Abstract
The emergence of multi-drug resistant bacteria is becoming a major health concern. New strategies to combat especially Gram-negative pathogens are urgently needed. Antimicrobial peptides (AMPs) found in all multicellular organisms act as a first line of defense in immunity. In recent years, AMPs have attracted increasing attention as potential antibiotics. Naturally occurring antimicrobial cyclic lipopeptides include colistin and daptomycin, both of which contain a flexible linker. We previously reported a cyclic AMP BSI-9 cyclo(Lys-Nal-Lys-Lys-Bip-O2Oc-Nal-Lys-Asn) containing a flexible linker, with a broad spectrum of activity against bacterial strains and low hemolytic activity. In this study, improvement of the antimicrobial activity of BSI-9, against the European Committee on Antimicrobial Susceptibility Testing (EUCAST) strains of S. aureus, E. coli, A. baumannii, and P. aeruginosa was examined. This led to synthesis of eighteen peptide analogues of BSI-9, produced in four individual stages, with a different focus in each stage; cyclization point, hydrophobicity, cationic side-chain length, and combinations of the last two. Specifically the modified compound 11, exhibited improved activity against Staphylococcus aureus and Pseudomonas aeruginosa with MIC of 4 µg/mL and 8 µg/mL, respectively, compared to the original BSI-9, which had an MIC of 16–32 µg/mL.
1. Introduction
The development of antibiotics is one of the greatest accomplishments in modern medicine, treating potentially fatal infections [1]. Nevertheless, the widespread use of antibiotics has led to the selection of resistant isolates, which in turn has resulted in the emergence of multi-drug resistant microorganisms capable of surviving treatment with most or all antibiotics [2]. Of dire importance is the development and prevalence of multidrug resistance in Gram-negative species, such as Klebsiella pneumonia, Acinetobacter baumannii, and Eschericia coli, where pan-resistance is now observed [3]. Therefore, the need for novel antibiotics is urgent, as drug-resistant microorganisms is a global health concern and antibiotic development has declined [4].
Interest in antimicrobial peptides (AMPs) as novel antibiotics, has increased in recent years. Despite their diversity, most AMPs share common features, including a net positive charge, amphipathic character, and short length (<50 amino acids). They have an ancient phylogenetic origin and play an essential role in the innate part of the immune system of multicellular organisms [5]. Their overall cationic charge attracts them to the anionic membrane of the bacterial cell, followed by membrane insertion, thanks to their amphipathic nature. There are various mechanisms which then lead to membrane permeabilization and membrane depolarization, accompanied by membrane disruption or leakage of essential intracellular metabolites, amongst others [6]. Some AMPs kill bacteria by targeting specific intracellular metabolic pathways, thereby disrupting important biological processes [7]. In general, development of resistance to membrane targeting AMPs is considered rare, compared to that of conventional antibiotics. This is likely the result of AMPs killing by disruption of the cellular membrane in a non-target specific way, i.e., the AMPs insert into and disrupt the structural organization of the membrane, as opposed to targeting a single specific target such as an enzyme [8].
The lipopeptide antibiotics colistin (polymyxin E) [9] and daptomycin [10] are currently the last resort drugs used for the treatment of multidrug resistant Gram-negative and Gram-positive bacteria, respectively. They both consist of a cyclic peptide part with a flexible linker. Colistin and daptomycin are highly active against P. aeruginosa and S. aureus, respectively. However, resistance to these lipopeptides has emerged [11,12], and while arising in highly diverse microorganisms, the resistance mechanisms share overlapping features. Resistance to colistin in Gram-negative species is associated with modification of the lipopolysaccharide found in the outer leaflet of the outer membrane not present in Gram-positive bacteria. Specifically these modifications change the net positive charge (to which polymyxins are attracted) through attachment of phosphoethanolamine (pEtN) or addition of 4-amino-4-deoxy-L-arabinose (L-Ara4N) to LPS [13,14]. Daptomycin resistance, likewise, result in changes to the net negative charge of the Gram-positive membrane, but by changing the content of phosphatidylglycerol (PG) to the more positively charged lysyl-phosphatidylglycerol (L-PG) [15].
Nonetheless, the broad target range, alternative modes of action and evolutionary robustness continue to make AMPs interesting drug candidates and an alternative to the traditional small molecule antibiotics. AMP development is hampered by AMP-specific problems of which in vivo stability, efficacy, and toxicity are the most prominent [16]. Systemic toxicity and stability issues often limit the use of AMPs and most of AMP drugs currently undergoing clinical trials are for topical applications [17]. However, work by Vaara and others, demonstrated how toxicity issues related to polypeptides (polymyxin) might be overcome by turning the molecule into a sensitizing agent, instead of a direct antimicrobial [18]. Thus, current AMP optimization efforts mainly focus on peptidomimetics, for improving stability and antimicrobial activity, while reducing toxicity [19].
BSI-9 cyclo(Lys-Nal-Lys-Lys-Bip O2Oc-Nal-Lys-Asn) is a cyclic AMP developed by Oddo et al. [20] in a study examining the effects of flexible residues on bioactivity and toxicity in cyclized amphipathic peptides. The flexible residue 8-amino-3,6-dioxaoctanoic acid (O2Oc) offers BSI-9, a combination of size, flexibility, and amphipathicity resulting in activity against E. coli, P. aeruginosa, and drug resistant strains of A. baumannii and S. aureus, with the minimum inhibitory concentration (MIC) values ranging from 16 µM–64 µM. In addition, BSI-9 displayed low hemolytic activity against red blood cells at 150 µM [20]. In this study, 18 cyclic peptide analogues of BSI-9 were synthesized in four stages (Scheme 1). The cyclic peptides were tested against the bacterial quality control strains set by the European Committee on Antimicrobial Susceptibility Testing (EUCAST); strains E. coli ATCC 25922, S. aureus ATCC 29213, A. baumannii ATCC 19606, and P. aeruginosa ATCC 27853.
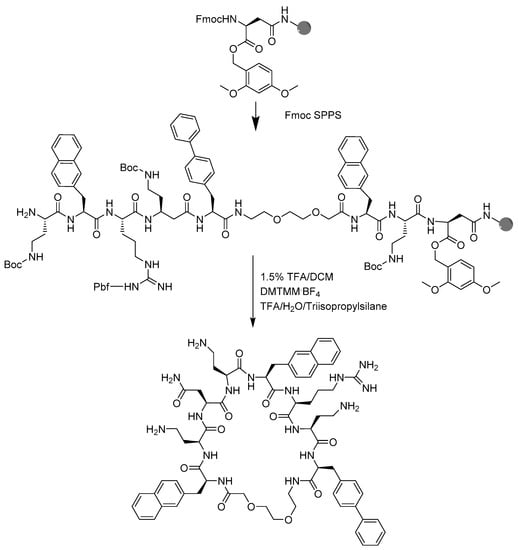
Scheme 1.
Strategy for solid-phase peptide synthesis of the lead compound 11.
2. Results and Discussion
Charge, overall hydrophobicity, and amphipathicity are important parameters that influence the biological activity and toxicity of AMPs [21]. Studies optimizing linear AMPs often aim to change one or more of these parameters with varying results [22,23,24,25]. Structure–activity studies were also conducted on cyclic peptides [26,27], peptide macrocycles [28], and cyclic peptides, with a flexible linker [29]. In this study, the antimicrobial activity of BSI-9 [20] was improved. We synthesized eighteen peptide analogues of BSI-9 in four stages, focusing on the cyclization point, hydrophobicity, cationic side-chain length, and the combinations of the last two (analogues presented in Table 1).

Table 1.
Minimum inhibitory concentration (µg/mL) and percent hemolytic activity against red blood cells.
2.1. Stage 1. The Impact of Cyclization Point on Antimicrobial Activity—Replacing Lys with Asn.
The amphipathicity of a peptide is determined by the number of polar and hydrophobic residues, and their position with respect to each other [30]. Therefore, we hypothesized that moving the asparagine—the cyclization point—to different positions in the BSI-9 sequence, would affect bioactivity. In BSI-9, the asparagine (polar, uncharged) was positioned between two lysines (polar, charged). This position was chosen for arbitrary reasons and might not be optimal. Thus, four peptide analogues (1–4) synthesized in stage 1 (S1) aimed to evaluate the importance of the cyclization point by substituting lysine residues with asparagine at different positions. The MIC and hemolysis results of the S1 peptides are displayed in Table 1. While the peptides displayed varying antimicrobial activity, all four peptides had an equal or higher MIC value compared to BSI-9, against all bacterial strains. We observed a similar level of antimicrobial activity for 1 against A. baumannii, compared to BSI-9. In addition, most S1 peptide analogues had a retention time (RT) higher than BSI-9 (Table S1), indicating increased hydrophobicity/amphipathicity. Since stage 1 analogues showed no significant antimicrobial activity, we did not test the hemolytic activity.
2.2. Stage 2. The Impact of Manipulating Lipophilicity on Antimicrobial Activity. Replacing Nal (3-(2-naphtyl)-L-alanine) and Bip (L-biphenylananine) with Phe.
Previously, Staubitz et al. reported that reducing the lipophilicity of a peptide by substituting the lipophilic residues could reduce the hemolytic activity, as well as improve antimicrobial activity [22]. Since no significant improvement was observed in stage 1, BSI-9 was used as the lead compound for the synthesis in stage 2 (S2). Here, the (Nal) and (Bip) residues were substituted with the less hydrophobic Phe. Nal and Bip both contain two aromatic rings, while Phe only contains one. Five new analogues of BSI-9 were synthesized (Table 1): compounds 5–9. In peptide 5 (Nal2→Phe), 6 (Bip5→Phe), and 7 (Nal7→Phe) one amino acid was substituted. In compound 8, two amino acids were substituted (Nal2,7, Bip5→Phe). The total number of amino acids remained nine. In this stage all peptides had MICs of 32 μM or above against all bacterial strains. The hemolytic activity of all stage 2 analogues was below 10%.
2.3. Stage 3. Importance of Amino Acid Sidechain Length on Antimicrobial Activity—Replacing Lys with Dab or Arg.
Stage 3 (S3) investigated the importance of amino acid side chains in relation to antibacterial activity. Specifically, the side chain of the four charged lysine (Lys) residues in BSI-9, were exchanged with charged amino acids, 2,4-diaminobutanoic acid (Dab) and Arginine (Arg). Since Lys and Arg had longer side-chains than, e.g., Dab, these residues were able to insert more deeply into the membrane while the side chain charge was still able to interact with the lipid head groups on the surface; this is known as “snorkeling” [31]. Furthermore, Arg is capable of forming three hydrogen bonds while Lys and Dab can form only two. In compound 9, we changed all Lys residues to Dab. Peptide 9 inhibited S. aureus and P. aeruginosa at 8 g/mL and 4 g/mL, respectively, which was an 8-fold increase in activity compared to BSI-9. Although peptide 9 showed an increased hemolytic activity of 76%, compared to BSI-9 (33%), it was decided to use this peptide in the design of additional stage 3 analogues.
Based on the above results, compounds 10–13 were designed as a cationic Arg scan of peptide 9:10 (Dab1->Arg), 11 (Dab3->Arg), 12 (Dab4->Arg), and 13 (Dab8->Arg). In peptide 14, all Dab residues were replaced with Arg (Dab1,3,4,8 ->Arg). In 15 (Dab1,3->Arg) were substituted.
The MIC and hemolysis results of the Stage 3 peptides are displayed in Table 1. For peptide 10, we observed an MIC of 2 µg/mL against P. aeruginosa and S. aureus. Peptide 11 was still able to inhibit S. aureus at 8 μg/mL and P. aeruginosa at 4 µg/mL. For both 10 and 11, the activity against E. coli and A. baumannii remained unchanged or slightly improved, compared to 9. Peptide 12 and 13 showed an improvement compared to 9 against S. aureus, with an MIC of 1 µg/mL, but not against the other bacterial strains. The MIC of 14 and 15 did not improve, compared to 9, against any bacterial strains. Peptide 10 showed a lower hemolytic value compared to 9 (from 76% to 66% at 150 μM). Compound 12, 13, 14, and 15 showed hemolytic values of 100%, 80%, 68%, and 100% at 150 µM, respectively. Apart from 13, which had an RT of 17.9 min, compared to 9 at 17.8 min, the RT times of S3 peptides were similar but lower compared to 9. They were all higher than BSI-9.
2.4. Stage 4. Reducing Hydrophobicity of Stage 3 Lead Peptide by Replacing Dab with Arg and Bip with Phe.
Although 12 and 13 were more active against S. aureus compared to 11, they were also significantly more hemolytic (100% and 80%, respectively). Therefore, compound 11 was chosen as the lead compound for stage 4. In stage 4 (S4), we replaced Dab with Arg and Bip of 11 with the less hydrophobic Phe to synthesize three new analogues (16–18). In 16 (Dab1,3->Arg, Bip5->Phe) and 17 (Dab1->Arg, Bip5->Phe) two and three substitutions were done, respectively. In 18, one substitution was made (Bip5->Phe). The MIC of compound 16 did not improve compared to 9, against P. aeruginosa (16 µg/mL) and S. aureus (8–64 µg/mL). For all Stage 4 analogues, the activity against E. coli and A. baumannii remained unchanged or worsened (32 → 64 μg/mL) compared to 11. (Bip) is often used in structure–activity studies to replace Phe, for example, in the linear antimicrobial peptide Lys-Bip-Lys-Bip-Lys [32], or in the cyclic antimicrobial peptide Polymyxin B [26]. In line with these studies, we observed that the Bip residue was important for antimicrobial activity, which was reduced in the BiP->-Phe analogues (16–18).
An indirect correlation between MIC values, % hemolysis, and HPLC retention times (Table S1) was found. In stage 2, MICs were 32 → 64 µg/mL against S. aureus and P. aeruginosa and we observed a hemolysis below 10%. However, in stage 3, the MICs ranged between 1–32 µg/mL and lysis of red blood cells increased to 36–100%. This might be indicative of a membrane disruption mode of action. The most hemolytic compounds in stage 3 had HPLC retention times between 17.0–17.8 min, while the less hemolytic stage 2 and 4 compounds had values between 13.8–16.0 min. The above correlation was previously observed for both cyclic [33] and linear peptides [34].
Since none of the stage 4 analogues were more active than 11, this compound remained our best candidate.
3. In Vitro Killing Kinetics against P. aeruginosa and S. aureus
The killing kinetics of compound 11 (Figure 1A–D) were performed in two types of experiments on approximately 5 × 105 (exponential phase) and 5 × 107 (late exponential phase) colony forming units (CFU). The former was equivalent to MIC setup and equal to an optical density at 600 nm (OD600 ~ 0.0005). Colistin and chloramphenicol was used as control antibiotics against P. aeruginosa and S. aureus, respectively. Chloramphenicol was chosen against S. aureus because initial experiments indicated 11 to be bacteriostatic.
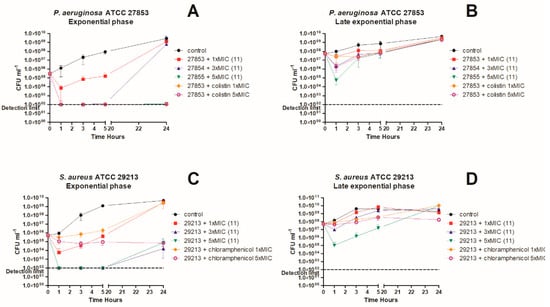
Figure 1.
Time-Kill Curves. Bactericidal activities of 11 and colistin at different concentrations against P. aeruginosa in the exponential phase (A) and late exponential phase (B), and chloramphenicol at different concentrations against S. aureus in the exponential phase (C) and late exponential phase (D).
A study by Bulitta et al. describes that colistin displays a rapid bactericidal activity against P. aeruginosa at low CFU/ mL but at higher CFU/mL, a higher colistin concentration was required to achieve activity [35]. Therefore, we decided to do time-kill experiments against late exponential phase cells (OD600 ~0.1 = 107 CFU) to determine if 11 had the same cell density dependency as most antimicrobial peptides [36]. Against P. aeruginosa in exponential phase, both compound 11 and colistin were bactericidal at different concentrations tested (Figure 1A). At 1× MIC of compound 11 we observed a ~2-log reduction in viable bacterial counts and at 3–5× MIC of 11 resulted in a >3 log reduction in CFU within the first hour. For 1–3xMIC concentrations of 11, the reduction in CFU was followed by regrowth, after the first hour of treatment. Against P. aeruginosa in late exponential phase (Figure 2B), both 11 and colistin had virtually no effect at 1× MIC, while we observed a 1-log reduction in CFU for 11 at 3× MIC and 5× MIC for colistin. The largest effect was seen for 11 at 5× MIC, where we observed a 3-log reduction in CFU. For all concentrations, regrowth occurred after 1 h.
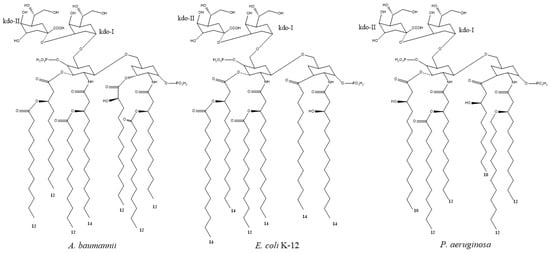
Figure 2.
Lipid A structure of (A) A. Baumannii, (B) E. coli, and (C) K. pneumonia. E. coli and K. pneumonia primarily contain hexa-acetylated Lipid A, while A. baumannii share much similarity but lipid A is primarily hepta-acetylated. P. aeruginosa differs from the other Gram-negative species in that, its hexa-acetylated Lipid A had a different distribution of Acyl chains and the acyl chains were generally shorter. Numbers below acyl chains indicated the length of the carbon chain. Figures are adapted from Raetz [37], Boll [38], Li [39], and Kim [40].
As experiments were performed at cell densities comparable to MIC, determination of killing kinetics indicated that our MIC measurements could be slightly underestimated since regrowth was seen at 1× MIC of compound 11 to comparable levels as untreated control sample. However, treatment with 1× MIC of colistin showed the same trend, therefore we believe that these differences are due to differences in physiological conditions in time-kill assays compared to MIC: time-kill assays were performed in blood glass tubes with vigorous agitation and optimal growth condition, whereas MICs were performed in small volumes in microtiter plates where aeration is less optimal.
For compound 11 at 1× MIC, 3× MIC, and 5× MIC against S. aureus in the exponential phase (Figure 1C), we observed a 2-log and >3-log reduction, respectively. Compound 11 exhibited a partially concentration-dependent killing profile, in which above 3× MIC no further reduction in CFU was observed. Killing appeared to be maximized at 1 h after treatment. However, regrowth was observed after 24 h. Against S. aureus in the late exponential phase, only 11 at 5× MIC had a significant effect, where a 3-log reduction in CFU was observed, again with regrowth after 24 h.
Overall, we observed a bactericidal activity of 11 against both P. aeruginosa and S. aureus, but with a transient activity that did not last. This might be due to physiological changes in the bacteria, such as enzymatic degradation of the compound, but we expected the major reason for this to be sequestration of the compound by dead cells and cell debris. This might be observed by dissolution of the pelleted debris and LC/MS analysis.
Importantly, we find that compound 11 had activity against P. aeruginosa and S. aureus, but no activity against E. coli and A. baumannii in MIC assays. Therefore, the activity was not directed towards bacterial structures associated with the typical Gram-negative (outer membrane), as P. aeruginosa shared much more similarity with E. coli than with S. aureus. This could imply that either the peptide did not disrupt the membrane or that membrane disruption was more dependent on specific structures shared by P. aeruginosa and S. aureus. We speculated that the difference in 11 activity between Gram-negative species related to differences in the membrane anchoring part of LPS, known as Lipid A. E. coli K-12 species and K. pneumoniae were described to predominantly contain hexa-acetylated Lipid A that was phosphorylated at the 1- and 4′ positions. A. baumannii had a similar lipid A structure, but with an extra acyl chain (hepta-acetylated) [13]. However, P. aeruginosa was different from E. coli, K. pneumonia, and A. baumannii. In P. aeruginosa, Lipid A was hexa-acetylated, similar to that of E. coli but the acyl chains were distributed differently (Figure 2). Furthermore P. aeruginosa Lipid A generally contain shorter acyl chains than that of the other species. Therefore, Gram-negative inter species differences might result from the acylation pattern on Lipid A or the length of acyl chains on Lipid A. However, these speculation remains to be experimentally verified. It was further speculated that the peptide was generally attracted to the bacterial surface through electrostatic interactions, as both Gram-positive and Gram-negative species have negatively charged membranes, however, the antimicrobial activity would depend on aforementioned differences in membrane composition. Therefore, it was also speculated that the peptide would generally show activity against Gram-positive species, which was unlike the nonapeptide described elsewhere [18,41], which had little antimicrobial activity by itself, but could sensitize bacteria towards other antibiotics.
4. Materials and Methods
4.1. Materials
Chemistry: Disposable 5-mL polypropylene reactors fitted with a PTFE filter were acquired from Thermo Scientific TentaGel® S RAM resin, trifluoroacetic acid (TFA), piperidine, and Fmoc-protected L-amino acids were purchased from Iris-Biotech GmbH. Disopropylamine (DIEA), Triisopropylamine (TIS), Phosphate-buffered saline (PBS), melitin, and 4-(4,6Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholiniumtetrafluoroborate (DMTMM.BF4) were purchased from Sigma-Aldrich. COMU ((1-Cyano-2-ethoxy-2oxoethylidenaminooxy)dimethylaminomorpholino-carbenium hexafluorophosphate) and Oxyma (Ethyl (hydroxyimino)cyanoacetate), HOAt (1-Hydroxy-7-azabenzotriazole), and HATU(1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxid hexafluorophosphate, N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexa-fluorophosphate N-oxide) were purchased from GL Biochem Shanghai. DMF (Dimethylformamide, synthesis grade), DCM (Dichloromethane, optical grade), and MeCN (Acetonitrile, optical grade) were obtained from VWR.
Peptides were purified by preparative Reverse Phase Analytical High Performance Liquid Chromatography (RP–HPLC) on a preparative WatersTM XBridgeTM BEH130 C18, HPLC column (5 µm 10 × 250 mm) equipped with a WatersTM Cartridge Holder PKG (10 × 10 mm).
MicroflexTM [Bruker Corporation] FlexControl software [Bruker Daltonik GmbH] was used to obtain the spectra and the data were processed using flexAnalysis [Bruker Daltonik GmbH]. All reagents and solvents were used without further purification.
Microbiology and Haemolysis: Non-cation adjusted Mueller–Hinton broth (MHB) media (BD BBLTM Beef extract powder, BD BactoTMCasamino acids, and DIFCO soluble starch were obtained from the Becton Dickinson and Company©, Franklin Lakes, NJ, USA. MHB media supplemented with 0.2% BSA was obtained from SigmaAldrich Co. and 0.01% acetic acid from VWR®. 96-well plates cell culture cluster, round-bottom, polypropylene plates polystyrene, flat-bottomed 96-well ELISA plate and V-shaped 96-well polypropylene plate were purchased from Corning® Inc., Costar®, Corning, NY, USA. Microseal® film was obtained from Bio-Rad Laboratories, Inc., Hercules, CA, USA. VersaMaxTM Tunable Microplate Reader (Molecular Devices LLC, Sunnyvale, CA, USA) and the data were evaluated on Softmax® Pro (Molecular Devices LLC).
4.2. Peptide Synthesis
Solid-phase peptide synthesis: Peptide analogues were synthesized in 4 stages, S1, S2, S3, and S4. Peptides were synthesized on a TentaGel® S RAM (90 µm) resin, with a loading of 0.22 mmol/g. The resin was weighed out into a disposable 5-mL polypropylene reactor, fitted with a PTFE filter and swelled in DMF for a minimum of two hours or overnight, and washed with DMF (3×), DCM (3×), and DMF (5×). The base-labile Fmoc group was removed by treatment with 4 mL 20% piperidine/DMF for four minutes, and reiterated three times with a DMF wash in between each treatment. After the third deprotection, the resin was washed with DMF (3×), DCM (3×), and DMF (5×). For BSI-9 and analogues 1–4, the amino acids were coupled successively and a single amino acid coupling used 5 equivalents of amino acid, COMU, and Oxyma each, and 2 equivalents DIEA. Fmoc-protected amino acids were dissolved in DMF at a concentration of 0.6 M. COMU and Oxyma were weighed out per coupling and dissolved in fresh DMF at a 0.6 M concentration, just prior to coupling. The amino acid solution, COMU/Oxyma solution, and DIEA were combined and immediately transferred to the syringe, covered in tin foil and put on a shaker for 1.5–2 h. For analogues 5–18, HATU, HOAt, and DIEA were used as coupling reagents, as described above. The coupling solution was discharged and the resin was washed with DMF (3×), DCM (3×), and DMF (5×). The Fmoc group was removed with 4 mL 20% piperidine/DMF for 3 × 4 min, as described here earlier, and washed with DMF (3×), DCM (3×), and DMF (5×). The next amino acid was then coupled.
4.3. Peptide Macrocyclization
The Fmoc group of the last amino acid was removed (4 mL 20% piperidine, 3 × 4 min). Next, the Dmb group was removed from the Asp by treatment with 3 mL 1.5% TFA/ DCM solution, 6 times for 5 min. The resin was washed with DMF, followed by DCM and finally ethanol (EtOH). The resin was freeze-dried and transferred to a reactor fitted with a PTFE filter and swelled in DMF for 2 h.
Three equivalent DMTMM·BF4 was dissolved in minimum DMF, 6 equivalents of DIEA were added, and the reaction was allowed to proceed for 8 h or overnight. The resin was washed with DMF (5×). Afterwards, 3 equivalents of DMTMM·BF4 and 3 equivalents of DIEA were added and the reaction was left for 8 h or overnight. The resin was washed with DMF (3×), DCM (3×), and EtOH (5×). Cleavage of a small amount of resin was done to confirm sequence completion and cyclization. The resin was placed in the freeze-dryer until dry.
4.4. Peptide Cleavage
Cleavage was performed on half or all of the resin with a TFA:H2O:TIS (95:2.5:2.5) solution for 2 h. The cleavage solution was collected and concentrated with a gentle stream of N2, down to about 300 µL. The peptide was precipitated and washed with 4 mL cold (−20 °C) diethyl ether, 3 times. The residual ether was left overnight to evaporate. Next, the crude product was dissolved in 90% water and 10% ACN and freeze-dried, ready for analysis and purification. Peptides were identified using Matrix Assisted Laser Desorption Ionization time of flight mass spectroscopy (MALDI–ToF MS). To this end, 1 µL peptide solution and 1 µL α-cyano4-hydroxycinnamic acid matrix (10 mg/ mL in ACN:H2O:TFA, 50:47.5:2.5) were spotted onto a target plate, followed by detection using a MicroflexTM. FlexControl software was used to obtain the spectra and the data were processed using flexAnalysis [Bruker Daltonik GmbH]. RP–HPLC was used to assess peptide purity. The system consisted of a WatersTM In-Line Degasser AF, a WatersTM 600 pump, a WatersTM 2996 Photodiode Array Detector, and a WatersTM Symmetry C18, 4.6 × 250 mm, 5 mm column. The data were processed using Empower3 software. Purity was ≥95% for all tested peptide. Peptide purification was done by dissolving the peptides in minimal H2O/can, and 300–500 µL was injected into the HPLC system to achieve separation.
4.5. Minimum Inhibitory Concentration Determination
Bacterial strains (E. coli ATCC 29522, S. aureus ATCC 29213, A. baumannii ATCC 19606, and P. aeruginosa ATCC 27853) were grown overnight on LB agar plates at 37 °C. Overnight cultures were prepared by inoculation of two to three colonies in 10 mL non-cation adjusted Mueller–Hinton broth (MHB) media (BD BBLTM Beef extract powder, BD BactoTMCasamino acids, and DIFCO soluble starch). Bacterial inoculum for MIC testing was prepared as balanced cultures, as previously described [25]. The bacterial suspension was adjusted using MHB to an OD600 of 0.1, approximately 1·108 colony forming units (CFU/mL), and then diluted 1:200 in MHB, to give a final bacterial suspension of 5·105 CFU/mL, as described by Wiegand et al. [42]. MIC plates were prepared by dissolving peptides and gentamycin samples at concentrations of 640 µM and 320 µM, respectively, in MHB media supplemented with 0.2% BSA and 0.01% acetic acid. For MIC determination, all peptides were measured in triplicates using the protocol described by Wiegand et al. [42] for “antimicrobial peptides that require the presence of acetic acid/BSA”. In brief, 20 µL peptide solution and gentamycin solution was transferred to column 1 of 96-well plates. Next, 10 µL 0.2% BSA; 0.01% acetic acid MHB media was transferred to column 2–11. Subsequently, peptides were serially diluted two-fold in the MIC plate and 90 µL bacterial suspension was transferred to all wells in column 1–11. Next, 100 µL bacterial suspension was transferred to column 12 D–F (growth control) and 100 µL media to column 12 A–C (sterility control). After an overnight incubation at 37 °C of the MIC plates, MIC values were determined as the lowest concentration at which there was no visible bacterial growth. For the inoculum control, 10 µL of solution from a positive-growth control well from each bacterial strain was dissolved in 990 µL phosphate-buffered saline (PBS) (1:100 dilution) in an Eppendorf tube. This solution was vortexed and 100 µL transferred to another Eppendorf tube containing 900 µL PBS (1:10 dilution) and vortexed. Then, 100 µL of each dilution was spread onto the MHB media plates, in triplicates, to achieve a CFU dilution of 1:1000 and 1:10,000, respectively. The plates were incubated overnight at 37 °C. The colonies were counted and 50 colonies were expected on the 1:10,000 plates and 500 on the 1:1000 plates.
4.6. Hemolysis
A total of 150 µL of 5 µM melittin was added to the positive control wells 1-6H of a V-shaped 96-well polypropylene plate and left overnight. The melittin solution was removed and the positive control wells were washed 3 times with 150 µL PBS. Afterwards, a 2.5 µM melittin solution was added to the positive control wells. For the assay, 0-negative blood in EDTA was used. Blood was washed by mixing 3 mL of PBS with 1 mL of whole blood, and centrifuged for 8 min at 3000 rpm. The supernatant was discarded and 4 mL of PBS added, mixed gently, and centrifuged. This wash cycle was repeated but the last centrifuge velocity was raised to 4000 rpm. Next, 40 µL red blood cells (RBC) was suspended in 8 mL of PBS to achieve an RBC suspension of 0.5% volume/volume (v/v). Peptide solutions were prepared at 600 µM, two times the highest concentration. A total of 150 µL peptide solution was transferred to row A. Next, 75 µL PBS was transferred to wells B1 to G12, as well as H7-12 (negative control), and the peptides were serially diluted two-fold. Then, 75 µL RBC suspension was added to all wells and the plate was covered with a Microseal® film and incubated for 1 h at 37 °C. Next, the plate was centrifuged for 10 min at 4000 rpm. Subsequently, 60 µL supernatant was transferred into the corresponding wells of a clear, polystyrene, flat-bottomed 96-well ELISA plate. The absorbance was read at λ = 414 nm with a VersaMaxTM Tunable Microplate Reader and the data were evaluated on Softmax® Pro. The results were normalized with respect to the average positive (100%) and negative (0%) controls, as described previously [43].
4.7. Time-Kill Kinetics
Time-kill experiments were performed on balanced exponentially growing cultures of S. aureus ATCC 29213 or P. aeruginosa ATCC 27853. We used balanced cultures, as described in Oddo et al. [44]. In brief, overnight cultures were prepared in MHB broth at 37 °C, with shaking. Overnight cultures, were back diluted and grown exponentially for no less than 8–10 generations, before experimentation. When experiments were done at OD600 = 0.0005 ~5 × 105, the cultures were back diluted into fresh MHB, heated to 37 °C from an exponentially growing culture. In the late exponential phase, cultures were simply kept in the exponential phase, until OD600 = 0.1, before experimentation.
5. Conclusions
The present study described the successful synthesis of 18 analogues of a cyclic peptide with a flexible linker. The analogues were synthesized in four stages, focusing on cyclization point, hydrophobicity, cationic side-chain length, and combinations of the last two. We found that compound 11 showed activity against P. aeruginosa and S. aureus, but showed no activity against E. coli and A. baumannii in MIC assays. The most active analogue 11, had a bactericidal activity against P. aeruginosa and bacteriostatic activity against S. aureus, as determined in exponential and late exponential phase time-kill experiments. At 5× MIC, 11 was just as effective as colistin in killing P. aeruginosa. For S. aureus, 11 was significantly better than chloramphenicol at 5× MIC. The results from this study might be helpful in the design of novel cyclic antimicrobial peptides. We are currently pursuing the synthesis of fatty acid analogues of 11 to mimic known cyclic lipopeptide antibiotics such as colistin and daptomycin.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/7/366/s1. Peptide structures, Analytical HPLC traces, and MALDI-TOF-MS spectra. Table S1. Peptide mass, and HPLC retention time and purity.
Author Contributions
Conceptualization, T.T.T., A.L.-O., A.O., and P.R.H; investigation, T.T.T, H.C.M., W.A.-M.; writing—original draft preparation, T.T., H.C.M. and P.R.H.; writing, review, and editing, T.T.T., H.C.M., W.A.-M., A.L.-O., A.O., P.R.H.; supervision T.T.T., A.L.-O., A.O., and P.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Novo Nordisk foundation; Novo Nordisk Fonden: NNF16OC0021700 (Challenge, A.L.-O.) and Novo Nordisk Fonden: NNF16OC0023482 (Tandem, A.L.-O.). Furthermore, we would like to thank the Kirsten and Freddy Johansens foundation (A.L.-O.), The Hørslev Foundation (P.R.H.), and the Augustinus Foundation (P.R.H.) for financial support.
Acknowledgments
Birgitte Simonsen is thanked for excellent technical help.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACN | Acetonitrile |
| Bip | L-biphenylalanine |
| COMU | 1-Cyano-2-ethoxy-2oxoethylidenaminooxy)-dimethylaminomorpholino-carbenium hexa-fluorophosphate |
| Dab | L-2,4-diaminobutyric acid |
| DCM | Dichloromethane |
| DIEA | Disopropylamine |
| DMF | Dimethylformamide, synthesis grade |
| (DMTMM·BF4) | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholiniumtetrafluoroborate |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| Fmoc | 9-fluorenylmethoxycarbonyl |
| HATU | 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxid hexafluoro-phosphate, N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b] pyridin-1-ylmethylene]-N-methylmethan-aminium hexa-fluorophosphate N-oxide |
| HOAt | 1-Hydroxy-7-azabenzotriazole |
| Nal | 3-(2-Naphthyl)-L-alanine |
| O2Oc | 8-amino-3,6-dioxaoctanoic acid |
| Oxyma | Ethyl (hydroxyimino)cyanoacetate |
| PBS | Phosphate-buffered saline |
| RAM | Rink amide Linker |
| RP-HPLC | Reverse Phase Analytical High Performance Liquid Chromatography |
| TFA | trifluoroacetic acid |
| TIS | Triisopropylamine |
References
- Rahbarnia, L.; Farajnia, S.; Naghili, B.; Ahmadzadeh, V.; Veisi, K.; Baghban, R.; Toraby, S. Current trends in targeted therapy for drug-resistant infections. Appl. Microbiol. Biotechnol. 2019, 103, 8301–8314. [Google Scholar] [CrossRef]
- Konaklieva, M.I. Addressing Antimicrobial Resistance through New Medicinal and Synthetic Chemistry Strategies. SLAS Discov. 2018, 24, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Brogden, K. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Greber, K.E.; Dawgul, M. Antimicrobial Peptides Under Clinical Trials. Curr. Top. Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Pollett, S.; Sakoulas, G. A Current Perspective on Daptomycin for the Clinical Microbiologist. Clin. Microbiol. Rev. 2013, 26, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Friedman, L.; Alder, J.D.; Silverman, J.A. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 2137–2145. [Google Scholar] [CrossRef]
- Trent, M.S.; Ribeiro, A.A.; Doerrler, W.T.; Lin, S.; Cotter, R.J.; Raetz, C.R.H. Accumulation of a Polyisoprene-linked Amino Sugar in Polymyxin-resistant Salmonella typhimurium and Escherichia coli: Structural Characterization and Transfer to Lipid A in the Periplasm. J. Biol. Chem. 2001, 276, 43132–43144. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Weiss, D.S. Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria. Antibiotics 2015, 4, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of drug resistance: Daptomycin resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Vaara, M. Polymyxin Derivatives that Sensitize Gram-Negative Bacteria to Other Antibiotics. Molecules 2019, 24, 249. [Google Scholar] [CrossRef]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef]
- Oddo, A.; Thomsen, T.T.; Britt, H.M.; Løbner-Olesen, A.; Thulstrup, P.W.; Sanderson, J.M.; Hansen, P.R. Modulation of Backbone Flexibility for Effective Dissociation of Antibacterial and Hemolytic Activity in Cyclic Peptides. ACS Med. Chem. Lett. 2016, 7, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Wiradharma, N.; Yang, Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014, 78, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Staubitz, P.; Peschel, A.; Nieuwenhuizen, W.F.; Otto, M.; Götz, F.; Jung, G.; Jack, R.W. Structure-function relationships in the tryptophan-rich, antimicrobial peptide indolicidin. J. Pept. Sci. 2001, 7, 552–564. [Google Scholar] [CrossRef]
- Bluhm, M.E.C.; Knappe, D.; Hoffmann, R. Structure-activity relationship study using peptide arrays to optimize Api137 for an increased antimicrobial activity against Pseudomonas aeruginosa. Eur. J. Med. Chem. 2015, 103, 574–582. [Google Scholar] [CrossRef]
- Ifrah, D.; Doisy, X.; Ryge, T.; Hansen, P. Structure-activity relationship study of anoplin. J. Pept. Sci. 2005, 11, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhu, N.; Zhu, Y.; Liu, T.; Gou, S.; Xie, J.; Yao, J.; Ni, J. Antimicrobial peptides conjugated with fatty acids on the side chain of D-amino acid promises antimicrobial potency against multidrug-resistant bacteria. Eur. J. Pharm. Sci. 2020, 141, 105123. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Wang, J.; Thompson, P.E.; Li, J. Teaching ‘Old’ Polymyxins New Tricks: New-Generation Lipopeptides Targeting Gram-Negative ‘Superbugs’. ACS Chem. Biol. 2014, 9, 1172–1177. [Google Scholar] [CrossRef]
- Kondejewski, L.H.; Lee, D.L.; Jelokhani-Niaraki, M.; Farmer, S.W.; Hancock, R.E.W.; Hodges, R.S. Optimization of Microbial Specificity in Cyclic Peptides by Modulation of Hydrophobicity within a Defined Structural Framework. J. Biol. Chem. 2002, 277, 67–74. [Google Scholar] [CrossRef]
- Oddo, A.; Münzker, L.; Hansen, P.R. Peptide Macrocycles Featuring a Backbone Secondary Amine: A Convenient Strategy for the Synthesis of Lipidated Cyclic and Bicyclic Peptides on Solid Support. Org. Lett. 2015, 17, 2502–2505. [Google Scholar] [CrossRef]
- Oddo, A.; Nyberg, N.T.; Frimodt-Moller, N.; Thulstrup, P.W.; Hansen, P.R. The effect of glycine replacement with flexible omega-amino acids on the antimicrobial and haemolytic activity of an amphipathic cyclic heptapeptide. Eur. J. Med. Chem. 2015, 102, 574–581. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipatic, a-helical anticrobial peptides. Pept. Sci. 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Uggerhøj, L.E.; Poulsen, T.J.; Munk, J.K.; Fredborg, M.; Sondergaard, T.E.; Frimodt-Moller, N.; Hansen, P.R.; Wimmer, R. Rational Design of Alpha-Helical Antimicrobial Peptides: Do’s and Don’ts. ChemBioChem 2015, 16, 242–253. [Google Scholar]
- Almaaytah, A.; Qaoud, M.T.; Khalil Mohammed, G.; Abualhaijaa, A.; Knappe, D.; Hoffmann, R.; Al-Balas, Q. Antimicrobial and Antibiofilm Activity of UP-5, an Ultrashort Antimicrobial Peptide Designed Using Only Arginine and Biphenylalanine. Pharmaceuticals 2018, 11, 3. [Google Scholar] [CrossRef]
- McCoy, L.S.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Velkov, T.; Li, J.; Tor, Y. Polymyxins and Analogues Bind to Ribosomal RNA and Interfere with Eukaryotic Translation in Vitro. ChemBioChem 2013, 14, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Simpkins, L.R.; Pérez-Payá, E.; Houghten, R.A. Influence of tryptophan residues on melittin’s hemolytic activity. Biochim. Biophys. Acta (BBA) 1993, 1202, 331–336. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Yang, J.C.; Yohonn, L.; Ly, N.S.; Brown, S.V.; Hondt, R.E.; Jusko, W.J.; Forrest, A.; Tsuji, B.T. Attenuation of Colistin Bactericidal Activity by High Inoculum of Pseudomonas Aeruginosa Characterized by a New Mechanism-Based Population Pharmacodynamic Model. Antimicrob. Agents Chemother. 2010, 54, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Luca, V.; Bocedi, A.; Massoud, R.; Park, Y.; Mangoni, M.L.; Stella, L. Cell-Density Dependence of Host-Defense Peptide Activity and Selectivity in the Presence of Host Cells. ACS Chem. Biol. 2017, 12, 52–56. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Boll, J.M.; Tucker, A.T.; Klein, D.R.; Beltran, A.M.; Brodbelt, J.S.; Davies, B.W.; Trent, M.S. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacter baumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. mBio 2015, 6, e00478-15. [Google Scholar] [CrossRef]
- Li, Y.; Yun, J.; Liu, L.; Li, Y.; Wang, X. Identification of Two Genes Encoding for the Late Acyltransferases of Lipid A in Klebsiella pneumoniae. Curr. Microbiol. 2016, 73, 732–738. [Google Scholar] [CrossRef]
- Kim, S.; Patel, D.S.; Park, S.; Slusky, J.; Klauda, J.B.; Widmalm, G.; Im, W. Bilayer Properties of Lipid A from Various Gram-Negative Bacteria. Biophys. J. 2016, 111, 1750–1760. [Google Scholar] [CrossRef]
- Ofek, I.; Cohen, S.; Rahmani, R.; Kabha, K.; Tamarkin, D.; Herzig, Y.; Rubinstein, E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob. Agents Chemother. 1994, 38, 374. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Hansen, P.R. Hemolytic Activity of Antimicrobial Peptides. Methods Mol. Biol. 2017, 1548, 427–435. [Google Scholar]
- Oddo, A.; Thomsen, T.T.; Kjelstrup, S.; Gorey, C.; Franzyk, H.; Frimodt-Møller, N.; Løbner-Olesen, A.; Hansen, P.R. An all-D amphipathic undecapeptide shows promising activity against colistin-resistant strains of Acinetobacter baumannii and a dual mode of action. Antimicrob. Agents Chemother. 2016, 60, 592–599. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).