Vapor Deposited Zeolitic Imidazolate Framework-8 Derived from Porous ZnO Thin Films
Abstract
1. Introduction
2. Materials and Methods
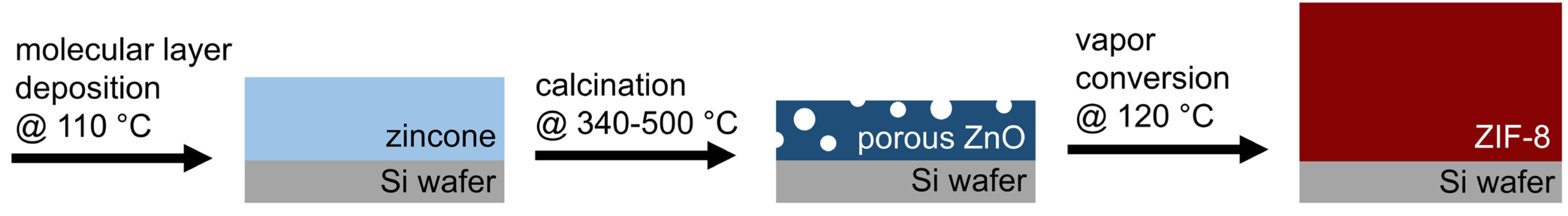
2.1. Sample Synthesis
2.2. Characterization Methods
2.3. Photocatalytic Tests
3. Results and Discussion
3.1. Investigation of Porous ZnO
3.2. Chemical and Crystallographic Characterization of ZIF-8
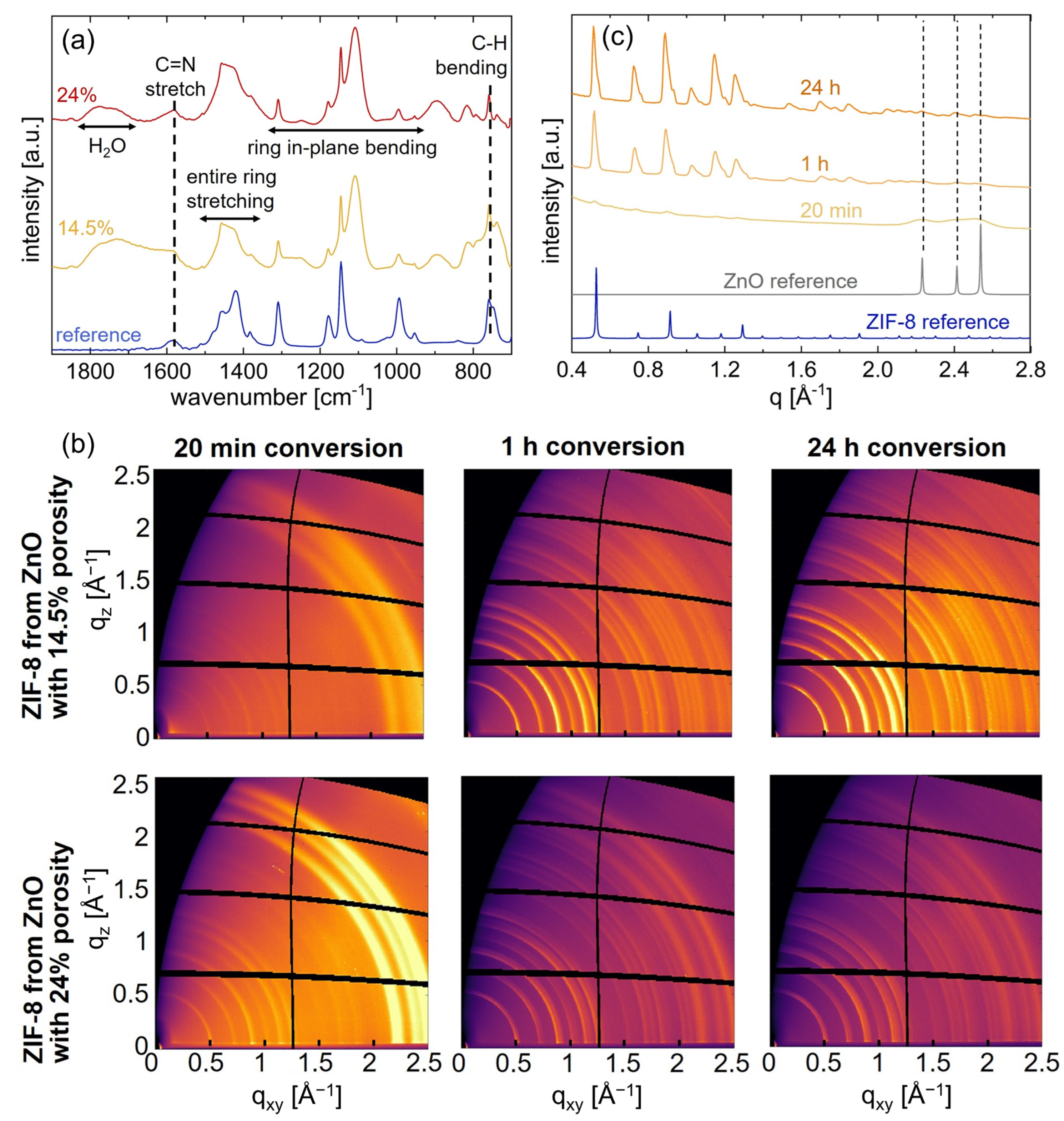
3.3. Growth and Topographical Development of ZIF-8
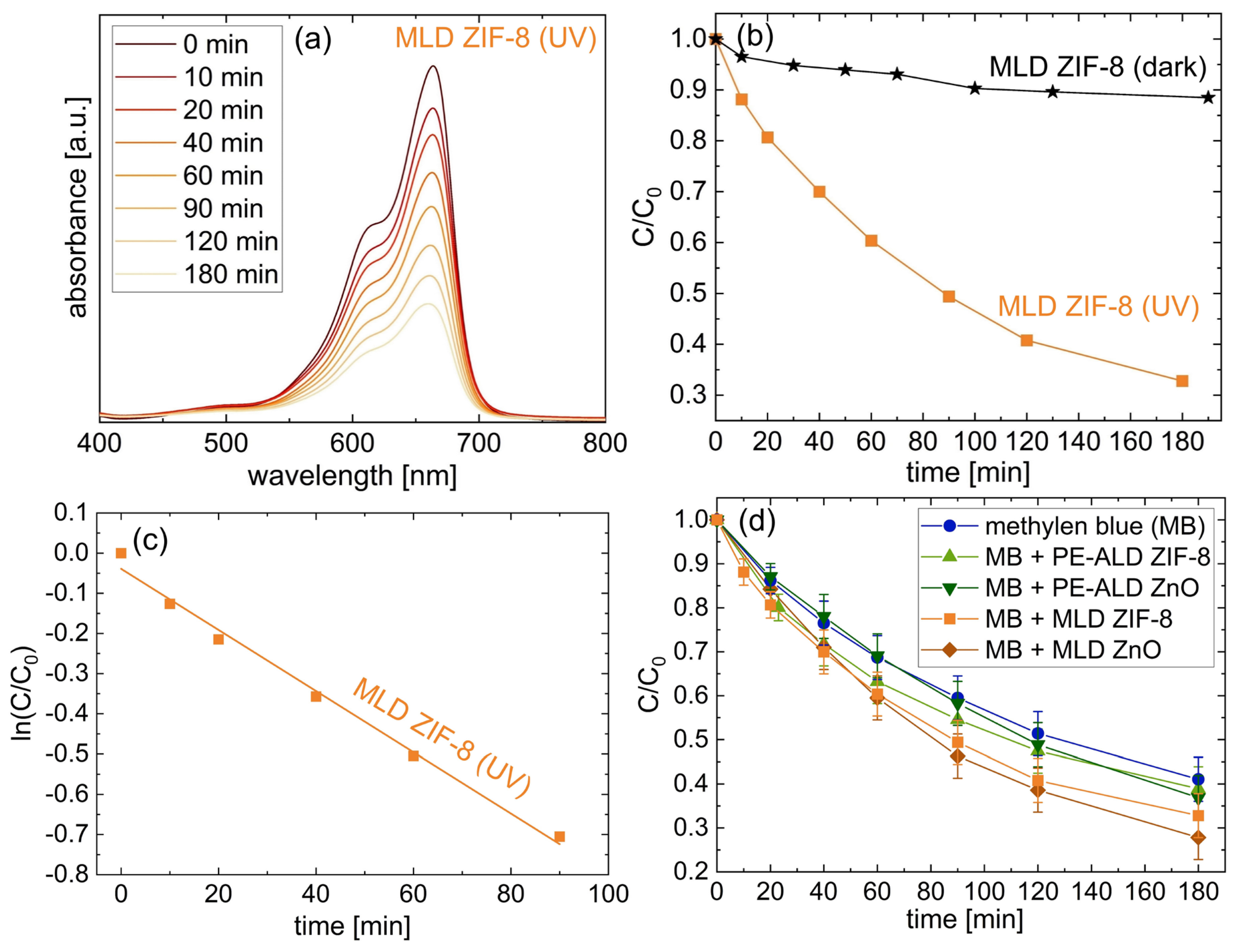
3.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stassin, T.; Stassen, I.; Wauteraert, N.; Cruz, A.J.; Kräuter, M.; Coclite, A.M.; De Vos, D.; Ameloot, R. Solvent-Free Powder Synthesis and Thin Film Chemical Vapor Deposition of a Zinc Bipyridyl-Triazolate Framework. Eur. J. Inorg. Chem. 2020, 2020, 71–74. [Google Scholar] [CrossRef]
- Gadipelli, S.; Travis, W.; Zhou, W.; Guo, Z. A thermally derived and optimized structure from ZIF-8 with giant enhancement in CO2 uptake. Energy Environ. Sci. 2014, 7, 2232–2238. [Google Scholar] [CrossRef]
- Stassin, T.; Verbeke, R.; Cruz, A.J.; Rodríguez-Hermida, S.; Stassen, I.; Marreiros, J.; Krishtab, M.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; et al. Porosimetry for Thin Films of Metal–Organic Frameworks: A Comparison of Positron Annihilation Lifetime Spectroscopy and Adsorption-Based Methods. Adv. Mater. 2021, 33, 2006993. [Google Scholar] [CrossRef]
- Zou, D.; Liu, D.; Zhang, J. From Zeolitic Imidazolate Framework-8 to Metal-Organic Frameworks (MOFs): Representative Substance for the General Study of Pioneering MOF Applications. Energy Environ. Mater. 2018, 1, 209–220. [Google Scholar] [CrossRef]
- Kajiwara, T.; Higuchi, M.; Watanabe, D.; Higashimura, H.; Yamada, T.; Kitagawa, H. A systematic study on the stability of porous coordination polymers against ammonia. Chem. Eur. J. 2014, 20, 15611–15617. [Google Scholar] [CrossRef]
- Su, Z.; Miao, Y.R.; Mao, S.M.; Zhang, G.H.; Dillon, S.; Miller, J.T.; Suslick, K.S. Compression-induced deformation of individual metal-organic framework microcrystals. J. Am. Chem. Soc. 2015, 137, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S. Solvothermal synthesis of porous hydrangea-like zeolitic imidazole framework-8 (ZIF-8)crystals. J. Solid State Chem. 2019, 276, 68–74. [Google Scholar] [CrossRef]
- Seoane, B.; Zamaro, J.M.; Tellez, C.; Coronas, J. Sonocrystallization of zeolitic imidazolate frameworks (ZIF-7, ZIF-8, ZIF-11 and ZIF-20). CrystEngComm 2012, 14, 3103–3107. [Google Scholar] [CrossRef]
- Lee, Y.R.; Jang, M.S.; Cho, H.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Tanaka, S.; Nagaoka, T.; Yasuyoshi, A.; Hasegawa, Y.; Denayer, J.F.M. Hierarchical Pore Development of ZIF-8 MOF by Simple Salt-Assisted Mechanosynthesis. Cryst. Growth Des. 2018, 18, 274–279. [Google Scholar] [CrossRef]
- Im, J.; Yim, N.; Kim, J.; Vogt, T.; Lee, Y. High-Pressure Chemistry of a Zeolitic Imidazolate Framework Compound in the Presence of Different Fluids. J. Am. Chem. Soc. 2016, 138, 11477–11480. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. 2011, 123, 698–701. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Van Gorp, H.; Vanderlinden, W.; De Feyter, S.; Falcaro, P.; De Vos, D.; Vereecken, P.; Ameloot, R. Chemical vapour deposition of zeolitic imidazolate framework thin films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sakamoto, K.; Inada, H.; Kawata, M.; Takasaki, G.; Imawaka, K. Vapor-Phase Synthesis of ZIF-8 MOF Thick Film by Conversion of ZnO Nanorod Array. Langmuir 2018, 34, 7028–7033. [Google Scholar] [CrossRef]
- Kräuter, M.; Cruz, A.J.; Stassin, T.; Rodríguez-Hermida, S.; Ameloot, R.; Resel, R.; Coclite, A.M. Influence of Precursor Density and Conversion Time on the Orientation of Vapor-Deposited ZIF-8. Crystals 2022, 12, 217. [Google Scholar] [CrossRef]
- Cruz, A.J.; Arnauts, G.; Obst, M.; Kravchenko, D.E.; Vereecken, P.M.; De Feyter, S.; Stassen, I.; Hauffman, T.; Ameloot, R. Effect of different oxide and hybrid precursors on MOF-CVD of ZIF-8 films. Dalt. Trans. 2021, 50, 6784–6788. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Turner, S.; Tu, M.; Wannapaiboon, S.; Schneemann, A.; Meyer, R.; Ludwig, A.; Van Tendeloo, G.; Fischer, R.A. Self-directed localization of ZIF-8 thin film formation by conversion of ZnO nanolayers. Adv. Funct. Mater. 2014, 24, 4804–4811. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, H.; Yang, Y.; Lin, X.; Cheng, H.; Zhang, R.; Jiang, X.; Wang, J. ZIF-8 derived porous ZnO with grain boundaries for efficient CO2 electroreduction. J. Nanoparticle Res. 2021, 23, 133. [Google Scholar] [CrossRef]
- Yang, R.; Yan, X.; Li, Y.; Zhang, X.; Chen, J. Nitrogen-Doped Porous Carbon-ZnO Nanopolyhedra Derived from ZIF-8: New Materials for Photoelectrochemical Biosensors. ACS Appl. Mater. Interfaces 2017, 9, 42482–42491. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, D.; Seo, J.Y.; Buyukcakir, O.; Coskun, A. Nanostructured ZnO as a structural template for the growth of ZIF-8 with tunable hierarchical porosity for CO2 conversion. CrystEngComm 2017, 19, 4147–4151. [Google Scholar] [CrossRef]
- Pérez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Chem. Eur. J. 2010, 16, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Matatagui, D.; Sainz-Vidal, A.; Gràcia, I.; Figueras, E.; Cané, C.; Saniger, J.M. Chemoresistive gas sensor based on ZIF-8/ZIF-67 nanocrystals. Sens. Actuators B Chem. 2018, 274, 601–608. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Shariatinia, Z. Applications of zeolitic imidazolate framework-8 (ZIF-8) in bone tissue engineering: A review. Tissue Cell 2021, 72, 101588. [Google Scholar] [CrossRef] [PubMed]
- Eslava, S.; Zhang, L.; Esconjauregui, S.; Yang, J.; Vanstreels, K.; Baklanov, M.R.; Saiz, E. Metal-organic framework ZIF-8 films as low-κ dielectrics in microelectronics. Chem. Mater. 2013, 25, 27–33. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Li, F.; Ondon, B.S. Zeolitic imidazolate framework-8 (ZIF-8) as robust catalyst for oxygen reduction reaction in microbial fuel cells. J. Power Sources 2019, 423, 9–17. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Zeolitic imidazolate frameworks (ZIF-8, ZIF-67, and ZIF-L) for hydrogen production. Appl. Organomet. Chem. 2021, 35, 25–29. [Google Scholar] [CrossRef]
- Jing, H.P.; Wang, C.C.; Zhang, Y.W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Sun, M.; Hanif, A.; Wu, H.; Gu, Q.; Ok, Y.S.; Tsang, D.C.W.; Li, J.; Yu, J.; et al. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde. Chem. Sci. 2020, 11, 6670–6681. [Google Scholar] [CrossRef]
- Si, Y.H.; Li, Y.Y.; Xia, Y.; Shang, S.K.; Xiong, X.B.; Zeng, X.R.; Zhou, J. Fabrication of novel ZIF-8@BiVO4 composite with enhanced photocatalytic performance. Crystals 2018, 8, 432. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abu-Elghait, M.; El-Shahat, M. Hybrid three MOFs composites (ZIF-67@ZIF-8@MIL-125-NH2): Enhancement the biological and visible-light photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104107. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Sheng, J.; Tang, F.; Zeng, K.; Wang, L.; Liang, K.; Hu, H.; Liu, Y.N. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2. Appl. Surf. Sci. 2019, 498, 143899. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Jin, C.; He, Q.; Wang, Y. Fabrication of ZIF-8@TiO2 micron composite via hydrothermal method with enhanced absorption and photocatalytic activities in tetracycline degradation. J. Alloy. Compd. 2020, 825, 154008. [Google Scholar] [CrossRef]
- Kräuter, M.; Abu Ali, T.; Stadlober, B.; Resel, R.; Unger, K.; Coclite, A.M. Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer Deposited “Zincones”. Materials 2022, 15, 6786. [Google Scholar] [CrossRef] [PubMed]
- Abu Ali, T.; Schäffner, P.; Belegratis, M.; Schider, G.; Stadlober, B.; Coclite, A.M. Smart Core-Shell Nanostructures for Force, Humidity, and Temperature Multi-Stimuli Responsiveness. Adv. Mater. Technol. 2022, 7, 2200246. [Google Scholar] [CrossRef]
- Schrode, B.; Pachmajer, S.; Dohr, M.; Röthel, C.; Domke, J.; Fritz, T.; Resel, R.; Werzer, O. GIDVis: A comprehensive software tool for geometry-independent grazing-incidence X-ray diffraction data analysis and pole-figure calculations. J. Appl. Crystallogr. 2019, 52, 683–689. [Google Scholar] [CrossRef]
- Necas, D.; Klapetek, P. Gwyddion. Available online: http://gwyddion.net/ (accessed on 20 August 2021).
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef]
- Sharma, S.K.; Utpalla, P.; Bahadur, J.; Goutam, U.K.; Pujari, P.K. Micrometer scale pore-interconnectivity in nanoporous ZIF-8 films with Zn enriched surface terminations. Microporous Mesoporous Mater. 2020, 307, 110519. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef]
- Cruz, A.J.; Stassen, I.; Krishtab, M.; Marcoen, K.; Stassin, T.; Rodríguez-hermida, S.; Teyssandier, J.; Pletincx, S.; Verbeke, R.; Rubio-Giménez, V.; et al. An integrated cleanroom process for the vapor phase deposition of large-area zeolitic imidazolate framework thin films. Chem. Mater. 2019, 31, 9462–9471. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1233–1236. [Google Scholar] [CrossRef]
- Perrotta, A.; Pilz, J.; Pachmajer, S.; Milella, A.; Coclite, A.M. On the transformation of “zincone”-like into porous ZnO thin films from sub-saturated plasma enhanced atomic layer deposition. Beilstein J. Nanotechnol. 2019, 10, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Leung, D.Y.C.; Kwong, P.C.W.; Xiong, J.; Zhang, L. Enhanced photocatalytic degradation of methylene blue under vacuum ultraviolet irradiation. Catal. Today 2013, 201, 189–194. [Google Scholar] [CrossRef]
- Jongnavakit, P.; Amornpitoksuk, P.; Suwanboon, S.; Ndiege, N. Preparation and photocatalytic activity of Cu-doped ZnO thin films prepared by the sol-gel method. Appl. Surf. Sci. 2012, 258, 8192–8198. [Google Scholar] [CrossRef]
- Pasichnyk, M.; Gaálová, J.; Minarik, P.; Václavíková, M.; Melnyk, I. Development of polyester filters with polymer nanocomposite active layer for effective dye filtration. Sci. Rep. 2022, 12, 973. [Google Scholar] [CrossRef]
- Basolite® Z1200-ZIF-8. Available online: https://www.sigmaaldrich.com/AT/de/product/aldrich/691348 (accessed on 25 May 2022).
- Han, J.; Qiu, W.; Gao, W. Potential dissolution and photo-dissolution of ZnO thin films. J. Hazard. Mater. 2010, 178, 115–122. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kräuter, M.; Unger, K.; Resel, R.; Coclite, A.M. Vapor Deposited Zeolitic Imidazolate Framework-8 Derived from Porous ZnO Thin Films. Coatings 2023, 13, 718. https://doi.org/10.3390/coatings13040718
Kräuter M, Unger K, Resel R, Coclite AM. Vapor Deposited Zeolitic Imidazolate Framework-8 Derived from Porous ZnO Thin Films. Coatings. 2023; 13(4):718. https://doi.org/10.3390/coatings13040718
Chicago/Turabian StyleKräuter, Marianne, Katrin Unger, Roland Resel, and Anna Maria Coclite. 2023. "Vapor Deposited Zeolitic Imidazolate Framework-8 Derived from Porous ZnO Thin Films" Coatings 13, no. 4: 718. https://doi.org/10.3390/coatings13040718
APA StyleKräuter, M., Unger, K., Resel, R., & Coclite, A. M. (2023). Vapor Deposited Zeolitic Imidazolate Framework-8 Derived from Porous ZnO Thin Films. Coatings, 13(4), 718. https://doi.org/10.3390/coatings13040718






