A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility
Abstract
1. Introduction
2. Additive Manufacturing of Orthopedic Implants: Advantages and Challenges
3. Requirements and Materials for Metal and Ceramic Coatings of Orthopedic Implants
3.1. Implant Surface Requirements
3.2. Requirements for In Vitro Testing
3.3. Porosity
3.4. Modulus of Elasticity
3.5. Surface Roughness
3.6. Materials
4. Thermal Plasma Spraying of Metal-Ceramic Coatings
- −
- To establish the correlation between the coating porosity and MPS parameters and to select specific MPS parameters to form porous coatings from Ti, Zr, Ta, and Ha on Ti substrates (with the desired porosity suitable for biomedical applications) with satisfactory adhesion of coatings to the substrate.
- −
- To improve and apply the technology of robotic MPS coatings on implants of complex shape in order to accurately maintain such critical process parameters as the speed of movement of the microplasmatron along the implant surface and the spraying distance.
- −
- To investigate the possibility of combining the technologies of robotic MPS and AM for the possible production of custom-designed implants with increased surface biocompatibility.
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baid, H.; Holland, J.; Pirro, F. Environmentally sustainable orthopaedics and trauma: Systems and behaviour change. Orthop. Trauma 2022, 36, 256–264. [Google Scholar] [CrossRef]
- Fontalis, A.; Kayani, B.; Thompson, J.W.; Plastow, R.; Haddad, F.S. Robotic total hip arthroplasty: Past, present and future. Orthop. Trauma 2022, 36, 6–13. [Google Scholar] [CrossRef]
- Pabinger, C.; Lothaller, H.; Portner, N.; Geissler, A. Projections of hip arthroplasty in OECD countries up to 2050. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2018, 28, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Swiss Society of Orthopaedics and Traumatology. Swiss National Hip & Knee Joint Registry Report. 2021; SIRIS: Basel, Switzerland, 2021; p. 152. [Google Scholar]
- Sharkey, S.; Pickles, E.; Palan, J. The role of hip arthroplasty in management of the hip fracture patient. Orthop. Trauma 2022, 36, 30–36. [Google Scholar] [CrossRef]
- Fischenko, A.V. Influence of the length of the lever of the forces of the abductors of the thigh on the function of walking of patients with coxarthrosis after the endoprosthetics. Trauma 2021, 19, 20–26. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Additive manufacturing applications in orthopaedics: A review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Andrade del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Xia, R.Z.; Zhai, Z.J.; Chang, Y.Y.; Li, H.W. Clinical Applications of 3-Dimensional Printing Technology in Hip Joint. Orthop. Surg. 2019, 11, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Tilton, M.; Lewis, G.S.; Manogharan, G.P. Additive Manufacturing of Orthopedic Implants. In Orthopedic Biomaterials: Progress. in Biology, Manufacturing, and Industry Perspectives; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–55. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Ghassemieh, E.; Voinarovych, S.; Russakova, A.; Kyslytsia, O.; Polovetskyi, Y.; Toxanbayeva, A. Characterisation of the microplasma spraying of biocompatible coating of titanium. J. Microsc. 2020, 279, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Alontseva, D.; Ghassemieh, E.; Voinarovych, S.; Kyslytsia, O.; Polovetskyi, Y.; Prokhorenkova, N.; Kadyroldina, A. Manufacturing and Characterization of Robot Assisted Microplasma Multilayer Coating of Titanium Implants. Biocompatible coatings for medical implants with improved density and crystallinity. Johns. Matthey Technol. Rev. 2020, 64, 12. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Khozhanov, A.R.; Gert, S.S.; Krasavin, A.L.; Prokhorenkova, N.V.; Kalyuzhny, S. Manufacturing and Characterization of Tantalum Microplasma Coatings for Biomedical Application. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020; pp. 813–816. [Google Scholar]
- Alontseva, D.L.; Khozhanov, A.R.; Voinarovich, S.; Kyslytsia, O.; Prokhorenkova, N.V.; Sadibekov, A.B.; Kalyuzhny, S.; Krasavin, A.L. Robotic Microplasma Spraying and Characterization of Zirconium Coatings. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020; pp. 817–821. [Google Scholar]
- Kussaiyn-Murat, A.; Krasavin, A.; Alontseva, D.; Kadyroldina, A.; Khozhanov, A.; Krak, I.; Escalona, P.M.d.; Dyomina, I. Development of an Intelligent Robotic System for Plasma Processing of Industrial Products with Complex Shape. In Proceedings of the 2021 11th IEEE International Conference on Intelligent Data Acquisition and Advanced Computing Systems: Technology and Applications (IDAACS), Cracow, Poland, 22–25 September 2021; pp. 572–579. [Google Scholar]
- Kadyroldina, A.; Alontseva, D.; Voinarovych, S.; Łatka, L.; Kyslytsia, O.; Azamatov, B.; Khozhanov, A.; Prokhorenkova, N.; Zhilkashinova, A.; Burburska, S. Microplasma spraying of hydroxyapatite coatings on additive manufacturing titanium implants with trabecular structures. Mater. Sci. 2022, 40, 28–42. [Google Scholar] [CrossRef]
- Zeidler, S. Additive Manufacturing in Orthopedics. SME 26 May 2020. Available online: https://www.sme.org/technologies/articles/2020/may/additive-manufacturing-in-orthopedics/ (accessed on 25 May 2023).
- Gharde, S.; Surendren, A.; Korde, J.M.; Saini, S.; Deoray, N.; Goud, R.; Nimje, S.; Kandasubramanian, B. Recent Advances in Additive Manufacturing of Bio-inspired Materials. In Biomanufacturing; Prakash, C., Singh, S., Singh, R., Ramakrishna, S., Pabla, B.S., Puri, S., Uddin, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, N.; Zhu, M.; Qiu, Q.; Zhao, P.; Zheng, C.; Bai, Q.; Zeng, Q.; Lu, T. The contribution of pore size and porosity of 3D printed porous titanium scaffolds to osteogenesis. Biomater. Adv. 2022, 133, 112651. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C. 2021, 131, 112499. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A Review of Research Progress in Selective Laser Melting (SLM). Micromachines 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ni, S.; Ma, L.; Li, M. Porous construction and surface modification of titanium-based materials for osteogenesis: A review. Front. Bioeng. Biotechnol. 2022, 10, 973297. [Google Scholar] [CrossRef]
- Han, J.; Li, Z.; Sun, Y.; Cheng, F.; Zhu, L.; Zhang, Y.; Zhang, Z.; Wu, J.; Wang, J. Surface Roughness and Biocompatibility of Polycaprolactone Bone Scaffolds: An Energy-Density-Guided Parameter Optimization for Selective Laser Sintering. Front. Bioeng. Biotechnol. 2022, 10, 888267. [Google Scholar] [CrossRef]
- Nandhakumar, R.; Venkatesan, K. A process parameters review on selective laser melting-based additive manufacturing of single and multi-material: Microstructure, physical properties, tribological, and surface roughness. Mater. Today Commun. 2023, 35, 105538. [Google Scholar] [CrossRef]
- Srivastava, M.; Rathee, S.; Patel, V.; Kumar, A.; Koppad, P.G. A review of various materials for additive manufacturing: Recent trends and processing issues. J. Mater. Res. Technol. 2022, 21, 2612–2641. [Google Scholar] [CrossRef]
- Saunders, J.; Elbestawi, M.; Fang, Q. Ultrafast Laser Additive Manufacturing: A Review. J. Manuf. Mater. Process. 2023, 7, 89. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Konovalov, S.; Su, C.; Fan, P.; Wang, Y.; Xiaoming, P.; Panchenko, I. A Review of Challenges for Wire and Arc Additive Manufacturing (WAAM). Trans. Indian. Inst. Met. 2023, 76, 1123–1139. [Google Scholar] [CrossRef]
- Zhai, X.; Jin, L.; Jiang, J. A survey of additive manufacturing reviews. MSAM 2022, 1, 21. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Norambuena, G.A.; Patel, R.; Karau, M.; Wyles, C.C.; Jannetto, P.J.; Bennet, K.E.; Hanssen, A.D.; Sierra, R.J. Antibacterial and Biocompatible Titanium-Copper Oxide Coating May Be a Potential Strategy to Reduce Periprosthetic Infection: An In Vitro Study. Clin. Orthop. Relat. Res. 2017, 475, 722–732. [Google Scholar] [CrossRef]
- Osękowska, M.; Wojcieszak, D.; Kaczmarek, D.; Mazur, M.; Obstarczyk, A.; Szponar, B. Multifunctional Nanocrystalline Cu–Ti Thin Films Enhance Survival and Induce Proliferation of Mouse Fibroblasts In Vitro. Coatings 2021, 11, 300. [Google Scholar] [CrossRef]
- Adamiak, B.; Wiatrowski, A.; Domaradzki, J.; Kaczmarek, D.; Wojcieszak, D.; Mazur, M. Preparation of multicomponent thin films by magnetron co-sputtering method: The Cu-Ti case study. Vacuum 2019, 161, 419–428. [Google Scholar] [CrossRef]
- Aissani, L.; Belgroune, A.; Saoudi, A.; Hmima, A.; Fellah, M.; Obrosov, A.; Alhussein, A. Tribo-mechanical performance and antibacterial activity in (Cu, Zr)-alloyed Ti(Al)N coatings synthesized by reactive magnetron sputtering. J. Mater. Sci. 2022, 57, 19612–19630. [Google Scholar] [CrossRef]
- Nicholson, W.; Titanium, J. Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- John, A.A.; Jaganathan, S.K.; Supriyanto, E.; Manikandan, A. Surface modification of titanium and its alloys for the enhancement of osseointegration in orthopaedics. Curr. Sci. 2016, 111, 1003–1015. [Google Scholar] [CrossRef]
- 194, T.C.I.T. ISO 10993-1:2018(en); Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2018; p. 28.
- 194, T.C.I.T. ISO 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. In Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009; p. 34.
- Olkowski, R.; Kaszczewski, P.; Czechowska, J.; Siek, D.; Pijocha, D.; Zima, A.; Ślósarczyk, A.; Lewandowska-Szumieł, M. Cytocompatibility of the selected calcium phosphate based bone cements: Comparative study in human cell culture. J. Mater. Sci. Mater. Med. 2015, 26, 270. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Keshavarz, T. 13—The challenge of biocompatibility evaluation of biocomposites. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 303–334. [Google Scholar] [CrossRef]
- Lewandowska-Szumieł, M.; Rumiński, S. 14—Cellular response to biocomposites. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 335–356. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. Scand. 2002, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J. Biomed. Mater. Res. 2002, 60, 420–433. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, W.-Q.; Qiu, J. Impact of exogenous metal ions on peri-implant bone metabolism: A review. RSC Adv. 2021, 11, 13152–13163. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Xia, Z.; Glyn-Jones, S.; Beard, D.; Gill, H.S.; Murray, D.W. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed. Mater. 2009, 4, 025018. [Google Scholar] [CrossRef]
- Hezil, N.; Aissani, L.; Fellah, M.; Abdul Samad, M.; Obrosov, A.; Timofei, C.; Marchenko, E. Structural, and tribological properties of nanostructured α + β type titanium alloys for total hip. J. Mater. Res. Technol. 2022, 19, 3568–3578. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Tobin, E.J. Recent coating developments for combination devices in orthopedic and dental applications: A literature review. Adv. Drug. Deliv. Rev. 2017, 112, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Beltrán, A.M.; Domínguez-Trujillo, C.; Garvi, M.D.; Lebrato, J.; Rodríguez-Ortiz, J.A.; García-Moreno, F.; Cauich-Rodriguez, J.V.; Guzman, J.J.; Torres, Y. Balancing Porosity and Mechanical Properties of Titanium Samples to Favor Cellular Growth against Bacteria. Metals 2019, 9, 1039. [Google Scholar] [CrossRef]
- Kalita, V.I.; Malanin, D.A.; Mamaev, A.I.; Mamaeva, V.A.; Novochadov, V.V.; Komlev, D.I.; Komlev, V.S.; Radyuk, A.A. 3D bioactive coatings with a new type of porous ridge/cavity structure. Materialia 2021, 15, 101018. [Google Scholar] [CrossRef]
- Matassi, F.; Botti, A.; Sirleo, L.; Carulli, C.; Innocenti, M. Porous metal for orthopedics implants. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2013, 10, 111–115. [Google Scholar]
- Tumilovich, M.V.; Savich, V.V.; Shelushina, A.I. Influence Of Shape And Size Of Particles On The Osseointegration Of Porous Implants Made Of Titanium Powder. Dokl. BGUIR 2016, 4, 115–119. [Google Scholar]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Kuibida, V.; Kokhanets, P.; Lopatynska, V. Mechanism of strengthening the skeleton using plyometrics. J. Phys. Educ. Sport. 2021, 21, 7. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully Porous 3D Printed Titanium Femoral Stem to Reduce Stress-Shielding Following Total Hip Arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Wang, B.J.X. The Effects of Surface Roughness on the Functionality of Ti13Nb13Zr Orthopedic Implants. Biomed. J. Sci. Tech. Res. 2021, 38, 9. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Trousdale, W.H.; Thaler, R.; Yao, J.J.; Xu, W.; Denbeigh, J.M.; Nair, A.; Kocher, J.P.; Dudakovic, A.; Berry, D.J.; et al. Surface Roughness of Titanium Orthopedic Implants Alters the Biological Phenotype of Human Mesenchymal Stromal Cells. Tissue Eng. Part. A 2021, 27, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J. 1—Metallic biomaterials: State of the art and new challenges. In Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–33. [Google Scholar] [CrossRef]
- Traumatology, T.E.F.o.N.A.o.O.a. EFORT Statement on Cobalt in Orthopaedic implants. Available online: https://www.efort.org/efort-statement-on-cobalt-in-orthopaedic-implants/ (accessed on 24 August 2021).
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in metals and alloys for joint replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Kulkarni, O.; Kakandikar, G. Formability Assessment with Microstructural Investigations for Zirconium 702 Thin Foils: Bio-Material Applications. Adv. Mater. Process. Technol. 2022, 8, 2367–2377. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Yang, H.-l.; Juaim, A.N.; Chen, X.-N.; Lu, C.; Zou, L.; Wang, Y.-Z.; Zhou, X.-W. Biocompatibility and osteogenic activity of Zr−30Ta and Zr−25Ta−5Ti sintered alloys for dental and orthopedic implants. Trans. Nonferrous Met. Soc. China 2023, 33, 851–864. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Kumari, R.; Yadav, K.B.; Barole, S.; Archana, K.; Besra, L.D. Microstructural characterisation and wettability behaviour of nano-HA coating on Ti-6Al-4V alloy by electrophoretic deposition method (EPD). Adv. Mater. Process. Technol. 2022, 8, 611–618. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4)-Based Bioceramics: Preparation, Properties, and Applications. Coatings 2022, 12, 1380. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Functionalized calcium orthophosphates (CaPO4) and their biomedical applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Nouri, A.; Sola, A. Powder morphology in thermal spraying. J. Adv. Manuf. Process. 2019, 1, e10020. [Google Scholar] [CrossRef]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray. Technol. 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Baizhan, D. Creation of Bioceramic Coatings on the Surface of Ti–6Al–4V Alloy by Plasma Electrolytic Oxidation Followed by Gas Detonation Spraying. Coatings 2021, 11, 1433. [Google Scholar]
- Alontseva, D.; Azamatov, B.; Voinarovych, S.; Kyslytsia, O.; Koltunowicz, T.N.; Toxanbayeva, A. Development of technologies for manufacturing medical implants using CNC machines and microplasma spraying of biocompatible coatings. Przegląd Elektrotechniczny 2020, 4, 3. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Abilev, M.B.; Zhilkashinova, A.M.; Voinarovych, S.G.; Kyslytsia, O.N.; Ghassemieh, E.; Russakova, A.; Łatka, L. Optimization of Hydroxyapatite Synthesis and Microplasma Spraying of Porous Coatings Onto Titanium Implants. Adv. Mater. Sci. 2018, 18, 79–94. [Google Scholar] [CrossRef]
- Łatka, L.; Pawlowski, L.; Chicot, D.; Pierlot, C.; Petit, F. Mechanical properties of suspension plasma sprayed hydroxyapatite coatings submitted to simulated body fluid. Surf. Coat. Technol. 2010, 205, 954–960. [Google Scholar] [CrossRef]
- Blum, M.; Sayed, M.; Mahmoud, E.M.; Killinger, A.; Gadow, R.; Naga, S.M. In Vitro Evaluation of Biologically Derived Hydroxyapatite Coatings Manufactured by High Velocity Suspension Spraying. J. Therm. Spray Technol. 2021, 30, 1891–1904. [Google Scholar] [CrossRef]
- Abir, M.M.M.; Otsuka, Y.; Ohnuma, K.; Miyashita, Y. Effects of composition of hydroxyapatite/gray titania coating fabricated by suspension plasma spraying on mechanical and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2022, 125, 104888. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K.; Zhao, L.; Zwick, J. Assessment of the Microplasma Spraying Process for Coating Application. Adv. Eng. Mater. 2006, 8, 635–639. [Google Scholar] [CrossRef]
- Voinarovych, S.G.; Alontseva, D.L.; Kyslytsia, O.N.; Kaliuzhnyi, S.; Khozhanov, A.R.; Krasavin, A.; Kolesnikova, T. Fabrication and Characterization of Zr Microplasma Sprayed Coatings for Medical Applications. Adv. Mater. Sci. 2021, 21, 93–105. [Google Scholar] [CrossRef]
- Arjun Dey, A.K.M. Microplasma Sprayed Hydroxyapatite Coatings, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D.; Bandyopadhyay, N.R. Nanoindentation study of microplasma sprayed hydroxyapatite coating. Ceram. Int. 2009, 35, 2295–2304. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D. Characterization of Microplasma Sprayed Hydroxyapatite Coating. J. Therm. Spray Technol. 2009, 18, 578–592. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K. In Vitro Dissolution, Microstructural and Mechanical Characterizations of Microplasma-Sprayed Hydroxyapatite Coating. Int. J. Appl. Ceram. Technol. 2014, 11, 65–82. [Google Scholar] [CrossRef]
- Junker, R.; Manders, P.J.; Wolke, J.; Borisov, Y.; Jansen, J.A. Bone-supportive behavior of microplasma-sprayed CaP-coated Junker, R.; Manders, P.J.; Wolke, J.; Borisov, Y.; Jansen, J.A. Bone-supportive behavior of microplasma-sprayed CaP-coated implants: Mechanical and histological outcome in the goat. Clin. Oral. Implant. Res. 2010, 21, 189–200. [Google Scholar] [CrossRef]
- ISO. ISO 13779; Implants for Surgery—Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite. In Implants for Surgery. The International Organization for Standardization: Geneva, Switzerland, 2018.
- Ohki, M.; Takahashi, S.; Jinnai, R.; Hoshina, T. Interfacial Strength of Plasma-sprayed Hydroxyapatite Coatings. J. Therm. Spray. Technol. 2020, 29, 1119–1133. [Google Scholar] [CrossRef]
- Voinarovych, S.G.; Alontseva, D.L.; Khozhanov, A.R.; Krasavin, A.L.; Kyslytsia, A.N.; Kalyuzhny, S.N. Effect of microplasma spraying parameters on the loss of sprayed Zr wire and coating porosity. Recent. Contrib. Phys. (Rec.Contr.Phys.) 2021, 4, 82–96. [Google Scholar] [CrossRef]
- ASTM F1147; Standard Test Method for Tension Testing of Calcium Phosphate and Metallic Coatings. ASTM International: West Conshohocken, PA, USA, 2017; p. 8.
- Dyman, M.; Moltasov, A.; Kaliuzhnyi, S.; Kyslytsia, O.; Tsymbalista, T. Research Of The Structure And Mechanical Properties Of Microplasm Porous Coatings For Biomedical Purposes. Ukr. J. Mech. Eng. Mater. Sci. 2022, 8, 7. [Google Scholar] [CrossRef]
- Szala, M.; Łatka, L.; Awtoniuk, M.; Winnicki, M.; Michalak, M. Neural Modelling of APS Thermal Spray Process Parameters for Optimizing the Hardness, Porosity and Cavitation Erosion Resistance of Al2O3-13 wt% TiO2 Coatings. Processes 2020, 8, 1544. [Google Scholar] [CrossRef]
- Moltasov, A.; Dyman, M.; Kaliuzhnyi, S.; Mossokovska, I.; Voinarovych, S.; Kyslytsia, O. Dependence of the elasticity modulus of microplasma coatings made of titanium grade VT1-00 and zirconium grade KTC-110 on their porosity. Ser. Biomech. 2022, 36, 11. [Google Scholar] [CrossRef]
- Alontseva, D.; Krasavin, A.; Abilev, M.; Zhilkashinova, A. Microplasma Deposition of Biocompatible Coatings Using an Intelligent Robotic System for Plasma Processing. Acta Phys. Pol. A 2019, 136, 3. [Google Scholar] [CrossRef]

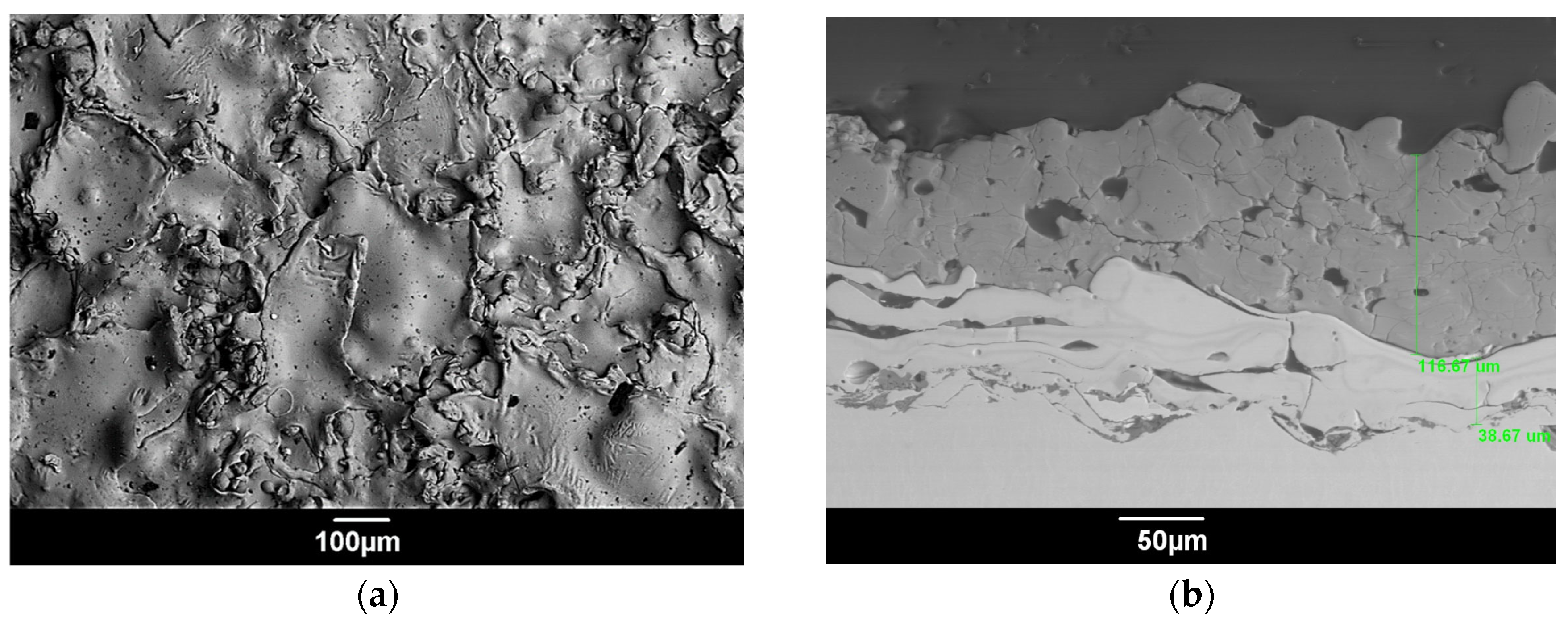
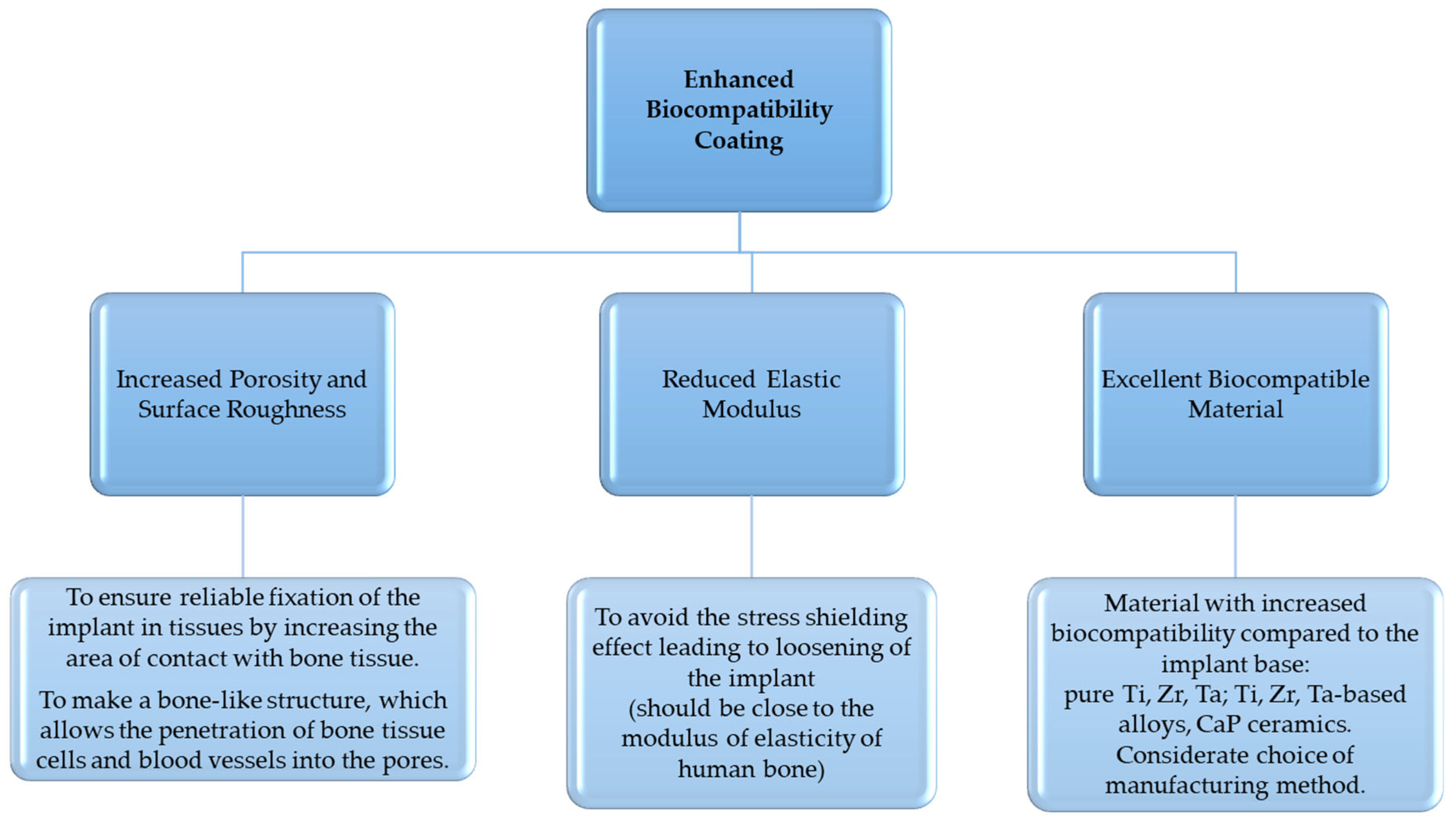
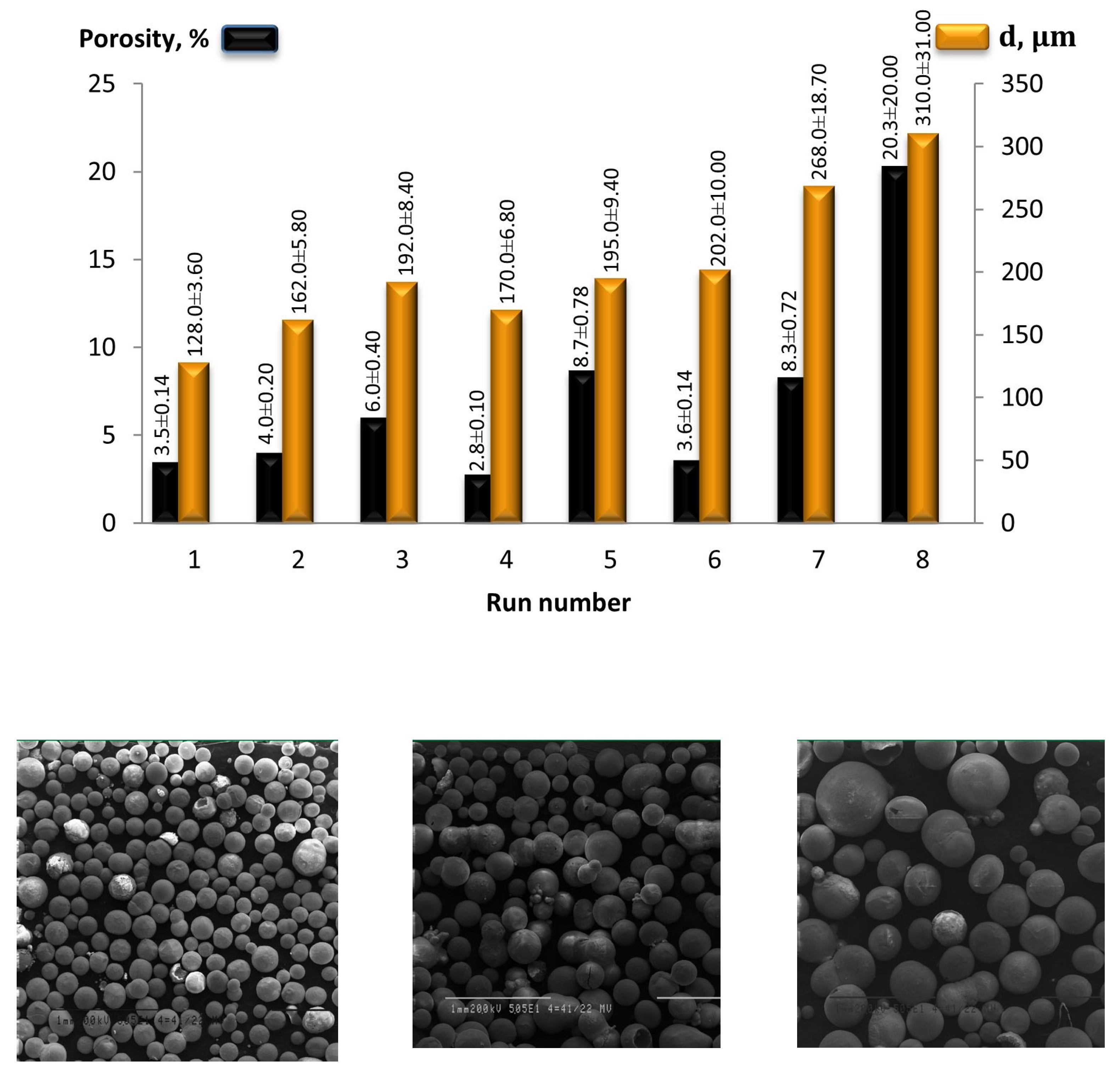
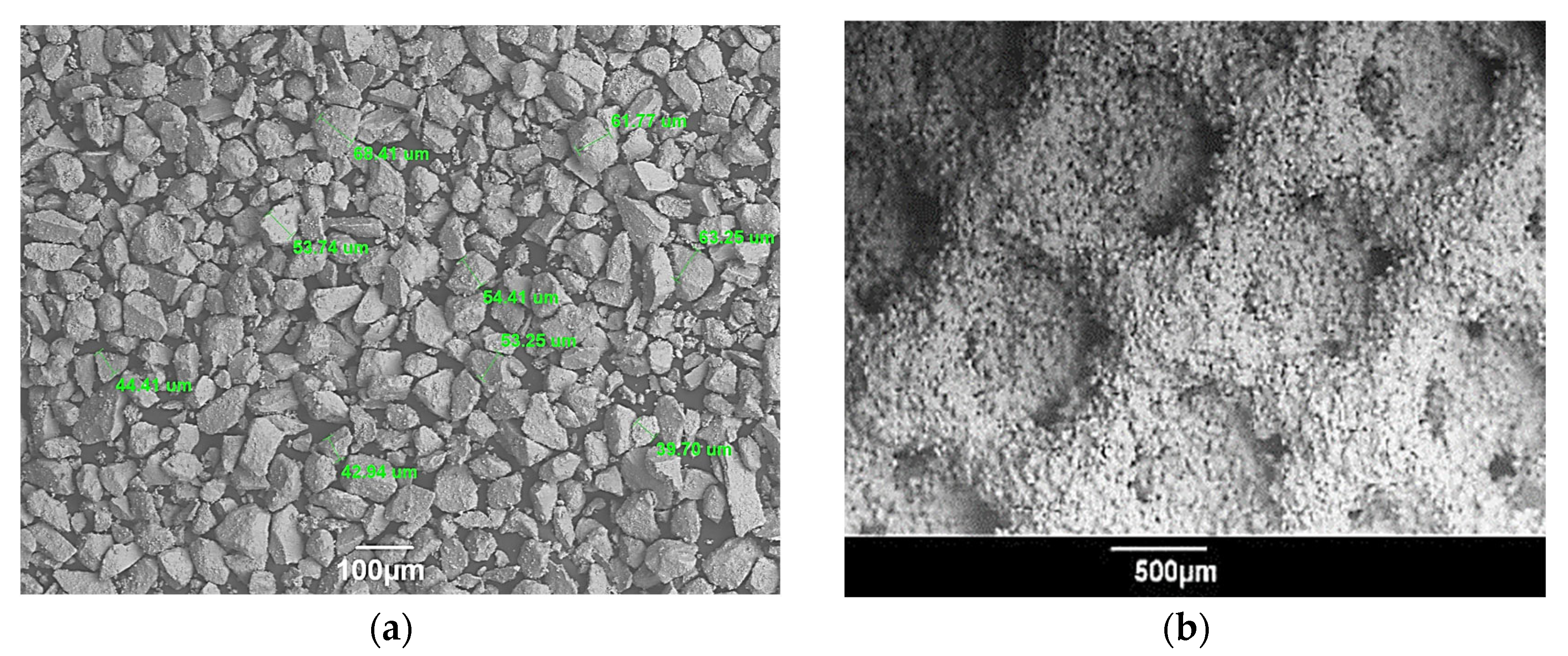
| Advantages | Challenges |
|---|---|
| High productivity and minimal waste. | The cost of equipment as well as alloy and ceramic powders is expensive. |
| The complexity of the parts is not limited. Possibility of prototyping 3D custom-designed implants with complex shapes. | High design costs. High time scale, depending on the complexity and size of the 3D model. Highly skilled staff are required. |
| Freedom of design. Rapid prototyping of porous scaffolds in a wide range of porosity, pore size, and structure regularity. | The complexity of porous implant design requires striking a balance between mechanical properties and effective bone formation capacity. The need to optimize the AM parameters in order to improve the melting of powder particles, provide the desired mechanical properties, surface roughness, etc. |
| The ability to use a wide range of hard-to-process materials, including metals, alloys, and ceramics with high melting points. | Rigidity in the choice of raw materials to be compatible with particular AM machines. |
| Ability to combine with other advanced technologies such as ultrafast lasers, robotic microplasma spraying (MPS), microarc oxidation (MAO), and wire and arc coating deposition. | In a new line of research, manufacturing reliability is not guaranteed. Interdisciplinary research is required in physics, materials science, robotics, artificial intelligence (AI), etc. |
| Porosity Range | Effect | Reference |
|---|---|---|
| 1000 µm | Promote adhesion, proliferation, and osteogenic differentiation of Bone Marrow-derived mesenchymal stem cells (BMMMSCs) | [22] |
| 300–500 µm | Induce oisteogenesis (improve the formation of new bone and capillaries, i.e., vascularization) | [25,55] |
| 200–400 µm | Promote adhesion, migration, and proliferation of osteoblasts | [25] |
| 100–200 µm | Promote cell adhesion and enhance antibacterial properties | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alontseva, D.; Azamatov, B.; Safarova, Y.; Voinarovych, S.; Nazenova, G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings 2023, 13, 1175. https://doi.org/10.3390/coatings13071175
Alontseva D, Azamatov B, Safarova Y, Voinarovych S, Nazenova G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings. 2023; 13(7):1175. https://doi.org/10.3390/coatings13071175
Chicago/Turabian StyleAlontseva, Darya, Bagdat Azamatov, Yuliya Safarova (Yantsen), Sergii Voinarovych, and Gaukhar Nazenova. 2023. "A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility" Coatings 13, no. 7: 1175. https://doi.org/10.3390/coatings13071175
APA StyleAlontseva, D., Azamatov, B., Safarova, Y., Voinarovych, S., & Nazenova, G. (2023). A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings, 13(7), 1175. https://doi.org/10.3390/coatings13071175





.JPG)

