat.; NSC KIPT Press: Kharkov, Ukraine, 2010. (In Russian) [Google Scholar]
- Shugurov, V.V.; Koval, N.N.; Krysina, O.V.; A Prokopenko, N. QUINTA equipment for ion-plasma modification of materials and products surface and vacuum arc plasma-assisted deposition of coatings. J. Phys. Conf. Ser. 2019, 1393, 012131. [Google Scholar] [CrossRef]
- Korsunsky, A.; McGurk, M.; Bull, S.; Page, T. On the hardness of coated systems. Surf. Coat. Technol. 1998, 99, 171–183. [Google Scholar] [CrossRef]
- Azarenkov, N.A.; Sobol’, O.V.; Beresnev, V.M.; Pogrebnyak, A.D.; Kolesnikov, D.A.; Turbin, P.V.; Toryanik, I.N. Vacuum-plasma coatings based on the multielement nitrides. Metallofiz. I Noveishie Tekhnologii 2013, 35, 1061–1084. Available online: http://dspace.nbuv.gov.ua/bitstream/handle/123456789/104178/07-Azarenkov.pdf?sequence=1 (accessed on 6 August 2023).
- Ivanov, Y.F.; Koval, N.N.; Akhmadeev, Y.H.; Uglov, V.V.; Shugurov, V.V.; Petrikova, E.A.; Krysina, O.V.; Prokopenko, N.A.; Azhazha, I.I. Structure and properties of multi-layer films of high-entropy alloys deposited by ion-plasma method. Rus. Phys. J. 2021, 64, 32–37. [Google Scholar] [CrossRef]
- Milman, Y.V. Plasticity characteristic obtained by indentation. J. Phys. D Appl. Phys. 2008, 41, 074013. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, Y.-T.; Grummon, D.S. Microscopic shape memory and superelastic effects under complex loading conditions. Surf. Coat. Technol. 2004, 177–178, 512–517. [Google Scholar] [CrossRef]
- Guillonneau, G.; Wheeler, J.; Wehrs, J.; Philippe, L.; Baral, P.; Höppel, H.W.; Göken, M.; Michler, J. Determination of the true projected contact area by in situ indentation testing. J. Mater. Res. 2019, 34, 2859–2868. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.; Braic, M. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).