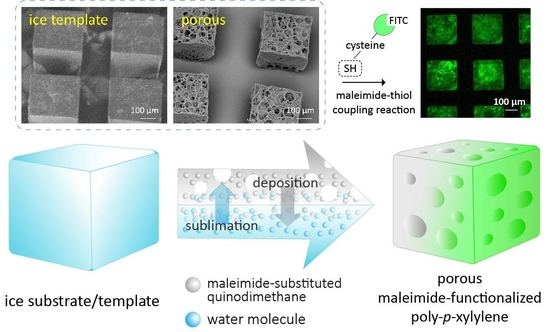
Vapor-Phase Fabrication of a Maleimide-Functionalized Poly-p-xylylene with a Three-Dimensional Structure
Abstract
1. Introduction
2. Materials and Methods
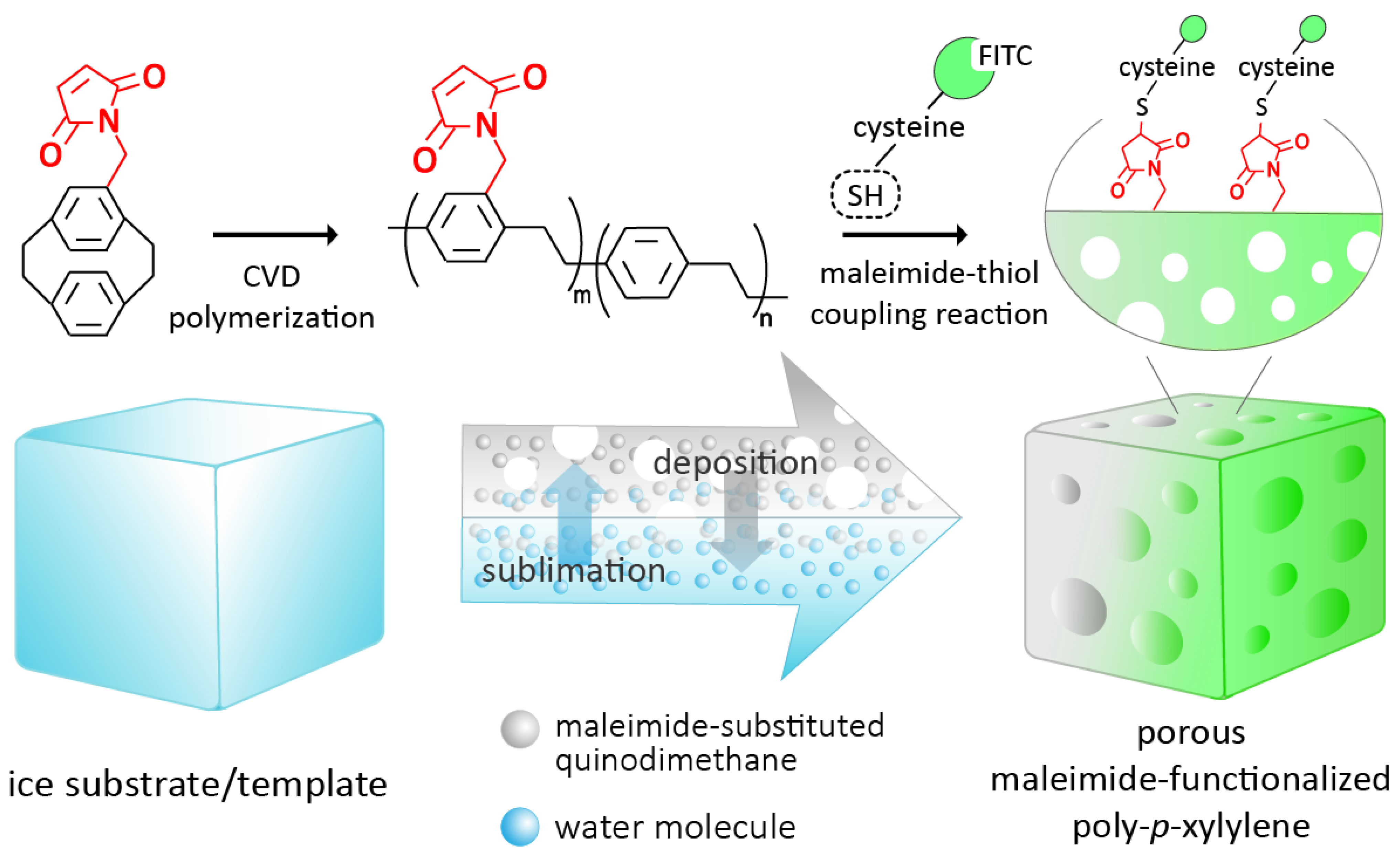
2.1. Fabrication Process
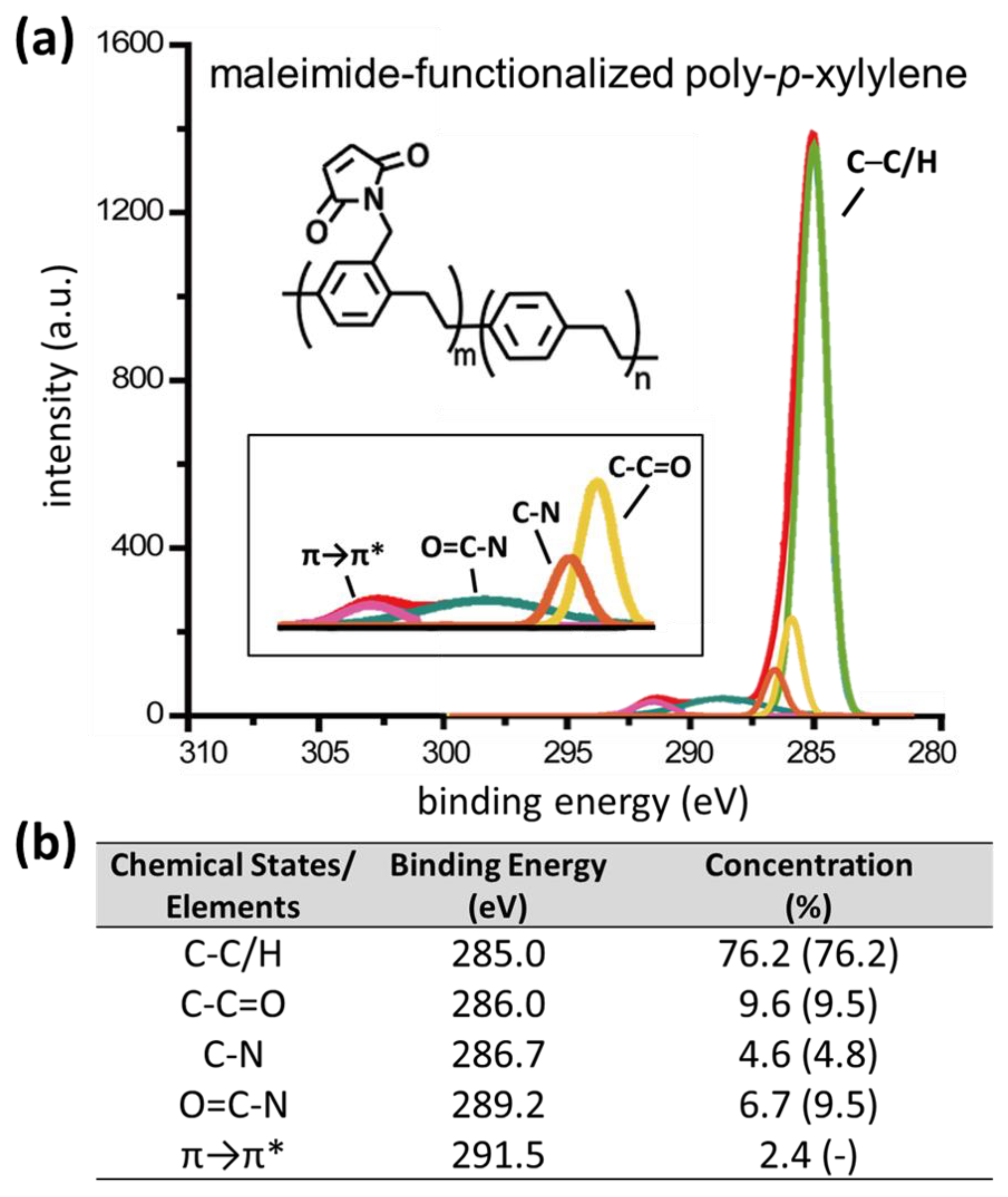
2.2. Characterizations
2.3. Conjugations
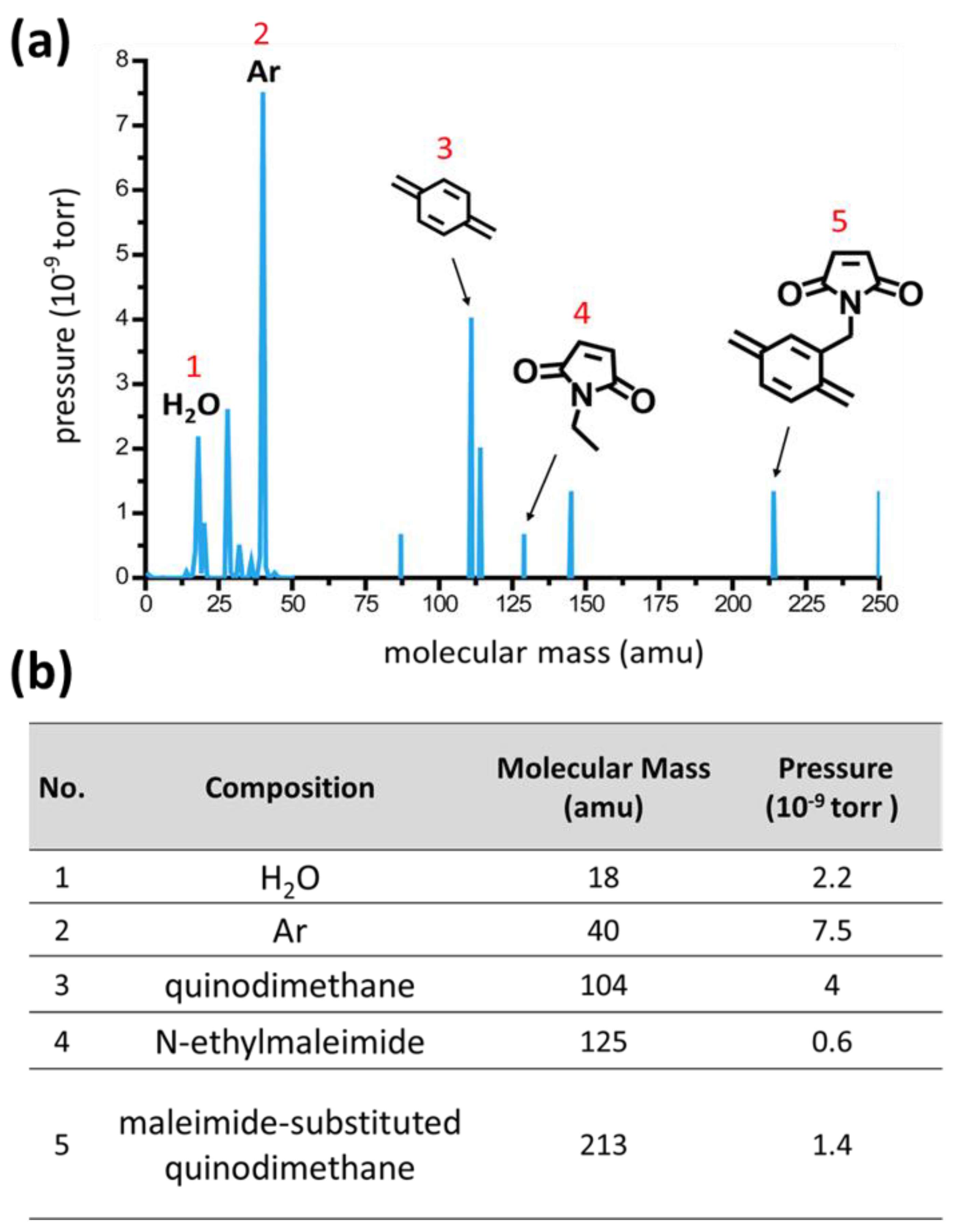
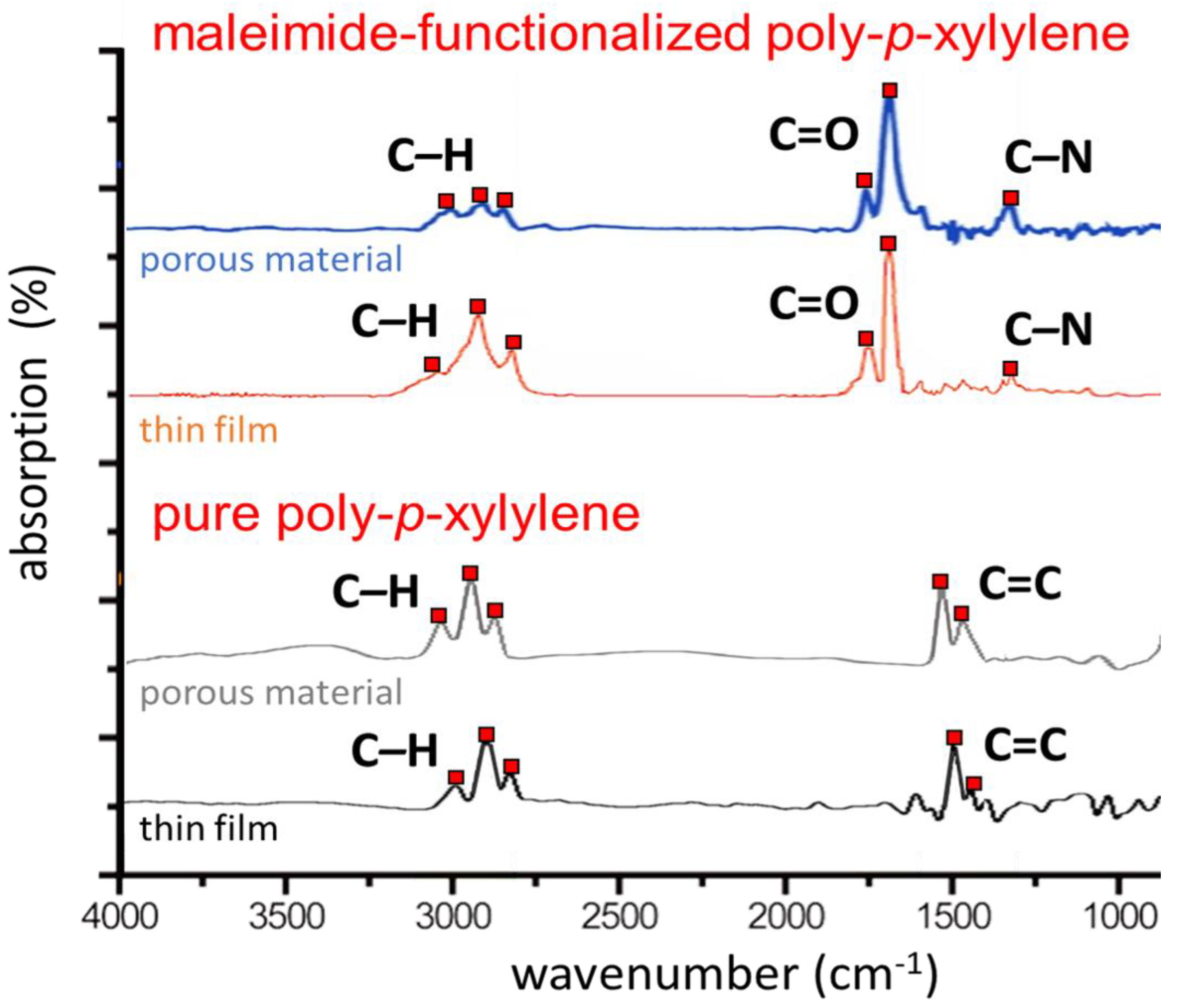
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, G.S.; Yoon, S.B.; Yu, J.-S.; Choi, J.-H.; Sung, Y.-E. Ordered Porous Carbons with Tunable Pore Sizes as Catalyst Supports in Direct Methanol Fuel Cell. J. Phys. Chem. B 2004, 108, 7074–7079. [Google Scholar] [CrossRef]
- Kosuge, K.; Kubo, S.; Kikukawa, N.; Takemori, M. Effect of Pore Structure in Mesoporous Silicas on VOC Dynamic Adsorption/Desorption Performance. Langmuir 2007, 23, 3095–3102. [Google Scholar] [CrossRef]
- Li, Y.Y.; Cunin, F.; Link, J.R.; Gao, T.; Betts, R.E.; Reiver, S.H.; Chin, V.; Bhatia, S.N.; Sailor, M.J. Polymer Replicas of Photonic Porous Silicon for Sensing and Drug Delivery Applications. Science 2003, 299, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas Sensing Using Porous Materials for Automotive Applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C. Porous Metal–Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Santos, H.A.; Mäkilä, E.; Airaksinen, A.J.; Bimbo, L.M.; Hirvonen, J. Porous Silicon Nanoparticles for Nanomedicine: Preparation and Biomedical Applications. Nanomedicine 2014, 9, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Yan, L.; Wang, G.; Liu, A. Recent Advances in the Synthesis of Spherical and Nanomof-Derived Multifunctional Porous Carbon for Nanomedicine Applications. Coord. Chem. Rev. 2019, 391, 69–89. [Google Scholar] [CrossRef]
- Pounder, R.J.; Stanford, M.J.; Brooks, P.; Richards, S.P.; Dove, A.P. Metal Free Thiol–Maleimide ‘Click’ Reaction as a Mild Functionalisation Strategy for Degradable Polymers. Chem. Commun. 2008, 5158–5160. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click Chemistry Reactions in Medicinal Chemistry: Applications of the 1, 3-dipolar Cycloaddition Between Azides and Alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Best, M.D. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry 2009, 48, 6571–6584. [Google Scholar] [CrossRef]
- Ciampi, S.; Böcking, T.; Kilian, K.A.; Harper, J.B.; Gooding, J.J. Click Chemistry in Mesoporous Materials: Functionalization of Porous Silicon Rugate Filters. Langmuir 2008, 24, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click Chemistry in Materials Science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- L’abbé, G. Decomposition and Addition Reactions of Organic Azides. Chem. Rev. 1969, 69, 345–363. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lahann, J. Designable Biointerfaces Using Vapor-Based Reactive Polymers. Langmuir 2010, 27, 34–48. [Google Scholar] [CrossRef]
- Tung, H.-Y.; Guan, Z.-Y.; Liu, T.-Y.; Chen, H.-Y. Vapor Sublimation and Deposition to Build Porous Particles and Composites. Nat. Commun. 2018, 9, 2564. [Google Scholar] [CrossRef]
- Tung, H.-Y.; Sun, T.-P.; Sun, H.-Y.; Guan, Z.-Y.; Hu, S.-K.; Chao, L.; Chen, H.-Y. Construction and Control of 3D Porous Structure Based on Vapor Deposition on Sublimation Solids. Appl. Mater. Today 2017, 7, 77–81. [Google Scholar] [CrossRef]
- Lin, C.-W.; Guan, Z.-Y.; Lu, M.; Wu, T.-Y.; Cheng, N.-C.; Chen, H.-Y.; Yu, J. Synergistically Enhanced Wound Healing of a Vapor-Constructed Porous Scaffold. ACS Appl. Bio Mater. 2020, 3, 5678–5686. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Guo, C.-L.; Yang, Y.-C.; Huang, C.-W.; Zeng, J.-Y.; Guan, Z.-Y.; Chiang, Y.-C.; Wang, P.-Y.; Chen, H.-Y. Parylene-Based Porous Scaffold with Functionalized Encapsulation of Platelet-Rich Plasma and Living Stem Cells for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 7193–7201. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Liu, H.-Y.; Huang, C.-W.; Yeh, S.-Y.; Cheng, N.-C.; Ding, S.-T.; Chen, H.-Y. Synergistically Controlled Stemness and Multilineage Differentiation Capacity of Stem Cells on Multifunctional Biointerfaces. Adv. Mater. Interfaces 2017. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chang, C.-W.; Yuan, R.-H.; Chiang, Y.-C.; Chen, J.-T.; Kang, D.-Y.; Chen, H.-Y. Multifunctional Nanoparticles with Controllable Dimensions and Tripled Orthogonal Reactivity. Nanoscale 2017, 9, 14787–14791. [Google Scholar] [CrossRef]
- Guan, Z.-Y.; Wu, C.-Y.; Chen, H.-Y. Stepwise and Programmable Cell Differentiation Pathways of Controlled Functional Biointerfaces. Acs Biomater. Sci. Eng. 2017, 3, 1815–1821. [Google Scholar] [CrossRef]
- Chen, H.-Y. Micro- and Nano-Surface Structures Based on Vapor-Deposited Polymers. Beilstein J. Nanotechnol. 2017, 8, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Huang, C.-W.; Guan, Z.-Y.; Wu, J.-T.; Yeh, S.-Y.; Su, C.-T.; Chang, C.-H.; Ding, S.-T.; Chen, H.-Y. Vapor-based Coatings for Antibacterial and Osteogenic Functionalization and the Immunological Compatibility. Mater. Sci. Eng. C 2016, 69, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-P.; Tai, C.-H.; Wu, J.-T.; Wu, C.-Y.; Liang, W.-C.; Chen, H.-Y. Multifaceted and Route-Controlled "Click" Reactions based on Vapor-Deposited Coatings. Biomater. Sci. 2016, 4, 265–271. [Google Scholar] [CrossRef]
- Guan, Z.-Y.; Wu, C.-Y.; Wu, J.-T.; Tai, C.-H.; Yu, J.; Chen, H.-Y. Multifunctional and Continuous Gradients of Biointerfaces Based on Dual Reverse Click Reactions. ACS Appl. Mater. Interfaces 2016, 8, 13812–13818. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Chen, Y.-C.; Lin, T.-J.; Hsu, Y.-C.; Lin, C.-Y.; Yuan, R.-H.; Yu, J.; Teng, M.-S.; Hirtz, M.; Chen, M.H.-C.; et al. Vapor-Based Multicomponent Coatings for Antifouling and Biofunctional Synergic Modifications. Adv. Funct. Mater. 2014, 24, 2281–2287. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Fang, C.-Y.; Lin, T.-J.; Chen, Y.-C.; Lin, C.-Y.; Ho, H.-Y.; Chen, M.H.C.; Yu, J.; Lee, D.-J.; Chang, C.-H.; et al. Thiol-Reactive Parylenes as a Robust Coating for Biomedical Materials. Adv. Mater. Interfaces 2014, 1, 1400093. [Google Scholar] [CrossRef]
- Su, C.-T.; Yuan, R.-H.; Chen, Y.-C.; Lin, T.-J.; Chien, H.-W.; Hsieh, C.-C.; Tsai, W.-B.; Chang, C.-H.; Chen, H.-Y. A Facile Approach Toward Protein-Resistant Biointerfaces Based on Photodefinable Poly-P-Xylylene Coating. Colloids Surf. B Biointerfaces 2014, 116, 727–733. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lin, T.-J.; Tsai, M.-Y.; Su, C.-T.; Yuan, R.-H.; Hsieh, C.-C.; Yang, Y.-J.; Hsu, C.-C.; Hsiao, H.-M.; Hsu, Y.-C. Vapor-Based Tri-Functional Coatings. Chem. Commun. 2013, 49, 4531–4533. [Google Scholar] [CrossRef]
- Wu, M.-G.; Hsu, H.-L.; Hsiao, K.-W.; Hsieh, C.-C.; Chen, H.-Y. Vapor-Deposited Parylene Photoresist: A Multipotent Approach toward Chemically and Topographically Defined Biointerfaces. Langmuir 2012, 28, 14313–14322. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-T.; Huang, C.-H.; Liang, W.-C.; Wu, Y.-L.; Yu, J.; Chen, H.-Y. Reactive Polymer Coatings: A General Route to Thiol-ene and Thiol-yne Click Reactions. Macromol. Rapid Commun. 2012, 33, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Lin, C.-Y.; Huang, C.-H.; Gu, J.-A.; Huang, S.-T.; Yu, J.; Chen, H.-Y. Vapor-based Synthesis of Maleimide-Functionalized Coating for Biointerface Engineering. Chem. Commun. 2012, 48, 10969–10971. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lahann, J. Vapor-Assisted Micropatterning in Replica Structures: A Solventless Approach towards Topologically and Chemically Designable Surfaces. Adv. Mater. 2007, 19, 3801–3808. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Wu, C.-Y.; Guan, Z.-Y.; Sun, H.-Y.; Cheng, N.-C.; Yeh, S.-Y.; Chen, H.-Y. Topologically Controlled Cell Differentiation Based on Vapor-Deposited Polymer Coatings. Langmuir 2017. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-Y.; Wu, C.-Y.; Chen, T.-Y.; Huang, S.-T.; Chiang, Y.-C.; Chen, H.-Y. Clickable and Photo-Erasable Surface Functionalities by Using Vapor-Deposited Polymer Coatings. ACS Biomater. Sci. Eng. 2019, 5, 1753–1761. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Sun, T.-P.; Su, C.-T.; Wu, J.-T.; Lin, C.-Y.; Yu, J.; Huang, C.-W.; Chen, C.-J.; Chen, H.-Y. Sustained Immobilization of Growth Factor Proteins Based on Functionalized Parylenes. ACS Appl. Mater. Interfaces 2014, 6, 21906–21910. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Langer, R. Novel Poly(p-xylylenes): Thin Films with Tailored Chemical and Optical Properties. Macromolecules 2002, 35, 4380–4386. [Google Scholar] [CrossRef]
- Deng, X.; Friedmann, C.; Lahann, J. Bio-orthogonal “Double-Click” Chemistry Based on Multifunctional Coatings. Angew. Chem. Int. Ed. 2011, 50, 6522–6526. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-Y.; Huang, C.-W.; Huang, M.-C.; Wu, C.-Y.; Liu, H.-Y.; Ding, S.-T.; Chen, H.-Y. Controlling Multi-Function of Biomaterials Interfaces Based on Multiple and Competing Adsorption of Functional Proteins. Colloids Surf. B Biointerfaces 2017, 149, 130–137. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-M.; Lee, C.-Y.; Chang, Y.-M.; Xiao, J.-Q.; Kusanagi, T.; Wu, T.-Y.; Chang, N.-Y.; Christy, J.; Chiu, Y.-R.; Huang, C.-W.; et al. Vapor-Phase Fabrication of a Maleimide-Functionalized Poly-p-xylylene with a Three-Dimensional Structure. Coatings 2021, 11, 466. https://doi.org/10.3390/coatings11040466
Hu S-M, Lee C-Y, Chang Y-M, Xiao J-Q, Kusanagi T, Wu T-Y, Chang N-Y, Christy J, Chiu Y-R, Huang C-W, et al. Vapor-Phase Fabrication of a Maleimide-Functionalized Poly-p-xylylene with a Three-Dimensional Structure. Coatings. 2021; 11(4):466. https://doi.org/10.3390/coatings11040466
Chicago/Turabian StyleHu, Shu-Man, Chin-Yun Lee, Yu-Ming Chang, Jia-Qi Xiao, Tatsuya Kusanagi, Ting-Ying Wu, Nai-Yun Chang, Jane Christy, Ya-Ru Chiu, Chao-Wei Huang, and et al. 2021. "Vapor-Phase Fabrication of a Maleimide-Functionalized Poly-p-xylylene with a Three-Dimensional Structure" Coatings 11, no. 4: 466. https://doi.org/10.3390/coatings11040466
APA StyleHu, S.-M., Lee, C.-Y., Chang, Y.-M., Xiao, J.-Q., Kusanagi, T., Wu, T.-Y., Chang, N.-Y., Christy, J., Chiu, Y.-R., Huang, C.-W., Yang, Y.-C., Chiang, Y.-C., & Chen, H.-Y. (2021). Vapor-Phase Fabrication of a Maleimide-Functionalized Poly-p-xylylene with a Three-Dimensional Structure. Coatings, 11(4), 466. https://doi.org/10.3390/coatings11040466