Abstract
The development of novel oxygen reduction electrodes with superior electrocatalytic activity and CO2 durability is a major challenge for solid oxide fuel cells (SOFCs). Here, novel cobalt-free perovskite oxides, BaFe1−xYxO3−δ (x = 0.05, 0.10, and 0.15) denoted as BFY05, BFY10, and BFY15, are intensively evaluated as oxygen reduction electrode candidate for solid oxide fuel cells. These materials have been synthesized and the electrocatalytic activity for oxygen reduction reaction (ORR) has been investigated systematically. The BFY10 cathode exhibits the best electrocatalytic performance with a lowest polarization resistance of 0.057 Ω cm2 at 700 °C. Meanwhile, the single cells with the BFY05, BFY10 and BFY15 cathodes deliver the peak power densities of 0.73, 1.1, and 0.89 W cm−2 at 700 °C, respectively. Furthermore, electrochemical impedance spectra (EIS) are analyzed by means of distribution of relaxation time (DRT). The results indicate that the oxygen adsorption-dissociation process is determined to be the rate-limiting step at the electrode interface. In addition, the single cell with the BFY10 cathode exhibits a good long-term stability at 700 °C under an output voltage of 0.5 V for 120 h.
1. Introduction
Perovskite-type materials are the promising cathodes for solid oxide fuel cells (SOFCs) [1,2,3]. Among the perovskite oxides, the Co-containing perovskite materials with mixed ionic-electronic conducting feature have been widely investigated due to their remarkable electrochemical performance for ORR [4,5,6]. However, Co-containing oxides show some other drawbacks, such as poor chemical stability, higher thermal expansion coefficient, and strong volatility, which inhibit their wide applications in SOFCs [7,8]. To address these issues, developing new cathodes with improved electrochemical performance and good chemical stability is an important trend. Recently, Fe-based perovskite materials exhibit attractive chemical compatibility and excellent electrocatalytic activity, such as SrFe1−xTixO3−δ, SrFe0.8Nb0.2−xTaxO3−δ, La1−xSrxFeO3−δ, and Ba1−xLaxFeO3−δ [9,10,11,12].
Among the Fe-based oxides, BaFeO3−δ presents attractive oxygen permeation flux and fast oxygen surface exchange kinetics [13,14]. This is mainly due to variable valence and excellent chemical stability of Fe ions, as well as lower valence and larger ionic radius of Ba2+, which facilitates the electrochemical performance and oxygen transport of the materials [15]. Cation-doping is commonly adopted to stabilize the cubic lattice of BaFeO3−δ with disordered oxygen vacancies, such as La3+, Sm3+, and Ca2+ in the A site and Nb5+, Sn4+, In3+, and Ni2+ in the B site [12,16,17,18]. Lu et al. found that In3+ doping in the B site of BaFeO3−δ can enhance oxygen permeation flux, in which BaFe0.9In0.1O3−δ presented the higher oxygen permeation flux of 1.11 mL cm−2 min−1 at 950 °C [19]. Song et al. reported an attractive cathode candidate of BaFe1−xBixO3−δ for SOFCs. The BaFe0.9Bi0.1O3−δ cathode exhibited a lower polarization resistance of 0.133 Ω cm2 at 750 °C and a high oxygen vacancy concentration of 0.408. However, the thermal expansion coefficient of BaFe0.9Bi0.1O3−δ is very large (26.697 × 10−6 K−1). Additionally, the oxygen permeability and oxygen non-stoichiometry of BaFe1−xYxO3−δ have been investigated by Liu et al. They found that Y-doping promotes the oxygen vacancy concentration and oxygen ion migration, which is believed to be favorable for transport of the oxygen ion in the bulk electrode, thereby resulting in the enhanced electrocatalytic performance [20].
In this work, Fe-based perovskite BaFe1−xYxO3−δ oxides have been investigated as the prominent cathodes for SOFCs. The crystalline structure, CO2 tolerance, and electrocatalytic activity for ORR of the BaFe1−xYxO3−δ cathodes are systematically investigated. The results provide an effective strategy for designing novel cathode electrocatalysts for SOFCs.
2. Experimental
2.1. Material Preparation
The BaFe1−xYxO3−δ (x = 0.05–0.15) samples were synthesized by the solid-state reaction. Stoichiometric amounts of BaCO3 (99.99%, Tianjin Guangfu Co. Ltd., Tianjin, China), Fe2O3 (99.99%, Tianjin Guangfu Co. Ltd.), and Y2O3 (99.99%, Tianjin Guangfu Co. Ltd.) were mixed and ground via the ball milling using ethanol as the dispersant. Afterwards, the mixture was pre-fired at 1000 °C for 10 h with a heating rate of 5 °C min−1 in air and then re-milled for 1 h, followed by calcining at 1300 °C for 12 h to obtain the final products.
2.2. Characterization
The crystal structure of BFYx cathodes were identified using X-ray diffraction (Bruker D8 advance) with filtered Cu-Kα radiation (λ = 1.5148 Å) source in a 2θ range of 10°–80°. The XRD data was analyzed to obtain the structural parameters by using Rietveld refinement method using the Rietica software (Version 1.7.7.8) program. The oxygen desorption property of the cathode catalysts was carried out by O2 temperature-programmed desorption (O2-TPD) with the TP-5076 instrument (Tianjin Xianquan, Co. Ltd., Tianjin, China). The electrical conductivity was measured between 100 and 800 °C in air by standard four-probe DC method with a Keithley 2400 SourceMeter Keithley Instruments Inc., Cleveland, OH, USA). The oxygen non-stoichiometry of the samples at room temperature was determined with the iodometric titration method, as described elsewhere [21,22]. The oxygen non-stoichiometry at high temperature was measured by thermogravimetric analysis (TGA, SETARAM, Caluire et Cuire, France).
2.3. Electrochemical Test
The dense Ce0.9Gd0.1O1.95 (CGO) electrolyte was fabricated by pressing CGO powders (SOFCMAN Co. Ltd., Ningbo, China) uniaxially at 220 MPa, and sintered at 1450 °C for 24 h. For the fabrication of symmetrical cells (BFYx|CGO|BFYx), the BFYx electrode powders were mixed with terpineol and ethyl cellulose to prepare the cathode slurry. The slurry was symmetrically coated on the Ce0.9Gd0.1O2−δ (CGO) electrolyte and sintered at 900 °C for 4 h. The electrochemical impedance spectroscopy (EIS) of symmetric cell was acquired by an Autolab PGSTAT302N workstation in the frequency range of 10−2–106 Hz under open voltage conditions at 500–700 °C. To explore the electrochemical process for ORR of the electrode, the EIS spectra were collected under different oxygen partial pressure (PO2).
The anode-supported half cell (NiO-YSZ|YSZ|CGO) was bought from Ningbo SuoFuRen Energy Co. Ltd. (Ningbo, China). The BFYx cathode slurry was printed onto the CGO barrier layer, and subsequently co-fired at 900 °C for 4 h. The electrochemical performance of anode-supported fuel cells was tested using electrochemical workstation (ZAHNER, IM6e, Kronach, Germany. The single cell was mounted on the alumina tube, while the humidified H2 (3% H2O) with a flow rate of 80 mL min−1 and ambient air were used as the fuel and oxidant, respectively.
3. Results and Discussion
Figure 1a displays the XRD patterns of BFYx samples. The diffraction profiles reveal that the BFYx oxides crystallize in a cubic perovskite structure with Pm-3m space group. The magnified XRD patterns between 29 and 33° are presented in Figure 1a. The diffraction peaks gradually shift to a lower angle direction with increasing the doping fraction, indicating the expansion of the lattice constants. To further obtain the lattice constants of the materials, Rietveld refined XRD data of BFY10 samples are given in Figure 1b. The refined lattice constants of BFYx samples are summarized in Table 1. The increase in cell volume from 67.220 Å3 (x = 0.05) to 69.148 Å3 (x = 0.15) is identified, which is attributed to the larger ionic radius of Y3+ (0.90 Å) relative to that of Fe3+ (0.55 Å). This phenomenon indicates that the Fe sites in BaFeO3 are partially replaced by the Y ions. Furthermore, to examine the chemical compatibility between electrode and electrolyte, the mixtures of BFYx and CGO were co-fired at 1000 °C for 12 h. Figure 1c presents the XRD patterns of the calcined BFYx-CGO mixtures. No obvious impurities can be detected, revealing the favorable chemical compatibility of the BFYx cathodes with CGO electrolyte at 1000 °C.
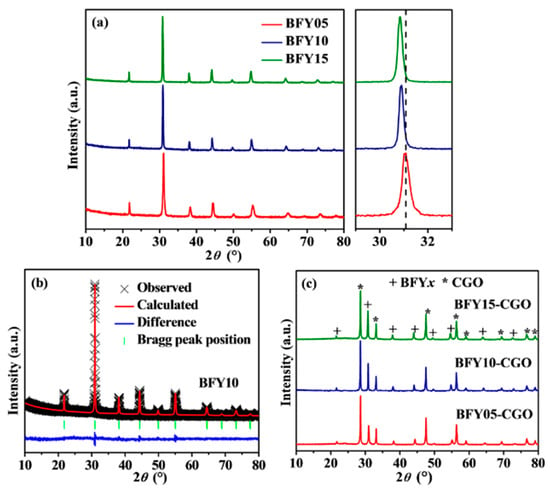
Figure 1.
(a) Room temperature XRD patterns of BFYx samples and the magnified view of the XRD patterns, 2θ = 29 to 33°; (b) Rietveld refinement plot of BFY10 XRD data; (c) XRD patterns of BFYx-CGO composites fired at 1000 °C for 12 h.

Table 1.
Lattice parameters of BFYx samples.
The oxygen mobility and reducibility of Fe ions for the BFYx samples were studied by O2-TPD. Figure 2a presents the representative O2-TPD profiles of the BFYx samples. The curves show two desorption peaks at around 200 and 650 °C in all samples. The first broad peak located at ~200–300 °C is associated with the desorption process of the chemisorbed oxygen on the material surface. The second oxygen desorption peak may be ascribed to the reduction of Fe4+ to Fe3+ at 300–650 °C [23]. It is noteworthy that the BFY10 material shows the largest area of second desorption peak among the BFYx samples, indicating the highest oxygen vacancy concentration and favorable oxygen mobility of the BFY10. The oxygen non-stoichiometry (δ) of the BFYx samples at elevated temperatures was explored by TGA in air, as presented in Figure 2b. The δ values were determined by TGA results and the initial oxygen non-stoichiometry (δ0) values at room temperature were obtained by the iodometric titration. The initial weight loss from room temperature to 300 °C is associated with the evaporation of adsorbed water. When the temperature is above 300 °C, the obvious weight loss is due to the reduction of Fe4+ to Fe3+. It can be seen that the δ values of the samples decreases with the doping content from room temperature to 600 °C. However, when increasing the temperature to 600 °C, the BFY10 possesses the largest oxygen vacancy concentration, meaning its excellent oxygen ions mobility and promoted catalytic activity for ORR.
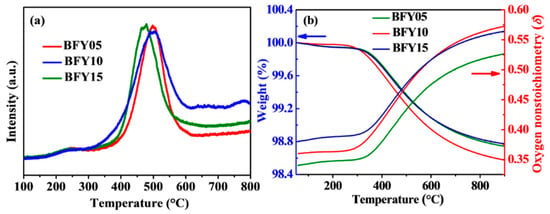
Figure 2.
(a) TPD curves of BFYx samples measured from 100 to 800 °C; (b) TG curves and oxygen non-stoichiometry (δ) of BFYx between 50 and 900 °C.
The temperature dependence of electrical conductivity for the BFYx samples within the temperature range of 100–800 °C in air is presented in Figure 3a. The electrical conductivity of all samples shows a similar trend as a function of temperature. When increasing the temperature, the electrical conductivity of BFYx initially increases and reaches a maximum value at about 400 °C, and subsequently decreases between 400 and 800 °C. This indicates a transition from semi-conducting behavior to metal-like conduction. In addition, it is observed that the electrical conductivity gradually decreases with increasing Y-doping fraction. This phenomenon may be due to the higher Y content leads to the reduction of Fe4+ to Fe3+ or Fe2+ for the charge compensation, resulting in a decrease in the electrical conductivity. The maximum values of electrical conductivity are 9.81, 5.03, and 1.67 S cm−1 for BFY05, BFY10 and BFY15, respectively. These values are comparable to those of other reported BaFeO3−δ-based cathodes, such as BaFe0.95Nb0.05O3−δ (9.5 S cm−1) [16], BaFe0.95Zr0.05O3−δ (7.5 S cm−1) [14] and BaFe0.8In0.2O3−δ (2.3 S cm−1) [19]. Furthermore, the Arrhenius plots of electrical conductivity are presented in Figure 3b. The calculated activation energies (Ea) of BFYx are 0.29, 0.38, and 0.43 eV for BFY05, BFY10 and BFY15, respectively.
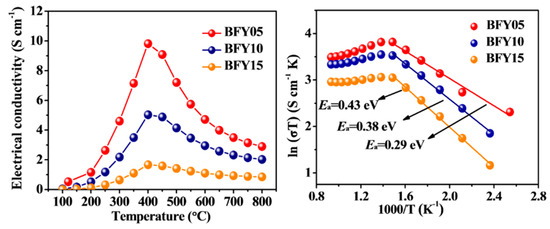
Figure 3.
(a) The electrical conductivity of BFYx samples at 100–800 °C; (b) Arrhenius plots of BFYx with temperature.
The EIS spectra were used to demonstrate electrocatalytic performance of the symmetric cells of BFYx|CGO|BFYx. Figure 4a displays the Nyquist plots of the BFYx cathodes at 700 °C in air. In general, the polarization resistance (Rp) value of the electrode is a crucial descriptor for the cathode performance, and the lower Rp value means a superior electrochemical activity for ORR. The Rp of BFY05, BFY10 and BFY15 cathodes are 0.136, 0.057, and 0.107 Ω cm2 at 700 °C, respectively, suggesting highly electrocatalytic performance of the BFYx cathodes. The Rp value (700 °C) of BFY10 cathode is smaller than that of the Fe-based perovskite electrodes (Figure 4b) [24,25,26,27]. Furthermore, the Arrhenius plots of the polarization resistance are presented in Figure 4c. The calculated Ea values are 1.40, 1.33 and 1.45 eV for BFY05, BFY10, and BFY15, respectively. Moreover, the BFY10 cathode presents the lowest Ea value, implying the highest electrocatalytic performance.
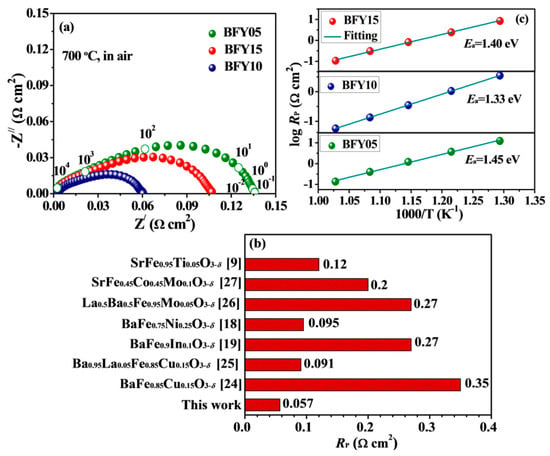
Figure 4.
(a) Impedance spectra of BFYx measured at 700 °C; (b) Comparison of RP for different Fe-based perovskite cathodes at 700 °C; (c) Arrhenius plots of Rp for BFYx cathodes.
To further clarify the electrochemical processes of the cathode, EIS spectra of the BFY10 electrode are systematically studied under varied Po2 at 700 °C, as shown in Figure 5a. Clearly, the impedance spectra are consisted of three separable high-frequency, intermediate-frequency and low-frequency arcs, respectively, suggesting that the three different electrode processes occur on the cathode. The EIS data are further fitted with an equivalent circuit using the model of [Rohm-(RH-CPEH)-(RM-CPEM)-(RL-CPEL)] (inset in Figure 5a) and analyzed by the distribution of relaxation time (DRT) method. Figure 5b displays the DRT results of BFY10 cathode under different Po2 at 700 °C. It can be seen that the typical DRT plots presents three distinct peaks, the high-frequency peak P1 (HF), and intermediate-frequency peak P2 (MF) and low-frequency peak P3 (LF), corresponding to charge transport process, adsorption-dissociation process of oxygen molecule, and gaseous diffusion, respectively [28,29]. Additionally, the relationship between Rp and PO2 can be expressed by the following formula: Rp = k(PO2)−m (1) [30,31]. The dependence of the RHF, RMF and RLF for the BFY10 cathode on the PO2 at 700 °C is presented in Figure 5c. One should note that the m values are 0.26, 0.48, and 1.03 in high-frequency, intermediate-frequency and low-frequency region, respectively, which are ascribed to the charge transfer process (, m = 1/4), the adsorption-dissociation of the oxygen molecule process (, m = 1/2) and the adsorption and diffusion of gaseous oxygen on the electrode surface (, m = 1) [32]. Furthermore, it can be found that the RMF is higher than RHF and RLF, implying that the rate-determining step for ORR is dominated by intermediate-frequency arc assigned to the oxygen adsorption-dissociation.
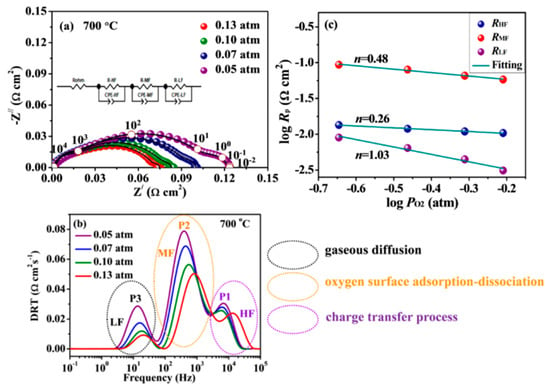
Figure 5.
(a) Impedance spectra and (b) DRT results of BFY10 cathode under different oxygen partial pressure at 700 °C; (c) RH, RM, and RL of BFY10 cathode as a function at 700 °C.
Some Ba-based cathodes for SOFCs show chemical instability under CO2-containing atmospheres because of the formation of BaCO3 on the cathode surface, diminishing oxygen reduction kinetics [33,34]. To evaluate the CO2 tolerance of the electrode, EIS spectra of the BFY10 cathode were acquired in CO2-containing air (3%, 5%, 10%) at 700 °C, as presented Figure 6a,b. It can be seen that the Rp value significantly increases with increasing the concentration of CO2. However, the Rp recovers to the initial value after removing CO2 atmosphere, which demonstrates the outstanding CO2 tolerance of the BFY10 cathode. Generally, the average metal oxide binding energy (ABE) is normally used to assess the CO2 durability of the cathode materials [35]. More negative ABE value indicates that oxide has excellent CO2 tolerance. The ABE is calculated based on the following equation [36]:
where xA and xB are the molar fraction of A and B metals; and are the sublimation heat of A and B metals; and are the formation heat of AmOn and BmOn oxides, and DO2 is the dissociation energy of O2. The ABE values of BFYx oxides are −287.18 kJ mol−1, −291.35 kJ mol−1, and −295.51 kJ mol−1, respectively, which are higher than cobalt-free perovskite cathodes, such as Ba0.5Sr0.5Co0.8Fe0.2O3−δ (−274 kJ mol−1) [37], Bi0.5Sr0.5FeO3−δ (−276.67 kJ mol−1) and Bi0.5Sr0.5Fe0.9Ta0.1O3−δ (−296.97 kJ mol−1) [26]. These results confirm that the BFYx cathodes have satisfactory CO2 tolerance.
ABE = ABE(A-O) + ABE(B-O)
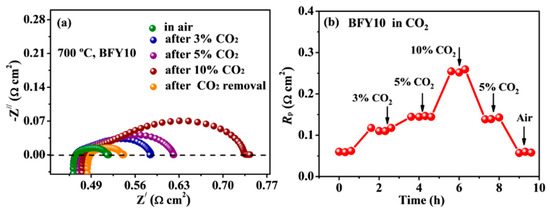
Figure 6.
(a) Impedance spectra and (b) short-term stability of BFY10 cathode in various CO2 concentrations at 700 °C.
To further demonstrate the practical application of the BFYx cathodes, the single cells were fabricated and tested. Figure 7a–c displays the I–V and I–P curves of the single cells with the BFYx cathodes 600–700 °C using humidified hydrogen and air as the fuel and oxidant, respectively. At 700 °C, the peak powder densities of 0.73, 1.1, and 0.89 W cm−2 are achieved in the BFY05, BFY10 and BFY15 cathodes, respectively. It should be noted that the single cell with the BFY10 cathode shows the highest peak powder density among the BFYx electrodes, which is associated with superior electrochemical performance for ORR. In addition, the peak powder density of single cell with the BFY10 cathode is higher than those of other cobalt-free perovskite cathodes [38,39,40,41,42,43,44]. Furthermore, Figure 7d displays the operating stability of the fuel cell with the BFY10 cathode during a period of 120 h. The single cell presents a stable current density and peak power density under an output voltage of 0.5 V without noticeable attenuation, suggesting that the BFY10 electrode has outstanding durability during the operating process. The remarkable electrocatalytic properties indicate that the BFY10 oxide is a highly attractive cathode candidate for SOFCs.
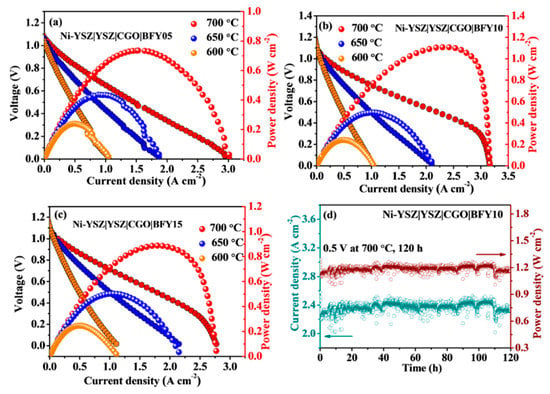
Figure 7.
(a–c) I–V and I–P curves of the single cells with BFYx cathodes at 600–700 °C; (d) Stability test of the single cell with BFY10 cathode under an output voltage of 0.5 V.
4. Conclusions
In summary, Fe-based perovskite BaFe1−xYxO3−δ oxides with excellent ORR performance and CO2 durability are evaluated as the cathode materials for SOFCs. Benefiting from cubic perovskite structure and large oxygen vacancy concentration, the BFY10 cathode presents outstanding electrochemical activity for ORR with a lower RP value of 0.057 Ω cm2 at 700 °C. In addition, the single fuel with the BFY10 cathode delivers a peak powder density of 1.1 W cm−2 at 700 °C, along with negligible attenuation over a period of 120 h. Furthermore, DRT study verifies that the adsorption-dissociation of the oxygen molecule process is the rate-limiting step on the cathode.
Author Contributions
D.M., Q.L., and H.Z. conceived and designed the experiments, and performed the experiments; J.G. and T.X. analyzed the data; L.S. and L.H. contributed reagents/materials/analysis tools; D.M. and Q.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by National Natural Science Foundation of China (51672072, 51972100) and Heilongjiang Provincial Fund for Distinguished Young Scholars (JC2018014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nat. Cell Biol. 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Liu, P.; Luo, Z.F.; Kong, J.R.; Yang, X.F.; Liu, Q.C.; Xu, H. Ba0.5Sr0.5Co0.8Fe0.2O3−δ-based dual-gradient cathodes for solid oxide fuel cells. Ceram. Int. 2018, 44, 4516–4519. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. PrBa0.5Sr0.5Co2O5+δ layered perovskite cathode for intermediate temperature solid oxide fuel cells. Electrochim. Acta 2010, 55, 3812–3816. [Google Scholar] [CrossRef]
- Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Chen, D.; Dong, F.; Park, H.J.; Kwak, C.; Shao, Z. Role of silver current collector on the operational stability of selected cobalt-containing oxide electrodes for oxygen reduction reaction. J. Power Sources 2012, 210, 146–153. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, Z.; Ran, R.; Jin, W.; Xu, N. A novel efficient oxide electrode for electrocatalytic oxygen reduction at 400–600 °C. Chem. Commun. 2008, 44, 5791–5793. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Long, W.; Jin, F.; He, T. Cobalt-free perovskite cathode materials SrFe1−xTixO3−δ and performance optimization for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2014, 123, 426–434. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Knibbe, R.; Khan, M.S.; Peterson, V.K.; Brand, H.E.A.; Li, Z.; Zhou, W.; Zhu, Z. Enhancing Oxygen Reduction Reaction Activity and CO2 Tolerance of Cathode for Low-Temperature Solid Oxide Fuel Cells by in Situ Formation of Carbonates. ACS Appl. Mater. Interfaces 2019, 11, 26909–26919. [Google Scholar] [CrossRef]
- Liou, Y.C.; Chen, Y.R. Synthesis and microstructure of (LaSr)MnO3 and (LaSr)FeO3 ceramics by a reaction-sintering process. Ceram. Int. 2008, 34, 273–278. [Google Scholar]
- Dong, F.; Chen, D.; Chen, Y.; Zhao, Q.; Shao, Z. La-doped BaFeO3−δ perovskite as a cobalt-free oxygen reduction electrode for solid oxide fuel cells with oxygen-ion conducting electrolyte. J. Mater. Chem. 2012, 22, 15071–15079. [Google Scholar] [CrossRef]
- Baiyee, Z.M.; Chen, C.; Ciucci, F. A DFT+U study of A-site and B-site substitution in BaFeO3−δ. Phys. Chem. Chem. Phys. 2015, 17, 23511–23520. [Google Scholar] [CrossRef]
- Wang, J.; Saccoccio, M.; Chen, D.J.; Gao, Y.; Chen, C.; Ciucci, F. The effect of A-site and B-site substitution on BaFeO3−δ: An investigation as a cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2015, 297, 511–518. [Google Scholar] [CrossRef]
- Dong, F.; Ni, M.; He, W.; Chen, Y.; Yang, G.; Chen, D.; Shao, Z. An efficient electrocatalyst as cathode material for solid oxide fuel cells: BaFe0.95Sn0.05O3−δ. J. Power Sources 2016, 326, 459–465. [Google Scholar] [CrossRef]
- Dong, F.; Chen, Y.; Ran, R.; Chen, D.; Tadé, M.O.; Liu, S.; Shao, Z. BaNb0.05Fe0.95O3−δ as a new oxygen reduction electrocatalyst for intermediate temperature solid oxide fuel cells. J. Mater. Chem. A 2013, 1, 9781–9791. [Google Scholar] [CrossRef]
- Wang, J.; Lam, K.Y.; Saccoccio, M.; Gao, Y.; Chen, D.; Ciucci, F. Ca and in co-doped BaFeO3−δ as a cobalt-free cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 324, 224–232. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, M.Z.; Xia, T.; Li, Q.; Li, T.S.; Zhao, H. Ni-doped BaFeO3−δ perovskite oxide as highly active cathode electrocatalyst for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2018, 289, 428–436. [Google Scholar]
- Lu, Y.; Zhao, H.L.; Cheng, X.; Jia, Y.B.; Du, X.F.; Fang, M.Y.; Du, Z.H.; Zheng, K.; Świerczek, K. Investigation of In-doped BaFeO3−δ perovskite-type oxygen permeable membranes. J. Mater. Chem. A 2015, 3, 6202–6214. [Google Scholar] [CrossRef]
- Liu, X.T.; Zhao, H.L.; Yang, J.Y.; Li, Y.; Chen, T.; Lu, X.G. Lattice characteristics, structure stability and oxygen permeability of BaFe1−xYxO3−δ ceramic membranes. J. Membr. Sci. 2011, 383, 235–240. [Google Scholar]
- Lu, Y.; Zhao, H.L.; Chang, X.W.; Du, X.F.; Li, K.; Ma, Y.H.; Yi, S.; Du, Z.H.; Zheng, K.; Świerczek, K. Novel cobalt-free BaFe1−xGdxO3−δ perovskite membranes for oxygen separation. J. Mater. Chem. A 2016, 4, 10454–10466. [Google Scholar]
- Gao, L.; Li, Q.; Sun, L.P.; Zhang, X.F.; Huo, L.H.; Zhao, H.; Grenier, J. A novel family of Nb-doped Bi0.5Sr0.5FeO3−δ perovskite as cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2017, 371, 86–95. [Google Scholar] [CrossRef]
- Lu, F.; Xia, T.; Li, Q.; Wang, J.; Huo, L.; Zhao, H. Heterostructured simple perovskite nanorod-decorated double perovskite cathode for solid oxide fuel cells: Highly catalytic activity, stability and CO2-durability for oxygen reduction reaction. Appl. Catal. B Environ. 2019, 249, 19–31. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, Z.; Xia, T.; Li, Q.; Huo, L.; Zhao, H. Cobalt-free perovskite BaFe0.85Cu0.15O3−δ cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 4784–4791. [Google Scholar] [CrossRef]
- Xia, W.W.; Li, Q.; Sun, L.P.; Huo, L.H.; Zhao, H. Enhanced electrochemical performance and CO2 tolerance of Ba0.95La0.05Fe0.85Cu0.15O3−δ as Fe-based cathode electrocatalyst for solid oxide fuel cells. J. Eur. Ceram. Soc. 2020, 40, 1967–1974. [Google Scholar] [CrossRef]
- Cai, H.D.; Xu, J.S.; Wu, M.; Long, W.; Zhang, L.; Song, Z.Y.; Zhang, L.L. A novel cobalt-free La0.5Ba0.5Fe0.95Mo0.05O3−δ electrode for symmetric solid oxide fuel cell. J. Eur. Ceram. Soc. 2020, 40, 4361–4365. [Google Scholar] [CrossRef]
- Zapata-Ramírez, V.; Mather, G.C.; Azcondo, M.T.; Amador, U.; Pérez-Coll, D. Electrical and electrochemical properties of the Sr(Fe,Co,Mo)O3−δ system as air electrode for reversible solid oxide cells. J. Power Sources 2019, 437, 226895. [Google Scholar] [CrossRef]
- Cai, W.; Guo, Y.; Zhang, T.; Guo, T.; Chen, H.; Lin, B.; Ou, X.; Liu, X. Characterization and polarization DRT analysis of a stable and highly active proton-conducting cathode. Ceram. Int. 2018, 44, 14297–14302. [Google Scholar] [CrossRef]
- Xia, J.; Wang, C.; Wang, X.F.; Bi, L.; Zhang, Y.X. A perspective of DRT analysis for electrodes in solid oxide cells. Electrochim. Acta 2020, 349, 136328. [Google Scholar]
- Li, S.L.; Zhang, L.K.; Xia, T.; Li, Q.; Sun, L.P.; Huo, L.H.; Zhao, H. Synergistic effect study of EuBa0.98Co2O5+δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Alloy. Comp. 2019, 771, 513–521. [Google Scholar] [CrossRef]
- Escudero, M.; Aguadero, A.; Alonso, J.; Daza, L. A kinetic study of oxygen reduction reaction on La2NiO4 cathodes by means of impedance spectroscopy. J. Electroanal. Chem. 2007, 611, 107–116. [Google Scholar] [CrossRef]
- Takeda, Y.; Kanno, R.; Noda, M.; Tomida, Y.; Yamamoto, O. Cathodic Polarization Phenomena of Perovskite Oxide Electrodes with Stabilized Zirconia. J. Electrochem. Soc. 1987, 134, 2656–2661. [Google Scholar] [CrossRef]
- Li, J.; Huo, J.; Lu, Y.; Wang, Q.; Xi, X.; Fan, Y.; Fu, X.Z.; Luo, J.L. Ca-containing Ba0.95Ca0.05Co0.4Fe0.4Zr0.1Y0.1O3−δ cathode with high CO2-poisoning tolerance for proton-conducting solid oxide fuel cells. J. Power Sources 2020, 453, 227909. [Google Scholar] [CrossRef]
- Bucher, E.; Egger, A.; Caraman, G.B.; Sitte, W. Stability of the SOFC cathode materials (Ba,Sr)(Co,Fe)O3−δ in CO2-containing atmospheres. J. Electrochem. Soc. 2008, 155, B1218–B1224. [Google Scholar] [CrossRef]
- Gu, H.; Sunarso, J.; Yang, G.; Zhou, C.; Song, Y.; Zhang, Y.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Turning Detrimental Effect into Benefits: Enhanced Oxygen Reduction Reaction Activity of Cobalt-Free Perovskites at Intermediate Temperature via CO2-Induced Surface Activation. ACS Appl. Mater. Interfaces 2020, 12, 16417–16425. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.; Chen, G.; Ran, R.; Zhou, W.; Shao, Z. Evaluation of the CO2 Poisoning Effect on a Highly Active Cathode SrSc0.175Nb0.025Co0.8O3−δ in the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 3003–3011. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Y.; Shao, Z. An Aurivillius Oxide Based Cathode with Excellent CO2 Tolerance for Intermediate-Temperature Solid Oxide Fuel Cells. Angew. Chem. 2016, 128, 9134–9139. [Google Scholar] [CrossRef]
- Wang, S.F.; Yeh, C.T.; Wang, Y.R.; Hsu, Y.F. Effect of (LaSr)(CoFeCu)O3−δ cathodes on the characteristics of intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 201, 18–25. [Google Scholar] [CrossRef]
- Shi, H.; Ding, Z.; Ma, G. Electrochemical Performance of Cobalt-free Nd0.5Ba0.5Fe1−xNixO3−δ Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. Fuel Cells 2016, 16, 258–262. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, Z.; Zhao, Y.; Chen, M.; Wang, S. Properties characterization of tungsten doped strontium ferrites as cathode materials for intermediate temperature solid oxide fuel cells. Electrochim. Acta 2017, 250, 203–211. [Google Scholar] [CrossRef]
- Song, X.Q.; Le, S.R.; Zhu, X.D.; Qin, L.; Luo, Y.; Li, Y.W.; Sun, K.N.; Chen, Y. High performance BaFe1−xBixO3−δ as cobalt-free cathodes for intermediate temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2017, 42, 15808–15817. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Guo, W.; Guo, R.; Liu, Z.; Sun, D.; Meng, L.; Zheng, M.; Wang, B. Synthesis and electrochemical properties of BaFe1−xCuxO3−δ perovskite oxide for IT-SOFC cathode. Fuel Cells 2016, 16, 829–838. [Google Scholar] [CrossRef]
- Ni, W.; Zhu, T.; Chen, X.; Zhong, Q.; Ma, W. Stable, efficient and cost-competitive Ni-substituted Sr (Ti,Fe)O3 cathode for solid oxide fuel cell: Effect of A-site deficiency. J. Power Sources 2020, 451, 227762. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).