Abstract
Colloidal microspheres could be used as raw materials to construct the photonic crystal modification layers on wood surfaces, and the wood would produce gorgeous structural color effect. The diameter, monodispersity and self-assembly of colloidal microspheres directly affect the well-structure order of photonic crystals. Therefore, poly(styrene-acrylic acid) (P(St-AA)) shell-core microspheres were prepared by emulsion polymerization. The effects of the reaction time, amount of initiator and emulsifier and reaction temperature on the particle size and dispersity of colloidal microspheres during polymerization were analyzed by single-factor experiments. The P(St-AA) colloidal microspheres self-assembled on wood surfaces and formed photonic crystals with structural color through thermally-assisted gravity deposition. The effects of the amount of emulsion coating per unit surface area and ambient temperature on the optical properties of the photonic crystals were investigated. It was found that the particle size of the synthesized microspheres increased with the increase of initiator amount and reaction temperature and the decrease of emulsifier; however, the effect of reaction time on microspheres was less regular. The best optical properties of the photonic crystals on wood surfaces were obtained using 0.408 μL/mm2 emulsion coating at a temperature of 50 °C. The photonic crystals were composed of both ordered and disordered layers.
1. Introduction
Wood is a kind of renewable material with outstanding environmental friendliness widely used in furniture production and architectural decoration [1]. Wood is composed of cells containing cellulose, hemicelluloses, and lignin. Wood presents specific patterns in macro structure, and in micro can be regarded as three-dimensional structures formed by the junction of cell cavity and wall. Due to the biomass composition and surface morphology of wood, defects such as decay, moth-eaten, mildew, and discoloration will occur in the process of wood use, which seriously affect the long-term utilization of wood [2,3,4]. Therefore, in the use process of wood, researchers often use physical chemical processing and protective means to prolong the life cycle of the product such as heat treatment [5], compression and compaction [6], coating [7,8], and laser [9,10]. Coating is the most commonly used wood protection method in the wood industry [11,12]. Wood surface coating can effectively isolate wood from the external environment, but by also adding dyes and pigments, change the color and decorative effect of wood. Thus the wood can be suitable for different environments and decorative decoration styles.
On the surface of biological bodies in nature, we can often see the bright structural color, which is formed by the interference, diffraction, or scattering of the submicron structure and light waves. Different from traditional pigments, structural color will not fade when the structure does not change [13]. Using structural color in the field of material modification can avoid water resource hazards caused by the use of pigments or dyes [14]. Photonic crystal has regular microstructure and photonic band gap effect which can produce structural color [15]. The brightly colored opal in nature is a typical example of photonic crystal structure, and organisms such as the weevil have similar structures [16,17].
Photonic crystals with well-ordered structures that show brilliant colors by imitating the microstructure of opal can be fabricated on the surface of wood from polystyrene (PSt)-based colloidal microspheres [18,19,20]. Unlike common wood color improvement techniques, such as dyeing and induced discoloration, modification effect of structural colors on wood surfaces are bright, gorgeous, and bionic. It is similar to the traditional Chinese craft of mother-of-pearl decorative wood products. Wood and wood products can obtain higher added value. According to Bragg’s law of diffraction, the structural color of three-dimensional photonic crystals is related to the lattice parameters of the crystal structure [21,22,23]. This allows structural color to be adjusted by changing the microsphere size and spacing between spheres, where the monodispersity of microspheres directly determines the well-order of photonic crystal [24,25,26].
Colloidal microspheres with excellent monodispersity can be obtained by emulsion polymerization [27]. In a previous study, we took styrene (St) as the main monomer and acrylate and acrylic acid (AA) as the copolymers, and synthesized six kinds of PSt-based colloidal microspheres [18]. The structure, morphology, surface functional groups, and chemical properties of microspheres could be adjusted by changing copolymers [28,29,30]. The addition of AA copolymer produced microspheres with a shell-core structure. When the microspheres squeezed each other during self-assembly, the soft shell deformed and made the photonic crystal more compact [31,32,33]. Moreover, carboxyl groups were distributed on the surface of poly(styrene-acrylic acid) (P(St-AA)) microspheres, which enhanced the hydrophilicity and bonding between microspheres, which was conducive to the formation of coatings with structural color. Although P(St-AA) microspheres can be synthesized by emulsion polymerization for construction of photonic crystals with structural color on wood surfaces, it is still not completely clear which factors affect the microsphere’s size and monodispersity in the synthesis process.
Therefore, in order to fabricate photonic crystals with excellent optical properties and different structural colors on wood surfaces, the aim of this study is to understand the effect of synthesis parameters on the size and monodispersity of P(St-AA) colloidal microspheres. The effects of polymerization time, amount of initiator and emulsifier, and reaction temperature of the emulsion polymerization on the size and monodispersity of P(St-AA) microspheres were investigated. P(St-AA) microspheres with excellent monodispersity were prepared, and the morphology, optical properties, and formation process of photonic crystals on wood surfaces were discussed.
2. Materials and Methods
2.1. Reagents and Materials
St and AA monomers for the copolymerization of colloidal microspheres, ammonium persulfate (APS) initiator, and sodium dodecylbenzene sulfonate (SDBS) emulsifier were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd., Shanghai, China. These reagents were chemically pure (CP) and used as received. Deionized water was used in all experiments. The wood species used to fabricate photonic crystal coatings on its surface was aspen (Populus tremuloides Michx.). The air-dried density of the wood was about 0.41 g/cm3, and the moisture content of the air-dried wood was in the range of 8%–12%. The surface used to construct photonic crystals was the radial section surface of sapwood. The color of raw wood samples was yellowish white. The surface dimensions of the wood samples were 50 mm × 50 mm, and the surface was polished with 320-mesh sandpaper to remove significant burrs and saw marks.
2.2. Synthesis of P(St-AA) Colloidal Microspheres by Emulsion Polymerization
The P(St-AA) colloidal microspheres were prepared by emulsion polymerization. The polymerization method and procedure were the same as we have previously reported [18]. Single-factor experiments were carried out to investigate variations in the microsphere size and monodispersity as a function of the polymerization reaction time, temperature, and the amount of initiator and emulsifier. For example, when discussing the effect of reaction time on the monodispersity and size of colloidal microspheres, the polymerization of P(St-AA) microspheres was carried out in a three-necked round-bottomed flask, equipped with a mercury thermometer, mechanical stirrer, and condenser tube. Furthermore, 100 mL deionized water, 40 mL absolute alcohol, 200 mg SDBS, and 150 mg APS were successively added into the round-bottomed flask. The mixture was stirred at 400 rpm and heated to 70 °C. Then, 15 g St and 1 g AA were added to the reaction vessel. The emulsion polymerization was continued for several hours. Finally, the optimum reaction conditions to obtain monodisperse colloidal microspheres were obtained within the range of factors set in this study. In the single-factor experiments, the amount of co-monomers was fixed at 15 g St and 1 g AA. The changes in other reaction variables are shown in Table 1. The reason why we set the variation intervals of these four factors was mainly based on previous similar studies and the preliminary studies of our group [18,19,20,28,29,30].

Table 1.
Single-factor variables of emulsion polymerization of P(St-AA) colloidal microspheres.
2.3. Fabrication of Photonic Crystals on Wood Surface
The P(St-AA) colloidal microspheres with the best monodispersity obtained from the emulsion polymerization in Section 2.2 were used as the raw material to construct photonic crystals with structural color on wood surfaces. The construction method was thermal-assisted gravity deposition. Variations in the optical properties of the photonic crystals with the amount of emulsion per unit area and the self-assembly temperature were analyzed, as shown in Table 2. Finally, the optimal self-assembly conditions of P(St-AA) microspheres were obtained within the range of factors set in this study. A 35 mm × 35 mm wireframe was drawn with a pencil on the radial section surface of the sanded wood before dripping the emulsion. Then, the synthesized microsphere emulsion was dropped by pipette and used to cover the wireframe. The wood samples coated with the emulsion were carefully moved into an air-drying oven. To ensure complete dryness, the drying time was changed depending on the amount of emulsion.

Table 2.
Single-factor variables of self-assembly of P(St-AA) microspheres on a wood surface.
2.4. Characterization of Colloidal Microspheres and Photonic Crystals on Wood Surfaces
The intensity-average hydrodynamic sizes and average diameter of colloidal microspheres synthesized by emulsion polymerization were determined by dynamic light scattering (DLS) and scanning electron microscopy (SEM). A laser particle sizer (Zetasizer Nano ZS90, Malvern Panalytical Ltd., Malvern, UK) was used to perform DLS. The colloidal microsphere emulsion obtained after polymerization was diluted with deionized water to a solids content of 1 wt% before DLS tests. This technique measures the diffusion of colloidal microspheres moving under Brownian motion and converts this to size and a size distribution using the Stokes–Einstein relationship. The instrument provided a constant temperature at 25 °C during the test. The hydrodynamic sizes of colloidal microspheres dispersed in emulsion and the polydispersity index (PDI) of microspheres were obtained by this method. SEM images were taken by a field emission SEM (FE-SEM, JSM-7600F, JEOL Ltd., Akishima, Japan). The FE-SEM samples were flake coatings made by dropping the emulsion obtained by polymerization onto the surface of a glass dish, followed by drying at 50 °C in an oven (DHG-9423A, Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China). All FE-SEM samples were coated with gold by ion sputtering. The acceleration voltage of FE-SEM was 15 kV. According to the FE-SEM images of colloidal microspheres synthesized under different polymerization conditions, the average diameter of dried colloidal microspheres was calculated by measuring more than 500 microspheres. To represent the size distribution of particles obtained under different conditions, the coefficient of variation of diameters was calculated by the ratio of the standard deviation to the average microsphere diameter. The microstructure of photonic crystals on wood surfaces was observed by FE-SEM. The morphology of microspheres was characterized by transmission electron microscopy (TEM, JEM2100F, JEOL Ltd., Akishima, Japan). The acceleration voltage of TEM was 100 kV. The reflectance spectra of photonic crystals on wood surfaces were obtained with a UV-Vis spectrometer (U3900, Hitachi Ltd., Tokyo, Japan), the incident light was perpendicular to the surfaces. The wavelength scanning mode was used, and the wavelength was 350–750 nm, which in the visible range. The scan speed was 300 nm/min, and the slit width was 2 nm. Photographs of photonic crystals with structural color were taken using a digital camera (D7000, Nikon, Nikon Imaging Sales Co., Ltd., Shanghai, China).
3. Results and Discussion
3.1. Influence of Polymerization Conditions on the Properties of P(St-AA) Colloidal Microspheres
3.1.1. Influence of Polymerization Time
The emulsion polymerization reaction time was taken as the single-factor variable, and the intensity-average hydrodynamic sizes and PDI of the synthesized P(St-AA) colloidal microspheres are shown in Figure 1A. When the reaction time was extended from 6 to 14 h, the diameter of microspheres in the emulsion increased before beginning to slowly decrease. This phenomenon indicated that the polymerization gradually completed, and this was similar to the research results of Shao’s group [29]. When the polymerization time was 6 h, the PDI was 0.098, which is a poor monodispersity. The particle diameter was not uniform. When the polymerization times were 8–14 h, the PDI decreased and then increased, and all of them were less than 0.05. When the polymerization time was 12 h, the lowest PDI was obtained (0.024), and the average microsphere diameter was 228.6 nm.
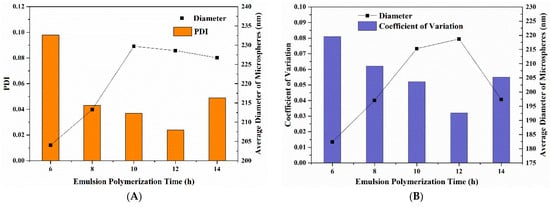
Figure 1.
The particle size analysis of P(St-AA) colloidal microspheres synthesized with different emulsion polymerization times: (A) DLS characterization; (B) FE-SEM images.
In this study, FE-SEM was used to characterize the morphology and particle size of P(St-AA) microspheres after drying under vacuum and gold spraying. The microspheres synthesized with different polymerization times were uniformly spherical (Figure 2). In this study, the microspheres self-assembled on a watch glass and formed ordered photonic crystals. When the polymerization time was 14 h (Figure 2E), the obtained microspheres did not form a long-range ordered structure, which may be related to the self-assembly forces of microspheres during emulsion drying. The sample formed by P(St-AA) microspheres synthesized with 12 h had the fewest micromorphological defects.
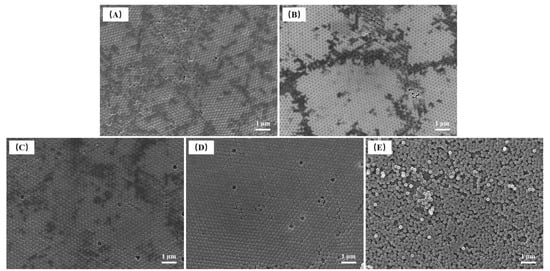
Figure 2.
FE-SEM images of P(St-AA) colloidal microspheres synthesized with different emulsion polymerization times: (A) 6 h; (B) 8 h; (C) 10 h; (D) 12 h; (E) 14 h.
As shown in Figure 1B, the diameters of 500 P(St-AA) microspheres obtained using different reaction times in different locations of the FE-SEM images were measured. The average diameter and coefficients of variation of the microspheres after gold spraying were obtained. By comparing the variation in average diameter of microspheres obtained by DLS and manual measurements, the average diameter showed similar trends but different inflection points. The manual measurement results showed that the largest average diameter of microspheres was with 12 h reaction time. This difference may be related to the degree of extrusion between microspheres during drying. The coefficient of variation decreased first and then increased, which was the same trend as PDI in Figure 1A. The lowest coefficient of variation was obtained at 12 h of polymerization. The average diameter of microspheres obtained by manual measurement (180–220 nm) was smaller than that obtained by DLS (200–230 nm). The main reason was that the P(St-AA) colloidal microspheres swelled when they were dispersed in the emulsion; however, during FE-SEM sample preparation, the emulsion dispersant evaporated, and the microspheres gradually shrank [34,35].
Figure 3 shows photos of the samples obtained after drying P(St-AA) microsphere emulsions. The dry samples appeared bright yellow-green under the irradiation of vertical white light when the angle between sight and incident light was 0°. The brilliant color was consistent with the ordered microspheres shown in Figure 2. The results indicated that the ordered photonic crystal formed by microspheres had a band gap in the yellow-green wavelength range. When the polymerization times were 6–12 h (Figure 3A–D), the color of samples shifted from yellow to yellow-green, which was consistent with that of the diameter of microspheres that increased gradually in the DLS and FE-SEM characterization. As shown in Figure 3E, when the reaction time was 14 h, the center of the sample was yellow-green, but the edge was transparent and colorless, and the overall color effect was poor. This was consistent with the FE-SEM images, which showed that the microspheres did not form long-range well-ordered structures (Figure 2E).
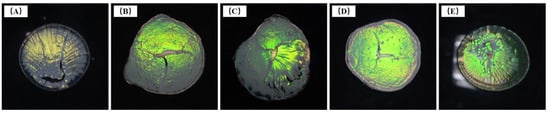
Figure 3.
Photos of dried test samples of P(St-AA) colloidal microspheres synthesized with different emulsion polymerization times: (A) 6 h; (B) 8 h; (C) 10 h; (D) 12 h; (E) 14 h.
As the emulsion dried on the watch glass, the spaces between microspheres gradually contracted, while the glass substrate did not undergo a corresponding deformation; therefore, the solid samples displayed striped cracking [22]. However, most of the samples formed a strong bond with the substrate, and the coffee ring effect was not obvious, which might be related to the presence of carboxyl groups and the good adhesion and film formation of polyacrylic acid (PAA).
3.1.2. Influence of Amount of Initiator (APS)
The amount of initiator will affect the particle size distribution and yield of polymer colloidal microspheres. APS is a typical initiator that thermally decomposes into two sulfate ion radicals, which then react with a monomer to form a monomer radical that diffuses into micelles from the aqueous phase to form polymers [36,37]. In this study, the average hydration diameter and PDI of P(St-AA) microspheres increased upon increasing the APS. The PDI was less than 0.05 in all cases, and the P(St-AA) microspheres displayed excellent monodispersity (Figure 4A). When the APS dosage was 125 mg, the lowest PDI was obtained (0.002). The average microsphere diameter was about 222.4 nm. The rate of free-radical formation increased upon increasing the initiator amount. The diffusion of free radicals from the aqueous phase into micelles increased, which increased the nucleation rate of microspheres and initiated more active chains. Many small P(St-AA) microspheres bonded to form larger ones, which was possibly why initiators increased the diameter of microspheres.
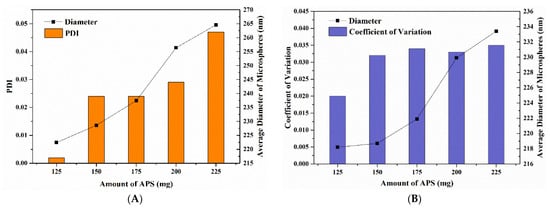
Figure 4.
The particle size analysis of P(St-AA) colloidal microspheres synthesized with different amounts of APS: (A) by DLS characterization; (B) by measuring FE-SEM images.
The FE-SEM images of P(St-AA) colloidal microspheres (Figure 5) synthesized with 125 and 150 mg APS, showed that the samples had only point and line defects; however, the samples with 175–225 mg APS had larger cave-like defects. The pore area increased upon adding more APS and significantly decreased the order, which may be related to increase of particle size and surface functional groups of microspheres [18].
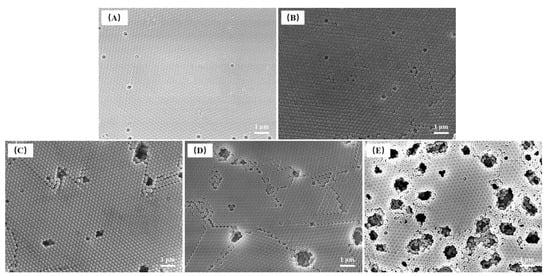
Figure 5.
FE-SEM images of P(St-AA) colloidal microspheres synthesized with different APS amounts: (A) 125 mg; (B) 150 mg; (C) 175 mg; (D) 200 mg; (E) 225 mg.
In this study, the coefficients of variation of P(St-AA) microspheres synthesized with different APS dosages were all less than 0.035 and showed a uniform distribution (Figure 4B). The average diameter of microspheres obtained by DLS and manual measurement was in the ranges of 220–265 nm and 218–234 nm, respectively. When the amount of APS was different, the dried emulsion samples showed different colors (Figure 6), which indicated that photonic crystals formed by microspheres changed the band gap. The diameters were similar when using 125 and 150 mg APS. The dried samples all appeared bright yellow-green (Figure 6A,B). When the amount of APS increased, the diameter of microspheres increased, and the dried samples appeared yellowish-orange, orange-red, and dark red, while the brightness of samples decreased. When 225 mg APS was used, the dark red color was observed only at the center of the sample, while the edge was gray. The gradual decrease in sample brightness was caused by the increase in cave-like defects of samples.
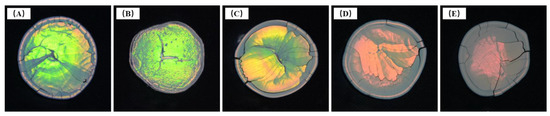
Figure 6.
Photos of dried samples of P(St-AA) colloidal microspheres synthesized with different amounts of APS: (A) 125 mg; (B) 150 mg; (C) 175 mg; (D) 200 mg; (E) 225 mg.
Different from the dried samples shown in Figure 4, which were assembled from microspheres with different polymerization times, the dried samples formed by microspheres synthesized with different amounts of APS showed large lamellar cracking, and the edges produced an obvious coffee ring effect. This may be related to the reactivity rates of St and AA during polymerization, which changed the properties of the final copolymerized microspheres.
3.1.3. Influence of the Amount of Emulsifier (SDBS)
Upon increasing the amount of SDBS, the average hydrodynamic diameters of P(St-AA) microspheres greatly decreased, similar to previous studies [19,28]. In this study, the PDIs were all less than 0.06 regardless of the amount of SDBS. When the SDBS dosage was 200 mg, the PDI was the smallest (Figure 7A). SDBS is an anionic emulsifier commonly used in emulsion polymerization. Since the hydrophilic group dissolves in water and the lipophilic group is pushed away by water toward the air, the water surface was covered with lipophilic groups. The monomers used in emulsion polymerization were incompatible with water. In the presence of an emulsifier, the monomer formed beads under stirring, and a layer of emulsifier was adsorbed on the surface of beads. The lipophilic groups extended into the droplet interior, and the hydrophilic groups remained in the aqueous phase. The anionic emulsifier gave the monomer bead surfaces a layer of negative charges, which caused electrostatic repulsion between the beads. This formed a stable emulsion system by preventing small droplets from joining with each other to form larger ones. Upon increasing the emulsifier content, more micelles were formed, and more latex particles were generated according to the micellar mechanism of emulsion polymerization [28,33]. The number of synthesized microspheres increased, while the average diameter of microspheres decreased.
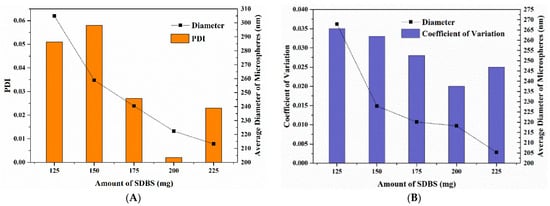
Figure 7.
Particle size analysis of P(St-AA) colloidal microspheres synthesized with different amounts of SDBS: (A) by DLS characterization; (B) by measuring FE-SEM images.
All colloidal microspheres in the FE-SEM images were orderly arranged during drying, and only point and line defects existed in each sample without disordered arrangement (Figure 8). This may be caused by steric hindrance between the ordered photonic crystals. The variation in the average diameter of P(St-AA) microspheres synthesized with different amounts of SDBS was consistent with the DLS results (Figure 7B).
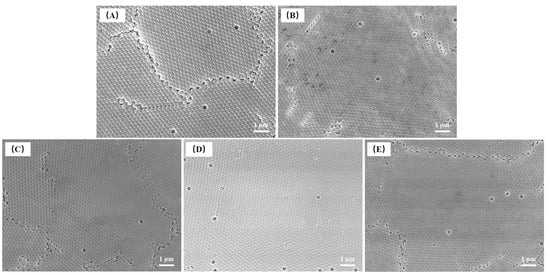
Figure 8.
FE-SEM images of P(St-AA) colloidal microspheres synthesized with different amounts of SDBS: (A) 125 mg; (B) 150 mg; (C) 175 mg; (D) 200 mg; (E) 225 mg.
Upon increasing the SDBS addition, and causing the diameter of microspheres to decrease gradually, the band gap position of the photonic crystal structure formed by the ordered microspheres underwent a blue shift. When using 125 mg SDBS, as shown in Figure 9A, the dried samples of microspheres appeared red with low brightness. This was mainly due to the large diameter of the microspheres (270 nm). The band gap of photonic crystals nearly exceeded the red visible light range. The dried samples of microspheres showed orange-yellow, yellow-green, green, and blue-green colors upon increasing the SDBS content (Figure 9B–E). When the SDBS dosage was 200 mg (Figure 9D), the structure of samples was smoother than others in the same group. Combined with the FE-SEM images, this indicated that the highly ordered microstructure of the samples affected their macroscopic morphology and optical effect.
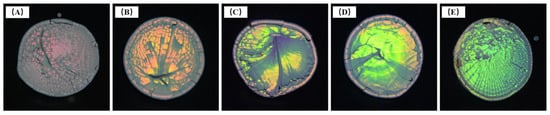
Figure 9.
Photos of dried test samples of P(St-AA) colloidal microspheres synthesized with different amounts of SDBS: (A) 125 mg; (B) 150 mg; (C) 175 mg; (D) 200 mg; (E) 225 mg.
3.1.4. Influence of Reaction Temperature
The average hydrodynamic diameters of P(St-AA) microspheres increased upon increasing the polymerization temperature (Figure 10A). When the temperature was 65 °C, the PDI was 0.098, and the microspheres had poor monodispersity. When the reaction temperature was increased to 70 °C, the PDI was 0.002, and the PDI increased with the temperature, and all values were less than 0.08. During emulsion polymerization, when the reaction temperature increased, the Brownian motion of latex particles was intensified, causing them to collide with each other. Moreover, the generation rate of free radicals increased, which accelerated the polymerization [38].
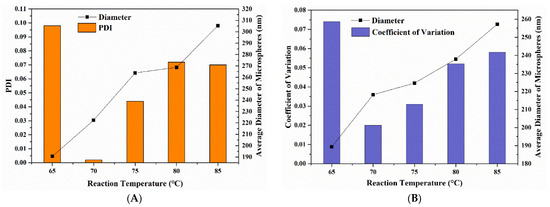
Figure 10.
The particle size analysis of P(St-AA) colloidal microspheres synthesized with different reaction temperatures: (A) by DLS characterization; (B) by measuring FE-SEM images.
When the reaction temperature was 65 °C (Figure 11), there were several large microspheres in the image. The morphology of the sample displayed only short-range order due to the poor monodispersity of microspheres. This was consistent with the DLS result. The microspheres synthesized at other reaction temperatures were well ordered after the drying process, and the photonic crystals had point and line defects. When the reaction temperature was 70 °C, the fewest structural defects were obtained. The manual measured average diameter range of microspheres was 185–260 nm (Figure 10B), and when the reaction temperature was 65 °C, the coefficient of variation was 0.074, indicating that non-uniform microspheres were obtained.
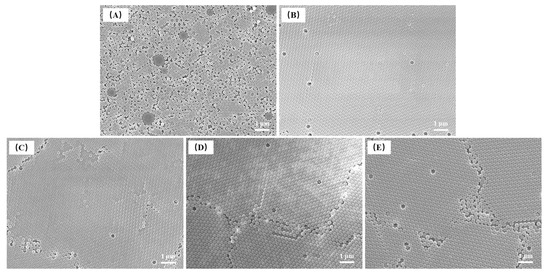
Figure 11.
FE-SEM images of P(St-AA) colloidal microspheres synthesized with different reaction temperatures: (A) 65 °C; (B) 70 °C; (C) 75 °C; (D) 80 °C; (E) 85 °C.
The dried emulsion samples appeared blue-green with low brightness, and the samples were cracked into small flakes when the reaction temperature was 65 °C, as shown in Figure 12A. This was mainly due to the existence of large microspheres and a disordered microstructure. Upon increasing the reaction temperature, the microsphere diameter gradually increased, and the dried samples appeared brilliant green, orange-yellow, red-orange, and red, respectively (Figure 12B–E).

Figure 12.
Photos of dried samples of P(St-AA) colloidal microspheres synthesized with different reaction temperatures: (A) 65 °C; (B) 70 °C; (C) 75 °C; (D) 80 °C; (E) 85 °C.
The effects of emulsion polymerization time, amount of initiator, amount of emulsifier, and reaction temperature on the size and monodispersity of P(St-AA) colloidal microspheres were discussed. When the reaction time was too long or too short, microspheres with good monodispersity cannot be obtained. The diameter of the colloidal microspheres decreased with the decrease of the amount of APS, the increase of SDBS, and the decrease of the reaction temperature. It was found that for the synthesis set up in this study, the reaction time of polymerization was set as 12 h, the amount of APS was 125 mg, the dosage of SDBS was 200 mg, and the reaction temperature was 70 °C based on the results of single-factor experiments to obtain the monodispersity of P(St-AA) colloidal microspheres.
3.2. Micromorphology Characteristics of P(St-AA)
Figure 13A and Figure 14 show the TEM images of the micromorphology of P(St-AA) colloidal microspheres synthesized under the optimum preparation conditions. The microspheres were regular balls with core-shell structure. The outer shell was composed of PAA with a thickness of about 30 nm by manual measurement. In the TEM images, the outer and core diameters of the P(St-AA) microspheres obtained under different conditions of emulsion polymerization were measured, respectively, then the thicknesses of the shells were calculated. Although there were slight differences in the particle sizes of microspheres measured by SEM and TEM, the size of microspheres changed with the reaction conditions was the same. When the size of microspheres changed with synthesis conditions, the outer and inner diameters would change accordingly. For example, when the emulsion polymerization temperature increased to 85 °C (Figure 13B), the outer diameters of the microspheres increased from 203.4 to 296.3 nm, and the diameter of the PSt core layers augment from 143.4 to 205.1 nm, while the PAA shell layers also thickened from 30 to 45.6 nm (Figure 13). In addition, the ratio of core diameters to the thickness of shell layers also increased significantly during this process. In other words, for a single microsphere, the hard-core parts became larger, while the soft-shell parts became thinner. Other factors also caused similar changes in the core shell structure of P(St-AA).
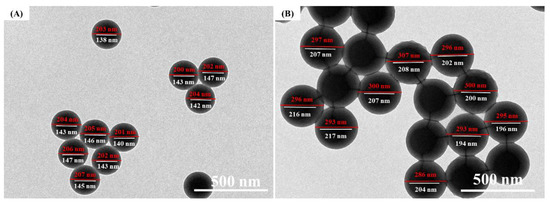
Figure 13.
TEM images of P(St-AA) colloidal microspheres synthesized at different temperatures to illustrate the structure and diameter of the core-shell microspheres. (A) 70 °C; (B) 85 °C.
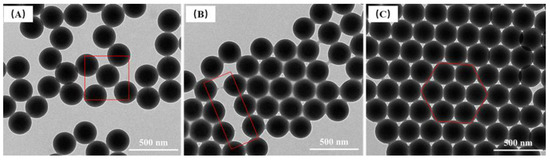
Figure 14.
TEM images of P(St-AA) colloidal microspheres to illustrate the contact pattern and arrangement structure. (A) the PSt core of the microspheres were connected to each other with a dark line; (B) the PAA shell was in a rubber-like state; (C) microspheres were arranged in a two-dimensional ordered hexagonal structure by self-assembly.
When P(St-AA) microspheres self-assembled, the shells layers were bonded together and the core of the microspheres were connected to each other and formed a dark line between two spheres in the TEM images (box line in Figure 14A); however, this phenomenon did not happen between every microsphere. Because acrylic acid contains rich carboxyl groups, when it polymerized on the PSt nucleus to form macromolecular chains, the PAA layer was in a rubber-like state. As shown in the frame of Figure 14B, when the distance between microspheres expanded and began to separate, necking and fracture traces of the PAA shell were observed. When many microspheres were arranged in a two-dimensional ordered hexagonal structure by self-assembly, it appeared as the (111) crystal plane of a face-centered cubic (fcc) lattice [39], namely, a two-dimensional photonic crystal structure (Figure 14C). In the photonic crystal structure, the microspheres fitted closely together, and the tangential surfaces of two microspheres were squeezed. The air gaps between microspheres had obvious differences at different degrees of squeezing, which affected the optical properties of the photonic crystals and the structural color.
3.3. Influence of Self-Assembly on the Optical Properties of Photonic Crystals
3.3.1. Influence of Amount of Emulsion
In this study, the solid content of the emulsion containing microspheres synthesized by the optimum parameters was about 9.5%. The emulsion was directly coated onto wood surfaces to form photonic crystals. The amount of emulsion coating in the 35 mm × 35 mm wireframe on the wood surface was set as 100–600 μL. Figure 14 shows the structural color images generated by photonic crystals on the wood surface. When the coating amount was more than 600 μL, the emulsion easily overflowed out of the wireframe.
When the emulsion amount was between 100 and 300 μL, as shown in Figure 15A–C, the photonic crystal showed yellow-green structural color but did not completely cover the coated area. When the amount of emulsion was 400–600 μL, the photonic crystal covered the entire coating area. At this time, the edges had different degrees of deviation. Some areas produced yellowish-brown with low brightness. This may be due to the slight dip angle and unevenness on the surfaces of the wood sample, which caused the emulsion to spill out of the wireframe. The microspheres gathered and self-assembled to the dip angle under the action of the emulsion flow. The best effect was obtained by using 500 μL of coating. The photonic crystals on the wood surface did not crack or warp, which was different from the samples obtained after emulsion drying on the glass surface. This phenomenon may be because wood is composed of biomacromolecules, such as cellulose, hemicelluloses, and lignin, which are rich with hydroxyl groups, as opposed to the glass dishes [40]. Hydrogen bonds may be formed between the microspheres and the wood, which can effectively alleviate the cracking and warping caused by microsphere shrinkage.
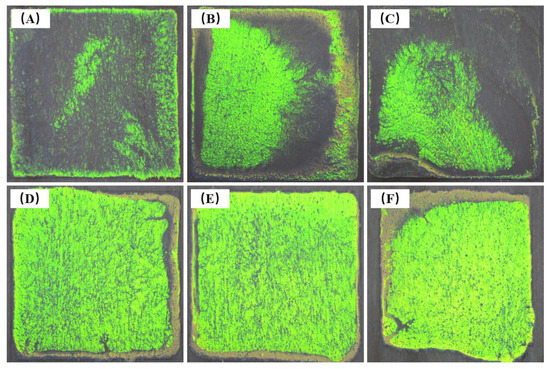
Figure 15.
Photos of photonic crystals formed by different amounts of P(St-AA) emulsions on 35 mm × 35 mm wood surface: (A) 100 μL; (B) 200 μL; (C) 300 μL; (D) 400 μL; (E) 500 μL; (F) 600 μL.
Visible light spectrophotometry was used to characterize the reflectance spectra of P(St-AA) photonic crystals on the wood surface (Figure 16). The photonic crystals had two reflection peaks at 425 nm and 524 nm. The peak at 524 nm was consistent with the particle size of colloidal microspheres (222.4 nm) and yellow-green structural color, which conformed to Bragg diffraction. The peak near 425 nm was the reflection peak of the P(St-AA) colloidal microspheres [41]. When the emulsion amounts were 100 and 200 μL, the spectra were different from that of a higher coating amount. When the coating amount was 100 μL, the curve was similar to uncoated wood, and only two extremely weak peaks were observed at 429 nm and 548 nm. There were fewer colloidal microspheres and the effective photonic crystal region was small. When the coating amount increased to 200 μL, the two peaks became more obvious, and both underwent a blue shift. The coating had photonic crystal structure but not enough to cover the entire surface. The reflectance spectra were similar upon increasing the coating amount. When the coating amount was 500 μL, the half-peak width of the coating was the smallest, which indicated that the coating had the best effect.
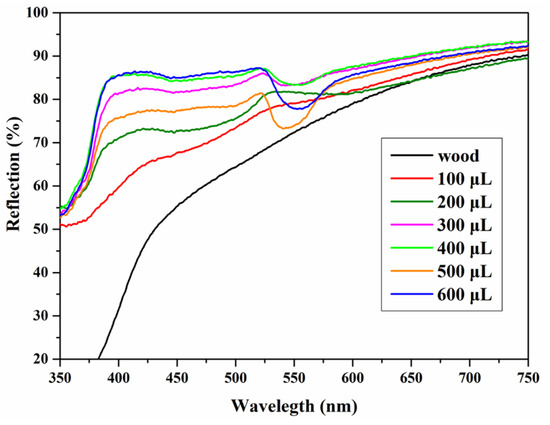
Figure 16.
Reflection spectra of photonic crystals formed by different amounts of the P(St-AA) emulsion on a 35 mm × 35 mm wood surface.
3.3.2. Influence of Self-assembly Temperature
When the self-assembly temperature was taken as the single-factor variable, the photonic crystals formed by P(St-AA) microspheres on the wood surface were not uniform. The coatings were brighter at their edges, and the final self-assembled parts were not covered with microspheres when the self-assembly temperature was 10 or 20 °C (Figure 17A,B). The bright areas showed striated textures, possibly because the microspheres firstly formed striped structures on the liquid surface and then gathered to form large photonic crystals during self-assembly. The uniform part of the photonic crystal gradually increased upon increasing the temperature [42]. When the self-assembly temperature was 50 °C, as shown in Figure 17E, the uniform part of the surface coating was the largest and had the highest smoothness. As the temperature continued to rise, the uneven part of the coatings gradually increased. By comparing the photos of coatings formed at 10 and 20 °C to other temperatures, it could be found that the yellowish-green part was flatter at a lower temperature.
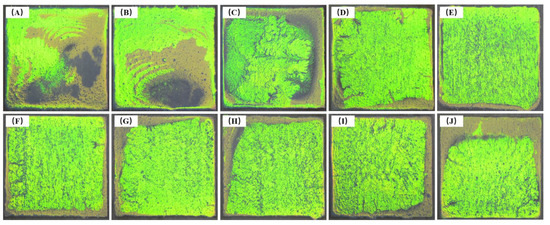
Figure 17.
Photos of photonic crystals formed by the self-assembly of P(St-AA) colloidal microspheres at different temperatures: (A) 10 °C; (B) 20 °C; (C) 30 °C; (D) 40 °C; (E) 50 °C; (F) 60 °C; (G) 70 °C; (H) 80 °C; (I) 90 °C; (J) 100 °C.
The reflectance spectra of the photonic crystals formed by P(St-AA) emulsion on wood surfaces changed obviously upon increasing the self-assembly temperature (Figure 18). When the self-assembly temperature was 10 or 20 °C, the reflectance spectra exhibited two reflection peaks at 399 and 506 nm with large half-peak widths. The two peaks shifted to 420 and 524 nm, respectively, when the self-assembly temperature was 30–100 °C, and the relative peak height and half peak width also changed significantly. This indicated that the band gap of the coatings was better at the corresponding wavelength (524 nm), and the response of photonic crystals to visible light was basically the same in this self-assembly temperature range. When the temperature was 50 °C, the half-peak width of the coating was the smallest; therefore, the amount of P(St-AA) emulsion coating in 35 mm × 35 mm area was set as 500 μL (about 0.408 μL/mm2), and the self-assembly temperature was set as 50 °C to fabricate photonic crystal coatings with structural color on the wood surface.
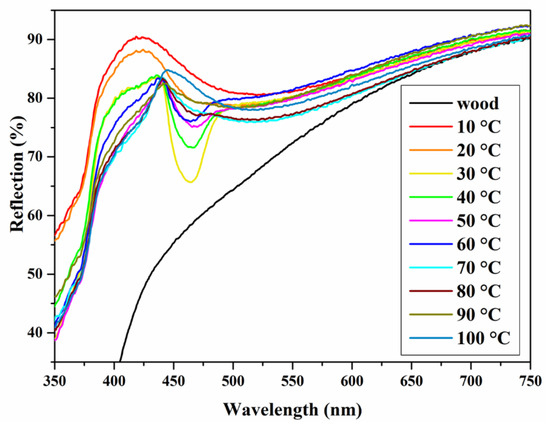
Figure 18.
Reflection spectra of photonic crystals formed by self-assembly of P(St-AA) colloidal microspheres at different temperatures.
In this study, a digital camera at different viewing angles was used to record the angle dependent (rainbow effect) of structural color of photonic crystal on wood surfaces (Figure 19). When this series of photos was taken, the white light was vertical incident into the photonic crystal, and the angle of reflection (β), which is between the camera and the normal, gradually increased from 0 to 60°. When β ≤ 20°, the structural color changed from bright yellow-green to dull green-blue. When the angle continued to increase, the structural color of photonic crystal became dim and difficult to observe.
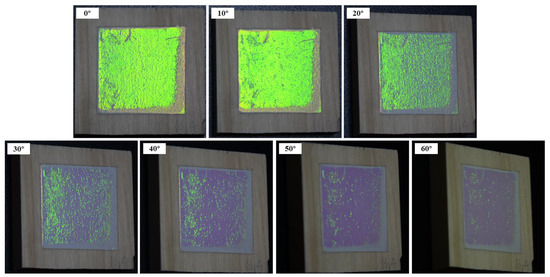
Figure 19.
Angle dependence of structural color of photonic crystal constructed by P(St-AA) colloidal microspheres on the wood surface.
3.4. Micromorphology and Structure of Photonic Crystals on Wood Surfaces
FE-SEM was used to characterize the microstructure and structural characteristics of the photonic crystals formed by P(St-AA) microspheres on wood surfaces. The coating formed using 0.408 μL/mm2 of emulsion covered the wood surface, and no wood cells were observed in the coated area. In Figure 20A, the photonic crystals formed by microspheres on the wood surface had an orderly arrangement, and the size of this structure was within the range of several hundred square microns. There were cracks between the well-ordered structures. In the high-magnification view (Figure 20B), microspheres with good monodispersity were closely bonded to each other to form a well-ordered hexagonal structure, similar to the (111) plane of an fcc lattice. It can be found that regardless of whether wood or glass was used as the substrate, the same well-ordered part of the microstructure was formed by the microspheres. Figure 20C shows the crack defects in photonic crystals. The sizes of cracks ranged from one to several microns, and there were still ordered colloidal microspheres in the cracks. These micron-scale cracks were mainly produced during the final drying stage. When the dispersant in the emulsion evaporated, the colloidal microspheres gradually changed from wet state to dry state, which caused them to shrink; however, the wood substrate did not shrink to the same degree, and the shrinkage difference resulted in microscale cracking. Both the point and line defects between microspheres and the microscale crack defects in the coatings weakened the band gap of photonic crystals, which reduced the structural color saturation of the wood surface [43].

Figure 20.
Top-down FE-SEM images of photonic crystals formed by the self-assembly of P(St-AA) colloidal microspheres on wood surfaces: (A) low-magnification view; (B) high-magnification view; (C) crack in the coating.
The sectional view FE-SEM images of photonic crystals formed by self-assembly of P(St-AA) colloidal microspheres on wood surfaces shows that the coating thickness was about 20–35 μm, which was comparable to the vessel diameter of aspen wood (Figure 21A). The wood shown in the image was radial section, which clearly shows the structural characteristics of aspen, such as conduits, wood fiber, and rays. There was obvious alternate pitting in the conduit. The surface of the coating formed by microspheres was not smooth. When the colloidal microspheres formed photonic crystals on the surface of the wood with microscale roughness, the microspheres filled the cell cavities and then stacked in layers; therefore, the uneven coating was inherited from the rough surface of the wood.
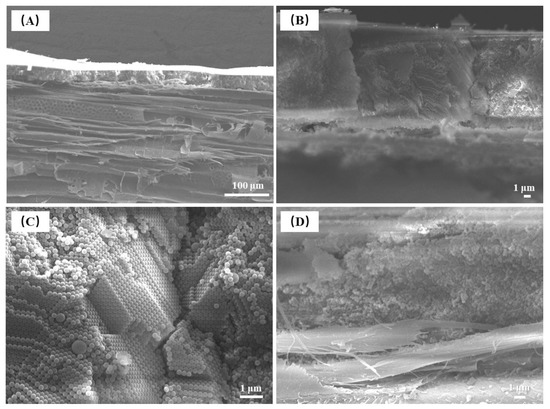
Figure 21.
Sectional view FE-SEM images of photonic crystals formed by the self-assembly of P(St-AA) colloidal microspheres on the wood surface: (A) micromorphology of photonic crystals and wood; (B) low-magnification view of the photonic crystal; (C) high-magnification view of the well-ordered part; (D) high-magnification view of the disordered part.
The coating microstructure consisted of two parts (Figure 21B). The photonic crystal near the coating surface was composed of ordered colloidal microspheres, while the amorphous structure was near the interface of the coating and wood. In the high-magnification image of the well-ordered part of the photonic crystals (Figure 21C), there were spheres with diameters that were significantly larger than the average diameter, but the orderly arrangement of microspheres was not significantly affected. The microspheres were arranged in two ways, one was a hexagonal structure, and the other was quadrilateral. The combination of these two structures formed ordered photonic crystals. Figure 21D shows an amorphous structure layer with disordered microspheres, which may be caused by the accumulation of colloidal microspheres after filling wood cells.
4. Conclusions
In this study, P(St-AA) colloidal microspheres were synthesized by emulsion polymerization. A series of single-factor experiments of emulsion polymerization with 15 g St and 1 g AA as the reaction raw materials, 100 mL water, and 40 mL absolute ethanol as the reaction dispersant were carried out. The optimal monodispersity of microspheres was obtained when the amounts of APS and SDBS were 125 and 200 mg, respectively, the reaction temperature was 70 °C, and the reaction time was 12 h. The particle size of P(St-AA) colloidal microspheres increased upon increasing the amount of APS initiator and reaction temperature, and it decreased upon increasing the amount of SDBS emulsifier. The core-shell microspheres were self-assembled on the wood surface by thermally-assisted gravity deposition. When the amount of emulsion was 0.408 μL/mm2 and the self-assembly temperature was 50 °C, the microstructure, optical properties, and structural color effect of the photonic crystals were the best, and the thickness of the photonic crystal was 20–35 μm. The photonic crystal consisted of an ordered layer and a disordered amorphous layer, and there were micron-scale crack defects. The colloidal microspheres on the photonic crystals surface were closely bonded to each other, forming a well-ordered continuous hexagonal structure with point and line defects. Ordered quadrilateral and hexagonal structures were formed in the cross-section of the photonic crystals. In this study, photonic crystals self-assembled from colloidal microspheres endowed wood with bright structural color, which provided theoretical basis for new wood surface decoration technology. In order to improve the binding strength and surface smoothness of the photonic crystal decorative layers, further in-depth research on the interface between wood and photonic crystal is needed, which will be the next focus of our research team.
Author Contributions
Conceptualization, Y.L., J.H., Z.W. and X.P.; methodology, Y.L., J.H., Z.W. and X.P.; data curation, Y.L.; writing—original draft preparation, Y.L. and J.H.; writing—review and editing, Y.L., J.H. and W.X.; visualization, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant numbers 20KJB220012), Postdoctoral research project of Zhejiang Province (271235), Qing Lan Project, the Youth Program of Science and Technology Innovation Fund of Nanjing Forestry University (grant numbers CX2019016) and Scientific Research Foundation of Nanjing Forestry University (grant numbers GXL 2018022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw and processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Acknowledgments
The authors acknowledge the valuable support from Zhejiang Shenghuayunfeng New Material Co., Ltd, Huzhou, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Z.; Zhang, X.; Lin, Q.; Zhu, N.; Gui, C.; Yong, Q. Development and investigation of a two-component adhesive composed of soybean flour and sugar solution for plywood manufacturing. Wood Mater. Sci. Eng. 2022, 1–9. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Sagaama, A.; Issaoui, N.; Malyar, Y.N.; Vasilieva, N.Y.; Borovkova, V.S. Theoretical and experimental study of guar gum sulfation. J. Mol. Model. 2021, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Hou, S.; Guo, M.; Fu, Y. Surface properties of spray-assisted layer-by-layer electrostatic self-assembly treated wooden take-off board. Appl. Sci. 2021, 11, 836. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, T.; Hou, S.; Fu, Y. Preparation and synergistic effect of chitosan/sodium phytate/MgO nanoparticle fire-retardant coatings on wood substrate through layer-by-layer self-assembly. Coatings 2020, 10, 848. [Google Scholar] [CrossRef]
- Hu, W.; Wan, H. Comparative study on weathering durability properties of phenol formaldehyde resin modified sweetgum and southern pine specimens. Maderas Cienc. Tecnol. 2022, 24, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yang, F.; Yang, L.; Wang, J.; Zhou, J.; Wang, J. A highly transparent compressed wood prepared by cell wall densication. Wood Sci. Technol. 2022, 56, 669–686. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, W.; Wang, L. Mechanism of thermochromic and self-repairing of waterborne wood coatings by synergistic action of waterborne acrylic microcapsules and fluorane microcapsules. Polymers 2022, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yan, X. Preparation of healable shellac microcapsules and color-changing microcapsules and their effect on properties of surface coatings on hard broad-leaved wood substrates. Coatings 2022, 12, 991. [Google Scholar] [CrossRef]
- Li, R.; He, C.; Wang, X. Effects of processing parameters on mass loss and coating properties of poplar plywood during CO2 laser modification. Eur. J. Wood Wood Prod. 2022, 80, 899–906. [Google Scholar] [CrossRef]
- Li, R.; He, C.; Chen, Y.; Wang, X. Effects of laser parameters on the width of color change area of poplar wood surface during a single irradiation. Eur. J. Wood Wood Prod. 2021, 79, 1109–1116. [Google Scholar] [CrossRef]
- Pan, P.; Yan, X.; Wang, L. Effects of thermochromic fluorane microcapsules and self-repairing waterborne acrylic microcapsules on the properties of water-based coatings on basswood surface. Polymers 2022, 14, 2500. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Y.; Yang, F.; Wu, X.; Ding, G. A novel waterborne polyurethane coating modified by highly dispersed nano-boron carbide particles. J. Appl. Polym. Sci. 2020, 138, 50214. [Google Scholar] [CrossRef]
- Takeoka, Y. Fusion materials for biomimetic structurally colored materials. Polym. J. 2015, 47, 106–113. [Google Scholar] [CrossRef]
- Zeng, Q.; Ding, C.; Li, Q.; Yuan, W.; Peng, Y.; Hu, J.; Zhang, K. Rapid fabrication of robust, washable, self-healing superhydrophobic fabrics with non-iridescent structural color by facile spray coating. RSC Adv. 2017, 7, 8443–8452. [Google Scholar] [CrossRef]
- Dziomkina, N.V.; Vancso, G.J. Colloidal crystal assembly on topologically patterned templates. Soft Matter 2005, 1, 265–279. [Google Scholar] [CrossRef]
- Parker, A.R.; Welch, V.L.; Driver, D.; Martini, N. Opal analogue discovered in a weevil. Nature 2003, 426, 786–787. [Google Scholar] [CrossRef]
- Zi, J.; Yu, X.; Li, Y.; Hu, X.; Xu, C.; Wang, X.; Liu, X.; Fu, R. Coloration strategies in peacock feathers. Proc. Natl. Acad. Sci. USA 2003, 100, 12576–12578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J. Investigation of polystyrene-based microspheres from different copolymers and their structural color coatings on wood surface. Coatings 2021, 11, 14. [Google Scholar] [CrossRef]
- Liu, Y. Self-assembly of poly(styrene-methyl methacrylate-acrylic acid) (P(St-MMA-AA)) colloidal microspheres on wood surface by thermal-assisted gravity deposition. Wood Sci. Technol. 2021, 55, 403–417. [Google Scholar] [CrossRef]
- Núñez-montenegro, A.; Crista DM, A.; Esteves da Silva JC, G. Structural coloration based on photonic crystals for coating applications on wood. Eur. J. Wood Wood Prod. 2020, 78, 293–300. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Yang, B. Self-assembly of photonic crystals from polymer colloids. Curr. Opin. Colloid Interface Sci. 2009, 14, 103–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Huang, Y.; Song YJiang, L. Fabrication of functional colloidal photonic crystals based on well-designed latex particles. J. Mater. Chem. 2011, 21, 14113–14126. [Google Scholar] [CrossRef]
- Galisteo-López, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Pérez, L.S.; Blanco, A.; López, C. Self-assembled photonic structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, B.; Wu, S.; Zhang, S. Facile synthesis of monodispersed polysulfide spheres for building structural colors with high color visibility and broad viewing angle. Small 2017, 13, 1602565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, H.; Chen, H. Preparation of monodisperse dye-doped copolymer spheres for photonic crystals. Mol. Cryst. Liq. Cryst. 2011, 534, 124–133. [Google Scholar] [CrossRef]
- Yang, X.; Ge, D.; Wu, G.; Liao, Z.; Yang, S. Production of structural colors with high contrast and wide viewing angles from assemblies of polypyrrole black coated polystyrene nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16289–16295. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Y.; Wang, J.; Ren, Y.; Song, Y.; Jiang, L. Ultra-fast fabrication of colloidal photonic crystals by spray coating. Macromol. Macromol. Rapid Commun. 2009, 30, 598–603. [Google Scholar] [CrossRef]
- Wang, J.; Wen, Y.; Ge, H.; Sun, Z.; Zheng, Y.; Song, Y.; Jiang, L. Simple fabrication of full color colloidal crystal films with tough mechanical strength. Macromol. Chem. Phys. 2006, 207, 596–604. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, Y.; Fu, G.; Zhou, L.; Fan, Q. Preparation of monodispersed polystyrene microspheres and self-assembly of photonic crystals for structural colors on polyester fabrics. J. Text. Inst. 2014, 105, 938–943. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, G.; Wu, Y.; Fan, Q.; Shao, J. The Synthesis of core-shell monodisperse P(St-MAA) microspheres and fabrication of photonic crystals structure with tunable colors on polyester fabrics. Fibers Polym. 2014, 15, 1112–1122. [Google Scholar] [CrossRef]
- McGrath, J.G.; Bock, R.D.; Cathcart, M.; Lyon, L.A. Self-assembly of “paint-on” colloidal crystals using poly(styrene-co-N-isopropylacrylamide) spheres. Chem. Mater. 2007, 19, 1584–1591. [Google Scholar] [CrossRef]
- Kohri, M.; Yamazaki, S.; Kawamura, A.; Taniguchi, T.; Kishikawa, K. Bright structural color films independent of background prepared by the dip-coating of biomimetic melanin-like particles having polydopamine shell layers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 564–569. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Wen, Y.; Song, Y.; Jiang, L. Hydrogen-bonding-driven wettability change of colloidal crystal films: From superhydrophobicity to superhydrophilicity. Chem. Mater. 2006, 18, 4984–4986. [Google Scholar] [CrossRef]
- Liu, G.; Shao, J.; Zhang, Y.; Wu, Y.; Wang, C.; Fan, Q.; Zhou, L. Self-assembly behavior of polystyrene/methacrylic acid (P(St-MAA)) colloidal microspheres on polyester fabrics by gravitational sedimentation. J. Text. Inst. 2015, 106, 1293–1305. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, L.; Fan, Q.; Chai, L.; Shao, J. The vertical deposition self-assembly process and the formation mechanism of poly(styrene-co-methacrylic acid) photonic crystals on polyester fabrics. J. Mater. Sci. 2016, 51, 2859–2868. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kanda, Y.; Higashitani, K. Molecular-scale observation of formation of nuclei in soap-free polymerization of styrene. Langmuir 2004, 20, 4400–4405. [Google Scholar] [CrossRef]
- Chern, C.S. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Taha, M. Synthesis of monodisperse styrene/methyl methacrylate/acrylic acid latex using surfactant-free emulsion copolymerization in air. J. Appl. Polym. Sci. 2009, 114, 1598–1605. [Google Scholar] [CrossRef]
- Moon, J.H.; Yi, G.R.; Yang, S.M.; Pine, D.J.; Park, S.B. Electrospray-assisted fabrication of uniform photonic balls. Adv. Mater. 2004, 16, 605–609. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Zhang, G.; Liu, G.; Fan, Q.; Shao, J. Study on the effects of the characteristics of textile substrates on the photonic crystal films and the related structural colors. Surf. Coat. Technol. 2017, 319, 267–276. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, Z. Fabrication of coatings with structural color on a wood surface. Coatings 2020, 10, 32. [Google Scholar] [CrossRef]
- Chai, L.; Zhou, L.; Liu, G.; Li, Y.; Fan, Q.; Shao, J. Interface-gravity joint self-assembly behaviors of P(St-MAA) colloidal microspheres on polyester fabric substrates. J. Mater. Sci. 2017, 52, 5060–5071. [Google Scholar] [CrossRef]
- Shen, Z.; Shi, L.; You, B.; Wu, L.; Zhang, D. Large-scale fabrication of three-dimensional ordered polymer films with strong structure colors and robust mechanical properties. J. Mater. Chem. 2012, 22, 8069–8075. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).