Effect of Calcium Carbonate Particle Size on the Scratch Resistance of Rapid Alkyd-Based Wood Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
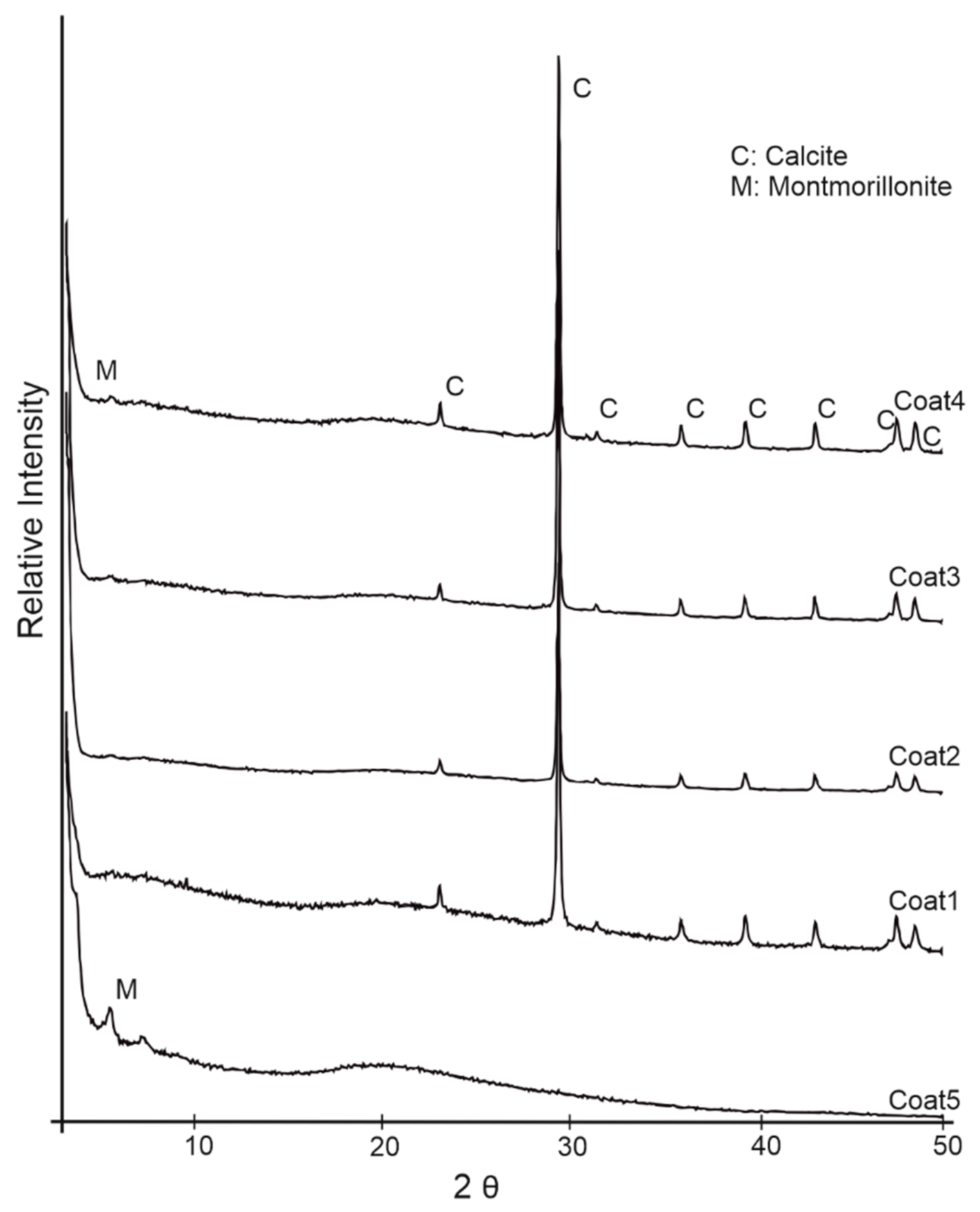
3.1. CaCO3 Powder and Coating Characteristics
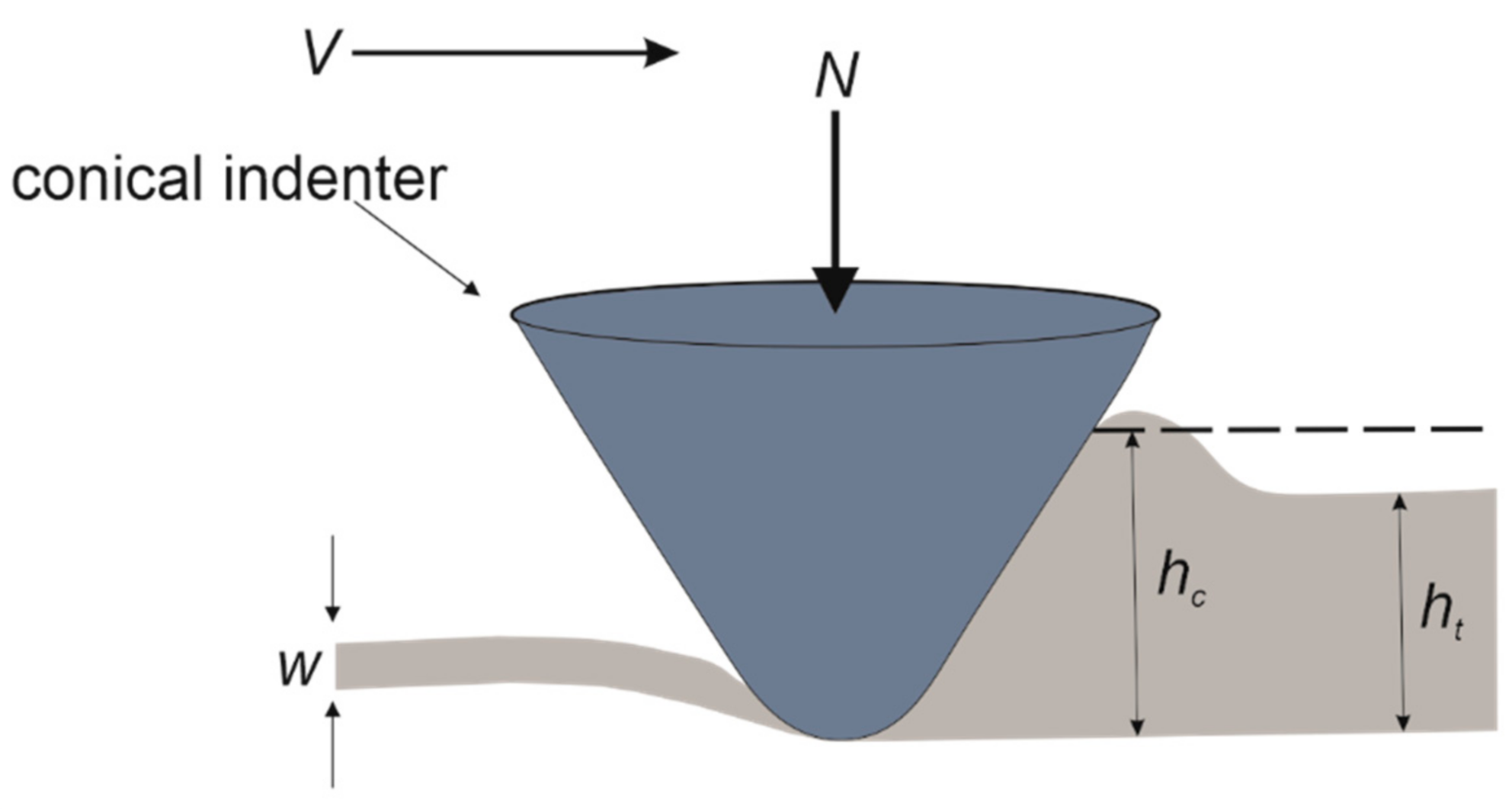
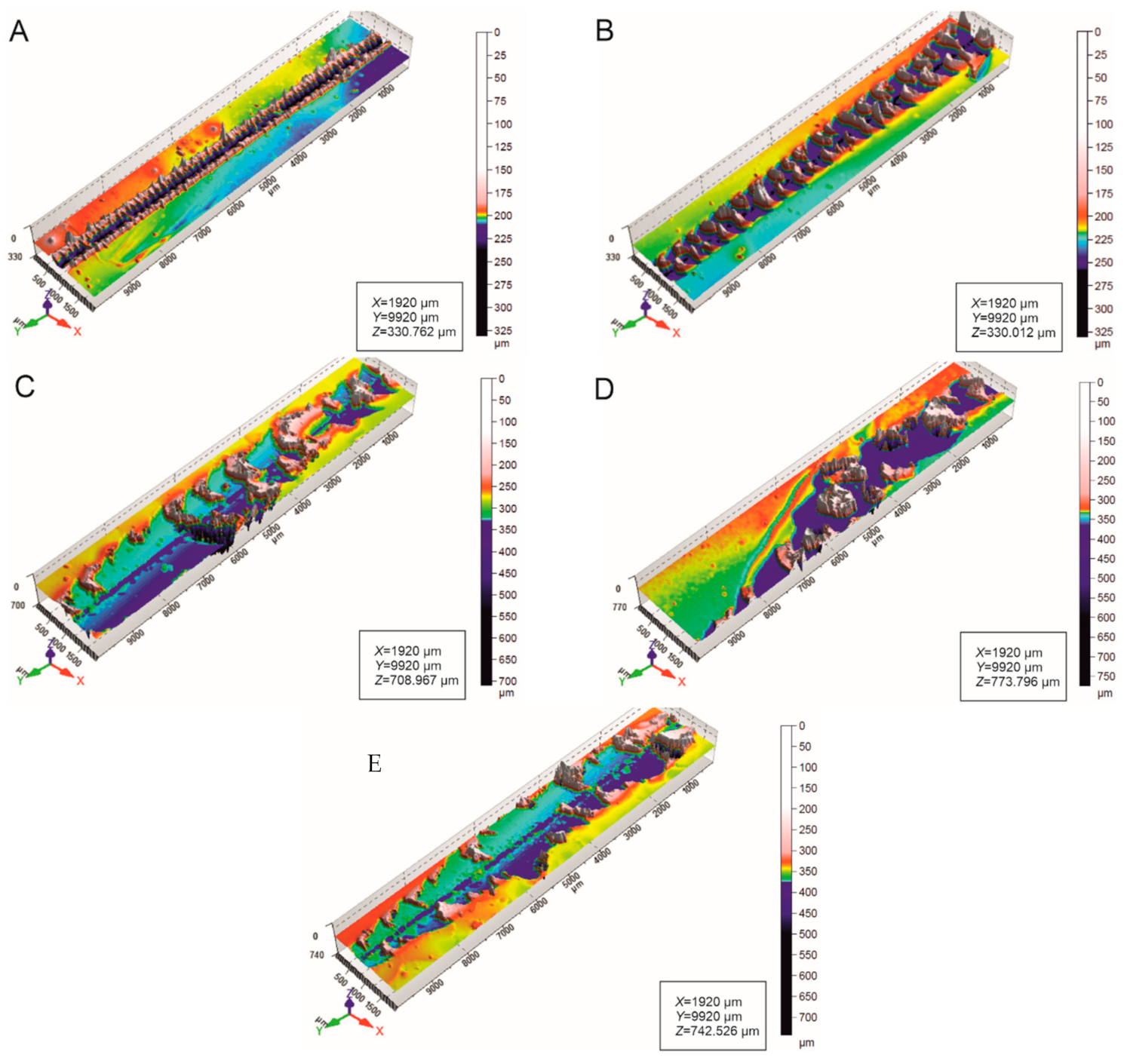
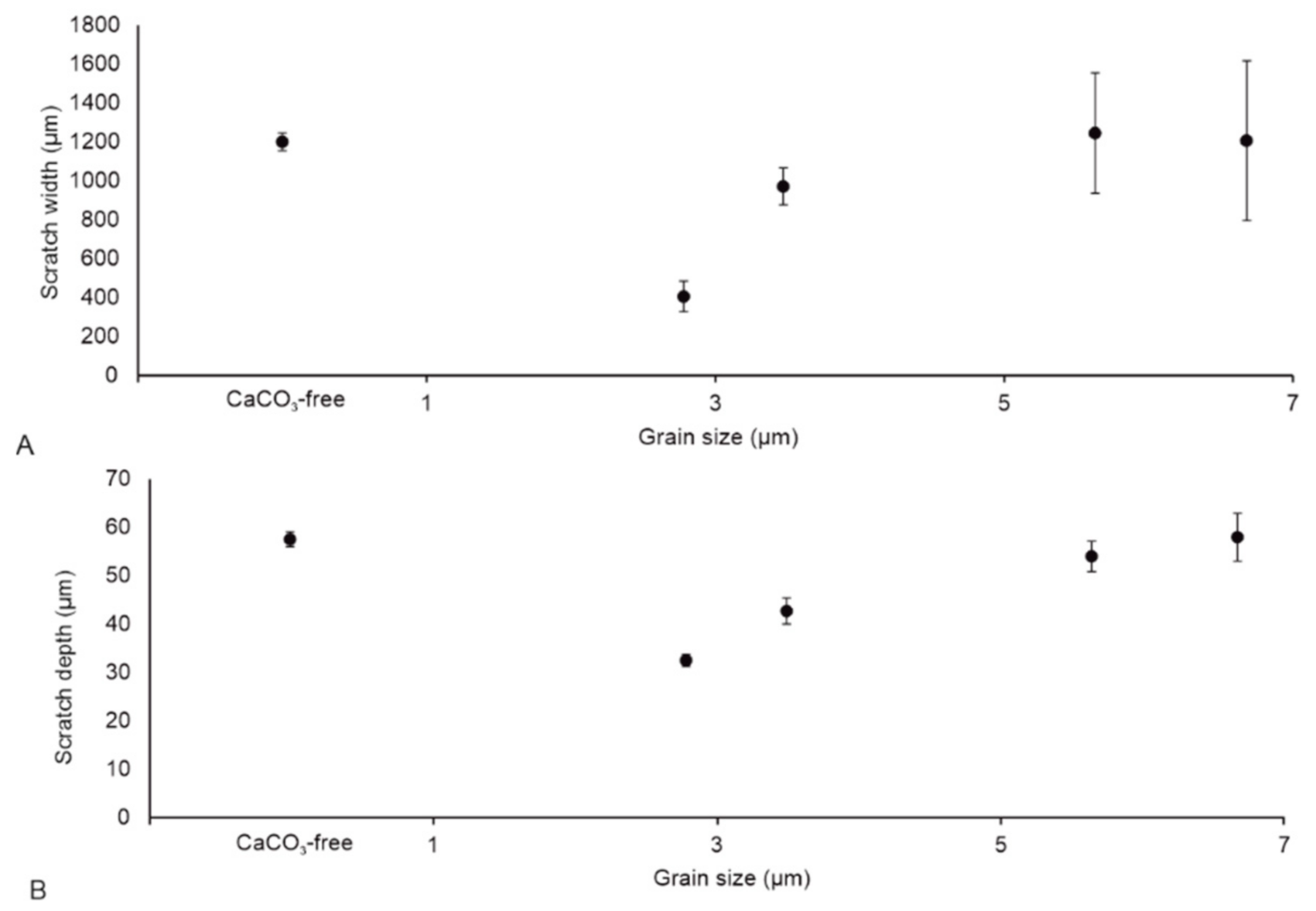
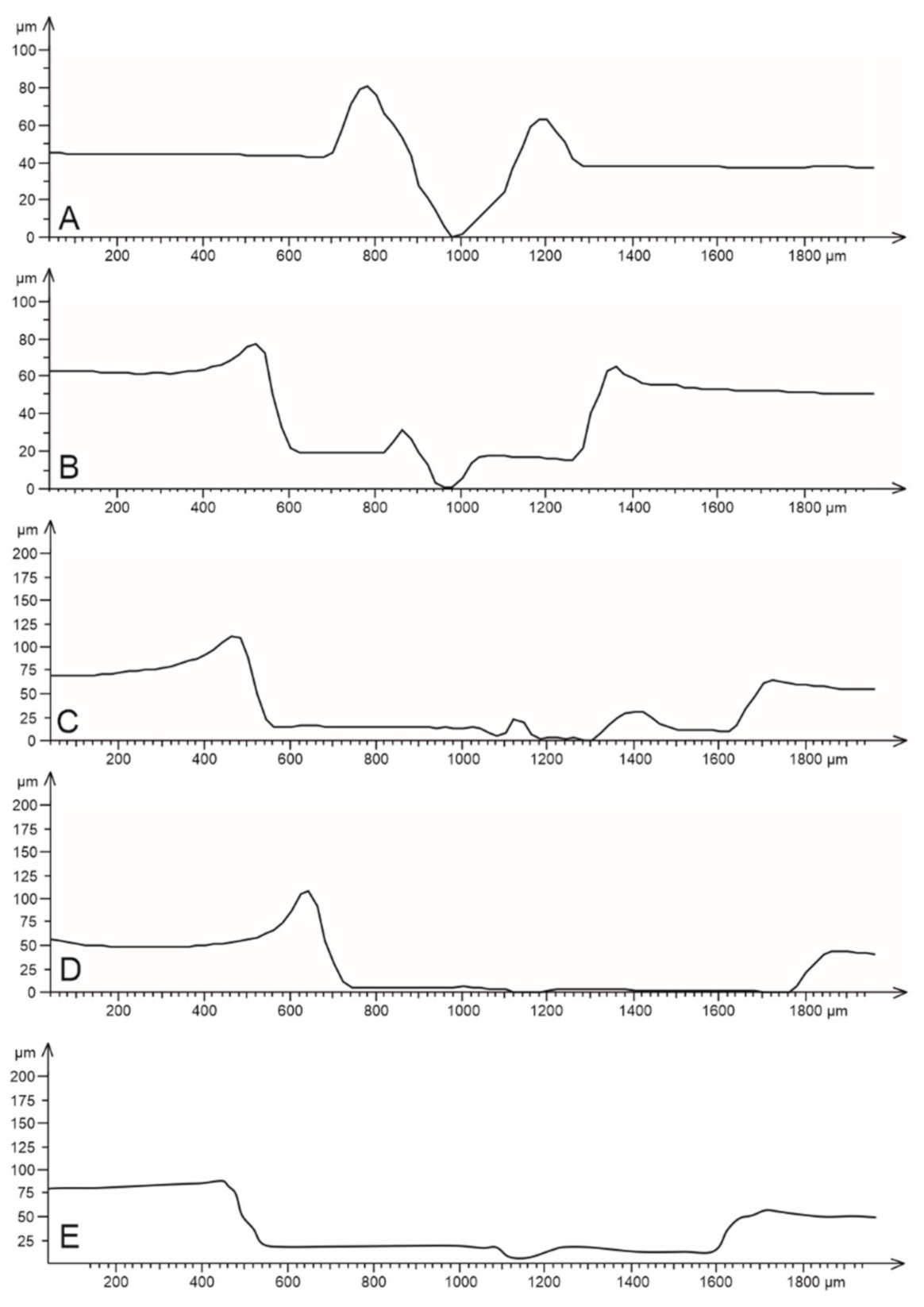
3.2. Scratch Morphology
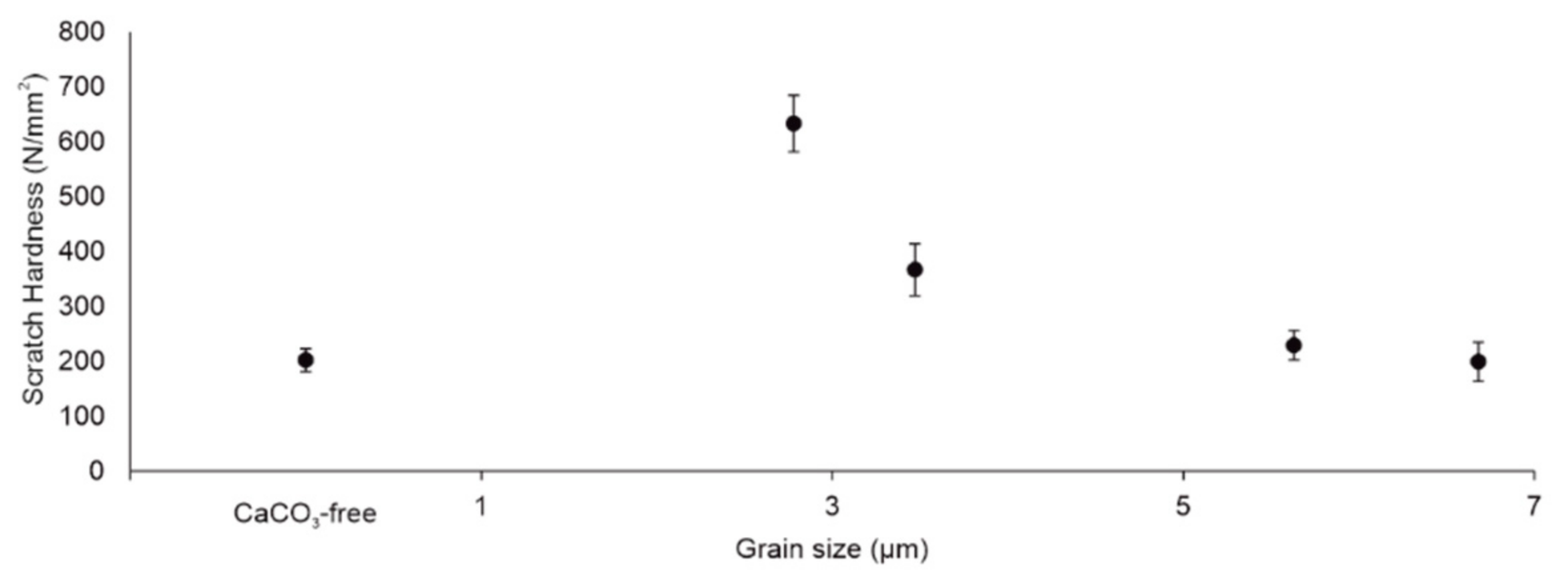
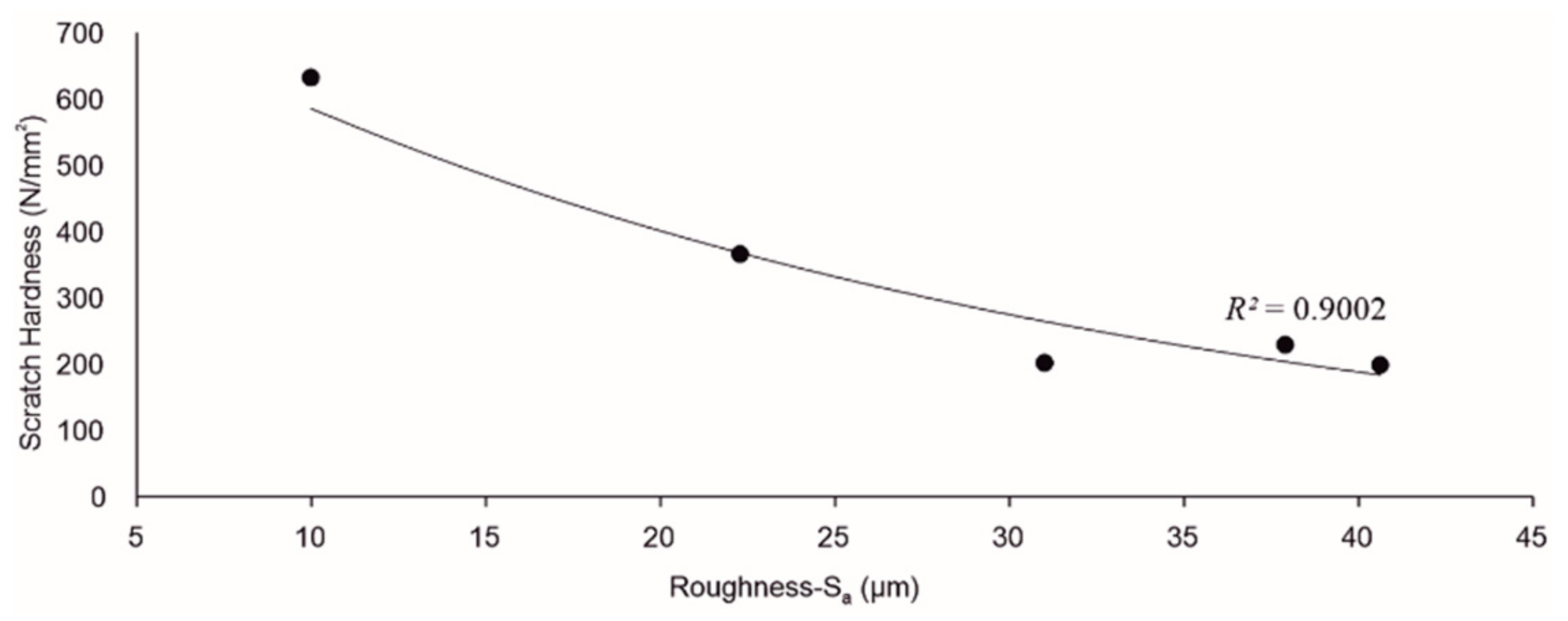
3.3. Scratch Hardness
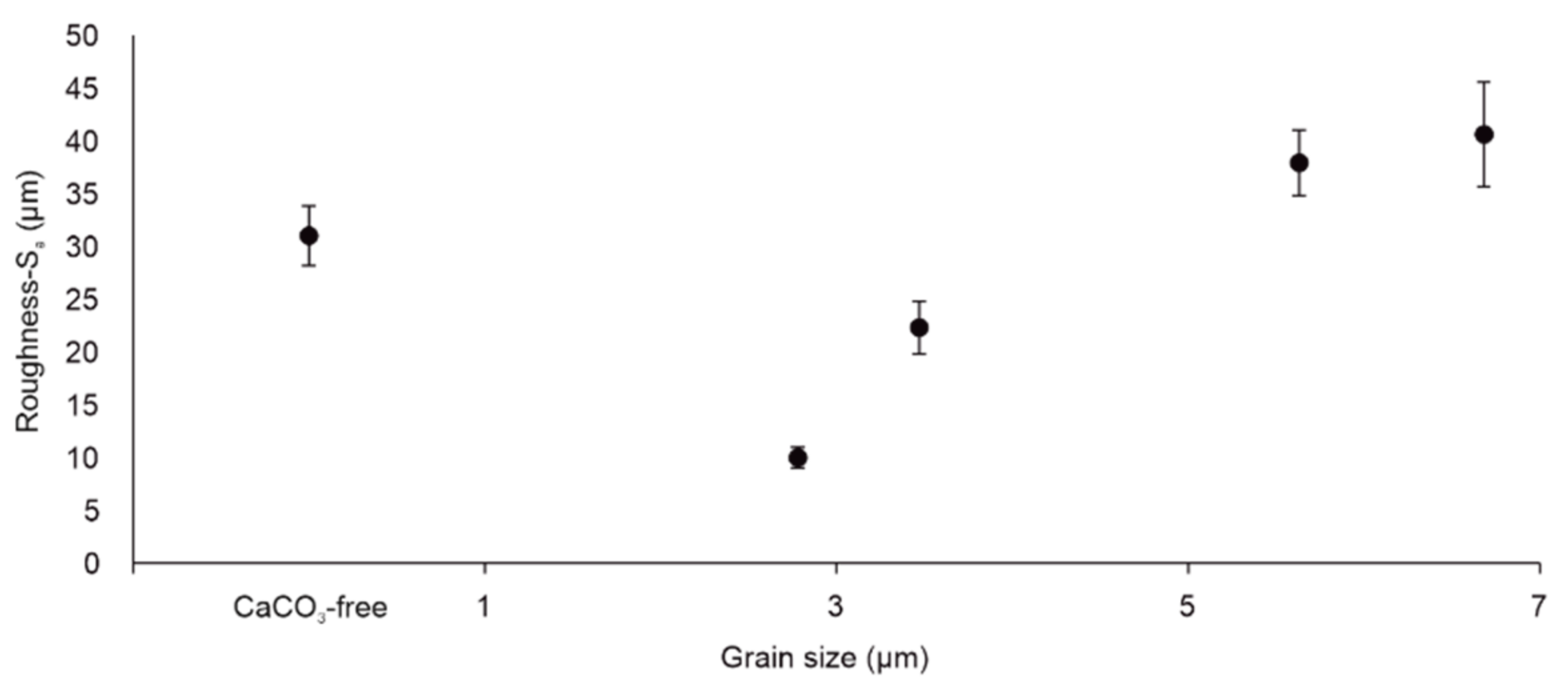
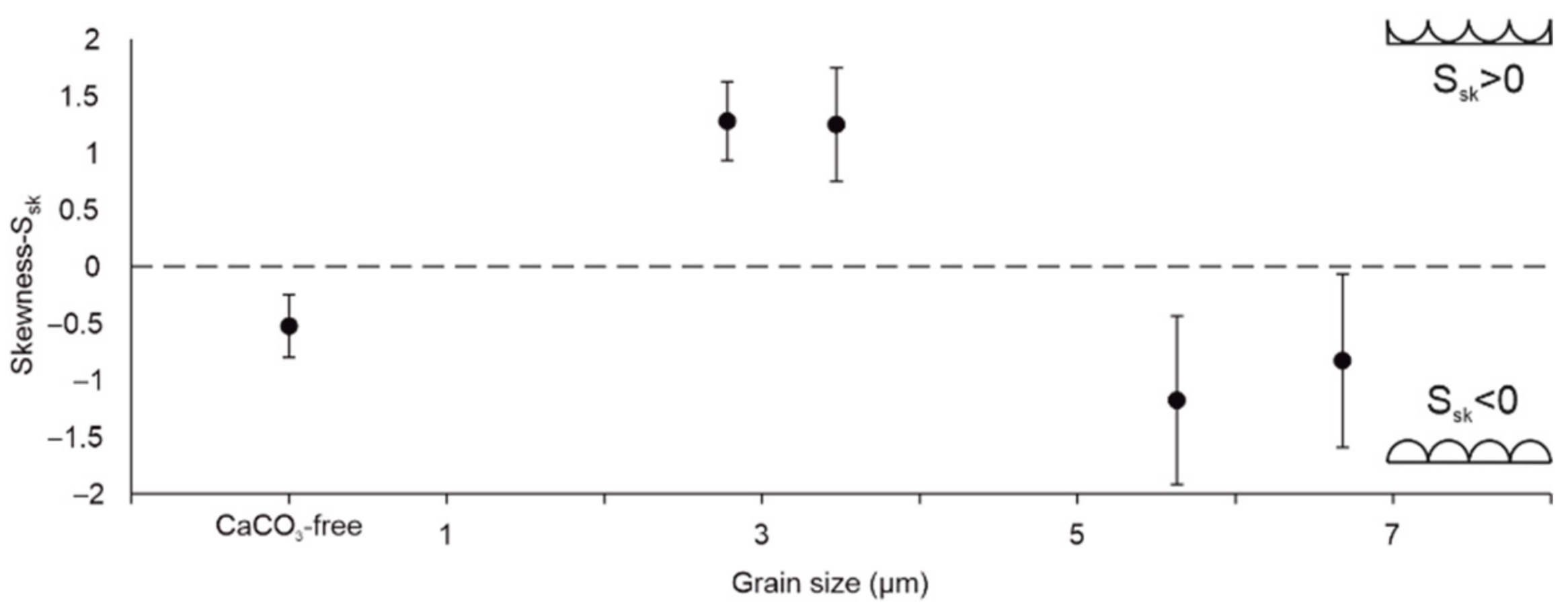
3.4. Roughness of Scratched Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolic, M.; Lawther, J.M.; Sanadi, A.R. Use of nanofillers in wood coatings: A scientific review. J. Coat. Technol. Res. 2015, 12, 445–461. [Google Scholar] [CrossRef]
- Flosbach, C.; Schubert, W. Zero etch clear—A new modular clear coat system with excellent scratch/mar performance. Prog. Org. Coat. 2001, 43, 123–130. [Google Scholar] [CrossRef]
- Asif, M. Sustainability of timber, wood and bamboo in construction. In Sustainability of Construction Materials; Khatib, J.M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 31–54. ISBN 9781845693497. [Google Scholar]
- Chardon, F.; Denis, M.; Negrell, C.; Caillol, S. Hybrid alkyds, the glowing route to reach cutting-edge properties? Prog. Org. Coat. 2021, 151, 106025. [Google Scholar] [CrossRef]
- Bauer, F.; Mehnert, R. UV curable acrylate nanocomposites: Properties and applications. J. Polym. Res. 2005, 12, 483–491. [Google Scholar] [CrossRef]
- Bauer, F.; Flyunt, R.; Czihal, K.; Buchmeiser, M.R.; Langguth, H.; Mehnert, R. Nano/micro particle hybrid composites for scratch and abrasion resistant polyacrylate coatings. Macromol. Mater. Eng. 2006, 291, 493–498. [Google Scholar] [CrossRef]
- Vu, C.; Laferté, O. All layers count: Silica nanoparticles in the optimisation of scratch and abrasion resistance of high performance UV multi-layer coatings. Eur. Coat. J. 2006, 6, 34. [Google Scholar]
- Xiang, C.; Sue, H.J.; Chu, J.; Masuda, K. Roles of additives in scratch resistance of high crystallinity polypropylene copolymers. Polym. Eng. Sci. 2001, 41, 23–31. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M. Scratch resistance enhancement of polymer coatings. Macromol. Mater. Eng. 2010, 295, 603–612. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Sinha, S.K. Scratch resistance and localised damage characteristics of polymer surfaces—A review. Materialwiss. Werkst. 2003, 34, 989–1002. [Google Scholar] [CrossRef]
- Jardret, V.; Morel, P. Viscoelastic effects on the scratch resistance of polymers: Relationship between mechanical properties and scratch properties at various temperatures. Prog. Org. Coat. 2003, 48, 322–331. [Google Scholar] [CrossRef]
- Hancock, M.; Rothon, R.N. Principal types of particulate fillers. In Particulate-Filled Polymer Composites, 1st ed.; Rothon, R.N., Ed.; Smithers Rapra Press: Shrewsbury, UK, 2000; pp. 53–100. ISBN 1-85957-382-7. [Google Scholar]
- Kurkcu, P.; Andena, L.; Pavan, A. An experimental investigation of the scratch behaviour of polymers—2: Influence of hard or soft fillers. Wear 2014, 317, 277–290. [Google Scholar] [CrossRef]
- Bautista, Y.; Gonzalez, J.; Gilabert, J.; Ibañez, M.J.; Sanz, V. Correlation between the wear resistance, and the scratch resistance, for nanocomposite coatings. Prog. Org. Coat. 2011, 70, 178–185. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, S.; Luo, J.L.; Xu, Z.H. Tribological and corrosion behaviors of Al2O3/polymer nanocomposite coatings. Wear 2006, 260, 976–983. [Google Scholar] [CrossRef]
- Amerio, E.; Fabbri, P.; Malucelli, G.; Messori, M.; Sangermano, M.; Taurino, R. Scratch resistance of nano-silica reinforced acrylic coatings. Prog. Org. Coat. 2008, 62, 129–133. [Google Scholar] [CrossRef]
- Douce, J.; Boilot, J.P.; Biteau, J.; Scodellaro, L.; Jimenez, A. Effect of filler size and surface condition of nano-sized silica particles in polysiloxane coatings. Thin Solid Films 2004, 466, 114–122. [Google Scholar] [CrossRef]
- Bauer, F.; Sauerland, V.; Gläsel, H.-J.; Ernst, H.; Findeisen, M.; Hartmann, E.; Langguth, H.; Marquardt, B.; Mehnert, R. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 3. Effect of filler particles and grafting agents. Macromol. Mater. Eng. 2002, 287, 546–552. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Verma, K.; Saren, P.; Oraon, R.; de Adhikari, A.; Nayak, G.C.; Kumar, V. Manipulating selective dispersion of reduced graphene oxide in polycarbonate/nylon 66 based blend nanocomposites for improved thermo-mechanical properties. RSC Adv. 2017, 7, 22145–22155. [Google Scholar] [CrossRef]
- Hadal, R.; Dasari, A.; Rohrmann, J.; Misra, R.D.K. Susceptibility to scratch surface damage of wollastonite- and talc-containing polypropylene micrometric composites. Mater. Sci. Eng. A 2004, 380, 326–339. [Google Scholar] [CrossRef]
- Chu, J.; Xiang, C.; Sue, H.J.; Hollis, R.D. Scratch resistance of mineral-filled polypropylene materials. Polym. Eng. Sci. 2000, 40, 944–955. [Google Scholar] [CrossRef]
- Lehmann, K.; Malacari, P.E. Polyolefin based compounds scratch resistance guaranteed by functional alliance of talc and additive used. In Proceedings of the Automotive TPO Global Conference, Detroit, MI, USA, 6–8 October 2008; SPE Detroit: Detroit, MI, USA, 2008; pp. 102–121. [Google Scholar]
- Dasari, A.; Rohrmann, J.; Misra, R.D.K. On the scratch deformation of micrometric wollastonite reinforced polypropylene composites. Mater. Sci. Eng. A 2004, 364, 357–369. [Google Scholar] [CrossRef]
- Zokaei, S.; Lesan Khosh, R.; Bagheri, R. Study of scratch resistance in homo- and co-polypropylene filled with nanometric calcium carbonate. Mater. Sci. Eng. A 2007, 445–446, 526–536. [Google Scholar] [CrossRef]
- Tanniru, M.; Misra, R.D.K.; Berbrand, K.; Murphy, D. The determining role of calcium carbonate on surface deformation during scratching of calcium carbonate-reinforced polyethylene composites. Mater. Sci. Eng. A 2005, 404, 208–220. [Google Scholar] [CrossRef]
- Tanniru, M.; Misra, R.D.K. Reduced susceptibility to stress whitening during tensile deformation of calcium carbonate-reinforced high density polyethylene composites. Mater. Sci. Eng. A 2006, 424, 53–70. [Google Scholar] [CrossRef]
- Azadi, M.; Olya, M.J.; Vahedi, S.; Sangani, N.F. The effect of the chemical composition and the volume of coated carbonate calcium on epoxy paint properties. Russ. J. Appl. Chem. 2017, 90, 1181–1187. [Google Scholar] [CrossRef]
- Yao, L.; Yang, J.; Sun, J.; Cai, L.; He, L.; Huang, H.; Song, R.; Hao, Y. Hard and transparent hybrid polyurethane coatings using in situ incorporation of calcium carbonate nanoparticles. Mater. Chem. Phys. 2011, 129, 523–528. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Lu, R.; Miyakoshi, T. Synergistic effect of addition of fillers on properties of interior waterborne UV-curing wood coatings. Coatings 2018, 8, 9. [Google Scholar] [CrossRef]
- Alvarez, V.; Paulis, M. Effect of acrylic binder type and calcium carbonate filler amount on the properties of paint-like blends. Prog. Org. Coat. 2017, 112, 210–218. [Google Scholar] [CrossRef]
- Bulian, F.; Graystone, J. Wood Coatings, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780444528407. [Google Scholar]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Di Gianni, A.; Amerio, E.; Monticelli, O.; Bongiovanni, R. Preparation of polymer/clay mineral nanocomposites via dispersion of silylated montmorillonite in a UV curable epoxy matrix. Appl. Clay Sci. 2008, 42, 116–124. [Google Scholar] [CrossRef]
- Piazza, D.; Lorandi, N.P.; Pasqual, C.I.; Scienza, L.C.; Zattera, A.J. Influence of a microcomposite and a nanocomposite on the properties of an epoxy-based powder coating. Mater. Sci. Eng. A 2011, 528, 6769–6775. [Google Scholar] [CrossRef]
- Pajarito, B.B.; Caguntas, A.J.F.; Felices, N.B.; Tubalinal, H.O.S.; Leuterio, G.L.D. Corrosion, wettability, and adhesion of acrylic coatings containing silane-treated mineral fillers on carbon steel. In Proceedings of the Materials Science Forum, Seoul, Korea, 20–22 October 2017; Argawal, R.K., Ed.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland; Volume 917, pp. 252–256. [Google Scholar] [CrossRef]
- Raper, S.; Skelhorn, D. Ground calcium carbonate versus feldspathic minerals. PCI Paint Coat. Ind. 2012, 28, 66–71. [Google Scholar]
- Kurkcu, P.; Andena, L.; Pavan, A. An experimental investigation of the scratch behaviour of polymers: 1. Influence of rate-dependent bulk mechanical properties. Wear 2012, 290–291, 86–93. [Google Scholar] [CrossRef]
- Krupička, A.; Johansson, M.; Hult, A. Use and interpretation of scratch tests on ductile polymer coatings. Prog. Org. Coat. 2003, 46, 32–48. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, J.C.M. Slip process of stick–slip motion in the scratching of a polymer. Mater. Sci. Eng. A 2003, 344, 182–189. [Google Scholar] [CrossRef]
- Gauthier, C.; Schirrer, R. Time and temperature dependence of the scratch properties of poly (methylmethacrylate) surfaces. J. Mater. Sci. 2000, 35, 2121–2130. [Google Scholar] [CrossRef]
- Wong, M.; Lim, G.T.; Moyse, A.; Reddy, J.N.; Sue, H.J. A new test methodology for evaluating scratch resistance of polymers. Wear 2004, 256, 1214–1227. [Google Scholar] [CrossRef]
- Rajesh, J.J.; Bijwe, J. Investigations on scratch behaviour of various polyamides. Wear 2005, 259, 661–668. [Google Scholar] [CrossRef]
- Hara, Y.; Mori, T.; Fujitani, T. Relationship between viscoelasticity and scratch morphology of coating films. Prog. Org. Coat. 2000, 40, 39–47. [Google Scholar] [CrossRef]
- Xiang, C.; Sue, H.J.; Chu, J.; Coleman, B. Scratch behavior and material property relationship in polymers. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 47–59. [Google Scholar] [CrossRef]
- Rodríguez, J.; Rico, A.; Soria, V. Tribological properties of commercial optical disks estimated from nanoindentation and scratch techniques. Wear 2007, 263, 1545–1550. [Google Scholar] [CrossRef]
- Browning, R.L.; Lim, G.T.; Moyse, A.; Sue, H.J.; Chen, H.; Earls, J.D. Quantitative evaluation of scratch resistance of polymeric coatings based on a standardized progressive load scratch test. Surf. Coat. Technol. 2006, 201, 2970–2976. [Google Scholar] [CrossRef]
- Hadal, R.S.; Misra, R.D.K. Scratch deformation behavior of thermoplastic materials with significant differences in ductility. Mater. Sci. Eng. A 2005, 398, 252–261. [Google Scholar] [CrossRef]
- Surampadi, N.L.; Pesacreta, T.C.; Misra, R.D.K. The determining role of scratch indenter radius on surface deformation of high density polyethylene and calcium carbonate-reinforced composite. Mater. Sci. Eng. A 2007, 456, 218–229. [Google Scholar] [CrossRef]
- Moghbelli, E.; Browning, R.L.; Boo, W.J.; Hahn, S.F.; Feick, L.J.E.; Sue, H.J. Effects of molecular weight and thermal history on scratch behavior of polypropylene thin sheets. Tribol. Int. 2008, 41, 425–433. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Evans, P.D.; Biswas, S.K.; Sinha, S.K. The hardnesses of poly (methylmethacrylate). Tribol. Int. 1996, 29, 93–104. [Google Scholar] [CrossRef]
- Wong, J.S.S.; Sue, H.J.; Zeng, K.Y.; Li, R.K.Y.; Mai, Y.W. Scratch damage of polymers in nanoscale. Acta Mater. 2004, 52, 431–443. [Google Scholar] [CrossRef]
- Brostow, W.; Cassidy, P.E.; Macossay, J.; Pietkiewicz, D.; Venumbaka, S. Connection of surface tension with multiple tribological properties in epoxy + fluoropolymer systems. Polym. Int. 2003, 52, 1498–1505. [Google Scholar] [CrossRef]
- Zarubica, A.; Miljkovic, M.; Purenovic, M.; Tomic, V. Colour parameters, whiteness indices and physical features of marking paints for horizontal signalization. Facta Univ. Ser. Phys. Chem. Technol. 2005, 3, 205–216. [Google Scholar] [CrossRef]
- ASTM International. ASTM D281-12 Standard Test Method for Oil Absorption of Pigments by Spatula Rub-out; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- ASTM International. ASTM D6677-18 Standard Test Method for Evaluating Adhesion by Knife; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- ASTM International. ASTM D3363-20 Standard Test Method for Film Hardness by Pencil Test; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- ASTM International. ASTM D4366-14 Standard Test Methods for Hardness of Organic Coatings by Pendulum Damping Tests; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Evans, P.D.; Pelillo, E.; Sinha, S.K. Scratching maps for polymers. Wear 1996, 200, 137–147. [Google Scholar] [CrossRef]
- Pelletier, H.; Mendibide, C.; Riche, A. Mechanical characterization of polymeric films using depth-sensing instrument: Correlation between viscoelastic-plastic properties and scratch resistance. Prog. Org. Coat. 2008, 62, 162–178. [Google Scholar] [CrossRef]
- International Organization for Standardization. Geometrical product specifications (GPS)—Surface texture: Areal; International Organization for Standardization (ISO): Geneva, Switzerland, 2012; ISO 25178. [Google Scholar]
- Fukumoto, M.; Nagai, H.; Yasui, T. Influence of surface character change of substrate due to heating on flattening behavior of thermal sprayed particles. J. Therm. Spray Technol. 2006, 15, 759–764. [Google Scholar] [CrossRef]
- Patel, K.; Doyle, C.S.; Yonekura, D.; James, B.J. Effect of surface roughness parameters on thermally sprayed PEEK coatings. Surf. Coat. Technol. 2010, 204, 3567–3572. [Google Scholar] [CrossRef]
- Bulaha, N. Calculations of surface roughness 3D parameters for surfaces with irregular roughness. In Proceedings of the Engineering for Rural Development, Jenglava, Latvia, 23–25 May 2018; Latvia University of Life Sciences and Technologies: Jenglava, Latvia, 2018; Volume 17, pp. 1437–1444. [Google Scholar]
- Stout, K.J.; King, T.G.; Whitehouse, D.J. Analytical techniques in surface topography and their application to a running-in experiment. Wear 1977, 43, 99–115. [Google Scholar] [CrossRef]
- Ersoy, O.; Aydar, E.; Gourgaud, A.; Bayhan, H. Quantitative analysis on volcanic ash surfaces: Application of extended depth-of-field (focus) algorithm for light and scanning electron microscopy and 3D reconstruction. Micron 2008, 39, 128–136. [Google Scholar] [CrossRef]
- Ersoy, O.; Gourgaud, A.; Aydar, E.; Chinga, G.; Thouret, J.C. Quantitative scanning-electron microscope analysis of volcanic ash surfaces: Application to the 1982–1983 Galunggung eruption (Indonesia). Bull. Geol. Soc. Am. 2007, 119, 743–752. [Google Scholar] [CrossRef]
- Orkun, E. Analyse morphologique quantitative des cendres des dépôts pyroclastiques d’origine hydrovolcanique et magmatique. Ph.D. Thesis, Universite Blaise Pascal–Clermont Ferrand II, Aubière, France, 2012. [Google Scholar]
- Chu, J.; Rumao, L.; Coleman, B. Scratch and mar resistance of filled polypropylene materials. Polym. Eng. Sci. 1998, 38, 1906–1914. [Google Scholar] [CrossRef]

| Material | % |
|---|---|
| Rapid alkyd resin | 60 |
| Solvent mixture (white spirit) | 13.2 |
| Lecithin | 0.4 |
| Antisetting agent (Texaphor) | 0.6 |
| Rheology additive (Viscogel ED) | 0.8 |
| CaCO3 | 25 |
| CaCO3 Sample | d50 (µm) | L* | a* | b* | Surface Area (m2/g) | DOP Oil Absorption (g/100 g) | Flax Oil Absorption (g/100 g) |
|---|---|---|---|---|---|---|---|
| Kal1 | 2.78 | 98.81 | −0.01 | 0.73 | 4.41 | 31.00 | 17.00 |
| Kal2 | 3.47 | 98.87 | −0.01 | 0.59 | 3.25 | 31.00 | 16.00 |
| Kal3 | 5.63 | 98.67 | 0.00 | 0.76 | 2.63 | 28.00 | 15.00 |
| Kal4 | 6.68 | 98.53 | 0.01 | 0.75 | 2.40 | 26.00 | 14.00 |
| Coating Sample | Filler | Viscosity (KU) | Viscosity (mPa·s) |
|---|---|---|---|
| Coat1 | Kal1 | 63.70 | 422 |
| Coat2 | Kal2 | 62.30 | 394 |
| Coat3 | Kal3 | 60.30 | 355 |
| Coat4 | Kal4 | 60.00 | 350 |
| Coat5 | CaCO3-free | 50.60 | 184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoy, O.; Fidan, S.; Köse, H.; Güler, D.; Özdöver, Ö. Effect of Calcium Carbonate Particle Size on the Scratch Resistance of Rapid Alkyd-Based Wood Coatings. Coatings 2021, 11, 340. https://doi.org/10.3390/coatings11030340
Ersoy O, Fidan S, Köse H, Güler D, Özdöver Ö. Effect of Calcium Carbonate Particle Size on the Scratch Resistance of Rapid Alkyd-Based Wood Coatings. Coatings. 2021; 11(3):340. https://doi.org/10.3390/coatings11030340
Chicago/Turabian StyleErsoy, Orkun, Sinan Fidan, Harun Köse, Dilek Güler, and Ömer Özdöver. 2021. "Effect of Calcium Carbonate Particle Size on the Scratch Resistance of Rapid Alkyd-Based Wood Coatings" Coatings 11, no. 3: 340. https://doi.org/10.3390/coatings11030340
APA StyleErsoy, O., Fidan, S., Köse, H., Güler, D., & Özdöver, Ö. (2021). Effect of Calcium Carbonate Particle Size on the Scratch Resistance of Rapid Alkyd-Based Wood Coatings. Coatings, 11(3), 340. https://doi.org/10.3390/coatings11030340