Abstract
To ensure the leak tightness of SiC/SiC composites cladding, niobium and tantalum have been retained as liner/coating materials for their high melting point, ductility and weldability; however, their chemical compatibility at high temperatures towards SiC remains to be assessed. In the literature, large discrepancies in the composition of the reaction zone and the kinetics were noticed between some metallic liners and SiC. In this work, diffusion couple experiments between Nb and Ta with SiC and SiC/SiC were conducted at high temperatures (1050–1500 °C) to determine the diffusion paths and the reaction kinetics in order to estimate the lifetime of such coatings in nominal conditions. A detailed analysis of the interaction area was conducted as a function of temperature by a combination of experimental characterizations and thermodynamic calculations. No significant difference in the sandwich cladding materials was observed. The interfacial reactivity was found to be strongly higher than expected from literature data. C and Si were evidenced as the main diffusing species in the Nb/SiC and Ta/SiC systems. From the reaction layer thickness extrapolation in gas-cooled fast reactor operating conditions, niobium but especially tantalum have been approved as liner material in hybrid CMC/metal cladding materials from a chemical compatibility point of view.
1. Introduction
Among the future nuclear reactor systems retained by the Generation IV Forum, the gas-cooled fast reactor (GFR) is one of the solutions to close the cycle [1]. Since the 1960s, this technology has already been studied [2]; however, its design is highly challenging due to severe conditions. Indeed, with the use of helium as a coolant, the cladding material has to operate at high nominal temperatures (850 °C) for years [3]. In addition, no fission product release for a clad temperature of 1600 °C for a few hours should occur, as well as maintaining the core-cooling capability up to 2000 °C [4,5]. The cladding material candidates withstanding those requirements are very limited. Thanks to its chemical inertness, its refractoriness and its low swelling under irradiation [6], especially at high temperatures, and benefiting from their use in high-temperature gas reactors as part of TRi-structural ISOtropic (TRISO) particles [7,8], silicon carbide (SiC) has gained interest for GFR application [9]. Due to its brittleness, SiC bulk material cannot be used for core applications, especially for cladding; therefore, SiC/SiC composites were considered for their high damage tolerance obtained thanks to the non-critical matrix multicracking mechanism under mechanical solicitation. Nevertheless, due to the matrix multicracking, the absence of leak-tightness cannot be guaranteed for this material under significant mechanical solicitation [10]. A concept called “sandwich” cladding was then patented. A thin metallic coating or layer, called a liner, is inserted between two layers of SiC/SiC composite, ensuring the gas-tightness and mechanical properties, respectively (Figure 1) [11]. To avoid any leak in operating conditions, the liner has to meet several criteria. It should be refractory to sustain accidental situations temperatures (≈2000 °C) [12]. It has to be more ductile than the SiC/SiC composite. The liner chemical compatibility has to be good with its environment: SiC and He coolant with its impurities.
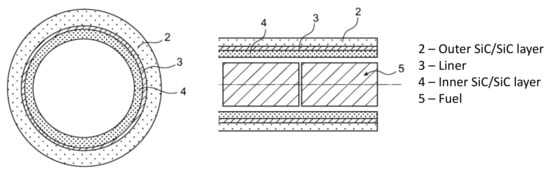
Figure 1.
Scheme of sandwich concept [11].
Literature data have shown that the growth rate of the reaction layer, formed at high temperature (T > 850 °C) between SiC and several metals (Ta, Nb, W, …), follows a parabolic law, highlighting a diffusion-controlled mechanism [10]. A compilation of parabolic reaction rate constants () for various metals, given by Equation (1), is presented in Figure 2 as a function of temperature.
where is the reaction layer thickness and the reaction time. Data show a wide dispersion. For a given element (W or Mo, for example), two orders of magnitude differences in were observed. This could be due to experiments performed with thin layers, of short durations and sometimes for different lower temperature intervals, leading to higher uncertainties and different mechanisms.
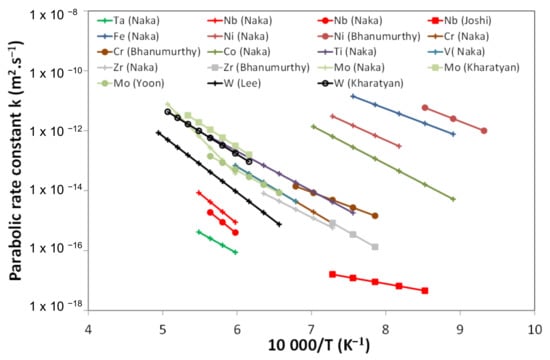
Figure 2.
Parabolic rate constants () for growth of reaction layer between various metals and SiC as a function of temperature gathered from the literature [10].
There is currently a growing interest in the high-temperature interactions of SiC with refractory metals because it is one of the solutions to solve the joining of SiC-based materials issue. Among others, diffusion bonding [13,14,15] or spark plasma sintering [16] techniques are based on the interdiffusion at the SiC/metal interface at high temperatures; therefore, there is a need to deeply characterize and fully assess the chemical interaction between refractory metals and SiC.
For the sandwich application, there is a need for this interaction to remain as low as possible to not interfere with the SiC/SiC or liner properties and keep the gas-tightness of the structure. From Figure 2, tantalum and niobium seem to be the less reactive metals toward SiC. Both are strongly refractory (melting temperature of 3287 K and 2740 K, respectively). They are resistant to corrosion at room temperature (except in the presence of water vapors or fluorides ions [17,18]) but not in air above 500 °C [19,20,21]. Moreover, the calculated neutron cross sections are moderate for the fast neutron spectrum (<0.1 barn at 1 MeV) [22]. Finally, niobium is cheaper and more abundant compared to tantalum.
Details regarding the ternary Nb–Si–C and Ta–Si–C phase diagrams, as well as the interfacial reactivity in the SiC/Nb and SiC/Ta systems, are presented in the following section. Then, high-temperature thermal treatments on both diffusion couples and manufactured sandwich cladding are discussed. Electron Probe MicroAnalysis (EPMA) and ion beam analysis were employed to determine the chemical composition of the interaction layer. The kinetics of interaction as a function of the holding time and temperature were determined. The latter results will be taken into account to determine the viability of the candidate liner materials in GFR operating conditions. Although being crucial for several demanding applications, it is the first time such an extensive work on both high purity SiC and SiC/SiC materials was conducted.
2. State-of-the-Art
2.1. Nb–Si–C System
The first study on the reactivity between Nb and Si, performed by Brewer [23] by mixing both element powders, has shown that NbSi0.55 and Nb5Si3 compounds form at 2100 K without the appearance of a liquid phase. NbSi0.55 was never again observed by the authors in further studies and did not appear in the Nb–Si phase diagram [24]. The authors observed that by adding C in the Nb–Si system, Nb2C forms. Similar results were obtained by Burykina [25] and Rahaman [26], where both carbides and silicides formed in the 1200–1800 °C temperature range. Above 1800 °C, Burykina only detected the presence of NbC. Brukl [27] found the Nb11Si8,1C2 ternary compound at 1300 °C, already reported in the literature [28,29], in equilibrium with Nb5Si3, NbSi2 and NbC1−x. Other authors reported the presence of Nb5Si4C [30,31] and Nb5Si3Cx [32,33], exhibiting a C-stabilized Na5Si3 hexagonal structure [34]. Metastable phases were also found to form in thin layer configurations (ex: Nb64C20Si16 [35]). The reaction between NbC and Si leads to the formation of NbSi2 and SiC, without the formation of a ternary compound [36,37,38]. The Nb3SiC2 MAX phase was predicted by thermodynamic calculations but was never experimentally evidenced [39]. From the Nb–Si–C ternary phase diagram at 1100 °C from the ASM (Figure 3a) [30], it can be concluded that SiC is not in thermodynamic equilibrium with Nb. The Nb5Si4C (T) compound is expected to form in equilibrium with Nb5Si3, NbSi2 and NbC1−x. The literature data summarized in Table 1 show strong discrepancies in the composition of the reaction layer formed in the Nb/SiC system as a function of the annealing temperature and the SiC type (sintered, CVD, …).
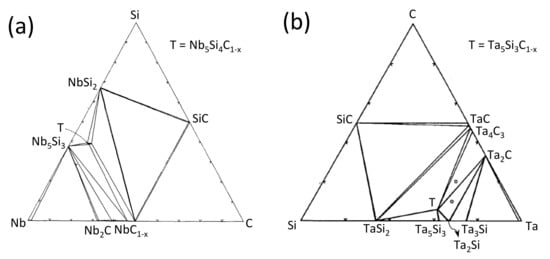
Figure 3.
(a) Isothermal sections of the ternary Nb–Si–C system at 1100 °C. Reprinted with permission from ref. [30]. 1990, Springer Nature; and (b) Ta–Si–C system at 1000 °C. Reprinted with permission from ref. [33]. 1993–1994, Elsevier.
The first study on the SiC/Nb interaction kinetics was conducted by Naka et al. [40]. Samples with high contents of free silicon (13% at.) in SiC were used. Nb5Si3 was found to form at 1200 °C, while at 1400 °C, the diffusion path is Nb/Nb5Si3/NbSi2/Si + SiC. When the Si content was reduced by the use of sintered SiC (containing a small percent of B and C) [41,42], then Nb5Si3 was the only observed phase and the interaction kinetics decreased. Using thin films of Nb (12.7 and 25 µm thick) [32,43,44], the reaction with sintered SiC (containing Al2O3 impurities) leads to the formation of carbides and Nb5Si3C for short durations. The following diffusion path SiC/NbC/Nb5Si3Cx/Nb5Si3/NbC + Nb2C/Nb2C/Nb, was observed, also confirmed in [45]. Nb thinner films (1–2 µm) on SiC at 1200 °C [35] lead to a close diffusion path: SiC/NbC/Nb64C20Si16/NbC/Nb2C/Nb. In another study [30], NbC was not found experimentally, but its presence is expected following thermodynamic consideration. The authors [35] proposed that Nb2C has to be sufficiently thick for NbCxSiy to form. Tests conducted on Hexalloy SiC evidenced the presence of SiO2 instead of carbides between SiC and NbCxSiy and Nb5Si3 layers between NbCxSiy and Nb2C layers. On dense SiC materials, two even (NbCx and NbCxSiy) layers and one uneven (Nb2C) layer in contact with Nb were observed at 1100 °C [30]. At lower temperatures (700–1000 °C) [46], a Nb thin layer (100 nm thick) on SiC leads to the following diffusion path: Nb/Nb carbides(Nb2C, Nb6C5)/Nb silicides (Nb3Si, Nb5Si3)/Nb5Si3/SiC, with even and uneven interfaces between carbides and silicides, which could be due to preferential diffusion at grain boundaries at a lower temperature. In this temperature range, metastable phases such as Nb3Si and Nb6C5 were also obtained. Figure 4a presents the parabolic rate constants as a function of temperature obtained from different studies, showing lack of data at high temperatures (T > 1200 °C) and discrepancies at low temperatures (800 °C < T < 1200 °C). Main experimental conditions and results are reported in Table 1.

Table 1.
Composition of the reaction zone in the Nb–Si–C system as a function of the system and the annealing conditions.
Table 1.
Composition of the reaction zone in the Nb–Si–C system as a function of the system and the annealing conditions.
| T (°C) | Duration | System | Formed Phases | Reference |
|---|---|---|---|---|
| 1827 | 15 to 60 min | SiC/Nb (powders) | Nb5Si3, NbSi0.55 | Brewer [23] |
| 1200 to 1800 | - | SiC/Nb (SiC compacted on dense metal) | NbC, NbSi2 | Burykina [25] |
| ≥1800 | 2 h | NbC | ||
| 1425 | - | SiC (nano)/Nb (powder) | Nb2C, Nb5Si3 | Rahaman [26] |
| 1300 | - | SiC/Nb (powders) | Nb11Si8.1C2, NbSi2, Nb5Si3, NbC1−x Nb2C | Brukl [27] |
| 1100 | 4 h | SiC (substrate)/Nb (1 to 2 µm) | Nb5Si4C, Nb5Si3, Nb2C | Yaney [30] |
| 1200 | 6 to 48 h | SiC/Nb (plates) | NbCx, NbSiyCz | Chou [31] |
| 1000 to 1200 | 4 h | SiC/Nb (1 µm) | NbC, Nb5Si4C, Nb5Si3, Nb2C | |
| 1100 to 1500 | 30 min to 6 h | Si + SiC/Nb (plates) | Nb5Si3 (1200 °C) Nb5Si3, NbSi2 (1400 °C) | Naka [40] |
| 1100 | 2 to 8 h | SiC/Nb (1 µm) | NbC, Nb2C, T(Nb 64 at.%–C 20 at.%–Si 16 at.%), NbC | Joshi [35] |
| 1200 | NbCx, T, NbCy | |||
| 1100 to 1500 | 30 min to 6 h | SiC(B,C)/Nb | Nb5Si3, NbSi2 | Naka [41] |
| 845 to 1500 | 30 min to 30 h | SiC(Al2O3)/Nb (12.7 and 25 µm) | NbC, Nb5Si3C, Nb5Si3, Nb2C | Naka [43] |
| 1500 | - | Si(Al2O3)/Nb (12.7 µm) | NbC, Nb5Si3C, Nb5Si3, Nb2C | Naka [32] |
| 1500 | 10 h | SiC(Al2O3)/Nb (plates) | NbC, Nb5Si3Cx, NbC, Nb2C | Naka [44] |
| 1000 to 1200 | 150 h | SiC (fibers or particles)/Nb (matrix) | NbC, Nb5Si3Cx, Nb5Si3, Nb2C | Colin [45] |
| 1300 | 8 to 100 h | NbC (sintered)/ Si (pure) | SiC, NbSi2 | Kao [36] |
2.2. The Ta–Si–C System
Thermal treatments at 1827 °C in the Ta–Si–C system performed by Brewer [23] by mixing Ta, Si and C powders have shown the formation of silicides (Ta4.5Si, also reported by Kieffer [47] but not reported in further studies, Ta2Si and Ta5Si3) in equilibrium with a ternary compound of hexagonal structure (Mn5Si3 type), Ta4.8Si3C0.5. As for the Nb–Si system, the Ta5Si3 phase may be stabilized by interstitial carbon; however, Burykina [25], who also studied the interactions in the Ta/SiC system by compacting dense Ta with SiC powders, has not detected the ternary compound between 1400 °C and 1500 °C but only TaC, Ta5Si3 and TaSi2 on the metal surface. At and above 1600 °C, the surface layers are only constituted of carbides. The isothermal section of the Ta–Si–C phase diagram at 1000 °C established by Schuster [33] shows that Ta5Si3C1−x is in equilibrium with TaC, Ta2C, Ta2Si, Ta5Si3 and TaSi2 (Figure 3b), in agreement with the isothermal section at 1827 °C [23]; therefore, SiC is not in equilibrium with Ta.
The Ta/SiC chemical interaction was mainly studied on thin films for electronic applications. At 400 °C, tantalum carbides may form [48], but some other authors did not observe any interaction below 800 °C [49]. Over this temperature, Ta reacts with SiC to form Ta5Si3Cx or tantalum silicides. For longer annealing durations, the Ta layer (320 nm) fully reacted with the SiC one (5 µm), deposited on Si substrate, to form TaC and TaSi2. A recent study on the sandwich cladding material with a tantalum liner has shown that in the as-fabricated material, an interaction already occurred with the formation of a layer of 1–3 µm thickness [50]. After annealing between 1000 and 1500 °C for 20 h, the SiC/TaC1−x/Ta5Si3/Ta2Si/Ta diffusion path has been identified from EPMA measurements. In addition, at 1500 °C, a carbon-rich zone between the two silicides was observed, but no specific species have been identified. A small amount of carbon was found in the bulk tantalum (5–6 at.%), which corresponds to the solubility limit at this temperature [51]. It was found in the form of TaCx particles in the bulk Ta after cooling.
The very few studies on interaction kinetics in the Ta/SiC system were essentially focused on thin-film experiments (Figure 4b). Geib [48] gave the following diffusion path between 400 and 800 °C: Ta/TaSi2/TaC/SiC. Chen [49] did not observe any reaction at 800 °C; however, at 1000 °C, the SiC/TaC/Ta5Si3Cx/Ta5Si3/Ta2C/Ta diffusion path was evidenced on a 320 µm thick Ta layer. At higher temperatures, the whole Ta layer reacted to form different compounds. Cao [52] found a slightly different diffusion path between 800 and 950 °C: SiC/Ta5Si3Cx + Ta2C/TaC/Ta. Only Feng [53,54] studied the kinetics of the reaction between Ta and SiC from 1400 to 1550 °C and determined the SiC/TaC/Ta5Si3Cx/Ta2C/Ta diffusion path. The growth kinetics of the reaction layer follows a parabolic law (diffusion-controlled process), with a growth constant of ; however, the experiments were conducted on a 7.5 µm Ta layer inserted between two layers of sintered SiC (containing a small percent of Al2O3), which does not correspond to a semi-infinite medium configuration. Parabolic rate constants deduced from experimental results obtained by Steinbrueck [50] are reported in Figure 4b. Large uncertainties can be obtained as the experiments were conducted for a single duration and as the metal may not be in close contact with the SiC/SiC composite; however, these values are more than two orders of magnitude higher than those reported by Feng [54]. These experimental studies on the Ta–Si–C system are gathered in Table 2.

Table 2.
Composition of the reaction zone as a function of the system and the annealing conditions in the Ta–Si–C system.
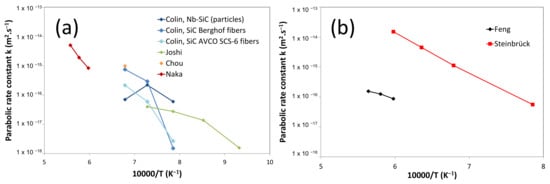
Figure 4.
Parabolic rate constants () as function of temperature in (a) the Nb/SiC. Data adapted from [31,35,43,45] and (b) the Ta/SiC systems. Data adapted from [50,54].
To conclude, the literature studies show large discrepancies and data are missing at high temperatures (T > 1200 °C). To guarantee the safety of nuclear systems, there is a need for reliable data on the growth kinetics and on the composition of the reaction zone in the Ta/SiC and Nb/SiC systems; therefore, diffusion couple experiments are performed at high temperatures (1050–1500 °C) for various durations and the reaction kinetics are compared to the kinetics obtained in the sandwich cladding configuration. Moreover, isothermal sections of the ternary Ta–Si–C and Nb–Si–C systems in the 1050–1500 °C temperature range are calculated with Thermocalc using the FUELBASE database.
3. Experimental Methods
Diffusion couples were made by putting into contact SiC and Ta or Nb plates. High purity CVD SiC (10 × 10 × 1 mm3), provided by Dow Chemical Co. (Marlborough, MA, USA), was used. For the metals, Ta and Nb from Plansee (Reutte, Austria), referred to as Ta_S (Sintered) and Nb_M (Melted) by the manufacturer, with a purity over 99.95% (100 ppm of Nb and 150 ppm of O) and 99.7% (3000 ppm of Ta and 150 ppm of O), respectively, were chosen. Then, 1 mm thick plates were cut to obtain 10 × 10 × 1 mm3 samples, which were mirror polished (down to 1 µm diamond paste). SiC plates were immersed for 1 min in a fluoride acid HF (10 vol%) solution to remove the native silicon oxide layer. The SiC and metal samples were placed in a graphite assembly and screwed with nuts and bolts (Figure 5).
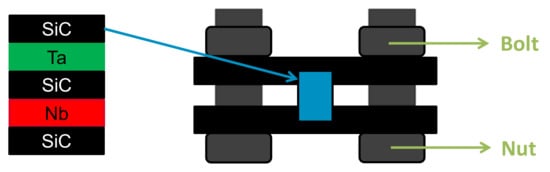
Figure 5.
Diffusion couple experiments scheme.
The sandwich concept was manufactured following the patent [11]. Hi-Nicalon type S (NGS Advanced Fibers Co., Toyama, Japan) fibers and Chemical Vapor Infiltration (CVI) were used to manufacture the high-purity SiC/SiC composites [56]. The inner layer is made of a single layer of filament winding SiC/SiC. It was ground to decrease its roughness (). The metal dimensions were adjusted to this layer outer diameter and put in contact with the SiC/SiC composite by drawing. The SiC/SiC outer layer is made of two layers of 2D braiding, also densified by CVI. The inner and outer SiC/SiC layers thicknesses are 300 and 600 µm, respectively. The same type of Ta (Ta_S) and Nb (Nb_M) were provided by Plansee for the metals. The tubes dimensions were previously adjusted to fit with the inner SiC/SiC diameter and the targeted thickness (70–100 µm) by High Pressure Tube Reduction (HPTR). Finally, recrystallization annealing for 30 min at 1200 °C was conducted.
Four annealing temperatures were chosen: 1050, 1200, 1350 and 1500 °C. These temperatures are above the nominal operating conditions of cladding material in GFR but can be reached in transient and/or accidental conditions. These temperatures were selected because they are expected to be sufficiently high to enhance the reaction kinetics and allow for a comparison with the literature data (Figure 4). Four holding times for each temperature were chosen to determine the growth kinetics of the reaction layer (assumed to follow a parabolic law): , , and . The value of depends on the annealing temperature. The isothermal holdings at 1050 and 1200 °C were conducted under vacuum and those at 1350 and 1500 °C under argon to avoid Si (to a lesser extent Si2C and SiC2) vaporization at high temperature. The experimental conditions are reported in Table 3.

Table 3.
Annealing treatment conditions were conducted on both sandwich cladding and diffusion couple experiments.
The thickness of the different layers was determined from Back-Scattered Electron Scanning Electron Microscope (BSE-SEM) images (Hirox SH-3500MB, Tokyo, Japan). Given the very close X-rays energies for Ta Mα and Si Kα energies (resp. 1.709 eV and 1.739 eV), Electron Probe MicroAnalysis (EPMA) (CAMECA SX-100, Gennevilliers, France) was preferred to measure the chemical composition of the phases. These measurements were conducted using a liquid nitrogen cold trap to properly evaluate the carbon concentration and to avoid contamination.
Ion beam analyses were also conducted to determine the reaction layer composition at CEA/IRAMIS facilities [57]. Rutherford Backscattering Spectrometry (RBS) and Medium Energy Ion Scattering (MEIS) were used for the “heavy” elements’ quantification (i.e., Si, Nb and Ta), while Particle Induced X-ray Emission (PIXE) was used to determine the C and O contents. Nuclear Reaction Analysis (NRA) was also applied to determine the C, O and Si contents in which deutons (2H2+) accelerated at 1.9 MeV were focused on a 3 × 3 µm2 area, which is close to the EPMA resolution (1 µm2).
4. Thermodynamic Calculations
Thermodynamic calculations using the CALPHAD (CALculation of PHAse Diagrams) method [48] were performed to predict the phase equilibria in the ternary Nb–Si–C and Ta–Si–C systems. The Thermo-Calc v. 2019a by Thermo-Calc Software AB (Solna, Sweden) and the FUELBASE database, developed for nuclear materials since 2005 [58], were used.
The thermodynamic calculations between 1050 and 1500 °C in the Nb–Si–C system show that Nb is not in equilibrium with SiC (Figure 6). In the model, the ternary compound Nb5Si3C phase was introduced as a stoichiometric compound due to the lack of thermodynamic data as a function of the carbon content. NbC1−x is in equilibrium with SiC and C along with Nb2C and Nb5Si3 for C-rich and Nb-rich compositions, respectively. When the carbon content decreases in NbC1−x, the carbide is in equilibrium with NbSi2 then Nb5Si3Cx and afterwards with Nb5Si3. The range of homogeneity of Nb2C1−x increases with temperature. At 1500 °C, a Si-rich liquid phase appears following the eutectic transformation in the Si–SiC–NbSi2 system. Following the Gibbs–Duhem equation, this liquid phase is limited to this area and should not appear in the SiC/Nb system. This is in agreement with the fact that no liquid phase was previously evidenced in the literature in this temperature range.
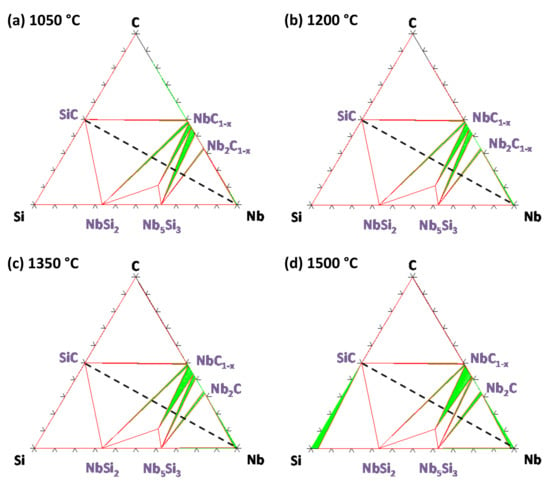
Figure 6.
Isothermal sections of the ternary Nb–Si–C system calculated with Thermo-Calc software and the FUELBASE database at (a) 1050 °C, (b) 1200 °C, (c) 1350 °C and (d) 1500 °C.
Calculated isothermal sections of the Ta–Si–C system are plotted in Figure 7. Ta is also not in equilibrium with SiC at these temperatures. The carbides TaC1−x, Ta2C1−x and Ta5Si3Cx are modeled as non-stoichiometric compounds, with x ranging from 0.45 to 1 in Ta5Si3Cx. Nevertheless, uncertainties remain regarding these phase equilibria due to the lack of experimental data. TaC1−x is in equilibrium with TaSi2 for almost its whole composition range, except for the lowest C concentration, where it is in equilibrium with Ta5Si3C. The ternary compound Ta5Si3Cx is in equilibrium with Ta2C (itself in equilibrium with Ta5Si3 and Ta2Si), while the rest of the domain extent of Ta2C1−x is in equilibrium with Ta3Si (between 1050 and 1350 °C) or with Ta3Si and Ta2Si (at 1500 °C). The main difference when the temperature increases is the formation of a liquid phase over 1400 °C in the Si-rich area. Again, the Gibbs–Duhem equation does not predict the formation of a liquid phase in the Ta/SiC system, which is corroborated by experimental data from the literature.
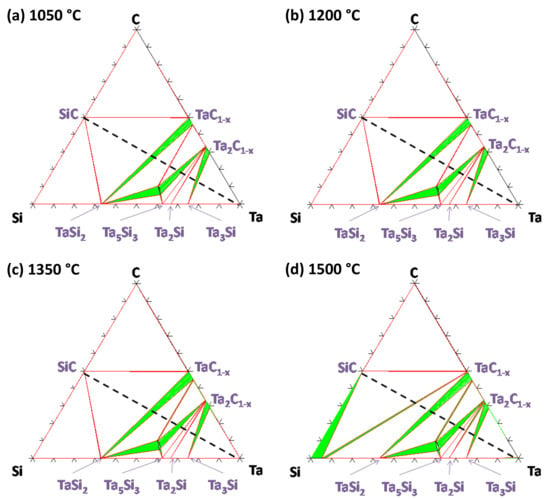
Figure 7.
Isothermal sections of the ternary Ta–Si–C system calculated with Thermo-Calc software and the FUELBASE database at (a) 1050 °C, (b) 1200 °C, (c) 1350 °C and (d) 1500 °C.
5. Results
In this section, first, the morphology and the chemical composition of the whole reaction product layer and of each of its sublayers, for both diffusion couple and sandwich cladding configurations, is assessed at 1200 °C from the EPMA and ion beam analyses. Afterward, the evolution of the composition of the reaction layers with the temperature for both configurations is given. From the SEM micrographs of the samples cross-sections, a detailed analysis of the layers’ thicknesses as a function of time at 1200 °C is provided in order to confirm if the growth kinetics follows a parabolic law (as it is expected from the literature data) and to determine if the behaviors of sandwich cladding materials and diffusion couples are the same. Finally, the evolution of the reactive systems with temperature is assessed and the interaction kinetics are compared with the literature data.
5.1. Reaction Zone Morphology and Composition at 1200 °C
Figure 8 shows the reaction layer in the sandwich cladding with Nb (Figure 8a) and Ta (Figure 8b) after 1000 h at 1200 °C, observed by BSE-SEM. The upper and lower areas correspond to the outer and inner SiC/SiC layers, respectively. In both cases, the reactivity is higher on the outer than on the inner side due to a better contact originating from the higher thermal expansion coefficients of metals (>7 · 10−6 K−1 for Nb [59] and >6.5 · 10−6 K−1 for Ta [60]) compared to that of β-SiC (<5.5.10−6 K−1 [61]) in the 20–1200 °C temperature range. The reaction layer thickness observed in the Ta/SiC system (≈30 µm) is significantly lower than in the Nb/SiC one (≈60 µm). Moreover, the cleavage between the reaction zone and SiC is observed on both faces after cooling down, which can lead to a decrease in the thermal conductivity, being detrimental to the cladding application, which has to act as a heat exchanger.
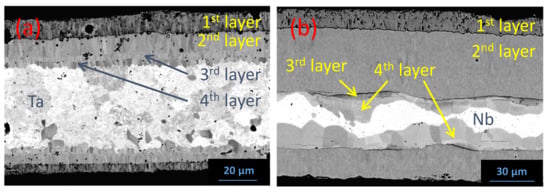
Figure 8.
BSE-SEM micrographs of the reaction zone after 1000 h at 1200 °C in the (a) Nb–SiC/SiC and (b) Ta–SiC/SiC sandwich claddings.
Regarding the Nb/SiC system, four layers are observed in the reaction zone. The first layer (from the SiC side), of ≈9 µm thickness, is in contact with a much thicker second layer (≈38 µm). The coalescence of vacancies during the interdiffusion process at the interface between the 1st and 2nd layers is observed, leading to the formation of porosities at this interface but also in the 1st layer. A non-continuous third thin layer (2 µm) only appears at some points of the sample. The thickness of the 4th layer presents a broad dispersion (9 ± 3 µm). No precipitates were detected in the metal. Finally, note that the 1st layer is almost not observed on the inner side of the sample, which could be related to the lack of contact between the metal and the SiC/SiC composite.
EPMA mapping and ion beam analyses of the Nb/SiC sandwich sample after 1000 h at 1200 °C (Figure 9) show that the 1st layer in contact with SiC is strongly depleted in Si and corresponds to NbC1−x. The measured value of x for the NbC1−x compound depends on the characterization technique (Table 4). The higher C content found by PIXE (48 at.%) than with EPMA (42 at.%) is probably due to the larger probed area for PIXE, including porosities, thus inducing C content increase. As far as the ternary compound is concerned, Nb5Si2.86C0.62 and Nb5Si3.01C0.84 compositions are found from EPMA and NRA/PIXE, respectively, leading to Nb5Si3Cy for the 2nd layer. This composition is different from the ternary compound reported in the literature in the Nb–Si–C system (Nb5Si4C) [30]. The exact composition of the 3rd layer could not be accurately determined due to its low thickness; however, it could correspond to another niobium carbide, noted NbC1−z, with z > x, i.e., less C content than NbC1−x. NRA shows two different C contents for the layers on the metal side, while EPMA analysis of the uneven layer in contact with the metal leads to Nb2C as the 4th layer. No binary silicides are detected in the Nb/SiC system.
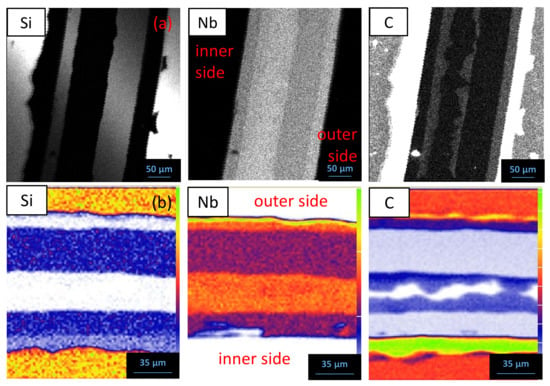
Figure 9.
EPMA mapping (a) and and ion beam analyses (b), NRA for Si and C and PIXE for Nb, of the reaction zone of a Nb sandwich cladding after 1000 h at 1200 °C.

Table 4.
Thickness and composition of the different reaction layers analyzed by EPMA and ion beam analyses (NRA for C and Si, PIXE for Ta and Nb) in the Nb/SiC and Ta/SiC systems after 1000 h at 1200 °C.
For Ta/SiC system (Figure 8b), the reaction product is also constituted of four layers of different compositions. The microstructures of the reaction layers are similar, with the presence of elongated grains oriented perpendicularly to the interface, highlighting columnar growth. Porosities are again observed in the metal, mainly on the inner side and between the 1st and 2nd layers from the SiC side (both ≈10 µm thick). The last two layers (on the Ta side) are thinner (≈3 and ≈6 µm thick for the 3rd and 4th layers, respectively).
Figure 10 shows the EPMA mapping and the ion beam analyses in the sandwich Ta/SiC system after 1000 h at 1200 °C. The measured chemical compositions are reported in Table 4. On the outer side, a strong Si depletion is observed: the 1st layer in contact with SiC matches the TaC1−x carbide phase. The 2nd layer corresponds to a ternary phase of compositions Ta5Si2.91C0.63 and Ta5Si2.90C0.89 determined by EPMA and ion beam techniques, respectively, in agreement with the Ta5Si3Cy compound. A depletion in C is then observed and two Si-rich phases are detected. The compound in contact with Ta5Si3Cx is Ta5.20Si3, corresponding to Ta5Si3 (3rd layer), and the one close to the metal is Ta2.07Si, corresponding to Ta2Si (4th layer). C X-ray mapping highlights the presence of precipitates within the metal, with a composition close to Ta2C; however, the fact that Ta2C is not present as a continuous layer strongly suggests that it could be a two-phase zone (Ta + Ta2C) inside this diffusion couple.
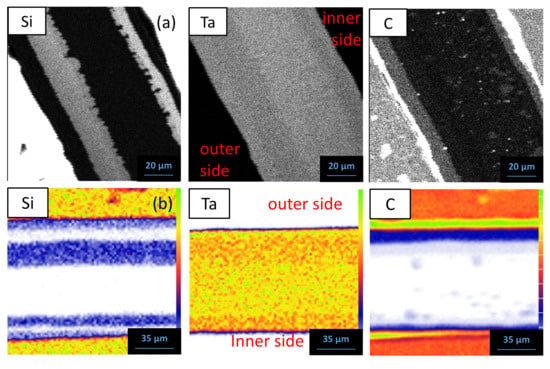
Figure 10.
EPMA mapping (a) and ion beam analyses (b), NRA for Si and C and PIXE for Ta, of the reaction zone of a Ta sandwich cladding after 1000 h at 1200 °C.
Based on the previous results, the following diffusion paths can be deduced for the Nb/SiC (a) and Ta/SiC (b) systems at this temperature:
- (a)
- SiC/NbC1−x/Nb5Si3Cy/NbC1−z/Nb2C/Nb
- (b)
- SiC/TaC1−x/Ta5Si3Cy/Ta5Si3/Ta2Si/Ta + (Ta2C)precipitates/Ta
The same microstructure was observed regardless of the annealing duration at 1200 °C; therefore, schematic presentations of the morphology of the reaction zone in the Nb/SiC (a) and Ta/SiC (b) diffusion couples at 1200 °C, based on the upper analysis and description, are given in Figure 11.
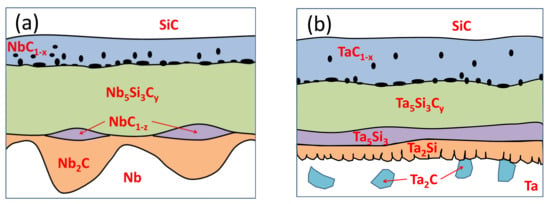
Figure 11.
Schematic illustration of the reaction zone in the Nb/SiC (a) and Ta/SiC (b) systems at 1200 °C.
5.2. Influence of Temperature
The same analyses of the reaction zone as those conducted at 1200 °C were performed after isothermal holdings at 1050, 1350 and 1500 °C. Diffusion paths observed at those temperatures are reported in Table 5.

Table 5.
Diffusion paths in the Nb/SiC and Ta/SiC systems as a function of the temperature.
In the Nb/SiC system, after 1000 h at 1050 °C (Figure 12a), the two first layers (NbC1−x and Nb5Si3Cy) are even and harmonious. The other phases form at the Nb5Si3Cy/Nb interface, where nucleation occurs, leading to an uneven interface. At each of these nucleation sites, two phases nucleate and grow: a very thin layer of Nb1−z in contact with Nb5Si3Cy, which seems to grow at the expense of the latter, and Nb2C of very irregular thickness and morphology. After 100 h at 1350 °C (Figure 12b), the same phases are detected with still a very thin and uneven layer of NbC1−z between Nb5Si3Cy and Nb2C, which is more regular; however, the porosities at the NbC1−x/Nb5Si3Cy interface, observed at 1200 °C, have shifted to the opposite interface (Nb5Si3Cy/NbC1−z or Nb5Si3Cy/Nb2C interface if NbC1−z is missing) and are less numerous. By increasing the temperature to 1500 °C (48 h) (Figure 12c), the same compositions of the reaction zone are still noted, but the Nb1−z layer is very thin and no porosities are observed.
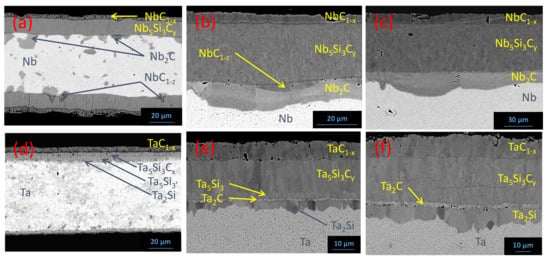
Figure 12.
Reaction layers in the Nb/SiC (a–c) and in the Ta/SiC (d–f) systems after 1000 h at 1050 °C, 100 h at 1350 °C and 48 h at 1500 °C, respectively.
In the Ta/SiC system at 1050 °C (Figure 12d), the reaction zone composition is identical to the one at 1200 °C (Figure 8b). At 1350 °C (100 h) (Figure 12e), the two phases formed on the SiC side are identical (TaC1−x and Ta5Si3Cy). EPMA highlights that the phase in contact with Ta5Si3Cy is still Ta5Si3; however, two layers are now observed between Ta5Si3 and Ta, whereas at 1200 °C, a single Ta2Si layer and a two-phase zone containing Ta2C precipitates inside Ta matrix are observed. The composition of the nearby Si-depleted additional layer cannot be determined because of its low thickness (1–2 µm), but EPMA results suggest that it should be Ta2C. Finally, a C-depleted layer, corresponding to Ta2Si, is in contact with the metal. After 48 h at 1500 °C (Figure 12f), only four layers are again observed. Ta5Si3 disappeared while the thin layer of Ta2C thickened. Moreover, porosities have switched from the TaC1−x/Ta5Si3Cy interface to the Ta2C/Ta2Si interface.
5.3. Diffusion Couple and Sandwich Geometry Comparison
The aim of performing tests in both geometries (diffusion couples and sandwich cladding) is to determine if the behavior is the same in both cases. In addition, for the highest temperature and/or longest duration investigated in this study, the whole liner thickness could have reacted with SiC, and therefore, the system could not be considered semi-infinite. Indeed, other phases could also form when the whole liner has reacted because one of the diffusion couple end-members is no longer identical.
Diffusion couples after isothermal holdings at 1050 °C could not be analyzed because the interaction thickness is too small; therefore, only the results on the sandwich concept are considered for this temperature. On the sandwich’s outer side, whatever the temperature, the same phases form as in the Nb/SiC or Ta/SiC diffusion couple systems. On the contrary, on the inner side, differences are noticed. For instance, at 1200 °C, Ta2C precipitates formed. The poor contact with SiC could decrease the reactivity. Carbon may diffuse through the layers and solubilize in Ta at 1200 °C. During cool down, carbon could then precipitate as Ta2C, due to the decrease in its solubility limit in Ta with decreasing temperature [51]. At 1350 °C, the layer compositions are identical on both sides; however, the minor phases (Ta2C and NbC1−z) are thicker on the inner side than on the outer one. At 1500 °C, similar results are observed on diffusion couples and on both sides of the sandwich cladding; therefore, it can be concluded that the same layers are obtained for both the diffusion couples and the outer side of the sandwich cladding. Note finally that the metal inner face reacts less with the composite, essentially at low temperature, due to poor contact.
5.4. Growth Kinetics of the Reaction Layer
The chosen isothermal holding times at 1200 °C were 63, 250, 570 and 1000 h for Nb/SiC and Ta/SiC systems for both diffusion couple and sandwich cladding configurations. For each experiment, the average thickness of each reaction layer was measured in several areas for a total of ≈2 mm wide. Note, however, that in the case of the Nb/SiC system, only the sum of the thicknesses of the 3rd and 4th layers (resp. NbC1−z and Nb2C) is given because the micrographs have revealed a concomitant growth of those layers (Figure 8a). Figure 13 shows the evolution of the total thickness of the reaction zone as well as the thickness of each layer as a function of time for the Nb/SiC (Figure 13a) and Ta/SiC (Figure 13b) systems obtained in the diffusion couple experiments. This figure clearly shows that, whatever the reaction time, the total thickness of the reaction zone for Nb/SiC system is about two times higher than in the Ta/SiC system. Thus, the Nb/SiC system is much more reactive than the Ta/SiC system. Moreover, for Nb/SiC system, the 2nd layer (ternary compound) is much thicker than the other layers, whereas, for the Ta/SiC system, the ternary compound layer is only slightly thicker than the 1st one (TaC1−x). In Figure 14, the average total thickness of the reaction zone is plotted versus the square root of the holding time. It clearly shows that for both Nb/SiC and Ta/SiC systems, the growth kinetics of the reaction layer follows a parabolic law and thus it is controlled by the diffusion process through the reaction layers. These results are in good agreement with those reported in the literature [31,35,43,45,50,54]. The same conclusion also holds for the 2nd layer (ternary compound) in both systems, as its thickness varies linearly with the square root of the reaction time (not presented in Figure 14); however, for the other reaction layers, given their small thicknesses, the high standard deviation and the small number of experimental measurements (four durations), it is not possible to definitely conclude whether their growth kinetics follows a parabolic law.
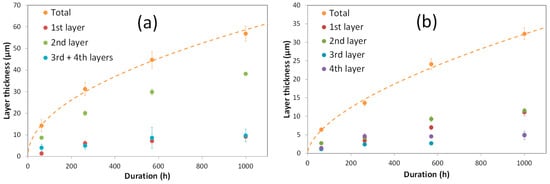
Figure 13.
Evolution of the total reaction layer and of each layer’s thicknesses as a function of time at 1200 °C in the Nb/SiC (a) and Ta/SiC (b) diffusion couples. The 1st, 2nd and 3rd + 4th layers correspond to NbC1−x, Nb5Si3Cy and NbC1−z + Nb2C in the Nb/SiC system, respectively. The 1st, 2nd, 3rd and 4th layers correspond to TaC1−x, Ta5Si3Cy, Ta5Si3 and Ta2Si in the Ta/SiC system, respectively.
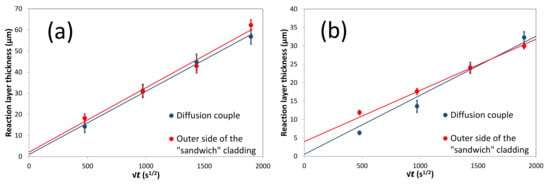
Figure 14.
Evolution of the reaction zone thickness at 1200 °C as a function of the square root of time and geometry in the (a) Nb/SiC and (b) Ta/SiC.
For comparison purposes, the total thickness of the reaction product corresponding to the outer side of the sandwich cladding is also presented in Figure 14. Note that for all experiments in the sandwich cladding configuration, strong variations of the total thickness of the reaction zone are observed on the inner side as the contact between the SiC/SiC and the metal is not good in some places; thus, no relation can be drawn for this side. Figure 14 shows that experimental results obtained for both configurations (diffusion couple and sandwich cladding) are quite similar.
Note that for Ta/SiC system, a good correlation is found between diffusion couples and the outer side of the sandwich cladding for long durations (570 and 1000 h at 1200 °C), whereas a higher reactivity (thicker reaction layer) is obtained for the sandwich configuration for shorter annealing times. For Nb/SiC system, the results for both configurations are almost identical for all conditions in time and temperature; the same slopes (parabolic rate constants) are obtained but with different intercepts. This phenomenon can be attributed to the manufacturing process of the sandwich cladding. In fact, the outer layers of SiC/SiC composites are densified by CVI, between 900 to 1050 °C, which induces a small but significant initial reactivity with the composite [50]. In these experiments, the initial reaction thicknesses after the manufacturing process of the sandwich cladding are ≈5 and ≈3 µm for Nb and Ta liners, respectively (Figure 15). Note that after the manufacturing process with both liners, the morphologies of the reaction layers are similar to those observed after the annealing at 1050 and 1200 °C, except for the fact that the 3rd layer (NbC1−z) in the Nb/SiC system is not observed. Finally, in the Ta/SiC system, porosities are also observed at the interface between the 1st and the 2nd layers, whereas there are fewer porosities in the case of Nb/SiC tubes.
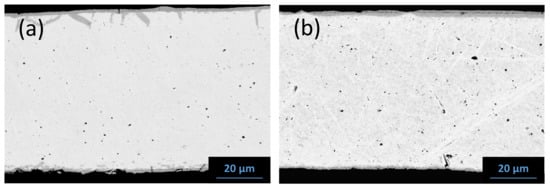
Figure 15.
Initial state of the liner material in the sandwich cladding made of (a) niobium and (b) tantalum after the manufacturing.
The total reaction layer thickness () was measured after all isothermal holdings between 1050 and 1500 °C. For each temperature (1050, 1200, 1350 and 1500 °C), the variation of the total thickness with the square root of time is plotted. In all cases, the growth kinetics follows a parabolic law (Equation (1)). The parabolic growth constants (), extracted from diffusion couples and sandwich cladding on the outer side, are plotted as a function of the inverse of temperature in Figure 16. This figure clearly shows that in all cases, a linear variation of with is obtained. The following expressions of with for both Nb/SiC and Ta/SiC systems are then obtained by using a linear regression analysis (Equations (2)–(5)):
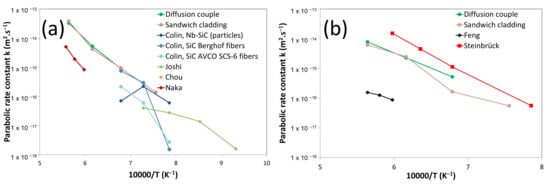
Figure 16.
Parabolic growth constants () as a function of the inverse of the temperature in comparison with literature data in the Nb/SiC (a) and Ta/SiC (b) systems.
For comparison purposes, Figure 16 shows some data from the literature on the growth kinetics in the Nb/SiC and Ta/SiC.
For the Nb/SiC system, Chou [31] and Joshi [35] reported the growth of both the (NbC1−z + Nb2C) and the ternary compound (Nb5Si4C in their work) as a function of time. A total growth constant () was extracted from those results. The results obtained in this study are close to the results obtained by Chou (only at 1200 °C) and Colin [45], on Berghof SiC fibers (between 1100 and 1200 °C) and on SiC particles (1000–1100 °C) in a Nb matrix. The lower reactivity of AVCO fibers is, according to the authors, due to the presence of free C on the fiber’s surface, decreasing their reactivity. Joshi’s experimental data obtained at a lower temperature (<1000 °C) are in agreement with results obtained in the present work; however, at higher temperature (1000, 1100 °C), the reactivity is underestimated in comparison with this study. Finally, the largest discrepancy is found in Naka’s work [32], who used sintered SiC containing a small percent of Al2O3, lowering the system reactivity.
A single study is available in the literature on the kinetics of the Ta/SiC interaction [54]. The results extracted from [50] come from only one duration for each temperature. The results of the present work highlight that the growth kinetics of the reaction zone is about 40 times higher than that reported by Feng [47] (Figure 16b). In the same way, the SiC quality (pressureless sintered SiC with a small percent of Al2O3) used by Feng could be accountable for this lower reactivity. Moreover, the parabolic growth constants obtained in this study are lower than those obtained by Steinbrueck using the same materials (sandwich cladding) [50]. This can be explained by the annealing at several durations for each temperature in this work, leading to higher precision. Note finally that in our study, the small reaction layer coming from the manufacturing process can be neglected (Figure 15).
Moreover, for both Ta/SiC and Nb/SiC systems, the evolution of the thickness of different layers was evaluated as a function of temperature in the same way as it has been achieved for the whole reaction layer. This treatment was first performed for some single-phase layers: TaC1−x, Ta5Si3Cy, NbC1−x and Nb5Si3Cy. For the other phases, an arbitrary group for each system was selected: (i) TSC group (corresponding to the Ta5Si3, Ta2C and Ta2Si ensemble)—phases from this group were gathered because their presence or absence depending on the temperature (Ta2C and Ta5Si3 are not detected at 1200 and 1500 °C, respectively)—and (ii) NbC group (i.e., NbC1−z and Nb2C ensemble).
The variation of the growth constant () of each layer (and group layers) as a function of the inverse of the temperature is given in Figure 17 for both sandwich cladding and diffusion couple experiments. This figure shows that for each layer, decreases linearly with 1/T. From Figure 17, the variation of the parabolic growth constant with T was determined according to the following equation:
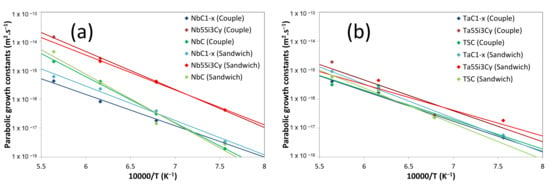
Figure 17.
Parabolic growth constants () of each layer as a function of the inverse of the temperature in Nb/SiC (a) and Ta/SiC (b) systems. TSC = group layer corresponding to the Ta5Si3, Ta2C and Ta2Si ensemble. NbC = group corresponding to NbC1−z and Nb2C ensemble.
From Arrhenius’ laws (Equation (6)), growth activation energies for each phase () were calculated (Table 6). Again, a good agreement is found between the sandwich cladding and the diffusion couples.

Table 6.
Arrhenius activation energies (kJ/mol) of the different phase formation in the Nb/SiC and Ta/SiC systems. TSC = group layer corresponding to the Ta5Si3, Ta2C and Ta2Si ensemble. NbC = group corresponding to NbC1−z and Nb2C ensemble. RL denotes the total reaction layer in each system.
In the Nb/SiC system, strong differences appear in the layers’ thicknesses and activation energies. The thickness of the Nb5Si3Cy layer is substantially greater than the other ones and is the major constituent of the reaction zone. The activation energy for the NbC1−x phase (217 kJ/mol) is close to the one of TaC1−x phase (213 kJ/mol). The Nb5Si3Cy phase has a slightly higher activation energy (240 kJ/mol) but is smaller than the NbC one (315 kJ/mol). This result suggests that the nucleation of the Nb2C phase could be difficult [62]. This difficulty in forming the Nb2C layer could lead to its strong irregularity. Thus, in some areas of the Nb/Nb5Si3Cy interface, nucleation of the Nb2C phase takes place at some preferential nucleation sites, thus allowing the Nb2C phase formation and growth. This phenomenon is evidenced in the micrographs after annealing at 1050 °C, showing that a uniform layer of Nb2C did not form even after 1000 h (Figure 12a). In the Ta/SiC system, the activation energies (around 200 kJ/mol) and parabolic growth constants of the different phases are close, highlighting a similar growth of the different layers. Note, however, that the activation energy for the whole reaction layer determined in this work (196 and 218 kJ/mol for the diffusion couples and sandwich cladding, respectively) is significantly higher than that determined by Feng [54] (143 kJ/mol), which could be attributed to the difference in the SiC quality.
These results are compared to the literature data in Figure 18. As for the whole layer, our results are in good agreement with Chou’s data at 1200 °C for NbC1−x and Nb5Si3Cy [31]. Joshi’s investigation of thin films strongly underestimated the ternary compound activation energy (84 kJ/mol) [35]. The agreement with Naka’s results strongly depends on the SiC type. The closest results were obtained with sintered SiC containing important quantities of free Si (13%) (Naka 1 [42]). When pressureless sintered SiC with sintered additives (C and B for Naka 2 [42] and Al2O3 for Naka 3 [43]) were used, the reactivity kinetics was slower. The corresponding activation energies for the Nb5Si3Cy phase (≈390 kJ/mol)) in their work are higher than those obtained here (≈240 kJ/mol), but the growth kinetics of the NbC1−x phase are similar (Figure 18); however, they also found activation energy of NbC group layers (also including NbC1−z and Nb2C layers) of 353 kJ/mol on the SiC with alumina sintering additive, which is close to that obtained in this work (315 kJ/mol).
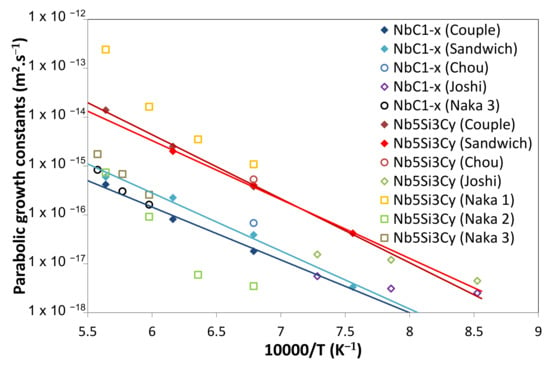
Figure 18.
Comparison of the parabolic growth constants () of the different layers obtained in this study with the literature data.
6. Discussion
In this section, some important points concerning the diffusion path, diffusing species and the relations between the species flux and their chemical potentials in both Nb/SiC and Ta/SiC systems are discussed. Finally, their consequences in the use of niobium or tantalum liners in sandwich cladding for an application in GFR are examined.
6.1. Diffusion Paths
In multiphase diffusion in binary and ternary solid-state systems, two main principles have to be fulfilled [63]. First, the mass balance, i.e., the diffusion path has to cross at least one time the SiC–Nb (or SiC–Ta) composition line in the ternary phase diagrams (Nb–Si–C or Ta–Si–C). If this is not clearly observed in a diffusion couple, one should verify that the solubility limits in the end members of the diffusion couple are sufficient enough in order to cross at least one time this composition line, otherwise, there is an error somewhere. Secondly, the diffusion of a given species always occurs in the direction of decreasing the chemical potential of that species, i.e., the chemical potential gradient is the driving force of the diffusion process. Note that in binary systems, the chemical potential gradient of a given species is always in the same direction as its concentration gradient, while in ternary and multicomponent systems, the chemical potential gradient of a given species could sometimes be in the opposite direction of its concentration gradient.
Based on the EPMA measurements (see Section 5.1 and Section 5.2), the diffusion paths at 1200, 1350 and 1500 °C are plotted on the isothermal sections of Nb–Si–C and Ta–Si–C systems in Figure 19. The diffusion paths at 1050 °C are not plotted because the results are similar to those obtained at 1200 °C. Regarding the Nb/SiC interaction, the diffusion path is also identical in the whole temperature range (SiC/Nb1−x/Nb5Si3Cy/Nb1−z/Nb2C/Nb), but the NbC1−z layer (between Nb5Si3Cy and Nb2C) is sometimes absent or too thin to be detected by EPMA. This explains why Nb5Si3Cy is directly in contact with Nb2C at 1500 °C. The segment between NbC1−x and Nb5Si3Cy crosses the SiC/Nb line, confirming the mass balance equation. Note that the chemical potentials of carbon and silicon both decrease from SiC to Nb end members of the diffusion couple. On the contrary, increases along the diffusion path from SiC to Nb.
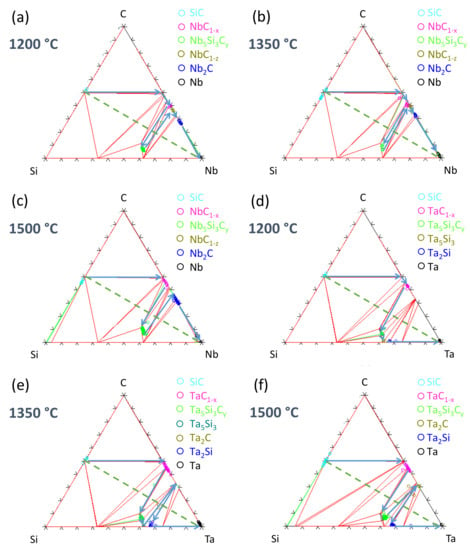
Figure 19.
Diffusion paths determined from EPMA measurements in the Nb/SiC ((a–c) and Ta/SiC (d–f)) systems at 1200 °C, 1350 °C and 1500 °C, respectively.
Starting from the SiC end member of the diffusion couple, SiC is in equilibrium with NbC1−x and NbSi2, i.e., is identical in these three phases (the same for ). Then, both and decrease in the NbC1−x phase until the NbC1−x/Nb5Si3Cy interface. Although not being modeled, the decrease in carbon concentration in the NbC1−x phase is observed on EMPA measurements, which is consistent with a decrease in . Afterward, the chemical potentials of C and Si decrease through the ternary Nb5Si3Cy phase until the Nb5Si3Cy/Nb1−z interface, then through the NbC1−z phase up to the NbC1−z/Nb2C interface, and finally through the Nb2C phase down to their limit values at the Nb2C/Nb interface.
The presence of tantalum silicides and carbides at all the temperatures also validates the mass balance in the Ta/SiC system even if the diffusion path evolves with the annealing temperature. According to the isothermal section of the Ta-Si-C system, the phases SiC/TaC, TaC/Ta5Si3Cy, Ta5Si3Cy/Ta5Si3, Ta5Si3/Ta2Si, Ta5Si3/Ta2C and Ta2Si/Ta2C are in equilibrium, so the observed transitions (Table 5) are thermodynamically possible; however, the Ta3Si phase was not detected between the Ta2Si layer and Ta liner at 1350 and 1500 °C. It could be explained by the formation of Ta2C instead of the Ta3Si phase, but the X-ray mapping does not evidence any increase in the carbon concentration at the Ta2Si/Ta interface. Another hypothesis is that the Ta3Si phase could be too thin to be detected. The exact composition of the carbide phase between Ta5Si3 and Ta2Si at 1350 °C could not be experimentally determined due to its limited thickness (2 µm), but Ta2C is the only phase in equilibrium with both Ta5Si3 and Ta2Si.
Therefore, except for the composition of the ternary compound (Figure 6) in the Nb–Si–C system, a good agreement between the diffusion paths and the calculated phase diagrams is found.
Figure 20 gives a schematic isothermal section of the ternary Nb–Si–C system. The composition range of the different phases is enlarged in order to better visualize the composition changes through all reaction layers as well as the diffusion path in the Nb/SiC diffusion couple. The pairs of points 1, 2, 3, 4 and 5 in Figure 20a correspond to the interfaces 1, 2, 3, 4 and 5 in Figure 20b, respectively. A similar schematic isothermal section can be drawn for the ternary Ta–Si–C system (not given here).
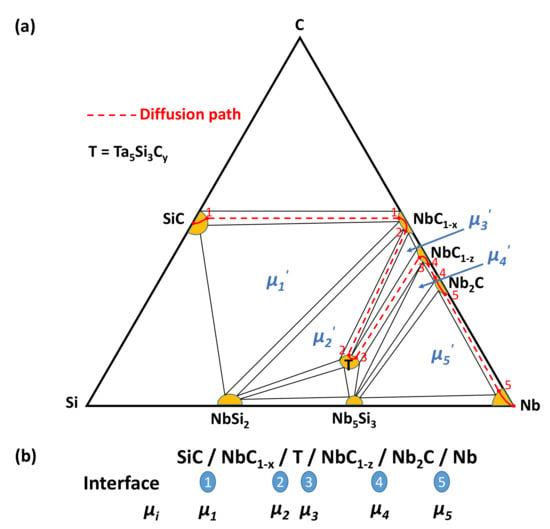
Figure 20.
Schematic isothermal section of the ternary Nb–Si–C system. (a) Diffusion path between 1200 and 1500 °C. (b) Chemical potentials at the different interfaces.
6.2. Diffusing Species
Carbon was determined as the diffusing species in the binary Nb–C [64,65] and Ta–C [66] systems, whereas in the binary Nb–Si [67] and Ta–Si [68] systems, both species were found to diffuse, even though the diffusion of the metal is slower than that of Si; however, to the best of our knowledge, there are no data on the mobilities of different components in the Nb–Si–C and Ta–Si–C ternary systems. In order to determine the fastest elements in the Nb/SiC and Ta/SiC systems, markers were placed at the SiC/metal diffusion couples’ interface before annealing for 1000 h at 1350 °C. Several marker types were tested. First, Al2O3 and Y2O3 submicronic powders placed between the plates led to bias because the optimal marker quantity cannot be determined. Thereby, refractory metal markers were used, such as tungsten (note that both Nb and Ta form continuous solid solutions with W).
After the experiments, EDX analyses were performed at each interface with Nb and SiC and in the reaction zone (zone 1 to 6) (Figure 21). W was only detected at the NbC/SiC interface (zones 5 and 6); therefore, Si and C are the fastest diffusing species and Nb mobility could be several orders of magnitudes lower than that of those elements. W markers were not applied to Ta/SiC systems, but the similarities in the reaction zone near SiC (MC1−x and M5Si3Cy with M corresponding to Nb or Ta) and the high melting temperatures of Nb and Ta allow us to reasonably think that C and Si are also the fastest diffusing species in the Ta/SiC system.
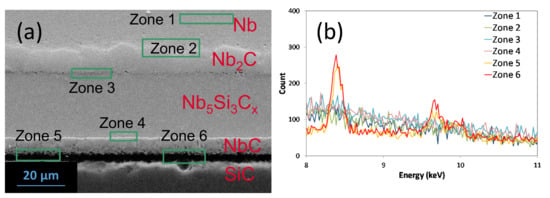
Figure 21.
BSE-SEM (a) and EDX spectra (b) of the tungsten (L-α and L-β) at several zones and interfaces in the Nb/SiC system.
Thanks to this result, by noting and , the diffusing C and Si atoms through different reaction layers and regarding the diffusion path in the different systems (Table 5), chemical reactions equations can be written from SiC decomposition Equation (7) at each interface in the Nb/SiC (Equations (8)–(11)) and Ta/SiC systems (Equations (12)–(18)).
| Interface | ||
| NbC1−x/SiC | (7) | |
| TaC1−x/SiC | ||
| Nb5Si3Cy/NbC1−x | (8) | |
| NbC1−z/Nb5Si3Cy | (9) | |
| Nb2C/NbC1−z | (10) | |
| Nb/Nb2C | (11) | |
| Ta5Si3Cy/TaC1−x | (12) | |
| Ta5Si3/Ta5Si3Cy (1050–1350 °C) | (13) | |
| Ta2C/Ta5Si3 (1350 °C) | (14) | |
| Ta2C/Ta5Si3Cy (1500 °C) | (15) | |
| Ta2Si/Ta5Si3 (1050–1200 °C) | (16) | |
| Ta2Si/Ta2C (1350–1500 °C) | (17) | |
| Ta/Ta2Si | (18) |
6.3. Evolution of a Reactive System-Chemical Potentials Gradients through Reactive Layers
Physicochemical growth models of the reactive layer in an A–B binary system are relatively well established in the case of growth limited by diffusion of the reactive element through the reaction product layer when one or two reaction layers grow and only one component is mobile (see for instance [69]). In this work, reactive diffusion in ternary systems with four or five reaction layers and two mobile species (Si and C) is studied. Thus, the evolution of such a system is relatively complex and no analytic expressions exist in the literature. Nevertheless, in the following, some general equations concerning the diffusion process are given and the driving forces of diffusion through each reaction layer are calculated for the Nb/SiC system.
In each phase (), the flux () of a given component () of the system is related to its concentration () gradient following Fick’s first law (Equation (19)).
Most of the diffusion coefficients of species in phase () along with the concentration of the different species in different phases are unknown. Moreover, in several cases, the variation of concentration of species in phase is very small (as is the case in this study) and often unknown; however, the flux () can be related to the chemical potentials gradient following Equation (20), where corresponds to the chemical potential of species in phase [63].
is the tracer diffusion coefficient of species in phase. The advantage of Equation (20) is that one can calculate the variation in the chemical potential of a given component through a given phase reaction layer in the case when the reactive interfaces are at thermodynamic equilibrium (i.e., diffusion-controlled growth process). This is performed in this work for both reactive systems and is given below.
The knowledge of and allows calculating and thus to establish the growth rate of a given phase reaction layer by writing the mass balance of species at both adjacent interfaces of this layer [69].
The variations of the C, Si, Nb and Ta chemical potentials through all observed reaction layers in Nb/SiC and Ta/SiC systems were calculated with the Thermo-Calc Software and the FUELBASE database along the SiC/Nb and SiC/Ta lines at 1200 °C and results are presented here only for the Nb/SiC system in Table 7.

Table 7.
Variation of the chemical potential gradient of C, Si and Nb through each reactive layer phase formed at the SiC/Nb interface at 1200 °C.
As an example, the variation of the chemical potential of a given component (C, Si, Nb) through the NbC1−x phase layer is given by the following expression:
6.4. Application for GFR
The aim of this study was to determine the diffusion kinetics in Nb/SiC and Ta/SiC systems in both the sandwich cladding and diffusion couple configurations. The results are close for both geometries within the studied temperature range (1050–1500 °C), even if a higher reactivity was observed on the sandwich configuration at the lowest temperatures. It appears that the parabolic growth rate constants in the Nb/SiC and Ta/SiC systems (Figure 16) are closer to each other than what was initially expected (Figure 2). In addition, the advantage of tantalum use might not be so significant considering its drawbacks (price, weight, neutron capture cross-section). Even though uncertainties still remain on the sandwich cladding thermal conductivity, the liner temperature should range between 550 and 950 °C in GFR nominal operating conditions [70]. The cladding has to operate for 3 to 4 years to optimize fuel use. Reaction layer thicknesses, extrapolated from Equations (3) and (5), are summarized in Table 8 for 3 or 4 years of operation at different temperatures; however, it has to be kept in mind that those results are only estimations. In fact, several phenomena can occur. First, the diffusion mechanisms can be different at lower temperatures, increasing the reactivity and, at the same time, the reaction thickness. In addition, at lower temperatures, the contact between SiC and the metal can be less pronounced, decreasing the reactivity and the thermal conductivity. Those results show that 1000 °C is already too high for Nb and almost close to the limit for Ta. Indeed, the maximum liner thickness should be 100 µm for neutron criteria consideration. It was also observed that at 1050 and 1200 °C, vacancies accumulation occurred at the MC1−x/M5Si3Cy interface, leading to strong porosities levels. Irradiation effects, still unknown on these materials, could also affect the reaction kinetics. The use of such liner materials may be limited during the whole operation of a GFR cladding. Hence, a buffer material may be placed between SiC and the liner to decrease the interaction.

Table 8.
Thickness extrapolation (µm) of the interaction layer on the outer side of the sandwich cladding as a function of time and temperature.
7. Conclusions
The niobium and tantalum reactivity towards SiC was studied in the 1050–1500 °C temperature range. No strong differences in the results obtained for both sandwich cladding materials outer side, considered for GFR application, and diffusion couples were evidenced. The reaction layer sequence and composition are identical on both geometries. Only the sandwich inner side composition differs, probably due to a poor contact between the metal and the SiC/SiC composite. EPMA and ion beam analyses were conducted to precisely determine the ternary compound composition in the Nb–Si–C system. It corresponds to Nb5Si3Cy, which is different from what is currently considered in the ternary phase diagram.
The phase sequence and their compositions in the reaction area were found to be identical within the studied temperature range in the Nb/SiC system, whereas it was changing for Ta/SiC system. Between 1050 and 1200 °C, a Ta5Si3 compound layer forms between Ta5Si3Cy and Ta2Si layers, whereas at 1350 °C, it forms between Ta5Si3Cy and Ta2C layers. Moreover, at 1350 °C, Ta2C forms between Ta5Si3 and Ta2Si layers, whereas at 1500 °C, it forms between Ta5Si3Cy and Ta2Si. Hence, the following diffusion paths were determined for both systems:
SiC/NbC1−x/Nb5Si3Cy/NbC1−z/Nb2C/Nb
SiC/TaC1−x/Ta5Si3Cy/Ta5Si3 (1050–1350 °C)/Ta2C (1350–1500 °C)/Ta2Si/Ta
A parabolic growth law, characteristic of a diffusion-controlled process, was evidenced for the whole reaction product layer as well as for each separate layer. Growth kinetics determined in the present work are higher than those reported in the literature. Tungsten markers experiments have shown that carbon and silicon are the fastest diffusing species. Reaction layer thicknesses are lower in the Ta/SiC system than in the Nb/SiC one. Tantalum is then preferentially considered for nuclear application. Nonetheless, considering that the diffusion modes are identical under normal operating conditions (up to 950 °C) and that strong contacts between the metal and the SiC/SiC composite occur, the Ta/SiC reaction can limit the use of this material for leak-tightness purposes during the whole cladding lifetime (3 to 4 years) for the considered reaction layer thicknesses (70 to 100 µm). On-going irradiation conducted on those materials for several years in SFR conditions (550 °C, hundreds of dpa) should also give information on the liner behavior under irradiation.
Author Contributions
Conceptualization, J.B., C.S., C.G. and F.B.-C.; methodology, J.B., C.S. and C.G.; software, J.B. and C.G.; validation, J.B., C.S., C.G., F.H. and F.B.-C.; formal analysis, J.B., C.S. and F.H.; investigation, J.B., C.G. and F.H.; resources, J.B. and C.G.; data curation, J.B. and C.G.; writing—original draft preparation, J.B.; writing—review and editing, C.S., C.G., F.H. and F.B.-C.; visualization, J.B., C.G. and F.H.; supervision, F.B.-C.; project administration, C.S. and F.B.-C.; funding acquisition, C.S. and F.B.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Caroline Raepsaet for her help with the ion beam analyses as well as the experimental setup.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertel, E. Advanced Fuel Cycles and Radioactive Waste Management. NEA News 2006, 24, 4–6. [Google Scholar]
- Van Rooijen, W.F.G. Gas-Cooled Fast Reactor: A Historical Overview and Future Outlook. Sci. Technol. Nucl. Install. 2009, 2009, e965757. [Google Scholar] [CrossRef]
- Čížek, J.; Kalivodová, J.; Janeček, M.; Stráský, J.; Srba, O.; Macková, A. Advanced Structural Materials for Gas-Cooled Fast Reactors—A Review. Metals 2021, 11, 76. [Google Scholar] [CrossRef]
- U.S. DOE Nuclear Energy Research Advisory Committee; The Generation IV International Forum. A Technology Roadmap for Generation IV Nuclear Energy Systems. 2002. Available online: https://www.gen-4.org/gif/jcms/c_40481/technology-roadmap (accessed on 1 April 2022).
- Idaho National Laboratory. Gas-Cooled Fast Reactor Research and Development Roadmap; Idaho National Laboratory: Idaho Falls, ID, USA, 2018.
- Snead, L.L.; Nozawa, T.; Katoh, Y.; Byun, T.-S.; Kondo, S.; Petti, D.A. Handbook of SiC Properties for Fuel Performance Modeling. J. Nucl. Mater. 2007, 371, 329–377. [Google Scholar] [CrossRef]
- López-Honorato, E.; Tan, J.; Meadows, P.J.; Marsh, G.; Xiao, P. TRISO Coated Fuel Particles with Enhanced SiC Properties. J. Nucl. Mater. 2009, 392, 219–224. [Google Scholar] [CrossRef]
- Seibert, R.L.; Jolly, B.C.; Balooch, M.; Schappel, D.P.; Terrani, K.A. Production and Characterization of TRISO Fuel Particles with Multilayered SiC. J. Nucl. Mater. 2019, 515, 215–226. [Google Scholar] [CrossRef]
- Dormeval, M. Sintering and Characterization of Ceramics for GFR Applications. In Proceedings of the ICAPP05, Seoul, Korea, 15–19 May 2005. Paper 5469. [Google Scholar]
- Sauder, C. Nuclear Applications. In Ceramic Matrix Composites; Bansal, N.P., Lamon, J., Eds.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zabiego, M.; Sauder, C.; Lorrette, C.; Guedeney, P. Improved Multilayer Tube Made from Ceramic-Matrix Composite Material, the Resulting Nuclear Fuel Cladding and Associated Production Methods. CEA Patent WO2013017621 A1, 7 February 2013. [Google Scholar]
- Weaver, K.D.; Toemeier, T.C.; Clark, D.E.; Feldman, E.E.; Hoffman, E.A.; Vilim, R.B.; Wei, T.Y.C.; Gan, J.; Meyer, M.K.; Gale, W.F.; et al. Gen IV Nuclear Energy Systems Gas-Cooled Fast Reactor (GFR) FY-04 Annual Report; Idaho National Engineering and Environmental Laboratory: Idaho Falls, ID, USA, 2004. [Google Scholar]
- Zhao, X.; Duan, L.; Wang, Y. Fast Interdiffusion and Kirkendall Effects of SiC-Coated C/SiC Composites Joined by a Ti-Nb-Ti Interlayer via Spark Plasma Sintering. J. Eur. Ceram. Soc. 2019, 39, 1757–1765. [Google Scholar] [CrossRef]
- Li, H.; Shen, W.; He, Y.; Zhong, Z.; Zheng, W.; Ma, Y.; Yang, J.; Wu, Y. Microstructural Evolution and Characterization of Interfacial Phases in Diffusion-Bonded SiC/Ta–5W/SiC Joints. Ceram. Int. 2020, 46, 22650–22660. [Google Scholar] [CrossRef]
- Camarano, A.; Narciso, J.; Giuranno, D. Solid State Reactions between SiC and Ir. J. Eur. Ceram. Soc. 2019, 39, 3959–3970. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, L.; Liu, W.; Wang, Y. Fast-Diffusion Joining of SiC-Coated Three-Dimensional C/SiC Composites with a Mo-W-Mo Interlayer by Spark Plasma Sintering. Ceram. Int. 2019, 45, 23111–23118. [Google Scholar] [CrossRef]
- Smallwood, R.E. Refractory Metals and Their Industrial Applications; ASTM Special Technical Publication; ASTM International: West Conshohocken, PA, USA, 1983; Volume 849. [Google Scholar]
- HSC: Chemistry–V6.12 (Thermochemical Database); Outotec Research Oy: Pori, Finland, 2007.
- Cowgill, M.G.; Stringer, J. The Effect of Oxygen Pressure on the High Temperature Oxidation of Tantalum. J. Less Common Met. 1960, 2, 233–240. [Google Scholar] [CrossRef]
- Strafford, K.N. A Comparison of the High Temperature Nitridation and Oxidation Behaviour of Metals. Corros. Sci. 1979, 19, 49–62. [Google Scholar] [CrossRef]
- Lyon, S.B. Corrosion of tantalum and niobium and their alloys. Shreir’s Corros. 2010, 3, 2135–2150. [Google Scholar] [CrossRef]
- Reffo, G.; Fabbri, F.; Wisshak, K.; Käppeler, F. Fast Neutron Capture Cross Sections and Related Gamma-Ray Spectra of Niobium-93, Rhodium-103 and Tantalum-181. Nucl. Sci. Eng. 1982, 80, 630–647. [Google Scholar] [CrossRef]
- Brewer, L.; Krikorian, O. Reactions of Refractory Silicides with Carbon and Nitrogen. J. Electrochem. Soc. 1956, 103, 38. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) System. JPE 1993, 14, 502–509. [Google Scholar] [CrossRef]
- Burykina, A.L.; Strashinskaya, L.V.; Evtushok, T.M. Investigation of the Interaction of Silicon Carbide with Refractory Metals and Oxides. Sov. Mater. Sci. A Transl. Fiz. Khimicheskaya Mekhanika Mater. Acad. Sci. Ukr. SSR 1968, 4, 220–223. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Huang, T.; Yao, A.; Bal, B.S.; Li, Y. SiC Nanoparticle-Reinforced Al2O3–Nb Composite as a Potential Femoral Head Material in Total Hip Arthroplasty. Mater. Sci. Eng. C 2010, 30, 1197–1203. [Google Scholar] [CrossRef]
- Brukl, C.E. Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems Part II. Ternary Systems Vol VII. The Ti-Si-C, Nb-Si-C and W-Si-C Systems; Aerojet General Corporation: Sacramento, CA, USA, 1965. [Google Scholar]
- Schachner, H.; Cerwenka, E.; Nowotny, H. Neue Silizide Vom M5Si3-Typ MitD 88-Struktur. Mon. Für Chem. Und Verwandte Teile And. Wiss. 1954, 85, 245–254. [Google Scholar] [CrossRef]
- Nowotny, H.; Lux, B.; Kudielka, H. Das Verhalten metallreicher, hochschmelzender Silizide gegenüber Bor, Kohlenstoff, Stickstoff und Sauerstoff. Mon. Für Chem. 1956, 87, 447–470. [Google Scholar] [CrossRef]
- Yaney, D.L.; Joshi, A. Reaction between Niobium and Silicon Carbide at 1373 K. J. Mater. Res. 1990, 5, 2197–2208. [Google Scholar] [CrossRef]
- Chou, T.C. High Temperature Interfacial Reactions of SiC with Metals. J. Vac. Sci. Technol. A Vac. Surf. Film. 1991, 9, 1525. [Google Scholar] [CrossRef]
- Naka, M.; Feng, J.C. Phase Reactions and Interface Strength of SiC/Nb Couples. In Strength of Materials; Japan Institute of Metals: Tokyo, Japan, 1994; Volume 4, pp. 235–238. [Google Scholar]
- Schuster, J.C. Silicon Carbide and Transition Metals: A Critical Evaluation of Existing Phase Diagram Data Supplemented by New Experimental Results. Int. J. Refract. Met. Hard Mater. 1993, 12, 173–177. [Google Scholar] [CrossRef]
- Birla, N.C.; Hoch, M. The Age Hardening Characteristics of Nb-Base Alloys Containing Carbon and/or Silicon: Part I.(Nb-15 At. Pct Hf). Metall. Trans. A 1975, 6, 1631–1643. [Google Scholar] [CrossRef]
- Joshi, A.; Hu, H.S.; Jesion, L.; Stephens, J.J.; Wadsworth, J. High-Temperature Interactions of Refractory Metal Matrices with Selected Ceramic Reinforcements. Metall. Trans. A 1990, 21, 2829–2837. [Google Scholar] [CrossRef]
- Kao, C.R.; Woodford, J.; Chang, Y.A. Reactive Diffusion between Silicon and Niobium Carbide: Application to the in-Situ Synthesis of a Silicon Carbide-Niobium Disilicide Composite. In Design Fundamentals of High Temperature Composites, Intermetallics, and Metal-Ceramics Systems; Lin, R.Y., Chang, Y.A., Reddy, R.G., Liu, C.T., Eds.; Minerals, Metals and Materials Society: Warrendale, PA, USA, 1995. [Google Scholar]
- Kao, C.R.; Woodford, J.; Chang, Y.A. A Mechanism for Reactive Diffusion between Si Single Crystal and NbC Powder Compact. J. Mater. Res. 1996, 11, 850–854. [Google Scholar] [CrossRef]
- Woodford, J.; Yang, C.Y.; Chang, Y.A. The Effect of NbC Porosity on Reaction-Layer Microstructure in NbC| Si Diffusion Couples. J. Mater. Res. 2000, 15, 248–252. [Google Scholar] [CrossRef]
- Grechnev, A.; Li, S.; Ahuja, R.; Eriksson, O.; Jansson, U.; Wilhelmsson, O. Layered Compound Nb3SiC2 Predicted from First-Principles Theory. Appl. Phys. Lett. 2004, 85, 3071. [Google Scholar] [CrossRef]
- Naka, M.; Saito, T.; Okamoto, I. Niobium Silicides at Interface between Niobium and SiC. J. Mater. Sci. Lett. 1987, 6, 875–876. [Google Scholar] [CrossRef]
- Naka, M.; Saito, T. Niobium Interlayer for Joining SiC to Stainless Steel. J. Mater. Sci. Lett. 1991, 10, 339–340. [Google Scholar] [CrossRef]
- Naka, M.; Saito, T.; Okamoto, I. Effect of a Silicon Sintering Additive on Solid State Bonding of SiC to Nb. J. Mater. Sci. 1991, 26, 1983–1987. [Google Scholar] [CrossRef]
- Naka, M.; Feng, J.C. Phase Reaction and Diffusion Path of SiC/Nb System. Trans. Mater. Res. Soc. Jpn. 1993, 16B, 1143–1146. [Google Scholar]
- Naka, M. Interfacial Reactions between Silicon Base Ceramics and Metals. Mater. Sci. Res. Int. 1996, 2, 273–274. [Google Scholar] [CrossRef]
- Colin, C. High Temperature Chemical Reactivity between Sic Reinforcements and Refractories Metallics or Intermetallics Matrix. Ph.D. Thesis, Université Claude Bernard, Lyon, France, 1993. [Google Scholar]
- Jung, K.; Sutou, Y.; Koike, J. Improved Microstructure and Ohmic Contact of Nb Electrode on N-Type 4H-SiC. Thin Solid Film. 2012, 520, 6922–6928. [Google Scholar] [CrossRef]
- Kieffer, R.; Benesovsky, F.; Nowotny, H.; Schachner, H. Beitrag Zum System Tantal-Silizium. Z. Met. 1953, 44, 242–246. [Google Scholar] [CrossRef]
- Geib, K.M.; Wilson, C.; Long, R.G.; Wilmsen, C.W. Reaction between SiC and W, Mo, and Ta at Elevated Temperatures. J. Appl. Phys. 1990, 68, 2796. [Google Scholar] [CrossRef]
- Chen, J.S.; Kolawa, E.; Nicolet, M.-A.; Ruiz, R.P.; Baud, L.; Jaussaud, C.; Madar, R. Reaction of Ta Thin Film with Single Crystalline (001) β-SiC. J. Appl. Phys. 1994, 76, 2169. [Google Scholar] [CrossRef]
- Steinbrueck, M.; Angelici Avincola, V.; Markel, I.J.; Stegmaier, U.; Gerhards, U.; Seifert, H.J. Oxidation of SiCf-SiC CMC Cladding Tubes for GFR Application in Impure Helium Atmosphere and Materials Interactions with Tantalum Liner at High Temperatures up to 1600 °C. J. Nucl. Mater. 2019, 517, 337–348. [Google Scholar] [CrossRef]
- Rudy, E.; Harmon, D.P. Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems—Part I. Related Binary Systems—Volume V. Ta-C System; Aerojet General Corporation: Sacramento, CA, USA, 1965. [Google Scholar]
- Cao, Y.; Pérez-García, S.A.; Nyborg, L. Interface Reactions and Electrical Properties of Ta/4H-SiC Contacts. Mater. Sci. Forum 2007, 556–557, 713–716. [Google Scholar] [CrossRef]
- Feng, J.C.; Naka, M.; Schuster, J.C. Phase Formation and Diffusion Path of SiC/Ta/SiC Joint. J. Mater. Sci. Lett. 1997, 16, 1116–1117. [Google Scholar] [CrossRef]
- Feng, J.C.; Naka, M.; Schuster, J.C. Interfacial Reaction and Strength of SiC/Ta/SiC Joint. J. Jpn. Inst. Met. 1997, 61, 456–461. [Google Scholar] [CrossRef][Green Version]
- Olowolafe, J.O.; Solomon, J.S.; Mitchel, W.; Lampert, W.V. Thermal and Electrical Properties of Au/B4C, Ni/B4C, and Ta/Si Contacts to Silicon Carbide. Thin Solid Film. 2005, 479, 59–63. [Google Scholar] [CrossRef]
- Braun, J.; Sauder, C.; Lamon, J.; Balbaud-Célérier, F. Influence of an Original Manufacturing Process on the Properties and Microstructure of SiC/SiC Tubular Composites. Compos. Part A Appl. Sci. Manuf. 2019, 123, 170–179. [Google Scholar] [CrossRef]
- Raepsaet, C.; Khodja, H.; Bossis, P.; Pipon, Y.; Roudil, D. Ion Beam Analysis of Radioactive Samples. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 2245–2249. [Google Scholar] [CrossRef]
- Guéneau, C.; Dupin, N.; Sundman, B.; Martial, C.; Dumas, J.-C.; Gossé, S.; Chatain, S.; Bruycker, F.D.; Manara, D.; Konings, R.J.M. Thermodynamic Modelling of Advanced Oxide and Carbide Nuclear Fuels: Description of the U–Pu–O–C Systems. J. Nucl. Mater. 2011, 419, 145–167. [Google Scholar] [CrossRef]
- Thurnay, K. Thermal Properties of Transition Metals; Forschungszentrum Karlsruhe: Karlsruhe, Germany, 1998; p. 137. [Google Scholar]
- Hidnert, P. Thermal expansion of tantalum. Bur. Stand. J. Res. 1929, 2, 887. [Google Scholar] [CrossRef]
- Li, Z.; Bradt, R.C. Thermal Expansion of the Cubic (3C) Polytype of SiC. J. Mater. Sci. 1986, 21, 4366–4368. [Google Scholar] [CrossRef]
- Li, Q.; Jun, Y.-S. The Apparent Activation Energy and Pre-Exponential Kinetic Factor for Heterogeneous Calcium Carbonate Nucleation on Quartz. Commun. Chem. 2018, 1, 56. [Google Scholar] [CrossRef]
- Van Loo, F.J.J. Multiphase Diffusion in Binary and Ternary Solid-State Systems. Prog. Solid State Chem. 1990, 20, 47–99. [Google Scholar] [CrossRef]
- Barzilai, S.; Raveh, A.; Frage, N. Inter-Diffusion of Carbon into Niobium Coatings Deposited on Graphite. Thin Solid Film. 2006, 496, 450–456. [Google Scholar] [CrossRef]
- Barzilai, S.; Frage, N.; Raveh, A. Niobium Layers on Graphite: Growth Parameters and Thermal Annealing Effects. Surf. Coat. Technol. 2006, 200, 4646–4653. [Google Scholar] [CrossRef]
- Brizes, W.F. Diffusion of Carbon in the Carbides of Tantalum. J. Nucl. Mater. 1968, 26, 227–231. [Google Scholar] [CrossRef]
- Prasad, S.; Paul, A. Growth Mechanism of Phases by Interdiffusion and Diffusion of Species in the Niobium–Silicon System. Acta Mater. 2011, 59, 1577–1585. [Google Scholar] [CrossRef]
- Roy, S.; Paul, A. Growth Mechanism of Tantalum Silicides by Interdiffusion. Philos. Mag. 2012, 92, 4215–4229. [Google Scholar] [CrossRef]
- Philibert, J. Reactive Diffusion. Defect Diffus. Forum 1991, 66–69, 995–1014. [Google Scholar] [CrossRef]
- Ingremeau, J.-J. Optimization Method Development of the Core Characteristics of a Fast Reactor in Order to Explore Possible High Performance Solutions (a Solution Being a Consistent Set of Fuel, Core, System and Safety). Ph.D. Thesis, Université Paris Sud-Paris XI, Bures-sur-Yvette, France, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).