Abstract
Ceftazidime-avibactam (CZA) is a novel beta-lactam beta-lactamase inhibitor combination approved for the treatment of complicated urinary tract infections, complicated intra-abdominal infections, and for hospital-acquired/ventilator-associated pneumonia. The aim of this systematic review (PROSPERO registration number: CRD42019128927) was to evaluate the effectiveness of CZA combination therapy versus CZA monotherapy in the treatment of severe infections. The databases included in the search, until 12 February 2020, were MEDLINE by PubMed, EMBASE, and The Cochrane Central Register of Controlled Trials. We included both randomized controlled trials (RCTs) and non-randomized studies published in peer-reviewed journals and in the English language. The primary outcome was all-cause mortality (longest follow-up) evaluated in patients with the diagnosis of infection with at least one pathogen; secondary outcomes were clinical and microbiological improvement/cure. Thirteen studies were included in the qualitative synthesis: 7 RCTs and 6 retrospective studies All the six retrospective studies identified carbapenamase-producing Enterobacteriaceae (CRE) as the cause of infection and for this reason were included in the network meta-analysis (NMA); the quality of the studies, assessed using the New Castle-Ottawa Scale, was moderate-high. In all the six retrospective studies included in the NMA, CZA was used in large part for off-label indications (mostly blood stream infections: 80–100% of patients included). No difference in mortality rate was observed in patients undergoing CZA combination therapy compared to CZA monotherapy [n = 503 patients, direct evidence OR: 0.96, 95% CI: 0.65–1.41].
1. Introduction
Ceftazidime-avibactam (CZA) is a combination of the third-generation cephalosporin ceftazidime and a novel non-beta-lactam beta-lactamase inhibitor avibactam. It is a first line therapy for difficult-to-treat infections due to Gram-negative bacilli (GNB) [1]. The prevalence of resistant GNB, including carbapenem-resistant Enterobacteriaceae (CRE) and Pseudomonas aeruginosa, is increasing worldwide. Infections due to CRE are associated with a mortality rate up to 50% [2]. CZA treatment indications are similar in the United States (US) and Europe; the U.S. Food and Drug Administration (FDA) approved CZA in 2015 for the treatment of complicated intra-abdominal infections (cIAI), in combination with metronidazole, and for complicated urinary tract infections (cUTI), including pyelonephritis, in adult patients with limited or no alternative treatment options [3]. The European Medicines Agency (EMA) approved CZA in 2016 for the treatment of adults with cUTIs, cIAIs (in association with metronidazole or an antibacterial agent active against Gram-positive pathogens), and hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP) [4]. Several studies reported the use of CZA in difficult-to-treat CRE infections, mostly bloodstream infections (BSIs), alone or in combination of other antibiotics [5,6,7,8]. The CZA off-label use in BSIs may be due to the lack of effective therapeutic options. Network meta-analysis (NMA), also known as multiple treatment meta-analysis, has been increasingly used in recent years with the aim to simultaneously compare the effects of multiple treatments on a health-related outcome [9,10]. We performed a systematic review and NMA to compare the effectiveness of CZA mono versus combination therapy with other antibiotics in terms of mortality in patients with CRE infections.
2. Methods
The protocol was prospectively registered in PROSPERO (CRD42019128927) on April 16, 2019 [11], after a search of the main electronic registries (Cochrane Database of Systematic Reviews, the JBI Database of Systematic Reviews and Implementation Reports and PROSPERO), to exclude overlap. The present systematic review was conducted according to PRISMA methodology [12].
2.1. Study Search
Table 1 shows the review question according to the PICOS format. The databases of the search included MEDLINE via PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The full search strategies are reported in the Table S1. The first search was performed until 4 April 2019; the search was re-run, updating the data collection definitively until 12 February 2020.

Table 1.
PICOS method for selecting clinical studies in the systematic reviews.
2.2. Study Selection
We included both randomized controlled trials (RCTs) and nonrandomized studies (both prospective and retrospective), published in English language and in peer-reviewed journals. No restriction on time of publication was applied.
Figure 1 shows the inclusion–exclusion process according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. After the searches, duplicates were removed using a citation management software (Endnote VX9. Clarivate Analytics, PA, USA) and a list of all included studies was created. Two authors (SDF and GI) independently performed a screening of retrieved articles based on titles and abstract. The same authors independently evaluated the full texts of the selected articles for final inclusion. The standardized reasons for exclusion were recorded.
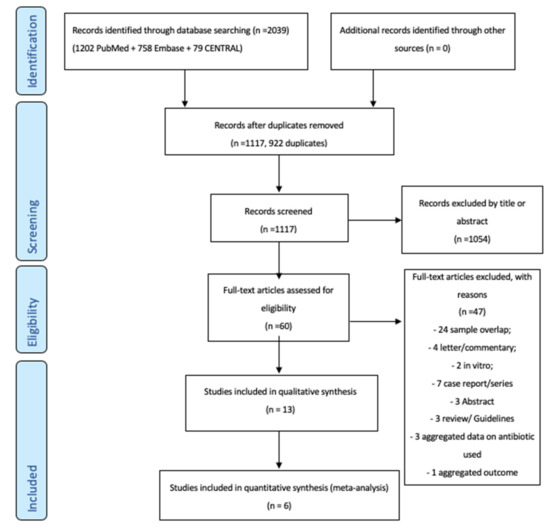
Figure 1.
The flow-chart of the study selection.
Any disagreements on study eligibility or data extraction were resolved according to a third reviewer’s opinion (AC).
2.3. Definition and Outcome
The primary outcome was all-cause mortality evaluated at the longest reported follow-up (if multiple time-points were considered by the authors of the included studies) in the microbiologically evaluable population. The microbiologically evaluable population included all patients who had a diagnosis of infection with at least one pathogen, as confirmed by the laboratory. We did not consider patients with no culture or negative culture. The secondary outcomes were the non-microbiological improvement and the non-clinical improvement.
2.4. Data Extraction and Quality Assessment
Data were extracted from studies included in the review by two reviewers independently (AC, MF) using the Cochrane data collection form for intervention reviews for RCTs and non-RCTs [13]. Two authors assessed the methodological quality of the included studies (AC, MF). The risk of bias of enrolled RCTs was evaluated using the Cochrane Collaboration Revised Assessment Tool (RoB 2) [14]. The quality of nonrandomized studies was assessed using the Newcastle-Ottawa Assessment Scale (NOS) [15].
2.5. Data Analysis
We used NMA for synthesizing information and pooled estimates based on the frequentist approach, using the statistical package ‘netmeta’ (version 1.2-0) in R [16]. For a given comparison (A vs B), direct evidence is usually provided by studies that compare these treatments directly. The NMA function provides a back-calculation method to derive indirect estimates from direct pairwise comparisons and combines both direct and indirect evidence across a network of studies into a single effect size. Q and I2 statistics were used to quantify heterogeneity among included studies, with I2 < 50% indicating no heterogeneity and I2 ≥ 50% indicating significant heterogeneity. A random-effects model was conducted in case of significant heterogeneity; otherwise, a fixed-effect model was applied. Q statistic was evaluated for both heterogeneity (within designs) and inconsistency (between designs). Plot of direct evidence proportion for each network estimate was proposed with minimal parallelism and mean path length metrics [17]. Furthermore, a net heat plot was used to evaluate cases of inconsistency between direct and indirect evidence [18].
The main outcome of this NMA and of each single study was mortality. This outcome was pooled as odds ratio (OR) with 95% confidence interval (CI). The ranking probability P-score was used to rank different treatments, where a larger value indicates better performance. This pooled outcome was furthermore analyzed using node splitting method which allows us to control for inconsistency in specific comparisons in our network and graphically represented with a forest plot. All analyses were performed using R software version 3.6.2.
3. Results
3.1. Study Selection and Characteristics
We retrieved 2039 articles from the database searches: 1202 on PubMed, 758 on EMBASE, and 79 on CENTRAL. After the removal of 922 duplicates, 1117 articles were identified as potentially relevant and screened as shown in the flow chart (Figure 1). After the screening of titles and abstracts, the full text of 60 articles were evaluated. We further excluded 47 studies for 8 main reasons (Figure 1). Thirteen studies were finally included in the qualitative synthesis (Table 2) and 6 studies in the final meta-analysis (Table 3).

Table 2.
Summary of the studies included in the qualitative synthesis.

Table 3.
Summary of the characteristics of enrolled studies in the meta-analysis.
Of the 13 studies included in the qualitative synthesis, of these, 7 were RCT and 6 retrospective cohort studies. The overall risk of bias of the 7 RCT, was low (Supplementary Table S2); the quality of the 6 retrospective studies, assessed using the New Castle-Ottawa Scale [15], was moderate-high (Supplementary Table S3). Only 2 of the 7 RCT evaluated mortality as outcome [19,20], with a total of 261 patients treated with CZA and 266 not treated with CZA. The microbiological outcome (microbiological non improvement/cure) and the clinical outcome (non clinical improvement/cure) were evaluated in all the 7 RCT. The microbiological outcome, in patients undergoing a pathogen-directed therapy, was evaluated in 2366 patients (1162 patients treated with a CZA-based therapy and 1204 patients treated with other antibiotic treatment). The clinical outcome was evaluated in a total of 2615 patients (1277 patients treated with a CZA-based therapy and 1338 patients treated with other antibiotic treatment).
Three of the 7 RCT enrolled patients with complicated intra-abdominal infections (cIAI) [21,22,23], two enrolled patients with complicated urinary tract infection (cUTI) [24,25], and one enrolled patient with nosocomial pneumonia (NP) [18]. One RCT enrolled patient with both cUTI or cIAI [19]. The 7 RCT enrolled patients infected by bacteria not exclusively belonging to CRE.
Conversely, in all the retrospective studies, the enrolled patients were infected by CRE only; in 4 retrospective studies, the authors enrolled patients with infections caused by all CRE [6,8,26,27] and in 2 studies, only Klebsiella pneumoniae carbapenemase (KPC)-producing [5,7], Klebsiella pneumoniae belongs to the tribe Klebsiellae, a member of the Enterobacteriaceae family [28]. In the studies included in the NMA, the authors have adopted a phenotypic definition of resistance to carbapenems (i.e., based on the antibiotic susceptibility pattern). The focus of infection in the 83% (419/503) of patients was bacteremia; only in 3 of these 6 retrospective studies bacteremia was aggregated with other focus of infection: In the study by King et al. [26], two fifths of the patients (23/60) had bacteremia, in the study by Sousa et al. [8], almost half of the patients (26/57) have bacteremia. In the study by Alraddadi et al. [27], more than a half of patients (22/38) have bacteremia.
For the quantitative evaluation, we decided to meta-analyze the studies in which the infection was caused by pathogens resistant to carbapenems.
3.2. NMA Heterogeneity and Inconsistency Evaluation
There is no statistical heterogeneity among included studies, with I2 0% (95% CI; 0–17.9%) and thereby a fixed-effect model was applied. The Q statistic was 0.62 (p = 0.89) for the within design and 1.48 (p = 0.69) for the between design indicating again no heterogeneity and consistency of model used.
As showed in Figure 2, more than 75% of each comparison is represented by direct proportion. Minimal parallelism is always higher than 1 and mean path length lower than 2, showing good metrics as reported by Konig and colleagues [17]. The net heat plot confirms the consistency of data and studies in this NMA [29] (Figure 3).
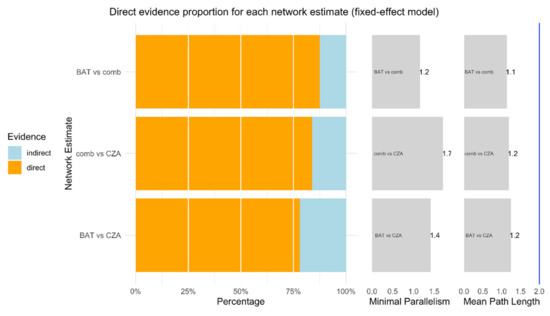
Figure 2.
This function plots relevant measures quantifying the percentage of direct and indirect evidence proportion, the aggregated minimal parallelism, and a mean path length of a frequentist network meta-analysis model [30].
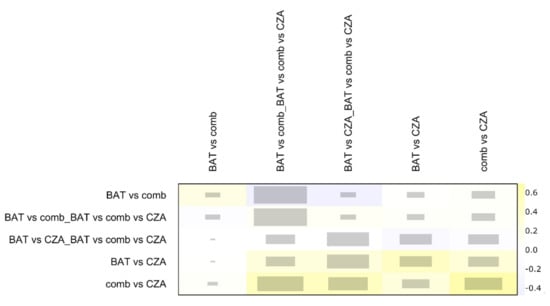
Figure 3.
The graph is a quadratic matrix in which each element in a row is compared to all other elements in the columns. Treatment comparison with only one kind of evidence (i.e., indirect or indirect evidence) are omitted in this plot. The grey boxes for each comparison of designs signify how important a treatment comparison is for the estimation of another treatment comparison. The bigger the box, the more important a comparison is. The colored backgrounds, ranging from blue to red, signify the inconsistency of the comparison in a row attributable to the design in a column. Red is highly inconsistent; blue is a lower value of inconsistency. BAT: Best antibiotic therapy; CZA: Ceftazidime-avibactam monotherapy; comb: Ceftazidime-avibactam combination therapy.
3.3. Mortality
We planned to analyze the longest follow-up, if the mortality was reported at different time-points; in 5 of 6 retrospective studies, included in the NMA, the longest follow-up was 30-day-mortality (Table 2).
The NMA, including 503 patients, did not show significant differences in CZA-combination therapy compared to CZA-monotherapy [OR: 0.96, 95% CI: 0.65–1.41]. A significant difference was observed in both CZA-monotherapy vs. best available antibiotic therapy (BAT) [OR: 0.68, 95% CI: 0.48–0.98] and CZA-combination vs. BAT [OR: 0.66, 95% CI: 0.48–0.89], the forest plot is shown in Figure 4. As a whole, the ranking probability P-score showed a larger value for CZA-combination therapy (0.7917) and CZA-monotherapy (0.6965) with the lowest rank score for the BAT (0.0118).
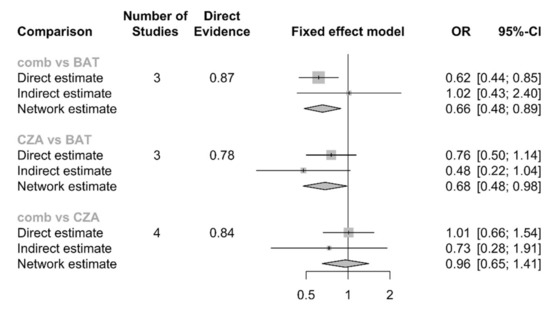
Figure 4.
Number of studies describe how many studies were analyzed for that comparison, with proportion of direct evidence. Direct estimate represents OR and 95%CI directly calculated from real comparison (for example CZA vs. BAT); Indirect estimation is a back-calculation based on other comparison (CZA vs. BAT estimated through CZA vs. comb and BAT vs. comb); Network estimation is a back-calculation based on direct and indirect evidence. BAT: Best antibiotic therapy; CZA: Ceftazidime-avibactam monotherapy; comb: Ceftazidime-avibactam combination therapy.
Non-clinical improvement was evaluated in only 4 studies [6,8,26,27], including 186 patients and microbiological failure was evaluated in only 4 studies [5,8,26,27], including 363 patients (Table 2). Therefore, due to the small number of patients, we did not proceed to quantitative synthesis for the secondary outcomes.
4. Discussion
The main findings of this study were that there were no significant differences in mortality in the treatment of CRE infections with CZA-combination therapy compared to CZA-monotherapy, based on available evidence.
Transmissible carbapenem-resistance in Enterobacteriaceae, among which the KPCs are the most notorious, has been reported since twenty years, but only recently it has been expressed as a public health problem with outbreaks reported worldwide [31,32,33,34]; CRE, due to the difficulty of effective treatment and the very high attributable mortality, are also known as “nightmare bacteria” [2]. Infections caused by these bacteria are associated with a mortality rate exceeding 50%. Since the burden of this problem is dramatic, the introduction of new antibiotics or the alternative use of existing antibiotics, as well as in our study exploring the off-label use of CZA in BSIs, is indispensable.
The Infectious Disease Society of America recommends the development of new antibiotic options through pathogen-directed studies, in which patients with multiple disease types are enrolled, rather than a single type of infection [35].
For these premises, we have meta-analyzed cumulates results from pathogen-directed studies, enrolling patients with different types of infection; for more than four-fifths of the patients, the infection focus was a BSI. Therefore, although the number of included patients is relatively low, the review cohort is homogeneous. We decided to explore as primary outcome the all-cause mortality because it is highly objective, accurate, and simple to measure [36], especially in case of low-quality evidence.
No difference in mortality rate was observed in patients undergoing CZA combination therapy compared to those who received CZA monotherapy for the treatment of CRE infections. This finding may be useful for optimizing the antibiotic treatment, with the potential to reduce the use of combination treatments [37].
Our results should be considered in light of some limitations. The outcome of mortality was not assessed in all studies at the same time point, although in the vast majority of studies (83%: 5/6), it was assessed at 30 days (Table 3). We used raw data from observational studies, and this approach is prone to the effect of potential confounders [38]. Our results cannot be extrapolated to infections other than due to CRE and should be carefully considered when treating patients with high severity of diseases. Moreover, local epidemiology should be considered when deciding to use CZA mono- or combination therapy. Due to the small number of studies and patients, we were not able to explore the secondary outcomes. Furthermore, we did not consider the side effect of antibiotics among the outcomes [11]. A limitation of our study is that a phenotypic diagnosis of resistance to carbapenems was adopted; there are many different mechanisms (i.e., genotypes) that can result in carbapenem resistance, while phenotypic tests are easy and cost-effective to perform, molecular diagnostic techniques can tailor treatment guidelines to optimize patient’s management [39].
Our results are in line with an unregistered systematic review and meta-analysis without network comparison but with a precedent search date [40].
In conclusion, in this systematic review and NMA CZA monotherapy was as effective as CZA combinations in reducing all-cause mortality in patients with infections by CRE (mostly KPC) but the quality of the available evidence and the overall number of patients from included studies was low.
Further clinical trials should evaluate the effectiveness and safety of different CZA combinations, especially in other infections and clinical settings. Moreover, further evidence is likely to change the outcome estimates.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/7/388/s1, Table S1: Search strategies for the databases utilized, Table S2: Risk-of-bias assessment in a systematic review of randomized trials, using version 2 of the Cochrane risk-of-bias tool, Table S3: Quality assessment in a systematic review of cohort studies, using the New Castle-Ottawa Scale.
Author Contributions
Conceptualization, M.F. and A.C.; formal analysis, V.S.; data curation, resources A.A.; data curation, resources S.D.F. and G.I.; writing—original draft preparation, M.F.; writing—review and editing, A.C.; supervision, M.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
V.S. was supported by the Programma VALERE, University of Campania “Luigi Vanvitelli”. We wish to thank the following authors for they provided additional data and/or full texts upon request: Adrian Sousa, Infectious Diseases Unit-Internal Medicine Department, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Spain. Mario Tamburello, Institute of Infectious Diseases, Fondazione Policlinico Universitario A. Gemelli - Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS)-Università Cattolica del Sacro Cuore, Rome, Italy
Conflicts of Interest
The authors declare no conflict of interest
References
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- (CDC), Centers for Disease Control and Prevention. Clinicians: Information about CRE. 2019. Available online: https://www.cdc.gov/hai/organisms/cre/cre-clinicians.html (accessed on 3 July 2020).
- Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206494Orig1s000SumR.pdf (accessed on 3 July 2020).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf (accessed on 3 July 2020).
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef]
- Castón, J.J.; Lacort-Peralta, I.; Martín-Dávila, P.; Loeches, B.; Tabares, S.; Temkin, L.; Torre-Cisneros, J.; Paño-Pardo, J.R. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int. J. Infect. Dis. 2017, 59, 118–123. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-00817. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Perez-Rodriguez, M.T.; Soto, A.; Rodriguez, L.; Perez-Landeiro, A.; Martinez-Lamas, L.; Nodar, A.; Crespo, M. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.; Richer, D.; Bonner, A.J.; Kibret, T.; Beyene, J. Network meta-analysis using R: A review of currently available automated packages. PLoS ONE 2014, 9, e115065. [Google Scholar] [CrossRef]
- Correction: Network meta-analysis using R: A review of currently available automated packages. PLoS ONE 2015, 10, e0123364. [CrossRef]
- Fiore, M.; Cortegiani, A. Ceftazidime-avibactam combination therapy compared to Ceftazidime-avibactam monotherapy for the treatment of severe infections. 2019/04/16 ed. 2019. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019128927 (accessed on 3 July 2020).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Data collection form for intervention reviews for RCTs and non-RCTs. Available online: https://dplp.cochrane.org/data-extraction-forms (accessed on 3 July 2020).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wells, G.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Rücker, G.; Krahn, U.; König, J.; Efthimiou, O.; Schwarzer, G. Netmeta: Network Meta-Analysis using Frequentist Methods (R Package Version 0.9-8). Available online: https://cran.r-project.org/web/packages/netmeta/index.html (accessed on 3 July 2020).
- Konig, J.; Krahn, U.; Binder, H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat. Med. 2013, 32, 5414–5429. [Google Scholar] [CrossRef]
- Krahn, U.; Binder, H.; König, J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 2013, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, Y.; Armstrong, J.; Laud, P.J.; Newell, P.; Stone, G.; Wardman, A.; Gasink, L.B. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): A randomised, pathogen-directed, phase 3 study. Lancet Infect. Dis. 2016, 16, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef]
- Lucasti, C.; Popescu, I.; Ramesh, M.K.; Lipka, J.; Sable, C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: Results of a randomized, double-blind, Phase II trial. J. Antimicrob. Chemother. 2013, 68, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Tran, B.G.; Kim, M.J.; Wang, L.; Nguyen, D.A.; Chen, Q.; Song, J.; Laud, P.J.; Stone, G.G.; Chow, J.W. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int. J. Antimicrob. Agents 2017, 49, 579–588. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Gasink, L.B.; Armstrong, J.; Broadhurst, H.; Stone, G.G.; Rank, D.; Llorens, L.; Newell, P.; Pachl, J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin. Infect. Dis. 2016, 62, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Gonzalez Patzan, L.D.; Stricklin, D.; Duttaroy, D.D.; Kreidly, Z.; Lipka, J.; Sable, C. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: Results of a prospective, investigator-blinded, randomized study. Curr. Med. Res. Opin. 2012, 28, 1921–1931. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Sobel, J.D.; Newell, P.; Armstrong, J.; Huang, X.; Stone, G.G.; Yates, K.; Gasink, L.B. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin. Infect. Dis. 2016, 63, 754–762. [Google Scholar] [CrossRef]
- King, M.; Heil, E.; Kuriakose, S.; Bias, T.; Huang, V.; El-Beyrouty, C.; McCoy, D.; Hiles, J.; Richards, L.; Gardner, J.; et al. Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Saeedi, M.; Qutub, M.; Alshukairi, A.; Hassanien, A.; Wali, G. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect. Dis. 2019, 19, 772. [Google Scholar] [CrossRef]
- Octavia, S.; Lan, R. The Family Enterobacteriaceae. In The Prokaryotes: Gammaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 225–286. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer: Cham, Switzerland, 2015; pp. 187–216. [Google Scholar] [CrossRef]
- Plot for direct evidence proportions in a network meta-analysis using netmeta. Available online: https://dmetar.protectlab.org/reference/direct.evidence.plot.html (accessed on 3 July 2020).
- Iovene, M.R.; Pota, V.; Galdiero, M.; Corvino, G.; Di Lella, F.M.; Stelitano, D.; Passavanti, M.B.; Pace, M.C.; Alfieri, A.; Di Franco, S.; et al. First Italian outbreak of VIM-producing Serratia marcescens in an adult polyvalent intensive care unit, August-October 2018: A case report and literature review. World J. Clin. Cases 2019, 7, 3535–3548. [Google Scholar] [CrossRef]
- Park, Y.; Choi, Q.; Kwon, G.C.; Koo, S.H. Emergence and transmission of New Delhi metallo-beta-lactamase-5-producing Escherichia coli Sequence Type 361 in a Tertiary Hospital in South Korea. J. Clin. Lab. Anal. 2019, e23041. [Google Scholar] [CrossRef] [PubMed]
- Dubendris, H.; MacFarquhar, J.; Kornegay, R.; Gable, P.; Boyd, S.; Walters, M.; Greene, S. Imipenemase-producing carbapenem-resistant Enterobacteriaceae transmission in a long-term-care facility during a community-wide multidrug resistant organism outbreak-North Carolina, 2017. Am. J. Infect. Control 2019. [Google Scholar] [CrossRef] [PubMed]
- Cuzon, G.; Bentchouala, C.; Vogel, A.; Hery, M.; Lezzar, A.; Smati, F.; Dortet, L.; Naas, T. First outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Constantine, Algeria. Int. J. Antimicrob. Agents 2015, 46, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Infectious Diseases Society of America; Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. 5), S397–S428. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; de Kraker, M.E.A.; Sommer, H.; Weiss, E.; Bettiol, E.; Wolkewitz, M.; Nikolakopoulos, S.; Wilson, D.; Harbarth, S.; On Behalf of the COMBACTE-NET Consortium. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: A perspective from COMBACTE’s STAT-Net. Intensive Care Med. 2017, 43, 1002–1012. [Google Scholar] [CrossRef]
- Leone, S.; Cascella, M.; Pezone, I.; Fiore, M. New antibiotics for the treatment of serious infections in intensive care unit patients. Curr. Med. Res. Opin. 2019, 35, 1331–1334. [Google Scholar] [CrossRef]
- Handbook-5-1. cochrane. Chapter 13: Including non-randomized studies. Available online: https://handbook-5-1.cochrane.org/index.htm#chapter_13/13_including_non_randomized_studies.htm (accessed on 3 July 2020).
- Naim, H.; Rizvi, M.; Gupta, R.; Azam, M.; Taneja, N.; Shukla, I.; Khan, H.M. Drug Resistance and Molecular Epidemiology of Carbapenem Resistant Gram-negative Bacilli Isolates. J. Glob. Infect. Dis. 2018, 10, 133–139. [Google Scholar] [CrossRef]
- Onorato, L.; Di Caprio, G.; Signoriello, S.; Coppola, N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: A meta-analysis. Int. J. Antimicrob. Agents 2019, 54, 735–740. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).