Abstract
The corrosion of galvanizing equipment parts by liquid zinc is an urgent problem that needs solving. In this work, FeB-30 wt.% Al0.25FeNiCoCr cermet coating was deposited on the surface of 316L stainless steel by AC-HVAF to protect galvanizing equipment parts from corrosion by liquid zinc. The microstructures and phase compositions of powders and the coating were determined by SEM, EDS, and XRD in detail. Additionally, the microhardness, fracture toughness, abrasion wear resistance, and corrosion resistance of the coating to liquid zinc were also studied. The results indicate that the abrasion wear resistance and corrosion resistance of the coating are much better than that of the 316L stainless steel substrate. The failure of the coating in liquid zinc is mainly due to the penetration of liquid zinc into macro-cracks, which causes the coating to peel off.
1. Introduction
Hot dip galvanizing is an effective method to prevent the atmospheric corrosion of steel [1,2]. However, liquid zinc is corrosive to almost all single metals and most alloys. Moreover, this corrosion is usually accompanied by wear. The corrosion and wear caused by liquid zinc are serious problems in the hot dip galvanizing industry [3]. During continuous hot dip galvanizing, important components such as stable rolls and sink rolls are corroded and worn by liquid zinc for a long time [4]. One of the widely used materials to produce these important parts is 316L stainless steel [5]. However, it was not particularly designed for galvanizing; thus, its corrosion and wear resistance need to be strengthened. To prolong its lifetime, MoB/CoCr [6,7] and WC/Co [8,9,10] cermets coatings are deposited on the steel using high-velocity oxygen fuel (HVOF) method [11,12,13,14]. There are still some issues to be resolved such as depositing even MoB/CoCr or WC/Co coating on 316L stainless steel. Except for the low-carbon WC/Co coating using Co3W3C and Co6W6C as bonding phases, most cermet coatings are composed of hard phases and metal bonding phases. On one hand, bonding phases, cobalt, nickel, and other metallic elements or alloys are more easily eroded by liquid zinc than hard phases, which is the main factor leading to failure in liquid zinc. On the other hand, the significant diversity in the thermal expansion coefficient (TEC) between a coating and a 316L stainless steel substrate is the major reason for the failure [15]. Therefore, the key to extending the usage life is to reduce or avoid the corrosion of a bonding phase by liquid zinc and to select the ceramic whose thermal expansion coefficient is close to that of 316L stainless steel as a hard phase.
The research results of Tsipas et al. showed that the corrosion resistance of FeB to liquid zinc is suitable [15]. Additionally, the TECs of FeB (αFeB = 23 × 10−6/K) [16] and 316L stainless steel (α316L = 19.3 × 10−6/K) [17] are close. However, brittleness and cracks form easily and expand, which lead to the failure of FeB in liquid zinc. Results showed that corrosion resistance of FeB alloy can be enhanced by alloying with Mo or W; however, it was still highly brittle [18,19]. The brittleness of FeB can be improved by using Co as the bonding phase, but it is easily eroded by liquid zinc, which is the primary reason for failure [20].
In recent years, high-entropy alloys have been fabricated to replace the traditional metal bonding phases of Ti(CN)-based [21], WC-based [22], and TiB2-based [23] cermets, and they are considered to be beneficial for the sintering of cermets [23,24,25]. In addition, high-entropy alloys [26,27,28,29] have better fracture toughness, corrosion resistance, and wear resistance than cobalt, nickel, and other metals or alloys. The majority of high-entropy alloys consists of Al, Fe, Ni, Co, Cr, and other common elements, and the preparation process of high-entropy alloys, including ball mill and vacuum sintering, is common. Therefore, adopting high entropy alloy instead of cobalt and nickel as a bonding phase is not only low-cost, but the fracture toughness, wear resistance, and corrosion resistance are also improved.
In this study, FeB-30 wt.% Al0.25FeNiCoCr cermet coating was prepared on 316L stainless steel substrate by activated combustion high-velocity air fuel (AC-HVAF). AC-HVAF is a technology, which uses compressed air and fuel to generate high-speed airflow to heat powders to a molten or semi-molten state, and at the same time accelerate the powders to more than 700 m/s, and then hit the treated substrate [30]. The powder particles impacted on the substrate surface are deformed by pressing to form a laminated sheet, which adheres to the substrate surface, and then cools and accumulates continuously, finally forming a layered coating. Compared with coatings prepared by HVOF, coatings deposited by AC-HVAF [30,31,32] have high density and low oxide content. In addition, the replacement of pure oxygen with compressed air can decrease the cost of preparation [33]. The cermet coating uses FeB as a hard phase, which has good corrosion resistance to liquid zinc, and Al0.25FeNiCoCr high-entropy alloy as a bonding phase to strengthen the fracture toughness. In general, the content of the bonding phase accounting for the content of cermets is 8–30 wt.% [34]. In order to improve the brittleness of FeB as much as possible, the content of the bonding phase was designed to be 30 wt.%. In this work, the fracture toughness, abrasion wear property, and corrosion behavior of FeB-30 wt.% Al0.25FeNiCoCr cermet coating in liquid zinc were studied.
2. Experiment
2.1. Preparation of FeB-30 wt.% Al0.25FeNiCoCr Powders
We added 2.9 wt.% aluminum, 24.06 wt.% iron, 25.35 wt.% nickel, 25.35 wt.% cobalt, 22.34 wt.% chromium powders with an average particle size of 2 μm, and 99.9 wt.% purity to a ball mill (YXQM-4L) (MITR, Changsha, China). Prior to ball milling, polyethylene glycol was added to mixed powders with a mass ratio to the powders of 1:100. The ball mill parameters are shown in Table 1. After ball milling, the powders were dried for 20 h in a drying chamber (DZ-1BCIV) (Taiste, Tianjin, China) at 100 °C. The powders were then sintered with a vacuum furnace (ZM-30-16) (Chenhua, Shanghai, China), ground with a mortar, and sieved with a 60-mesh stainless steel sieve. Afterward, 30 wt.% Al0.25FeNiCoCr powders and 70 wt.% FeB powders with a particle size ranging from 1 to 5 μm with 99.9 wt.% purity were ball-milled. FeB-30 wt.% Al0.25FeNiCoCr powders were prepared through the same approaches used for Al0.25FeNiCoCr powders, apart from the ball milling time of 3 h, a ball ratio of 3:1, and a sieve of 400 mesh. The vacuum sintering process of Al0.25FeNiCoCr powders and FeB-30 wt.% Al0.25FeNiCoCr powders is shown in Figure 1.

Table 1.
Ball mill process parameters of Al0.25FeNiCoCr powders.
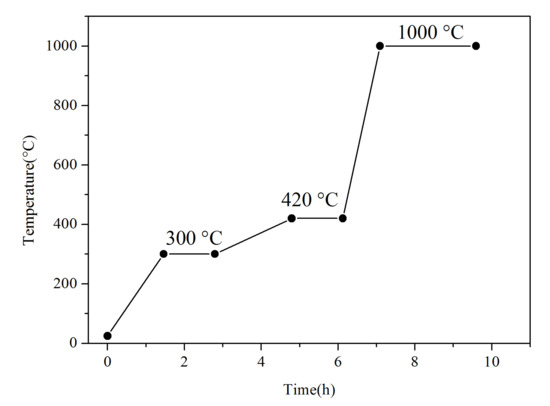
Figure 1.
Vacuum sintering process of Al0.25FeNiCoCr powders and FeB-30 wt.% Al0.25FeNiCoCr powders.
2.2. Preparation of FeB-30 wt.% Al0.25FeNiCoCr Coating
The 316L stainless steel was used as the substrate and its dimensions were 100 × 100 × 3 mm3. In order to enhance the bond strength between the substrate and the coating, the substrate was sandblasted with alumina sand to dislodge impurities on the substrate surface and improve its coarseness before spraying. The cermet powders were sprayed onto a cermet coating with AC-HVAF equipment (AK06) (Kermetico Inc., Benicia, CA, USA). The spraying process parameters are shown in Table 2. Usually, a thermal spray coating thickness ranges from 100 to 1000 μm [34]. If the coating is too thick, it will have complex stress state and cracks will form easily. If a coating is too thin, liquid zinc penetrates the coating more directly and quickly through pores, thus reducing the corrosion resistance to liquid zinc. Therefore, the thickness of the coating was designed to be 260 μm in this work.

Table 2.
Spraying process parameters of FeB-30 wt.% Al0.25FeNiCoCr coating.
2.3. Microhardness and Fracture Toughness Tests of the Coating
A Vickers microhardness tester (MH-5L) was used to measure the microhardness of the coating before and after immersion in liquid zinc. The load was 1.96 N and the loading time was 15 s. In order to ensure the accuracy of test results, the measurements were recorded at five different locations and the average microhardness values were obtained. The indentation method was used to measure the fracture toughness of the coating. After testing the Vickers microhardness of the coating, indentation and two cracks appeared on the surface of the coating, and then the length of two diagonal lines of the indentation and the length of the cracks were measured under a scanning electron microscope (SEM), and 10 sets of data were measured and averaged to obtain the fracture toughness value. The fracture toughness of the coating is calculated using the following equation [35]:
where KIC is the fracture toughness (MPa·m1/2), a1 and a2 are the half length of the indentation diagonal (mm), c1 and c2 are the half length of the cracks (mm), and HV is the Vickers hardness of the cermet (GPa).
2.4. Abrasion Wear Experiment of the Coating and the Substrate
The abrasion wear performance of FeB-30 wt.% Al0.25FeNiCoCr coating and 316L stainless steel substrate was detected by a wet rubber wheel abrasion test machine (MLS-225) (YIHUA, Jinan, China). The experiment parameters are shown in Table 3. Both the coating and the substrate were worn 6 times with each abrasion wear of 200 revolutions. After each abrasion wear experiment, the mass was recorded using an electronic scale (FA1104) (Liangping, Shanghai, China) with a precision of 0.1 mg. The mass loss after each abrasion wear test was obtained by weighing the mass before and after each experiment.

Table 3.
Abrasive wear test parameters of FeB-30 wt.% Al0.25FeNiCoCr coating and the substrate.
2.5. Immersion Experiment in Liquid Zinc
FeB-30 wt % Al0.25FeNiCoCr coating samples were processed into cuboids with a size of 16 × 16 × 3.2 mm3. The heat was supplied by the furnace (SG2-7.5-10) (Rongfeng, Shanghai, China), and the static immersion experiments in liquid zinc were conducted in graphite crucibles placed in the furnace. In order to study corrosion behavior of the coating in liquid zinc, samples were immersed in liquid zinc at 450 °C for 1, 3, 5, and 10 days. The average thickness of specimens before and after the immersion test were gauged with eight measurements. The corrosion rate was calculated using the following equation [36]:
where R is the corrosion rate, a is the original thickness of the coating, b is the coating thickness after immersion in liquid zinc, and t is the immersion time.
2.6. Material Characterizations
The phase compositions of specimens were determined using X-ray powder diffraction (XRD) analysis (Rigaku Ultima IV, Tokyo, Japan; 40 kV and 40 mA with Cu Kα radiation. Among them, The samples were scanned in the 2θ ranging from 20° to 90° with the step space of 0.02° and scanning speed of 2 °/min. The microstructures of the specimens were observed using a scanning electron microscope (SEM, JSM-6360LV) (JEOL Ltd., Tokyo, Japan). The extra high tension (EHT) was 20 kV, I probe was 400 PA, and spot size was 450. Energy dispersive spectroscopy (EDS, OXFORD INCA) (High Wycombe, U.K.) was also used to identify the chemical compositions of specimens.
3. Results and Discussion
3.1. Morphologies and Phase Compositions of the Powders
Figure 2a, b shows the morphologies of Al0.25FeNiCoCr powders after ball milling and vacuum sintering, respectively. We found that the particle size of Al0.25FeNiCoCr powders became larger and denser after vacuum sintering. Figure 2c shows the phase compositions of the Al0.25FeNiCoCr powders as determined by XRD. After ball milling, the powders consisted of FCC solid solution and BCC solid solution. However, BCC solid solution transformed into FCC solid solution after vacuum sintering.
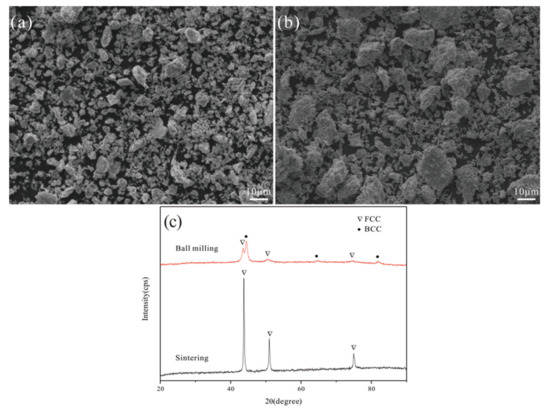
Figure 2.
Morphologies and phase compositions of Al0.25FeNiCoCr powders after ball milling 50 h and vacuum sintering: (a) ball milling, (b) vacuum sintering, and (c) phase compositions.
Figure 3a,b shows the morphologies of FeB-30 wt.% Al0.25FeNiCoCr powders after ball milling and vacuum sintering, respectively. After ball milling, the powders had a multi-angular shape; after sintering, the powders became more compact and more spherical, which were beneficial to thermal spraying. The phase compositions of FeB-30 wt.% Al0.25FeNiCoCr powders after ball milling and vacuum sintering are shown in Figure 3c. No new phase formed during the ball milling. After vacuum sintering, the powders comprised (Fe, Al, Ni, Co, Cr)B and (Fe, Al, Ni, Co, Cr)2B solid solutions, as shown in Figure 3c. A part of FeB was transformed to Fe2B during the vacuum sintering. Moreover, the elements in this high-entropy alloy were completely dissolved into FeB and Fe2B. The bonding phase did not exist in the powders, which had two influences on the coating’s performance: cracks were more likely to occur and grow, and the problem of the binder being easily corroded by liquid zinc was solved.
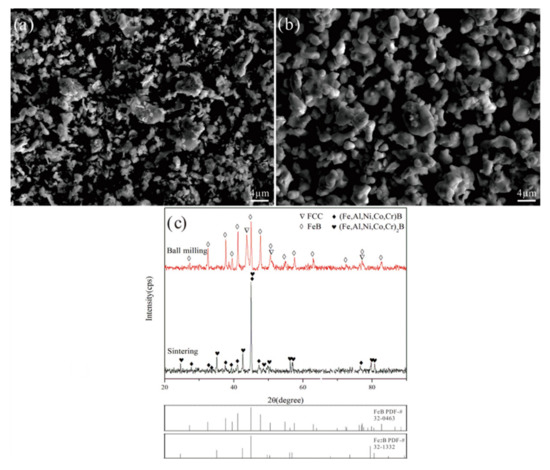
Figure 3.
Morphologies and phase compositions of FeB-30 wt.% Al0.25FeNiCoCr powders after ball milling and vacuum sintering: (a) ball milling, (b) vacuum sintering, and (c) phase compositions.
3.2. Microstructure Characterizations and Phase Compositions of the Coating
As shown in Figure 4a, there are some sandwiched alumina between the coating and the substrate. This might have been caused by sandblasting. The sprayed powders presented a molten or semi-melted state at high temperatures. When it quickly struck the substrate, it rapidly solidified and plastically deformed. It also led to some pores on the coating, as shown in Figure 4b. In Figure 4c, XRD shows that the coating was still composed of (FeAlNiCoCr)2B and (FeAlNiCoCr)B phases, indicating that no phase transitions had occurred during thermal spraying.

Figure 4.
Morphologies and phase compositions of FeB-30 wt.% Al0.25FeNiCoCr coating after ball milling and vacuum sintering: (a) ball milling, (b) vacuum sintering, and (c) phase compositions.
3.3. Microhardness and Fracture Toughness of the Coating
The microhardness values of the coating ranged from 827 to 859 HV, which is much lower than that of FeB (approximately 2100 HV) and Fe2B (approximately 1800 HV) [37], which was caused by the addition of high-entropy alloy elements and the existence of pores formed during the thermal spraying process. According to Equation (1), the fracture toughness values of the coating ranged from 5.27 to 5.89 MPa·m1/2. Since the elements in this high-entropy alloy were completely dissolved in FeB and Fe2B, the fracture toughness of the coating was significantly higher than that of FeB (1.79 ± 0.70 MPa·m1/2) and Fe2B (2.42 ± 0.66 MPa·m1/2) [38].
3.4. Abrasion Wear of the Coating and the Substrate
As shown in Figure 5a, furrow-like wear marks appeared on the coating after the abrasion wear test because a small amount of particles in the coating peeled off due to abrasive particles, and some holes formed on the surface of the coating. These particles further wore the coating and formed more furrow-like wear marks. Figure 5b shows the surface morphology of the 316L stainless steel substrate after the abrasion wear test. It can be seen that there are more furrow-like wear marks on the substrate than on the coating. Figure 6 is a graph showing the relationship between their mass loss after each wear and the revolutions. Since the coating and substrate initially had high surface roughness, their mass loss was large after the first wear, and then gradually reduced. Compared with the substrate, the abrasion wear resistance of the coating was significantly improved, being three times as much as that of the substrate.
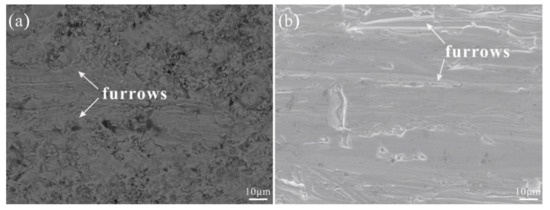
Figure 5.
Surface morphologies of FeB-30 wt.% Al0.25FeNiCoCr coating and 316L stainless steel substrate after abrasive wear for 1200 revolutions: (a) coating, (b) 316L stainless steel.
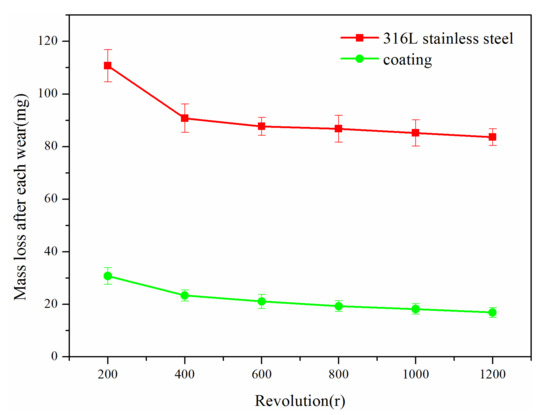
Figure 6.
Comparison of abrasive wear properties between the coating and 316L stainless steel.
3.5. Corrosion Behavior of the Coating in Liquid Zinc
According to Equation (2), the corrosion rate of the coating was calculated by measuring the thickness change of the coating immersed in 450 °C liquid zinc for 1, 3, 5, and 10 days. Figure 7 is a diagram of the relationship between thickness loss, corrosion rate, and corrosion time. The corrosion rate of this coating in liquid zinc bath at 450 °C was 24.19 μm/day, which is lower than that of 316L stainless steel in liquid zinc bath at 460 °C with a value of 115.44 μm/day [39]. Figure 7 shows that the coating thickness did not change in the initial period, indicating that the coating still maintained good resistance to liquid zinc. However, with the immersion time extended to 3 days, the thickness loss and corrosion rate of the coating began to increase. In the period of 5 to 10 days, the thickness loss of the coating increased significantly, and the corrosion rate increased gradually. At this time, the corrosion resistance to liquid zinc was considerably reduced.
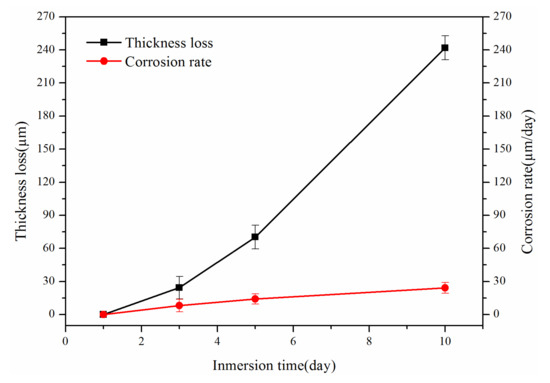
Figure 7.
Corrosion resistance of FeB-30 wt.% Al0.25FeNiCoCr coating immersed in liquid zinc at 450 °C.
Figure 8 shows the interface morphology of the coating immersed in liquid zinc for various times. During immersion in liquid zinc, as shown in Figure 9, the relationship between the microhardness of the coating near the liquid zinc and immersion time is closely related to the corrosion process near cracks. After immersion for 1 day, liquid zinc and the coating maintained a good boundary, but micro-cracks were generated under the influence of residual stress and thermal stress, and the microhardness of the coating near the liquid zinc was almost the same as that of the coating before immersion in liquid zinc. Micro-cracks grew into macro-cracks, and liquid zinc more rapidly invaded into the coating along macro-cracks after being immersed for three days. In addition, liquid zinc was filled in these macro-cracks, causing local stress concentration and promoting the propagation of cracks. In turn, this caused more coating to peel off. A small amount of elements such as Fe in the coating were dissolved by liquid zinc to form porous corrosion products like δ phase (91.3 wt.% Zn, 6.62 wt.% Fe, 0.21 wt.% Al, 0.68 wt.% Ni, 0.61 wt.% Co, and 0.58 wt.% Cr), which can fix peeled coatings to resist the corrosion by liquid zinc. The porosity of the coating near the liquid zinc increased and the microhardness value of the coating decreased. After soaking for 5 days, the speed at which the coating peeled off was accelerated, and more coating fell off and flowed into liquid zinc. The pores of the coating near the liquid zinc continued to increase, and the microhardness value further decreased. After immersing for 10 days, the coating was almost completely eroded by liquid zinc, and the remaining coating was too thin to test its microhardness value, but the substrate was not affected by liquid zinc and remained intact.
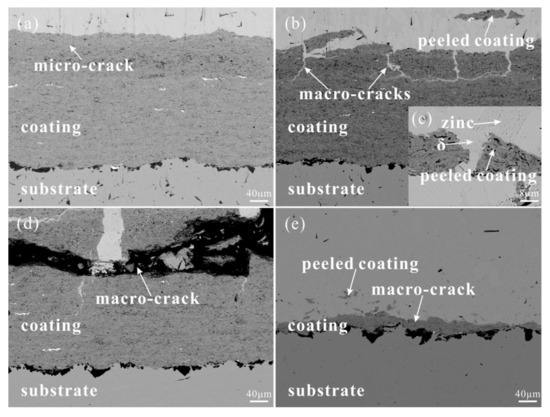
Figure 8.
Cross-section morphologies of the coating after the immersion experiment in liquid zinc at 450 °C for different times (a) 1, (b) 3, (c) 3, (d) 5, and (e) 10 days.
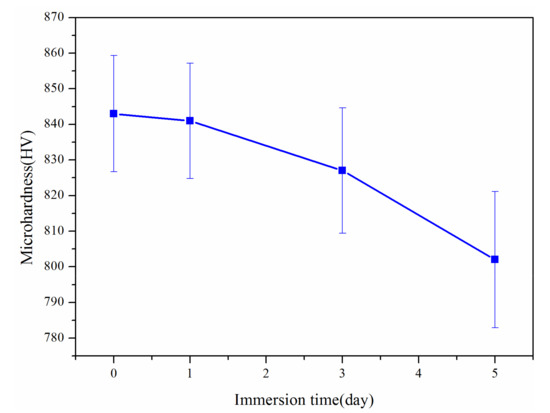
Figure 9.
The relationship between the microhardness of the coating near the liquid zinc and immersion time.
In order to further study the corrosion behavior of the coating in liquid zinc, area scanning analysis was performed of Fe and Zn elements at the corrosion interface between the coating and liquid zinc after 3 days of corrosion, as shown in Figure 10. It can be seen that Fe was mainly distributed in the substrate and the coating, including spalled coatings; however, Zn was mainly distributed in liquid zinc, corrosion products, and macro-cracks. Liquid zinc penetrated into the coating through macro-cracks, and the distribution of Zn in the coating was minimal, indicating that (Fe, Al, Ni, Co, Cr)B and (Fe, Al, Ni, Co, Cr)2B solid solutions were not easily corroded by liquid zinc. Therefore, the failure of the coating in liquid zinc was dominated by the penetration of liquid zinc into macro-cracks, and it was accompanied by the dissolution of elements in the coating by liquid zinc.

Figure 10.
Area scanning analysis of Fe and Zn of the coating immersed in liquid zinc for 3 days.
4. Conclusions
FeB-30 wt.% Al0.25FeNiCoCr coating was prepared by AC-HVAF, which consists of (Fe, Al, Ni, Co, Cr)B and (Fe, Al, Ni, Co, Cr)2B solid solutions. The microhardness values of the coating range from 827 to 859 HV, and the fracture toughness values of the coating range from 5.27 to 5.89 MPa·m1/2. The abrasion wear resistance of the coating is three times higher than that of 316L stainless steel. Compared with the 316L stainless steel substrate, the corrosion resistance of the coating to liquid zinc was significantly enhanced by about five times. The durability of the coating with a thickness of 260 μm in liquid zinc at 450 °C exceeds 10 days. Under the effect of residual stress and thermal stress, micro-cracks form in the coating when the coating is immersed in liquid zinc. With the increase in immersion time, micro-cracks propagate to macro-cracks. A small amount of elements such as Fe in the coating are dissolved by liquid zinc to form porous corrosion products such as δ phase, which increases the porosity of the coating near the liquid zinc and decreases the microhardness value of the coating. Liquid zinc penetrates the coating along cracks, leading to local stress concentration and further propagation of cracks, causing the coating to peel off and fail.
Author Contributions
Conceptualization, B.Y. and X.X.; methodology, B.Y. and X.X.; formal analysis, X.X. and B.Y.; data curation, X.O.; writing—Original draft preparation, X.X.; writing—Review and editing, F.Y. and B.Y.; supervision, B.Y. and F.Y.; project administration, B.Y. and F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Foundation of the China (Nos. 52001268, 51771160, and 11904307), the Scientific Research Fund of Hunan Provincial Educational Department (Nos. 2018JJ4057 and 18C0111), the Natural Science Foundation of Hunan province (Grant No. 2019JJ50576), and the Hunan Postgraduate Research and Innovation Project (No. CX20190478).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, X.; Huang, Y.; Li, Y.; Zhao, L. Production process and technology development of hot-dip galvanizing. Appl. Mech. Mater. 2014, 488, 61–65. [Google Scholar] [CrossRef]
- Cook, T. Hot-dip galvanizing technology. Met. Finish. 2000, 98, 19–28. [Google Scholar] [CrossRef]
- Shibli, S.; Meena, B.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Ren, X.; Mei, X.; She, J.; Ma, J. Materials resistance to liquid zinc corrosion on surface of sink roll. J. Iron Steel Res. Int. 2007, 14, 130–136. [Google Scholar] [CrossRef]
- Bobde, S.; Kshirsagar, S. Improving the sink roll life in galvalume using material AT101 & the various thermal-spray coating on SS3L6L roll surface. Int. J. Soft Comput. Eng. 2013, 3, 282–286. [Google Scholar]
- Lv, H.; Nie, P.; Yan, Y.; Wang, J.; Sun, B. Microstructure and interfacial adhesion of high velocity oxy-fuel-sprayed mob-cocr alloy coating on 316L stainless steel. Surf. Interface Anal. 2009, 41, 725–729. [Google Scholar] [CrossRef]
- Lv, H.; Wang, J.; Yan, Y.; An, Q.; Nie, P.; Sun, B. Characterisation of detonation sprayed Mo–Co–Cr–B alloy coatings. Mater. Sci. Technol. 2010, 26, 950–955. [Google Scholar] [CrossRef]
- López, A.; Rams, J. Protection of carbon steel against molten aluminum attack and high temperature corrosion using high velocity oxygen-fuel WC–Co coatings. Surf. Coat. Technol. 2015, 262, 123–133. [Google Scholar] [CrossRef]
- Mi, P.; Zhao, H.; Wang, T.; Ye, F. Sliding wear behavior of hvof sprayed WC-(Nano-WC–Co) coating at elevated temperatures. Mater. Chem. Phys. 2018, 206, 1–6. [Google Scholar] [CrossRef]
- Tomita, T.; Takatani, Y.; Kobayashi, Y.; Harada, Y.; Nakahira, H. Durability of WC/Co sprayed coatings in molten pure zinc. ISIJ Int. 1993, 33, 982–988. [Google Scholar] [CrossRef]
- Karaoglanli, A.; Oge, M.; Doleker, K.; Hotamis, M. Comparison of tribological properties of HVOF sprayed coatings with different composition. Surf. Coat. Technol. 2017, 318, 299–308. [Google Scholar] [CrossRef]
- Aw, P.; Tan, A.; Tan, T.; Qiu, J. Corrosion resistance of tungsten carbide based cermet coatings deposited by high velocity oxy-fuel spray process. Thin Solid Films 2008, 516, 5710–5715. [Google Scholar] [CrossRef]
- Peat, T.; Galloway, A.; Toumpis, A.; Harvey, D. Evaluation of the synergistic erosion-corrosion behaviour of HVOF thermal spray coatings. Surf. Coat. Technol. 2016, 299, 37–48. [Google Scholar] [CrossRef]
- Wielage, B.; Wank, A.; Pokhmurska, H.; Grund, T.; Rupprecht, C.; Reisel, G.; Friesen, E. Development and trends in HVOF spraying technology. Surf. Coat. Technol. 2006, 201, 2032–2037. [Google Scholar] [CrossRef]
- Tsipas, D.; Triantafyllidis, G.; Kiplagat, J.; Psillaki, P. Degradation behaviour of boronized carbon and high alloy steels in molten aluminium and zinc. Mater. Lett. 1998, 37, 128–131. [Google Scholar] [CrossRef]
- Ozdemir, O.; Usta, M.; Bindal, C.; Ucisik, A. Hard iron boride (Fe2B) on 99.97 wt.% pure iron. Vacuum 2006, 80, 1391–1395. [Google Scholar] [CrossRef]
- Mizuno, H.; Junya, K. MoB/CoCr cermet coatings by HVOF spraying against erosion by molten Al-Zn alloy. J. Ther. Spray Technol. 2007, 16, 404–413. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, G.; Yin, F.; Liu, Y.; Zhao, M. Effect of molybdenum on the microstructures of as-cast Fe-B alloys and their corrosion resistance in liquid zinc. Corrosion 2017, 73, 942–952. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Yin, F.; Ouyang, X.; Li, Z. Effects of tungsten addition on the microstructure and corrosion resistance of Fe-3.5B alloy in liquid zinc. Materials 2017, 10, 399. [Google Scholar] [CrossRef]
- Ye, P.; Yin, F.; Liu, Y.; Ouyang, X.; Xie, X. Corrosion resistance of liquid zinc FeB/Co cermet coating deposited by AC-HVAF. Chin. J. Nonferrous Met. 2018, 28, 782–791. [Google Scholar]
- Fang, Y.; Chen, N.; Du, G.; Zhang, M.; Zhao, X.; Cheng, H.; Wu, J. High-temperature oxidation resistance, mechanical and wear resistance properties of Ti(C,N)-based cermets with Al0.3CoCrFeNi high-entropy alloy as a metal binder. J. Alloy. Comp. 2020, 815, 152486. [Google Scholar] [CrossRef]
- Velo, I.; Gotor, F.; Alcalá, M.; Real, C.; Córdoba, J. Fabrication and characterization of WC-HEA cemented carbide based on the CoCrFeNiMn high entropy alloy. J. Alloy. Comp. 2018, 746, 1–8. [Google Scholar] [CrossRef]
- Fu, Z.; Koc, R. Processing and characterization of TiB2–TiNiFeCrCoAl high-entropy alloy composite. J. Am. Ceram. Soc. 2017, 100, 2803–2813. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Tang, Q.; Wang, W.; Yan, X.; Dai, P. Microstructure and mechanical properties of FeCoCrNiMnAlx high-entropy alloys prepared by mechanical alloying and hot-pressed sintering. J. Alloy. Comp. 2019, 775, 742–751. [Google Scholar] [CrossRef]
- Ye, Q.; Yang, G.; Yang, B. Effect of aging on microstructure and property of AlCoCrFeMo0.05Ni2 high entropy alloy. Mater. Sci. Eng. A 2019, 760, 1–6. [Google Scholar] [CrossRef]
- Tsai, M.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Tang, Z.; Gao, M.; Dahmen, K.; Liaw, P.; Lu, Z. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys (HEAs) and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. Microstructural characteristics and corrosion behavior of HVAF- and HVOF-sprayed Fe-based coatings. Surf. Coat. Technol. 2017, 318, 365–373. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.; Chen, Q.; Yang, Y.; Li, N.; Liu, L. Study of structure and corrosion resistance of Fe-based amorphous coatings prepared by HVAF and HVOF. Corros. Sci. 2011, 253, 2351–2356. [Google Scholar] [CrossRef]
- Bolelli, G.; Berger, L.; Börner, T.; Koivuluoto, H.; Lusvarghi, L.; Lyphout, C.; Markocsan, N.; Matikainen, V.; Nylén, P.; Sassatelli, P.; et al. Tribology of HVOF- and HVAF-sprayed WC–10Co4Cr hardmetal coatings: A comparative assessment. Surf. Coat. Technol. 2015, 265, 125–144. [Google Scholar] [CrossRef]
- Jacobs, L.; Hyland, M.; Bonte, M. Study of the influence of microstructural properties on the sliding-wear behavior of HVOF and HVAF sprayed WC-cermet Coatings. J. Therm. Spray Technol. 1999, 8, 125–132. [Google Scholar] [CrossRef]
- Ikeda, H.; Yanagimoto, K. Surface coating material for liquid zinc bath member, production method thereof, and molten zinc bath member. U.S. Patent 8927111, 6 January 2015. [Google Scholar]
- Xie, J. Study on TiB2-TaC Ceramic Tool Material and Its Oxidation Resistance. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2019. (In Chinese). [Google Scholar]
- Ma, S.; Xing, J.; Fu, H.; He, Y.; Yu, B.; Li, Y.; Bai, Y. Interface characteristics and corrosion behaviour of oriented bulk Fe2B alloy in liquid zinc. Corros. Sci. 2014, 78, 71–80. [Google Scholar] [CrossRef]
- Allaoui, O.; Bouaouadja, N.; Saindernan, G. Characterization of boronized layers on a XC38 steel. Surf. Coat. Technol. 2006, 201, 3475–3482. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Liu, X.; Barbero, E.; Xu, J.; Burris, M.; Chang, K.; Sikka, V. Liquid metal corrosion of 316L, Fe3Al, and FeCrSi in molten Zn–Al baths. Metall. Mater. Trans. A 2005, 36, 2049–2058. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).