Abstract
The objective of the study was to evaluate the capacity of GERH®-derived local resistance maps (LRMs) to predict antibiotic susceptibility profiles and recommend the appropriate empirical treatment for ICU patients with nosocomial infection. Data gathered between 2007 and 2016 were retrospectively studied to compare susceptibility information from antibiograms of microorganisms isolated in blood cultures, lower respiratory tract samples, and urine samples from all ICU patients meeting clinical criteria for infection with the susceptibility mapped by LRMs for these bacterial species. Susceptibility described by LRMs was concordant with in vitro study results in 73.9% of cases. The LRM-predicted outcome agreed with the antibiogram result in >90% of cases infected with the bacteria for which GERH® offers data on susceptibility to daptomycin, vancomycin, teicoplanin, linezolid, and rifampicin. Full adherence to LRM recommendations would have improved the percentage adequacy of empirical prescriptions by 2.2% for lower respiratory tract infections (p = 0.018), 3.1% for bacteremia (p = 0.07), and 5.3% for urinary tract infections (p = 0.142). LRMs may moderately improve the adequacy of empirical antibiotic therapy, especially for lower respiratory tract infections. LRMs recommend appropriate prescriptions in approximately 50% of cases but are less useful in patients with bacteremia or urinary tract infection.
1. Introduction
Healthcare-associated infections are those developed by patients as result of care received in hospital (known as nosocomial infections) or any other healthcare setting [1]. Nosocomial infections are those that appear at least 72 h after hospital admission and were not previously present or incubating [2,3]. They indicate the care quality delivered by hospitals and are related to increased morbidity and mortality and a longer hospital stay, representing an important public health problem and increasing healthcare costs [4]. Nosocomial infections are estimated to affect 5–10% of patients admitted to hospital [5], although their prevalence varies among departments and hospitals, being three- to five-fold higher in intensive care units (ICUs) than in other hospital areas [6,7]. They are considered to be the sixth cause of death in Europe and the USA, but around one-third of them could be prevented by infection control programs and adequate hygiene measures [8]. The magnitude of the attributable mortality is controversial and depends on the type of infection, the severity of the patient (APACHE II scale), and the length of hospital stay, among many other factors [9,10]. However, the earliest possible prescription of an appropriate empirical antibiotic treatment of nosocomial infections is known to be a key factor to improve the survival of ICU patients [11].
In ICU patients, nosocomial infections are often produced by multi-resistant microorganisms [12], complicating prescribing decisions. The scant development of new active ingredients has prompted novel strategies to extend the usefulness of existing antibiotics against severe infections [13], including the local study of bacterial resistance phenotypes. This allows a more precise selection of antibiotics based on local knowledge of the microorganisms most frequently responsible for infection in each hospital area [14].
Since 2012, Spanish hospitals have implemented programs to optimize the use of antibiotics (Programas de Optimización del Uso de Antimicrobianos (PROA)). The main objectives are to improve the clinical outcomes of patients with infections; minimize antimicrobial-related adverse effects, including the development and spread of resistance; and promote more effective treatments with lower health costs. PROA recommendations include accessibility to microbiological data, knowledge of bacterial resistance percentages (especially in the hospital area), and the implementation of measures to assist prescribing decisions. Computerized clinical decision support systems have proven useful to meet these objectives [13].
PROA implementation in our hospital (Torrecardenas Hospital Complex, Almeria, Spain) led to the development in 2014 of a computerized clinical decision support program for the prescription of antimicrobials, named in Spanish the Guía Electrónica de Resistencias Hospitalarias (GERH®). Using this system, updated identification and susceptibility data from microbiological studies in all hospitalized patients can be rapidly communicated to physicians using a secure hospital intranet system. Two GERH®-based applications were launched: local resistance maps (LRMs) and preliminary microbiological reports with therapeutic recommendations (PMRTRs). To date, these applications have only been available to ICU physicians.
The objective of this study was to evaluate the capacity of LRMs to predict antibiotic susceptibility and resistance profiles and improve the appropriateness of empirical treatments for ICU patients with nosocomial infections, including bacteremia and/or lower respiratory or urinary tract infections.
2. Methods
GERH® is based on the Microsoft.NET Framework with Visual C# and SQL and Open Database Connectivity (ODBC) to the laboratory information system (Sistema de Información de Laboratorio (SIL)) of the hospital microbiology laboratory. It is installed on a central server and has been used by ICU physicians since 2014 to consult all susceptibility results stored in the SIL since 2006. The data are organized according to the hospital department, date/date interval, sample type, microorganism/s isolated, and antimicrobials tested, and graphs are created for the ready visualization and interpretation of these data [15].
2.1. Local Resistance Maps (LRMs)
LRMs (Figure 1) graphically depict information on the frequency of isolated microorganisms (Figure 1A), bacterial susceptibility (Figure 1B–D), and antibiotic activity (Figure 1E). The physicians select and access the graphs via touch screens connected to the hospital intranet. Data are automatically updated every 24 h to include new records from the central GERH® server [15].
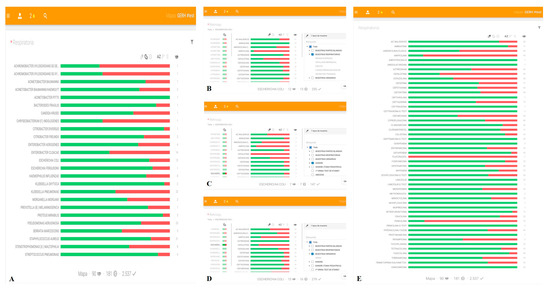
Figure 1.
GERH®-derived local resistance maps (LRMs). The search criteria in LRMs offer different types of graph. As an example, this figure depicts the following: (A) graph showing the frequency of isolation of each bacterial species in ICU patients with lower respiratory tract infection between 1 January and 31 December 2015 alongside the accumulated susceptibility; (B) graph showing the accumulated susceptibility of E. coli in isolates obtained in respiratory samples (bronchial aspirate, bronchial brushing, sputum, bronchoalveolar lavage or tracheal secretion) of ICU patients between 1 January and 31 December 2015; (C) graph showing the accumulated susceptibility of E. coli in isolates obtained in blood samples of ICU patients between 1 January and 31 December 2015; (D) graph showing the accumulated susceptibility of E. coli in isolates obtained from urine samples of ICU patients between 1 January and 31 December 2015; and (E) graph showing the activity of various antimicrobials, considering the group of microorganisms isolated in respiratory samples of ICU patients between 1 January and 31 December 2015, in which green indicates the percentage of microorganisms of the total tested in which each antibiotic was active. In (A–D), the percentage of susceptible bacteria is represented using a color code: green for the percentage of susceptible isolates and red for the percentage of isolates with intermediate or resistant clinical category.
The LRMs were derived from analyses of the outcomes obtained in all in vitro susceptibility assays for bacteria isolated in ICU patients with bacteremia, lower respiratory tract infection, or urinary tract infection within a defined time interval, commonly the 12-month period before the consultation. The graphs depict accumulated information for the selected time period on the antibiotic susceptibility profile of isolated bacteria, indicating the likelihood that the infection in question is caused by specific bacteria as well as the expected activity of antibiotics against them. This allows the ICU physician to make informed decisions about the treatment of cases based on the local bacterial epidemiology and on the predicted susceptibility profile of the bacteria isolated.
This instrument followed the recommendation of the Spanish Society of Infectious Diseases and Clinical Microbiology (Spanish abbreviation: SEIMC) for accumulated reports on antimicrobial susceptibility to include solely microorganisms obtained from human clinical samples with susceptibility results verified by clinical microbiologists. When the same microorganisms are isolated multiple times in the same patient, those with a change in their resistance phenotype to one or more antibiotics are considered [16].
2.2. Study Design
This retrospective study compared the concordance obtained each year from 2007 to 2016 between susceptibility data from the antibiograms of microorganisms isolated in blood cultures, respiratory samples (bronchial aspirate, bronchial brushing, sputum, bronchoalveolar lavage, and/or tracheal secretion), and/or urine samples from all ICU patients meeting clinical criteria for infection and the susceptibility data depicted by LRMs for the same bacterial species, based on accumulated data for the whole year. In the LRMs, bacteria were defined as susceptible to an antibiotic when at least 75% of clinical isolates of this bacterial species were susceptible according to in vitro tests [15]. Concordance was defined as agreement between the susceptibility evaluated by LRMs and the susceptibility obtained in the in vitro study in the microbiology laboratory, i.e. when bacteria were considered as susceptible or resistant by both methods, including “intermediate resistance” within the “resistant” category. There was no concordance when the bacteria were considered susceptible by one approach and resistant by the other. Duplicate bacteria with the same identification and antibiogram were excluded, whether obtained from the same sample or isolated in multiple samples from the same patient.
The adequacy of empirical antibiotic treatments was also retrospectively studied, considering them appropriate when active against the bacterial pathogen causing the infection [13]. Accordingly, we determined whether each antibiotic prescribed was active against the bacteria isolated in the different clinical samples from each patient, based on the antibiogram data. The percentage adequacy of empirical prescriptions if they had been based on LRM recommendations was calculated as the number of times that LRM results for the susceptibility of a bacterium to each antibiotic agreed with the result of the susceptibility study as a percentage of the total number of isolates tested. The adequacy of the actual empirical treatment prescribed by physicians was evaluated with reference to the activity of the antibiotic(s) against each bacterium isolated in the different clinical samples according to the corresponding antibiograms. Data on the antibiotics prescribed in ICU patients were retrospectively obtained from the Spanish national nosocomial infection surveillance program (Spanish abbreviation: ENVIN).
Although the instrument is available in the ICU, therapeutic decisions do not have to be based on the data it provides. For this reason, we did not consider or gather data on the compliance of prescribed treatments with LRM recommendations.
2.3. Bacteria Selection
Study inclusion criteria for bacteria were: (i) isolation in blood, respiratory, or urine samples; (ii) definitive microbiological species identification; and (iii) belonging to one of the following bacterial groups: (a) Enterobacteriaceae (Citrobacter spp., Enterobacter spp., Escherichia spp., Klebsiella spp., Morganella spp., Proteus spp., Providencia spp., or Serratia spp.); (b) non-fermenting Gram-negative bacilli (Acinetobacter spp., Pseudomonas spp., and Stenotrophomonas spp.); (c) coagulase-positive staphylococci (Staphylococcus aureus), and coagulase-negative staphylococci (Staphylococcus auricularis, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus intermedius, Staphylococcus saprophyticus, Staphylococcus simulans, and Staphylococcus warneri); (d) Streptococcus pneumoniae; (e) enterococci (Enterococcus faecalis and Enterococcus faecium); or (f) Haemophilus spp.
2.4. Antibiotic Selection
The following antibiotics were included in relation to the above bacteria: (a) amikacin, amoxicillin-clavulanic acid, aztreonam, cefepime, cefotaxime, ceftazidime, ceftriaxone, cefuroxime, ciprofloxacin/levofloxacin, gentamicin, imipenem, meropenem, piperacillin-tazobactam, and tobramycin in Enterobacteriaceae; (b) amikacin, cefepime, ceftazidime, ciprofloxacin/levofloxacin, colistin, gentamicin, imipenem, meropenem, piperacillin-tazobactam, and tobramycin in non-fermenting Gram-negative bacilli; (c) ciprofloxacin/levofloxacin, clindamycin, daptomycin, erythromycin, gentamicin, linezolid, oxacillin, rifampicin, teicoplanin, tobramycin, and vancomycin in staphylococci; (d) cefotaxime, levofloxacin, linezolid, and penicillin in S. pneumoniae; (e) ampicillin, levofloxacin, daptomycin, linezolid, teicoplanin, and vancomycin in enterococci; and (f) amoxicillin-clavulanic acid, ampicillin, cefotaxime, ciprofloxacin/levofloxacin, and erythromycin in Haemophilus spp.
2.5. Study Variables
Data gathered from antibiogram results, LRMs, and the ENVIN platform were: type of infection, date of sample gathering, type of sample (blood, respiratory, or urine), bacteria identified in each sample, antibiogram of the microorganism(s) isolated, concordance between antibiogram and LRM data, empirical antibiotic treatment prescribed, and concordance with the antibiogram result.
2.6. Statistical analysis
In the statistical analysis, the chi-square test was used to compare the adequacy of the actual empirical treatment with the adequacy of the LRM-recommended treatment, considering p < 0.05 to be significant.
3. Results
3.1. Concordance Between LRMs and Susceptibility In Vitro
Table 1 compares the susceptibility data for each bacterium and antibiotic according to in vitro studies with those provided by LRMs after analyzing the information accumulated during the previous year. During the study period (2007–2016), the results of 22,520 in vitro trials were compared to the LRM data, obtaining an average concordance of 73.9%. In other words, the susceptibility or resistance described by LRMs for each bacterium–antibiotic association agreed with the in vitro study results in 73.9% of cases.

Table 1.
Percentage concordance between bacterial susceptibility shown by LRMs and susceptibility obtained from antibiograms by year, bacterial group, and antibiotic.
For enterobacteria, the concordance ranged from a mean of 95.9% for amikacin over the study period (range 89.7–100%) to mean of 63.6% (range 47.6–86.7%) for ciprofloxacin/levofloxacin. In other words, when an enterobacterium was isolated, the expected susceptibility outcome was the same according to both the LRM and antibiogram in 95.9% of cases for amikacin and in 63.6% of cases for ciprofloxacin/levofloxacin. An intermediate degree of concordance was obtained for the other antibiotics studied.
For non-fermenting Gram-negative bacilli, the concordance widely varied among different antibiotics, obtaining the highest percentage agreement for colistin (88.3%; range 87.5–97.3%) and tobramycin (86.2%; range 58.2–100%) and lower degrees of concordance for amikacin (76.3%; range 42.9–100%), cefepime (54.9%; range 31.3–86.4%), ceftazidime (53.1%; range 28.2–68.8%), ciprofloxacin/levofloxacin (68.8%; range 43.2–88.3%), gentamicin (62.1%; range 34.3–85.0%), piperacillin-tazobactam (60.6%; range 20.0–96.6%), imipenem (49.4%; range 32.4–62.3%), and meropenem (50,7%; range 36.0–71.4%).
For staphylococci, the highest concordance was obtained for daptomycin (99.7%; range 99.0–100%), followed by vancomycin (98.7%; range 95.2–100%), linezolid (92.9%; range 79.4–100%), teicoplanin (92.2%; range 82.1–100%), and rifampicin (90.9%; range 53.6–99.0%). A lower percentage agreement was found for ciprofloxacin/levofloxacin (64.6%; range 54.8–88.6%), clindamycin (60.2%; range 39.6–79.7%), erythromycin (71.0%; range 63,9–84.1%), gentamicin (68.1%; range 53.4–83.1%), oxacillin (71.9%; range 59.5–91.7%), and tobramycin (59.1%; range 50.5–69.7%).
For the remaining bacteria under study (S. pneumoniae, enterococci and Haemophilus spp.), there were few comparative data and the mean percentage agreement was generally high but showed a very wide range.
Considering all bacteria and antibiotics included in the 22,520 comparisons conducted during the study period, the highest percentage concordance between LRMs and antibiograms was observed for daptomycin (99.7%; range 99.0–100%), vancomycin (98.7%; range 95.5–100%), teicoplanin (92.5%; range 83.0–100%), linezolid (92.3%; range 79.3–100%), and rifampicin (90.9%; range 53.6–99.0%). In summary, the susceptibility data offered by LRMs for bacteria on which the GERH® has this information agrees with the antibiogram result in >90% of cases.
Table 2 compares the in vitro and LRM susceptibility data for each bacterium and antibiotic by year and by infection type. The mean percentage concordance was 73.5% in lower respiratory tract infections (range 66.5–80.0%), 69.3% in urinary tract infections (range 54.7–81.6%), and 76.1% in bacteremia (range 68.9–80.8%). These findings indicate that the susceptibility information provided by LRMs for these infections is in agreement with the actual susceptibility of the isolated bacteria in 73.5%, 69.3%, and 76.1% of cases, respectively.

Table 2.
Percentage of concordance between bacterial susceptibility depicted by LRMs and that obtained from antibiograms by year and type of infection.
3.2. Adequacy of Actual Empirical Prescription and Susceptibility Obtained in the Antibiogram
Table 3 displays the percentage adequacy of LRM-recommended empirical prescriptions for bacteria in relation to the actual susceptibility observed for them. Antibiotics with a percentage adequacy >80% in the empirical antibiotic prescription were amikacin, colistin, daptomycin, linezolid, teicoplanin, and vancomycin. Thus, in relation to amikacin, out of 981 isolates of enterobacteria or non-fermenting Gram-negative bacilli isolated in samples, 904 were susceptible to this antibiotic and 77 were resistant, while its use was recommended by LRMs in 854 of cases, giving a percentage adequacy of 87.1%. Accordingly, if amikacin had been used as empirical treatment when recommended by LRMs, this treatment would have been appropriate in 87.1% of cases in which enterobacteria or non-fermenting Gram-negative bacilli were isolated. For daptomycin, linezolid, teicoplanin, and vancomycin the LRM-recommended treatment would have been appropriate in 99.7%, 92.0%, 92.2%, and 98.7% of cases in which a Gram-positive coccus (staphylococcus, enterococcus, or pneumococcus) was isolated.

Table 3.
Percentage adequacy of the empirical prescription of an antibiotic if based on LRM recommendations with reference to the actual susceptibility of isolated bacteria.
Table 4 exhibits the percentage adequacy of empirical antibiotic prescriptions if they had followed LRM recommendations, being 57.6% for lower tract respiratory infections, 41.4% for bacteremia, and 54.9% for urinary tract infections. Table 5 lists the percentage adequacy of the empirical antibiotics actually prescribed, being 55.4% for lower respiratory tract infections, 38.3% for bacteremia, and 49.6% for urinary tract infections. Hence, if LRM recommendations had always been followed in the ICU, the percentage adequacy of prescriptions would have been improved by 2.2% for lower respiratory tract infections (57.6% vs. 55.4%; p = 0.018), 3.1% for bacteremia (41.4% vs. 38.3%; p = 0.070), and 5.3% for urinary tract infections (54.9% vs. 49.6%; p = 0.142).

Table 4.
Percentage adequacy of the empirical prescription of an antibiotic if based on LRM recommendations with reference to the actual susceptibility of isolated bacteria by year and by type of infection.

Table 5.
Percentage adequacy of empirical antibiotic treatment by year and by type of infection.
4. Discussion
A major factor in the emergence of bacterial resistances is the inappropriate prescription of antibiotics [17], estimated to represent 30–50% of all antibiotic prescriptions [18]. For this reason, analysis of the antibiotic susceptibility of microorganisms is not only of major epidemiological and clinical importance but provides invaluable support for prescribing decisions. The use of computerized systems based on laboratory susceptibility results assists physicians in the selection of treatments without replacing their own clinical judgement. Various studies have demonstrated that these systems can improve healthcare, reduce inappropriate prescriptions and pharmaceutical costs, monitor antibiotic resistances, and diminish the morbidity and mortality of patients [15,19,20,21].
GERH® is integrated within the routine clinical workflow of our ICU, offering a predictive model that provides timely recommendations [13] and is designed to increase the percentage of patients who receive appropriate empirical antibiotic therapy, as recommended in previous studies [22,23]. According to the present findings, LRM-recommended prescriptions would have been appropriate in terms of the susceptibility of isolated bacteria in 57.6% of lower respiratory tract infection cases, 41.4% of bacteremia cases, and 54.9% of urinary tract infection cases. Higher percentages were published for these infections in the ENVIN study (2018), ranging between 63% and 72% [24]. Nevertheless, the use of LRMs in our ICU would have significantly improved the adequacy of empirical treatment prescriptions in lower respiratory tract infections by 2.2% (p = 0.018), although no significant improvement would have been achieved in the cases of bacteremia (3.1%; p = 0.070) or urinary tract infection (5.3%; p = 0.142). These improvements are modest but similar to previous reports [25,26,27], contributing to evidence that these systems can assist clinical decision-making and improve the adequacy of empirical antibiotic treatments, as previously affirmed [28].
Therapeutic recommendations are provided by LRMs before the responsible microorganism has been defined, and their percentage adequacy is less than when recommendations are made after identifying the etiological agent but before testing its susceptibility [29]. This is the case with PMRTRs, another GERH® instrument, whose prescription recommendations were reported to be appropriate in >82% of cases and to achieve an improvement of 40% in the adequacy of prescriptions for each clinical situation, as we noted in a previous publication [15].
The main study limitation was that it did not consider whether or not physicians had consulted LRMs (available since 2014) before prescribing antibiotics, preventing assessment of the impact of LRM consultations over time on the adequacy of empirical antibiotic therapies. LRMs were designed to inform clinicians about the local epidemiology related to nosocomial infections, allowing them to base empirical antibiotic prescriptions on the likelihood of infection with a specific microorganism and on the accumulated activity of different antibiotics against bacteria isolated in a given focus. The aim was not to replace the judgment of clinicians, which may or may not coincide with LRM recommendations. For this reason, clinicians were not asked to state whether or not their prescription followed these recommendations, thereby preserving their prescribing autonomy. As a novel instrument, an adaptation period can be expected before it is accepted and implemented by physicians, who are also influenced by the perception of resulting improvements in antibiotic prescribing and outcomes.
As currently designed, LRMs do not yield information in relation to other PROA objectives such as the improvement in clinical outcomes and the reduction in antibiotic resistance rates, adverse effects, pharmacological interactions, antibiotic consumption, or pharmaceutical costs. These instruments could be improved by the incorporation of new functionalities that monitor and respond to these objectives and tailor empirical therapy recommendations to the clinical situation of each patient. For instance, prescription decisions could be further supported by integrating data for each patient on clinical observations, laboratory results (biochemistry and microbiology), radiology findings, and/or the concentrations of antibiotics in each tissue sample [30], along with the antibiotic susceptibility data.
5. Conclusions
Although GERH®-derived LRMs proved to have a high capacity to predict antibiotic susceptibility and resistance profiles, they produce only a moderate improvement in the adequacy of empirical antibiotic therapy, which is significantly greater in cases of lower respiratory tract infections. According to these findings, LRMs are useful to recommend appropriate prescriptions in approximately 50% of cases but less so in patients with bacteremia or urinary tract infections.
Author Contributions
Conceptualization, J.G.-F., M.A.R.-M., and A.S.-P.; Methodology, P.N.-G., M.A.R.-M., and M.C.O.-P.; Formal Analysis, M.C.O.-P.; Investigation, P.N.-G. and M.A.R.-M.; Validation, J.G.-F., M.A.R.-M., and A.S.-P.; Writing—Original Draft Preparation: PNG, A.S.-P.; and Writing—Review and Editing: J.G.-F., M.A.R.-M. and A.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation received no specific grant from agencies of public, commercial, or nonprofit sectors.
Conflicts of Interest
The authors declare no conflict of interest
References
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care—Associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, R.; Ramírez, P.; López-Pueyo, M.J. Nosocomial infections in intensive care units. Enferm. Infecc. Microbiol. Clin. 2014, 32, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Sydnor, E.R.; Perl, T.M. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef]
- Raka, L.; Mulliqi-Osmani, G. Infection control in developing world. In Infection Control—Updates, 1st ed.; Sudhakar, C., Ed.; IntechOpen: London, UK, 2012; pp. 65–78. [Google Scholar]
- Capdevila Morell, J.A. Empiric use of antibiotics in nosocomial infections. Rev. Clin. Esp. 2008, 208, 423–425. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, L.D.; Ponce de León Rosales, S.; Rangel Frausto, M.S. The burden of nosocomial infection in the intensive care unit: Effects on organ failure, mortality and costs. A nested case-control study. Arch. Med. Res. 2006, 37, 370–375. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Olaechea, P.M.; Álvarez-Lerma, F.; Palomar, M.; Gimeno, R.; Gracia, M.P.; Mas, N.; Rivas, R.; Seijas, I.; Nuvials, X.; Catalán, M.; et al. Characteristics and outcomes of patients admitted to Spanish ICU: A prospective observational study from the ENVIN-HELICS registry (2006-2011). Med. Intensiva 2016, 40, 216–229. [Google Scholar] [CrossRef]
- Alvarez-Lerma, F.; Alvarez, B.; Luque, P.; Ruiz, F.; Dominguez-Roldan, J.M.; Quintana, E.; Sanz-Rodriguez, C.; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: A prospective observational study. Crit. Care 2006, 10, R78. [Google Scholar] [CrossRef]
- López-Pueyo, M.J.; Barcenilla-Gaite, F.; Amaya-Villar, R.; Garnacho-Montero, J. Antibiotic multiresistance in critical care units. Med. Intensiva 2011, 35, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Paño-Pardo, J.R.; Alvarez-Rocha, L.; Asensio, Á.; Calbo, E.; Cercenado, E.; Cisneros, J.M.; Cobo, J.; Delgado, O.; Garnacho-Montero, J.; et al. Programs for optimizing the use of antibiotics (PROA) in Spanish hospital: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Farm. Hosp. 2012, 36, e1–e30. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H. Inadequate antimicrobial treatment: An important determinant of outcome for hospitalized patients. Clin. Infect. Dis. 2000, 31, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Maresca, M.; Sorlózano, A.; Grau, M.; Rodríguez-Castaño, R.; Ruiz-Valverde, A.; Gutiérrez-Fernández, J. Implementation of a computerized decision support system to improve the appropriateness of antibiotic therapy using local microbiologic data. Biomed. Res. Int. 2014, 2014, 395434. [Google Scholar] [CrossRef] [PubMed]
- Calvo Montes, J.; Canut Blasco, A.; Martínez-Martínez, L.; Rodríguez Díaz, J.C. Preparación de informes acumulados de sensibilidad a los antimicrobianos. In Procedimientos en Microbiología Clínica, 2nd ed.; Cercenado Mansilla, E., Cantón Moreno, R., Eds.; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC): Madrid, Spain, 2014. [Google Scholar]
- Cantón, R.; Horcajada, J.P.; Oliver, A.; Garbajosa, P.R.; Vila, J. Inappropriate use of antibiotics in hospitals: The complex relationship between antibiotic use and antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. 2013, 31 (Suppl. 4), 3–11. [Google Scholar]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- Evans, R.S.; Pestotnik, S.L.; Classen, D.C.; Clemmer, T.P.; Weaver, L.K.; Orme, J.F., Jr.; Lloyd, J.F.; Burke, J.P. A computer-assisted management program for antibiotics and other antiinfective agents. N. Engl. J. Med. 1998, 338, 232–238. [Google Scholar] [CrossRef]
- Pestotnik, S.L. Expert clinical decision support systems to enhance antimicrobial stewardship programs: Insights from the society of infectious diseases pharmacists. Pharmacotherapy 2005, 25, 1116–1125. [Google Scholar] [CrossRef]
- Thursky, K.A.; Buising, K.L.; Bak, N.; Macgregor, L.; Street, A.C.; Macintyre, C.R.; Presneill, J.J.; Cade, J.F.; Brown, G.V. Reduction of broad-spectrum antibiotic use with computerized decision support in an intensive care unit. Int. J. Qual. Health Care 2006, 18, 224–231. [Google Scholar] [CrossRef]
- Forrest, G.N.; Van Schooneveld, T.C.; Kullar, R.; Schulz, L.T.; Duong, P.; Postelnick, M. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin. Infect. Dis. 2014, 59, S122–S133. [Google Scholar] [CrossRef]
- Simões, A.S.; Maia, M.R.; Gregório, J.; Couto, I.; Asfeldt, A.M.; Simonsen, G.S.; Póvoa, P.; Viveiros, M.; Lapão, L.V. Participatory implementation of an antibiotic stewardship programme supported by an innovative surveillance and clinical decision-support system. J. Hosp. Infect. 2018, 100, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC). Grupo de Trabajo de Enfermedades Infecciosas y Sepsis (GTEIS). Estudio Nacional de Vigilancia de Infección Nosocomial en Servicios de Medicina Intensiva. Available online: http://hws.vhebron.net/envin-helics/ (accessed on 23 September 2019).
- Linder, J.A.; Schnipper, J.L.; Tsurikova, R.; Yu, D.T.; Volk, L.A.; Melnikas, A.J.; Palchuk, M.B.; Olsha-Yehiav, M.; Middleton, B. Electronic health record feedback to improve antibiotic prescribing for acute respiratory infections. Am. J. Manag. Care 2010, 16, e311–e319. [Google Scholar] [PubMed]
- Mainous, A.G., 3rd; Lambourne, C.A.; Nietert, P.J. Impact of a clinical decision support system on antibiotic prescribing for acute respiratory infections in primary care: Quasi-experimental trial. J. Am. Med. Inform. Assoc. 2013, 20, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mullett, C.J.; Thomas, J.G.; Smith, C.L.; Sarwari, A.R.; Khakoo, R.A. Computerized antimicrobial decision support: An offline evaluation of a database-driven empiric antimicrobial guidance program in hospitalized patients with a bloodstream infection. Int. J. Med. Inform. 2004, 73, 455–460. [Google Scholar] [CrossRef]
- Carracedo-Martinez, E.; Gonzalez-Gonzalez, C.; Teixeira-Rodrigues, A.; Prego-Dominguez, J.; Takkouche, B.; Herdeiro, M.T.; Figueiras, A.; Galician Pharmacoepidemiology Research Group. Computerized clinical decision support systems and antibiotic prescribing: A systematic review and meta-analysis. Clin. Ther. 2019, 41, 552–581. [Google Scholar] [CrossRef]
- Leibovici, L.; Gitelman, V.; Yehezkelli, Y.; Poznanski, O.; Milo, G.; Paul, M.; Ein-Dor, P. Improving empirical antibiotic treatment: Prospective, nonintervention testing of a decision support system. J. Intern. Med. 1997, 242, 395–400. [Google Scholar] [CrossRef][Green Version]
- Navarro-Gómez, P.; Sorlózano-Puerto, A.; Olmo-Navas, M.M.; Nieto-Guindo, P.; Dueñas-Alcalá, R.; Gutiérrez-Fernández, J.; Romero-González, R.; Rodriguez-Maresca, M.A. Assessment of adherence to antibiotic treatment in Primary Care by determining levels of the drug using a liquid chromatography technique. Rev. Esp. Quimioter. 2017, 30, 341–349. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).