Abstract
The water is a vital compound for all known forms of life, but it can also cause detrimental consequences to our daily routine if by natural means becomes pathogenic bacterial carrier or transforms into ice. Imaginative by necessity, the surrounding environment has stimulated the mankind to emulate natural-design solutions and invent the so-called super-nonwettable coatings. Undisputedly, these coatings have revolutionized the modern industry by providing “a vehicle” for potential eco-friendly water purification, passive icing protection, suppression of the solid surface-associated spreading of bacterial infections and enhanced cryopreservation of living matter. Regrettably, the wide domestic use of liquid impermeable coatings (surfaces) is yet limited, since the current market trends impose the possession of fabrication scalability and multifunctionality, which is not covered by most of the available non-wettable products. This viewpoint article intends to outline the most significant scientific achievements within the past five years related to the release of anti-wetting coatings with multiple applications. Design and performance efficiencies in light of the physical chemistry of the surface are demonstrated, emphasizing on the likelihood of integrating icephobicity and anti-biofouling capacity within a single interfacial nanostructure.
1. Introduction
For decades, the perfect symbiosis among the physical, chemical and biological processes in our ecosystem has served as a driving force to the human race for life-changing scientific inventions and technological progress [1]. An instance of classy biomimicry is the currently intensive fabrication of liquid-repellent coatings, directly provoked by the eternally dry and clean leaves of the sacred Lotus [2].
The aim of endowing a surface-of-interest with exceptional resistance to wetting is dictated by the necessity of minimizing the solid-liquid contact area and promoting the creation of self-cleaning and drag-reducing surfaces, passive anti-icing systems, anti-microbial and anti-corrosive coatings, enhanced pervaporative separation membranes, water purification devices, biological sensors for human semen quality analysis or new technologies for cryopreservation of living matter [3,4,5]. For instance, it is crucially important to keep different industrial facilities such as high-voltage power lines or telecommunication towers free of ice/snow, since the ice/snow accretion may block their operability and thus the people’s access to electricity, telecommunications and internet [6,7]. In parallel, up to 26% of all hospital-associated infections occur via biofouling of the medical equipment, which can be alleviated using coatings with custom surface topographies [8,9]. However, the increasing demand of economic profitability and scalability of a given commercial anti-wetting coating implies that the latter needs to be flexible and combine several functionalities. Therefore, the global scientific community has steadily shifted its recent efforts towards designing multifunctional super-nonwettable coatings [10,11,12].
The inherent meaning of “multifunctional” implies simultaneous possession of more than two relevant properties with different physicochemical origin (e.g., icephobicity, anti-bioadhesiveness, self-healing, etc.). Regrettably, in many cases this expression is somehow inappropriately used to take into account the self-cleaning and anti-corrosion capabilities of the surface [13,14,15]. Its non-wetting state is interrelated to the above-mentioned features, since the weak interfacial attraction forces lead to liquid droplet contact angle exceeding 150°, contact angle hysteresis below 5° and rolling angle less than 10°, converting the substrate to quasifrictionless and forming a slippery interface [16,17,18,19,20]. Despite being dually roughened, hierarchical or composed of randomly distributed micro- nanoparticles, every super-nonwettable surface is expected to exhibit self-cleaning and to a certain extent anti-corrosion performance [14]. In contrast, and although the suppression of the ice nucleation and biofilm spreading could be governed by the degree of water repellency [21,22], the contrivance of anti-icing and anti-microbial coatings is not a straightforward procedure and requires careful consideration of some additional parameters [4,23].
The idea of this viewpoint article is to serve as a useful information platform for those scholars working in the field of wetting phenomena, whose research is dedicated to the fabrication of multifunctional non-wettable coatings. Thus, the discussion is focused on the main principles and difficulties related to the design of universal and versatile liquid-repellent interfaces, considering the findings described in specially-selected scientific publications reporting multifarious surface properties. A few hypotheses concerning the future incorporation of icephobic and anti-bioadhesive characteristics within a single functional coating are presented too in the context of well-established physicochemical mechanisms.
2. Importance of the Physicochemical Profile
A significant factor contributing to the development of diversified non-wettable thin films is the choice of appropriate chemicals and roughness modification protocols, since the appearance of hydrophobic micro-nanoprotrusions reduces the interfacial contact, leading to weaker solid-liquid attraction per unit area (compared to a flat surface with the same non-polar chemistry) and amplification of the hydrophobic effect [16]. While combining non-polar chemistry and micro-nanoscale surface asperities is suitable for anti-wetting applications [16], the assignment of fluorine-containing compounds may lead to superamphiphobicity (repelling both water and oil) [24], impeding the oil filtration efficiency of the interface. Therefore, many researchers prefer to employ fluorine-free strategies for surface patterning, because they ensure non-wettability to a variety of liquids such as water, glycerol, ethylene glycol, blood, tea, juice, milk, etc., but the surface is still wettable by oils (decisive when developing oil-water separation membranes) and the fabrication is environmentally benign [10,11,12,25,26,27,28]. Commonly used chemicals are carboxymethyl cellulose (CMC), hydroxyapatite nanowires (HAP), ZnO nanoparticles [10], fly ash [12,26], dymethyloctadecyl ammonium chloride [7], TiO2-PDMS [12,28], tetrabutyl-titanate-dymethylformamide-acetic acid-n Octyltrietoxysilane [25] or tetraethoxysilane hexamethyldisilazane [27], whose different combinations may endow superior mechanical durability of the coated surface and “a bouquet” of functionalities.
Embedding fly ash or activated carbon in the matrix of TiO2-PDMS is demonstrated to render self-healing, oil-water separation, UV protective, photocatalytic and even flame-retardant properties of the coatings [12,28], shown concisely in Figure 1 and Figure 2, which is a good alternative to the more sophisticated CuFe2O4-TiO2 nanocomposite supported reduced graphene oxide photocatalysts [29]. It is argued that the self-healing ability is due to self-migration of PDMS molecules towards the surface by heating, leading to recovered superhydrophobicity [28]. In comparison, the flame-retardancy is attributed to the incombustibility of the fly ash and its covalent bonding to the TiO2-PDMS [12].
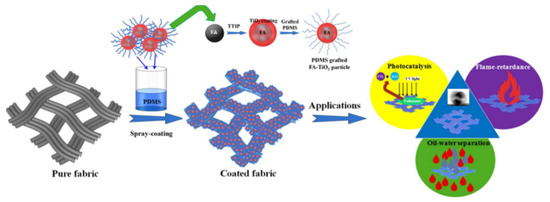
Figure 1.
Multifunctional properties of a fly ash-TiO2-PDMS spray coated superhydrophobic fiber. Reproduced with permission from [12]. Copyright 2020 Elsevier.
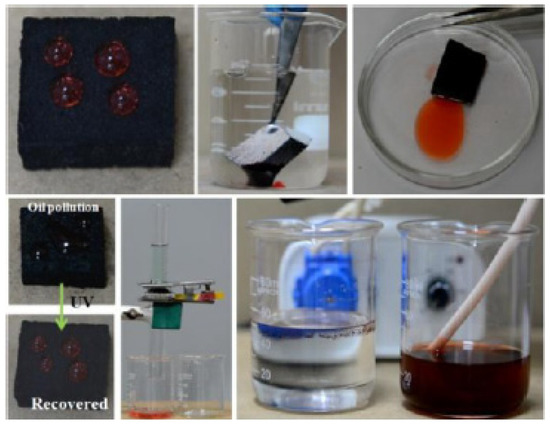
Figure 2.
Illustration of the applicability of an activated carbon-TiO2-PDMS coated superhydrophobic sponge. Reproduced with permission from [28]. Copyright 2020 ACS Publications.
On the other hand, the resistivity to burning and the self-healing capacity can be gained by adding inherently fire-resistant hydroxyapatite and thermally stable ZnO, which also yields a bactericidal effect of the solid surface [10], revealed in Figure 3.
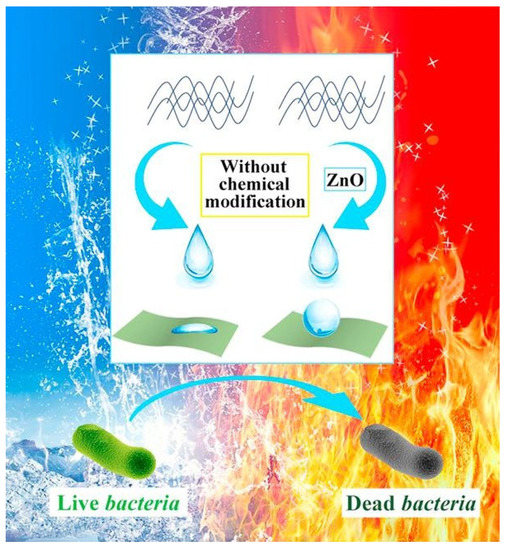
Figure 3.
Bactericidal effect of modifier-free superhydrophobic and flame-resistant paper. Reproduced with permission from [10]. Copyright 2018 Elsevier.
The surface porosity, morphology and topography are another three parameters of practical significance in terms of the anti-microbial, oil- and sound absorption effectiveness of the interface [27,30]. An innovative technique of adjusting them is via compression and strict monitoring of the curing temperature, since in such a way the object’s thickness and particles’ size are accurately controlled [27]. Namely, Figure 4 depicts how a superhydrophobic latex sponge is fabricated by immersing it into a preliminary prepared colloidal silica sol composed of tetraethoxysilane, hexamethyldisilazane, ethanol and de-ionized water, further homogeneously mixed with epoxy resin and polyamide by stirring. The sponge is then dried in air for 24 h and during this procedure, the samples could be free-standing or strongly bonded to the substrate depending on whether the specimens are flipped or no [27]. Upon compression, a sheet-like superhydrophobic material with desired thickness might be obtained. Meanwhile, if epoxy spherical structures with dimensions exceeding 5 μm are required, the curing temperature should be 15–25 °C, while at 60 °C, the size decreases below 2 μm. The authors explain these observations with the epoxy resin’s solubility, which enhances at high temperatures, inducing shrinking of the gel [27]. Moreover, as described elsewhere [25], the hot pressing of cotton fabrics at 200 °C leads to an UV protection factor of 45 (10 times higher than pristine cotton fabrics) owing to the undergone crystal phase transition. Furthermore, tuning the quantity of the chemical components in any coating would reflect on the corresponding wettability, filtration characteristics and UV protection, based on the observations in ref. [10]. The excessive concentration of ZnO, for example, increases the number of hydrophilic surface sites and the superhydrophobicity and UV resistivity are degraded due to the formation of oxygen vacancies resulting from electron-hole pairs generation and reaction of the holes with the oxygen sites [10], which may trigger the absorption of UV-light-forming excitons [31].
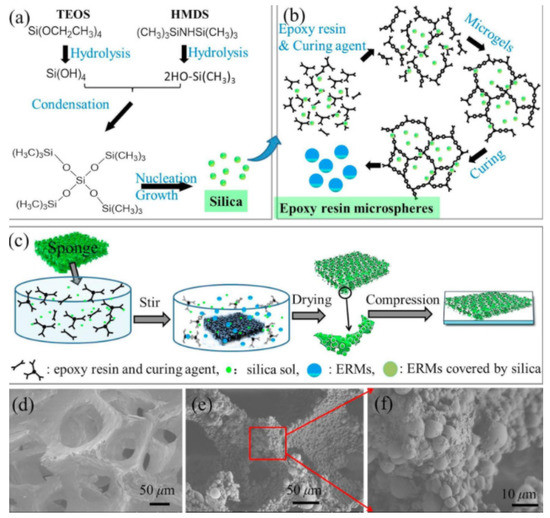
Figure 4.
Formation mechanism, synthesis and morphology of water-repellent latex sponges. (a) Speculated formation pathway of silica. (b) Speculated formation pathway of ERMs. (c) Schematic illustration of the one-pot synthesis procedure of ERMs@Latex-T. SEM images of latex porous structures before (d) and after (e) ERMs adhering. (f) The high magnification image of (e). The reaction temperature of the sample was 25 °C. Reproduced with permission from [27]. Copyright 2019 Elsevier.
Altogether, to engineer liquid-repellent materials manifesting contemporaneously more than two functional properties, one needs to ensure that the synthesis method will provide a coating with non-polar chemical composition and micro-nanoscale surface roughness, thus, amplifying the effect of hydrophobicity and minimizing the solid-liquid interactions [16]. In the meantime, the interface has to maintain photocatalytic activity upon UV irradiation (e.g., for decomposing the absorbed organic pollutants) or self-replenishment of the lost/disintegrated hydrophobic molecules for sustainable long-term non-wettability. The current state-of-the-art in the field proposes TiO2 and PDMS as suitable compounds fulfilling the above requirements [12,28], however, it will be wise to look for existing alternatives including silver gallium sulfide (AgGaS2) and zinc sulfide (ZnS) due to the proven bactericidal behavior of Ag and Zn. Besides, it is pertinent to attentively consider the scalability of the experimental approach, since the industrial physicochemical processing must be feasible at large scales, irrelevantly of the complexity and geometry of the solid substrate. Nowadays, the combustion flame synthesis, dip coating and spray deposition are three prominent techniques with undoubtful fabrication scalability [11,12,25,26,27,28,32].
3. Icephobicity and Anti-Bioadhesiveness, Are They Unifiable?
At present, and despite the exponential progress of nanotechnologies, the scientific literature seems to suffers from the profound lack of even speculative assumptions on how to design super-nonwettable surfaces, possibly unifying anti-icing and anti-microbial capabilities. On one hand, there is substantial disagreement within the scientific community about the similarities between water and ice repellency, mainly because of the complexity of the heterogeneous ice nucleation, involving the impact of the surface morphology/topography, porosity, roughness, air cushion (plastron) distribution, degree of non-wettability and film thickness [4,21,33]. At the same time, the development of interfaces non-stickable to living biomass (bacteria, fungi, algae, plants, biomolecules, etc.) is very challenging. It depends on the type of microorganisms, their size, shape and cell walls’ charge, as well as on the optimal nanopillar radius, spacing and length determining the cell’s binding affinity and eventual mechanical rupture [30,34,35]. Interestingly, the super-nonwettable coatings may mitigate the icing by reducing the heat transfer rate and weakening the ice adhesion strength (if the phase transition occurs) as a consequence of the negligible solid fraction in contact with the freezing water and the availability of trapped air gaps [4]. Instead, the biomass attachments are suppressed by increasing the substrate-to-foulant separation distance and the kinetic threshold for bioadhesion [36] or rupturing the cells through the tensile stress exerted by the nanopillars [35]. In other words, these are implicitly distinct mechanisms, whose intersection is unclear so far and the author will try to briefly hypothesize on the integration of icephobicity and anti-bioadhesiveness in an individual coating.
One of the rare cases where the aforementioned anti-icing and anti-microbial pathways are noticed independently of one another refers to the carbon soot, as illustrated in Figure 5 and Figure 6.
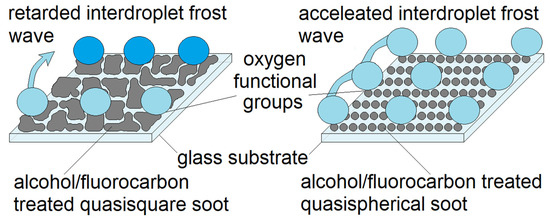
Figure 5.
Delaying the interdroplet ice bridging using super-nonwettable carbon soot coatings. The opaque blue and sky blue droplets represent, the frozen and unfrozen subcooled condensates, respectively. The quasisquares (left side) and spheres (right side) denote the surface morphology of the soot. The lines below the frozen droplets aim to depict the oxygen functional groups serving as ice nucleation (active) sites. The abundance of active sites on the quasispherical soot (right side) accelerates the freezing events and the velocity of the interdroplet freezing [37].
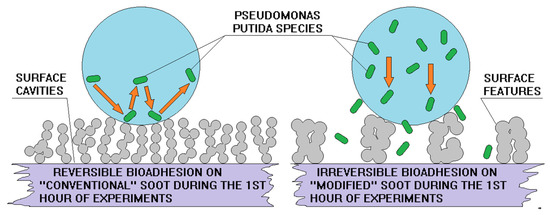
Figure 6.
Reversible (left part) and irreversible (right part) bioadhesion on super-nonwettable carbon soot coatings. Reproduced with permission from [24]. Copyright 2020 Elsevier.
Some newest studies divulge that the incipiency of ice nuclei on the soot is regulated by the amount and spatial arrangement of its oxygen active sites serving as nucleation centers that regulate the hydrogen bonding with the surrounding water vapor and the subsequent interdroplet frost wave [37]. It is found that the degree of soot oxidation and the space between the individual active sites can be successfully manipulated via alcohol modifications causing retardation or acceleration of the freezing front velocity regardless of the nanoparticles’ size and shape (i.e., the surface morphology) [37]. However, if the surface roughness curvature (the nanocavities associated with the roughening) is closer or larger than the critical nucleus’ radius, the thermodynamic barrier for heterogeneous nucleation might still be decreased despite the material’s weak oxidation [21]. Conversely, the bacterial accumulation on sooted substrates is highly sensitive to the soot particle/aggregate morphology and if the dimensions of the surface protrusions and the distance among them are commensurable with the length scale of the adhering living matter, the solid-cell contact area increases, morphologically-triggered partial wetting occurs and the cells binding becomes irreversible [30,34]. Obviously, at a first glance, the icephobicity and the anti-bioadhesiveness are functional features with no identifiable abutment, so it is quite provocative and complicated to invent an interfacial coating with synergistic ice repellent and anti-bioadhesion performance.
Providing a more general view, because this is the primary objective herein, a universal surface (coating) accommodating the above properties may have to consist of solid particles with as small as possible size (nanometer scale) in order to increase the Gibbs thermodynamic free energy barrier and limit the locally-wetted solid fractions. Hence, the liquid-solid phase transitions would be delayed up to a few hours [38] and if immersed in biocontaminated solutions, the surface could stay free of attached biomass [30,34]. Other mandatory requirement might be to set an aggregate-to-aggregate (pillar-to-pillar) distance lower than the size of the biospecies, but large enough to ensure the appearance of steady air gaps (i.e., plastron), enhancing the thermal resistance of the material (slowing the heat transfer rate) and guaranteeing sufficient tensile stress experienced by the colonizing microorganisms, as a result of the sagging among the protuberances, and their eventual physical damage [35]. Finally, avoiding the presence of oxygen functional groups on the surface is highly beneficial for preventing the hydrogen bonds formation and canceling the chemistry-mediated freezing events [37].
Quantitatively, suppose the target is to inhibit the nascency of ice embryos at negative temperatures and simultaneously regulating the spontaneous adherence of Gram-positive (e.g., Escherichia coli) or Gram-Negative (e.g., Pseudomonas putida) bacterial strains. In that case, and taking into account the size of the strains [34], it would hypothetically be wise to employ a laboratory patterning technique allowing the production of pillars with length and diameter less than 800 nm and 500 nm, accordingly, and a pillar pitch of approximately 900–1500 nm. One of the best ways of accomplishing such a task is via photolithography, “where a layer is illuminated through a patterned mask”, facilitating the preparation of well-defined surfaces in multiple identical copies [16]. Of course, this is a costly method, so the templating or chemical etching are relatively more cost-effective options [16], additionally to the adjustment of the physicochemical features by immersion in short alkyl chain alcohols and fluorocarbon emulsions, as recently reported for carbon soot and wax-based coatings [37,39]. Finally, and since the soot is a graphite-like material [32,40], the above hypothetical considerations might be applicable also to graphene and related materials, so future research efforts in such a direction seem reasonable.
4. Conclusions and Outlook
The launch of reliable and multifunctional non-wettable materials is shown to go beyond the cliché of applying low surface energy chemical treatment to highly roughened solid substrates. Finding a subtle balance among the particles’ morphology, surface topography, porosity and ratio of the chemical reagents during the synthesis procedure is the key for supporting self-replenishing, chemically non-polar interfaces, successfully absorbing and decomposing hazardous organic oil wastes, while exhibiting UV shielding, fire resistivity and anti-microbial behavior. A synergistic anti-icing and anti-bioadhesive performance is speculated to be achievable if special attention is devoted to the opportunities of optimizing the geometrical distribution of the nanopillars making up the surface, because of the necessity of having sustainable air cushion for optimal heat transfer reduction and pillar-induced tensile stress that breaks the cell membrane and kills the attached biospecies. Enriching the knowledge in surface sciences and advancing the development of mechanically robust and broadly applicable liquid impervious surfaces, in author’s opinion, goes hand-in-hand with elucidating the mixed impact of the collective physicochemical components; an aspect scantily addressed in the scientific literature up to now.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rinaldi, A. Naturally better. Science and technology are looking to nature’s successful design for inspiration. EMBO Rep. 2007, 8, 995–999. [Google Scholar] [CrossRef]
- Lathe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of Lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef]
- Valipour, M.N.; Birjandi, F.C.; Sargolzaei, J. Super-non-wettable surfaces: A review. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 93–106. [Google Scholar] [CrossRef]
- Esmeryan, K.D. From extremely water-repellent coatings to passive icing protection—Principles, limitations and innovative application aspects. Coatings 2020, 10, 66. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Lazarov, Y.; Stamenov, G.S.; Chaushev, T.A. When condensed matter physics meets biology: Does superhydrophobicity benefiting the cryopreservation of human spermatozoa? Cryobiology 2020, 92, 263–266. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lv, X.-F.; Zhao, X.-T. Overhead transmission lines deicing under different incentive displacement. J. Appl. Mater. 2014, 2014, 872198. [Google Scholar] [CrossRef]
- Mulherin, N.D. Atmospheric icing and communication tower failure in the United States. Cold Reg. Sci. Technol. 1998, 27, 91–104. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef]
- Wen, G.; Gao, X.; Tian, P.; Zhong, L.; Wang, Z.; Guo, Z. Modifier-free fabrication of durable and multifunctional superhydrophobic paper with thermostability and anti-microbial property. Chem. Eng. J. 2018, 346, 94–103. [Google Scholar] [CrossRef]
- Wang, F.; Pi, J.; Song, F.; Feng, R.; Xu, C.; Wang, X.L.; Wang, Y.Z. A superhydrophobic coating to create multi-functional materials with mechanical/chemical/physical robustness. Chem. Eng. J. 2020, 381, 122539. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, S.; Shi, X.; Lan, Y.; Zeng, G.; Zhang, K.; Li, X. A fluorine-free method for fabricating multifunctional durable superhydrophobic fabrics. Appl. Surf. Sci. 2020, 505, 144621. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Multifunctional surfaces produced by femtosecond laser pulses. J. Appl. Phys. 2015, 117, 033103. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Chen, H.-C.; Chuang, K.-S.; Zheng, J.-H.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Guo, Z.; Luo, J.; Xue, H.; Gao, J. Durable and multifunctional superhydrophobic coatings with excellent joule heating and electromagnetic interference shielding performance for flexible sensing electronics. ACS Appl. Mater. Interfaces 2019, 11, 34338–34347. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Volpe, C.D.; Holysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef]
- Bormashenko, E. Physics of solid-liquid interfaces: From the Young equation to the superhydrophobicity (Review article). Low Temp. Phys. 2016, 42, 622. [Google Scholar] [CrossRef]
- Schmitt, M.; Heib, F. A more appropriate procedure to measure and analyse contact angles/drop shape behaviors. In Advances in Contact Angle, Wettability and Adhesion III; Mittal, K.L., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2018. [Google Scholar]
- Amirfazli, A.; Neumann, A.W. Status of the three-phase line tension: A review. Adv. Colloid Interface Sci. 2004, 110, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of icing and rational design of surfaces with extraordinary icephobicity. Langmuir 2015, 31, 4807–4821. [Google Scholar] [CrossRef]
- Li, B.; Ouyang, Y.; Haider, Z.; Zhu, Y.; Qiu, R.; Hu, S.; Niu, H.; Zhang, Y.; Chen, M. One-step electrochemical deposition leading to superhydrophobic matrix for inhibiting abiotic and microbiologically influenced corrosion of Cu in seawater environment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126337. [Google Scholar] [CrossRef]
- Vladkova, T.G.; Staneva, A.D.; Gospodinova, D.N. Surface engineered biomaterials and ureteral stents inhibiting biofilm formation and encrustation. Surf. Coat. Technol. 2020, 404, 126424. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, Y.; Wang, J.; Sun, Y.; Zhang, J.; Li, Y. Superamphiphobic aluminum alloy with low sliding angles and acid-alkali liquids repellency. Mater. Des. 2020, 188, 108479. [Google Scholar] [CrossRef]
- Gao, S.; Huang, J.; Li, S.; Liu, H.; Li, F.; Li, Y.; Chen, G.; Lai, Y. Facile construction of robust fluorine-free superhydrophobic TiO2@fabrics with excellent anti-fouling, water-oil separation and UV-protective properties. Mater. Des. 2017, 128, 1–8. [Google Scholar] [CrossRef]
- Pang, B.; Qian, J.; Zhang, Y.; Jia, Y.; Ni, H.; Pang, S.D.; Liu, G.; Qian, R.; She, W.; Yang, L.; et al. 5S multifunctional intelligent coating with superdurable, superhydrophobic, self-monitoring, self-heating and self-healing properties for existing construction application. ACS Appl. Mater. Interfaces 2019, 11, 29242–29254. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-S.; Quan, Y.-Y.; Mao, J.-J.; Wang, Y.-L.; Lai, Y.; Zheng, J.; Chen, Z.; Wei, K.; Li, H. Multifunctional superhydrophobic composite materials with remarkable mechanochemical robustness, stain repellency, oil-water separation and sound-absorption properties. Chem. Eng. J. 2019, 358, 1610–1619. [Google Scholar] [CrossRef]
- Shi, X.; Lan, Y.; Peng, S.; Wang, Y.; Ma, J. Green fabrication of a multifunctional sponge as an absorbent for highly-efficient and ultrafast oil-water separation. ACS Omega 2020, 5, 14232–14241. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Karthik, P.; Anpo, M.; Neppolian, B. Facile construction of ternary CuFe2O4-TiO2 nanocomposite supported reduced graphene oxide (rGO) photocatalysts for the efficient hydrogen production. Appl. Surf. Sci. 2018, 449, 772–779. [Google Scholar] [CrossRef]
- Bruzaud, J.; Tarrade, J.; Celia, E.; Darmanin, T.; de Givenchy, E.T.; Guittard, F.; Herry, J.M.; Guilbaud, M.; Bellon-Fontaine, M.N. The design of superhydrophobic stainless steel surfaces by controlling nanostructures: A key parameter to reduce the implantation of pathogenic bacteria. Mater. Sci. Eng. C 2017, 73, 40–47. [Google Scholar] [CrossRef]
- Schmitt, M. Synthesis and testing of ZnO nanoparticles for photo-initiation: Experimental observation of two different non-migration initiators for bulk polymerization. Nanoscale 2015, 7, 9532–9544. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bressler, A.H.; Abolghasemibizaki, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Sojoudi, H.; Wang, M.; Boscher, N.D.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Avramova, I.A.; Castano, C.E.; Ivanova, I.A.; Mohammadi, R.; Radeva, E.I.; Stoyanova, D.S.; Vladkova, T.G. Early stage anti-bioadhesion behavior of superhydrophobic soot based coatings towards Pseudomonas putida. Mater. Des. 2018, 160, 395–404. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Watson, J.A.; Zhou, Z.; Li, X.; Cheung, G.S.P.; Gellender, M. A simple model for binding and rupture of bacterial cells on nanopillar surfaces. Adv. Mater. Interfaces 2019, 6, 1801646. [Google Scholar] [CrossRef]
- Yoon, S.H.; Rungraeng, N.; Song, W.; Jun, S. Superhydrophobic and superhydrophilic nanocomposite coatings for preventing Escherichia coli K-12 adhesion on food contact surface. J. Food Eng. 2014, 131, 135–141. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Gyoshev, S.D.; Castano, C.E.; Mohammadi, R. Anti-frosting and defrosting performance of chemically modified super-nonwettable carbon soot coatings. J. Phys. D Appl. Phys. 2021, 54, 015303. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A.M.; Korolev, V.V.; Pashinin, A.S. Effect of wettability on sessile drop freezing: When superhydrophobicity stimulates an extreme freezing delay. Langmuir 2014, 30, 1659–1668. [Google Scholar] [CrossRef]
- Esmaeili, A.E.; Mir, N.; Mohammadi, R. A facile, fast and low-cost method for fabrication of micro/nano-textured superhydrophobic surfaces. J. Colloid Interface Sci. 2020, 573, 317–372. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).