Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Test Organism and Culture Conditions
4.2. Surface Preparation
4.3. Biofilm Assays
4.4. Offline Quantification of Biofilm Cells
4.5. Optical Coherence Tomography (OCT)
4.6. Scanning Electron Microscopy (SEM)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm activity as a health issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.; Yokoe, D.S. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Hancock, V.; Ferrières, L.; Klemm, P. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 2006, 267, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef]
- Koseoglu, H.; Aslan, G.; Esen, N.; Sen, B.H.; Coban, H. Ultrastructural stages of biofilm development of Escherichia coli on urethral catheters and effects of antibiotics on biofilm formation. Urology 2006, 68, 942–946. [Google Scholar] [CrossRef]
- Shunmugaperumal, T. Biofilm Eradication and Prevention: A Pharmaceutical Approach to Medical Device Infections; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Trautner, B.W.; Lopez, A.I.; Kumar, A.; Siddiq, D.M.; Liao, K.S.; Li, Y.; Tweardy, D.J.; Cai, C. Nanoscale surface modification favors benign biofilm formation and impedes adherence by pathogens. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 261–270. [Google Scholar] [CrossRef][Green Version]
- Stamm, W.E.; Hooton, T.M. Management of urinary tract infections in adults. N. Engl. J. Med. 1993, 329, 1328–1334. [Google Scholar] [CrossRef]
- Buijssen, K.; Oosterhof, J.; Basil, L.; Waters, M.; Duits, M.; Busscher, H.; van der Mei, H.; van der Laan, B. Influence of surface roughness on silicone rubber voice prostheses on in vitro biofilm formation and clinical lifetime in laryngectomised patients. Clin. Otolaryngol. 2017, 42, 1235–1240. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhão, F.J.M. SEM analysis of surface impact on biofilm antibiotic treatment. Scanning 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Teodósio, J.S.; Simões, M.; Melo, L.F.; Mergulhão, F.J.M. Flow cell hydrodynamics and their effects on E. coli biofilm formation under different nutrient conditions and turbulent flow. Biofouling 2011, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.R.; Araújo, J.D.P.; Miranda, J.M.; Simões, M.; Melo, L.F.; Mergulhão, F.J.M. The effects of surface properties on Escherichia coli adhesion are modulated by shear stress. Colloids Surf. B Biointerfaces 2014, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, R.G. Biomaterials: Stable antifouling surfaces. Nat. Mater. 2003, 2, 207–208. [Google Scholar] [CrossRef]
- Rodríguez-Emmenegger, C.; Brynda, E.; Riedel, T.; Houska, M.; Šubr, V.; Alles, A.B.; Hasan, E.; Gautrot, J.E.; Huck, W.T. Polymer brushes showing non-fouling in blood plasma challenge the currently accepted design of protein resistant surfaces. Macromol. Rapid Commun. 2011, 32, 952–957. [Google Scholar] [CrossRef]
- Rodríguez-Emmenegger, C.; Houska, M.; Alles, A.B.; Brynda, E. Surfaces resistant to fouling from biological fluids: Towards bioactive surfaces for real applications. Macromol. Biosci. 2012, 12, 1413–1422. [Google Scholar] [CrossRef]
- Ma, H.; Li, D.; Sheng, X.; Zhao, B.; Chilkoti, A. Protein-resistant polymer coatings on silicon oxide by surface-initiated atom transfer radical polymerization. Langmuir 2006, 22, 3751–3756. [Google Scholar] [CrossRef]
- Gautrot, J.E.; Trappmann, B.; Oceguera-Yanez, F.; Connelly, J.; He, X.; Watt, F.M.; Huck, W.T. Exploiting the superior protein resistance of polymer brushes to control single cell adhesion and polarisation at the micron scale. Biomaterials 2010, 31, 5030–5041. [Google Scholar] [CrossRef]
- Rodríguez-Emmenegger, C.; Decker, A.; Surman, F.; Preuss, C.M.; Sedláková, Z.; Zydziak, N.; Barner-Kowollik, C.; Schwartz, T.; Barner, L. Suppressing Pseudomonas aeruginosa adhesion via non-fouling polymer brushes. RSC Adv. 2014, 4, 64781–64790. [Google Scholar] [CrossRef]
- De los Santos Pereira, A.; Sheikh, S.; Blaszykowski, C.; Pop-Georgievski, O.; Fedorov, K.; Thompson, M.; Rodriguez-Emmenegger, C. Antifouling polymer brushes displaying antithrombogenic surface properties. Biomacromolecules 2016, 17, 1179–1185. [Google Scholar] [CrossRef]
- Lopez-Mila, B.; Alves, P.; Riedel, T.; Dittrich, B.; Mergulhão, F.J.M.; Rodríguez-Emmenegger, C. Effect of shear stress on the reduction of bacterial adhesion to antifouling polymers. Bioinspiration Biomimetics 2018. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, J. Synthesis and characterization of poly(N-hydroxyethylacrylamide) for long-term antifouling ability. Biomacromolecules 2011, 12, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Tauhardt, L.; Pretzel, D.; Kempe, K.; Gottschaldt, M.; Pohlers, D.; Schubert, U.S. Zwitterionic poly(2-oxazoline)s as promising candidates for blood contacting applications. Polym. Chem. 2014, 5, 5751–5764. [Google Scholar] [CrossRef]
- Surman, F.; Riedel, T.; Bruns, M.; Kostina, N.Y.; Sedláková, Z.; Rodríguez-Emmenegger, C. Polymer brushes interfacing blood as a route toward high performance blood contacting devices. Macromol. Biosci. 2015, 15, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Emmenegger, C.; Brynda, E.; Riedel, T.; Sedlakova, Z.; Houska, M.; Alles, A.B. Interaction of blood plasma with antifouling surfaces. Langmuir 2009, 25, 6328–6333. [Google Scholar] [CrossRef]
- Riedel, T.; Riedelová-Reicheltová, Z.; Májek, P.; Rodríguez-Emmenegger, C.; Houska, M.; Dyr, J.E.; Brynda, E. Complete identification of proteins responsible for human blood plasma fouling on poly(ethylene glycol)-based surfaces. Langmuir 2013, 29, 3388–3397. [Google Scholar] [CrossRef]
- Tischer, T.; Rodríguez-Emmenegger, C.; Trouillet, V.; Welle, A.; Schueler, V.; Mueller, J.O.; Goldmann, A.S.; Brynda, E.; Barner-Kowollik, C. Photo-patterning of non-fouling polymers and biomolecules on paper. Adv. Mater. 2014, 26, 4087–4092. [Google Scholar] [CrossRef]
- Rodríguez-Emmenegger, C.; Janel, S.; de los Santos Pereira, A.; Bruns, M.; Lafont, F. Quantifying bacterial adhesion on antifouling polymer brushes via single-cell force spectroscopy. Polym. Chem. 2015, 6, 5740–5751. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Horn, H.; Reiff, H.; Morgenroth, E. Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol. Bioeng. 2003, 81, 607–617. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z. Critical review on biofilm methods. Critical Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.R.; Ponmozhi, J.; Campos, J.; Miranda, J.M.; Mergulhão, F.J.M. Micro-and macro-flow systems to study Escherichia coli adhesion to biomedical materials. Chem. Eng. Sci. 2015, 126, 440–445. [Google Scholar] [CrossRef]
- Gomes, L.C.; Moreira, J.M.R.; Araújo, J.D.P.; Mergulhão, F.J.M. Surface conditioning with Escherichia coli cell wall components can reduce biofilm formation by decreasing initial adhesion. AIMS Microbiol. 2017, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Mosayyebi, A.; Lange, D.; Yann Yue, Q.; Somani, B.; Zhang, X.; Manes, C.; Carugo, D. Reducing deposition of encrustation in ureteric stents by changing the stent architecture: A microfluidic-based investigation. Biomicrofluidics 2019, 13, 014101. [Google Scholar] [CrossRef] [PubMed]
- Mosayyebi, A.; Yue, Q.Y.; Somani, B.K.; Zhang, X.; Manes, C.; Carugo, D. Particle accumulation in ureteral stents is governed by fluid dynamics: In vitro study using a “stent-on-chip” model. J. Endourol. 2018, 32, 639–646. [Google Scholar] [CrossRef]
- Stewart, P.S. Biophysics of biofilm infection. Pathog. Dis. 2014, 70, 212–218. [Google Scholar] [CrossRef]
- Nauman, E.A.; Ott, C.M.; Sander, E.; Tucker, D.L.; Pierson, D.; Wilson, J.W.; Nickerson, C.A. Novel quantitative biosystem for modeling physiological fluid shear stress on cells. Appl. Environ. Microbiol. 2007, 73, 699–705. [Google Scholar] [CrossRef]
- Rodesney, C.A.; Roman, B.; Dhamani, N.; Cooley, B.J.; Touhami, A.; Gordon, V.D. Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc. Natl. Acad. Sci. USA 2017, 114, 5906–5911. [Google Scholar] [CrossRef]
- Gomes, L.C.; Moreira, J.M.R.; Teodósio, J.S.; Araújo, J.D.P.; Miranda, J.; Simões, M.; Melo, L.F.; Mergulhão, F.J.M. 96-well microtiter plates for biofouling simulation in biomedical settings. Biofouling 2014, 30, 535–546. [Google Scholar] [CrossRef]
- Shah, K.J.; Cherabuddi, K.; Shultz, J.; Borgert, S.; Ramphal, R.; Klinker, K.P. Ampicillin for the treatment of complicated urinary tract infections caused by vancomycin resistant Enterococcus spp (VRE): A single-center university hospital experience. Int. J. Antimicrob. Agents 2017. [Google Scholar] [CrossRef]
- Halperin, A.; Fragneto, G.; Schollier, A.; Sferrazza, M. Primary versus ternary adsorption of proteins onto PEG brushes. Langmuir 2007, 23, 10603–10617. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lee, J.; Andrade, J.; De Gennes, P. Protein—Surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. [Google Scholar] [CrossRef]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Karunakaran, E.; Biggs, C.A. Using a multi-faceted approach to determine the changes in bacterial cell surface properties influenced by a biofilm lifestyle. Biofouling 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Vila, J.; Sáez-López, E.; Johnson, J.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.; Naas, T.; Carattoli, A.; Martínez-Medina, M. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef]
- Gomes, L.C.; Silva, L.; Simões, M.; Melo, L.F.; Mergulhão, F.J.M. Escherichia coli adhesion, biofilm development and antibiotic susceptibility on biomedical materials. J. Biomed. Mater. Res. Part A 2015, 103, 1414–1423. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Vorobii, M.; Rodríguez-Emmenegger, C.; Mergulhão, F.J.M. The potential advantages of using a poly(HPMA) brush in urinary catheters: Effects on biofilm cells and architecture. Colloids Surf. B Biointerfaces 2020, 191, 110976. [Google Scholar] [CrossRef]
- Yao, Z.; Kahne, D.; Kishony, R. Distinct single-cell morphological dynamics under β-lactam antibiotics. Mol. Cell 2012, 48, 705–712. [Google Scholar] [CrossRef]
- Martinez, O.V.; Gratzner, H.G.; Malinin, T.I.; Ingram, M. The effect of some β-lactam antibiotics on Escherichia coli studied by flow cytometry. Cytom. J. Int. Soc. Anal. Cytol. 1982, 3, 129–133. [Google Scholar] [CrossRef]
- Ionescu, M.; Belkin, S. Overproduction of exopolysaccharides by an Escherichia coli K-12 rpoS mutant in response to osmotic stress. Appl. Environ. Microbiol. 2009, 75, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hengge-Aronis, R.; Lange, R.; Henneberg, N.; Fischer, D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 1993, 175, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.F.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005, 187, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L.; Nilson, J.L.; Barnes, A.M.T.; Dunny, G.M. Restructuring of Enterococcus faecalis biofilm architecture in response to antibiotic-induced stress. NPJ Biofilms Microbiomes 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Uzoechi, S.C.; Abu-Lail, N.I. The Effects of β-Lactam Antibiotics on Surface Modifications of Multidrug-Resistant Escherichia coli: A Multiscale Approach. Microsc. Microanal. 2019, 25, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.; Keevil, C. A simple artificial urine for the growth of urinary pathogens. Letters in Applied Microbiology 1997, 24, 203–206. [Google Scholar] [CrossRef]
- Vagos, M.R.; Moreira, J.M.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Mergulhão, F.J.M. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym. Compos. 2019, 40, E1697–E1704. [Google Scholar] [CrossRef]
- Velraeds, M.M.; van de Belt-Gritter, B.; van der Mei, H.; Reid, G.; Busscher, H. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J. Med. Microbiol. 1998, 47, 1081–1085. [Google Scholar] [CrossRef]
- Barbosa, J.; Cuppini, M.; Flach, J.; Steffens, C.; Cansian, R.L.; Toniazzo, G. Removal of Escherichia coli in boning knives with different sanitizers. LWT Food Sci. Technol. 2016, 71, 309–315. [Google Scholar] [CrossRef]
- Winkelströter, L.K.; Gomes, B.C.; Thomaz, M.R.S.; Souza, V.M.; De Martinis, E.C.P. Lactobacillus sakei 1 and its bacteriocin influence adhesion of Listeria monocytogenes on stainless steel surface. Food Control 2011, 22, 1404–1407. [Google Scholar] [CrossRef]
- Romeu, M.J.; Alves, P.; Morais, J.; Miranda, J.M.; de Jong, E.D.; Sjollema, J.; Ramos, V.; Vasconcelos, V.; Mergulhão, F.J.M. Biofilm formation behaviour of marine filamentous cyanobacterial strains in controlled hydrodynamic conditions. Environ. Microbiol. 2019, 21, 4411–4424. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Pereira, M.O.; Vieira, M. The role of hydrodynamic stress on the phenotypic characteristics of single and binary biofilms of Pseudomonas fluorescens. Water Sci. Technol. 2007, 55, 437–445. [Google Scholar] [CrossRef] [PubMed]
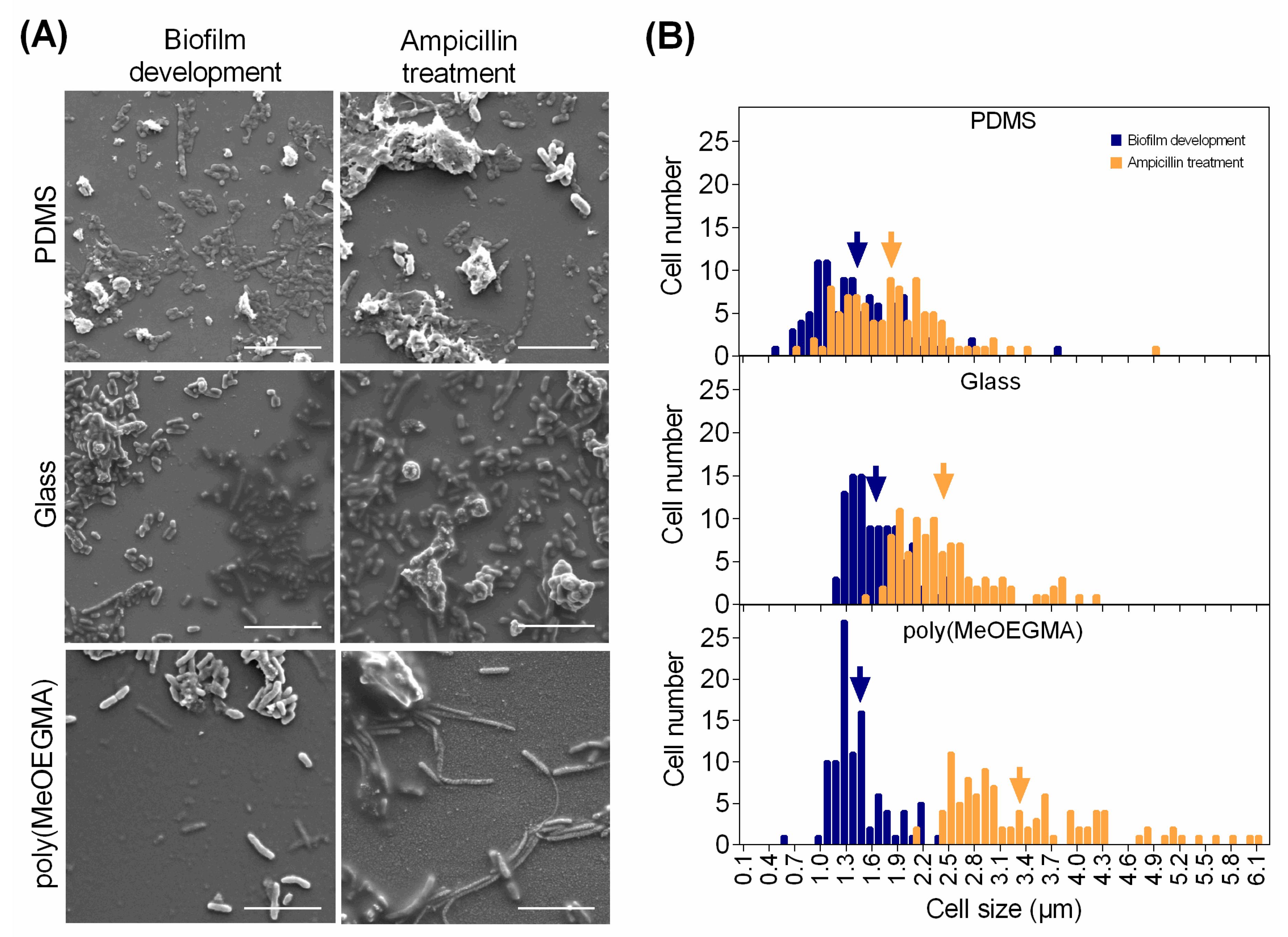
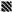 , respectively), glass (■ and
, respectively), glass (■ and  , respectively), and poly(MeOEGMA) brush (■ and
, respectively), and poly(MeOEGMA) brush (■ and 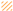 , respectively). Standard deviations for three independent samples are presented. Statistical significance for all the surfaces at each time point was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, and ** for p < 0.01).
, respectively). Standard deviations for three independent samples are presented. Statistical significance for all the surfaces at each time point was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, and ** for p < 0.01).
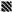 , respectively), glass (■ and
, respectively), glass (■ and  , respectively), and poly(MeOEGMA) brush (■ and
, respectively), and poly(MeOEGMA) brush (■ and 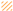 , respectively). Standard deviations for three independent samples are presented. Statistical significance for all the surfaces at each time point was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, and ** for p < 0.01).
, respectively). Standard deviations for three independent samples are presented. Statistical significance for all the surfaces at each time point was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, and ** for p < 0.01).
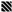 , respectively), glass (■ and
, respectively), glass (■ and  , respectively), and poly(MeOEGMA) brush (■ and
, respectively), and poly(MeOEGMA) brush (■ and 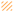 , respectively). Standard deviations for three independent samples are presented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01 and * for p < 0.05).
, respectively). Standard deviations for three independent samples are presented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01 and * for p < 0.05).
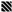 , respectively), glass (■ and
, respectively), glass (■ and  , respectively), and poly(MeOEGMA) brush (■ and
, respectively), and poly(MeOEGMA) brush (■ and 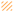 , respectively). Standard deviations for three independent samples are presented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01 and * for p < 0.05).
, respectively). Standard deviations for three independent samples are presented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01 and * for p < 0.05).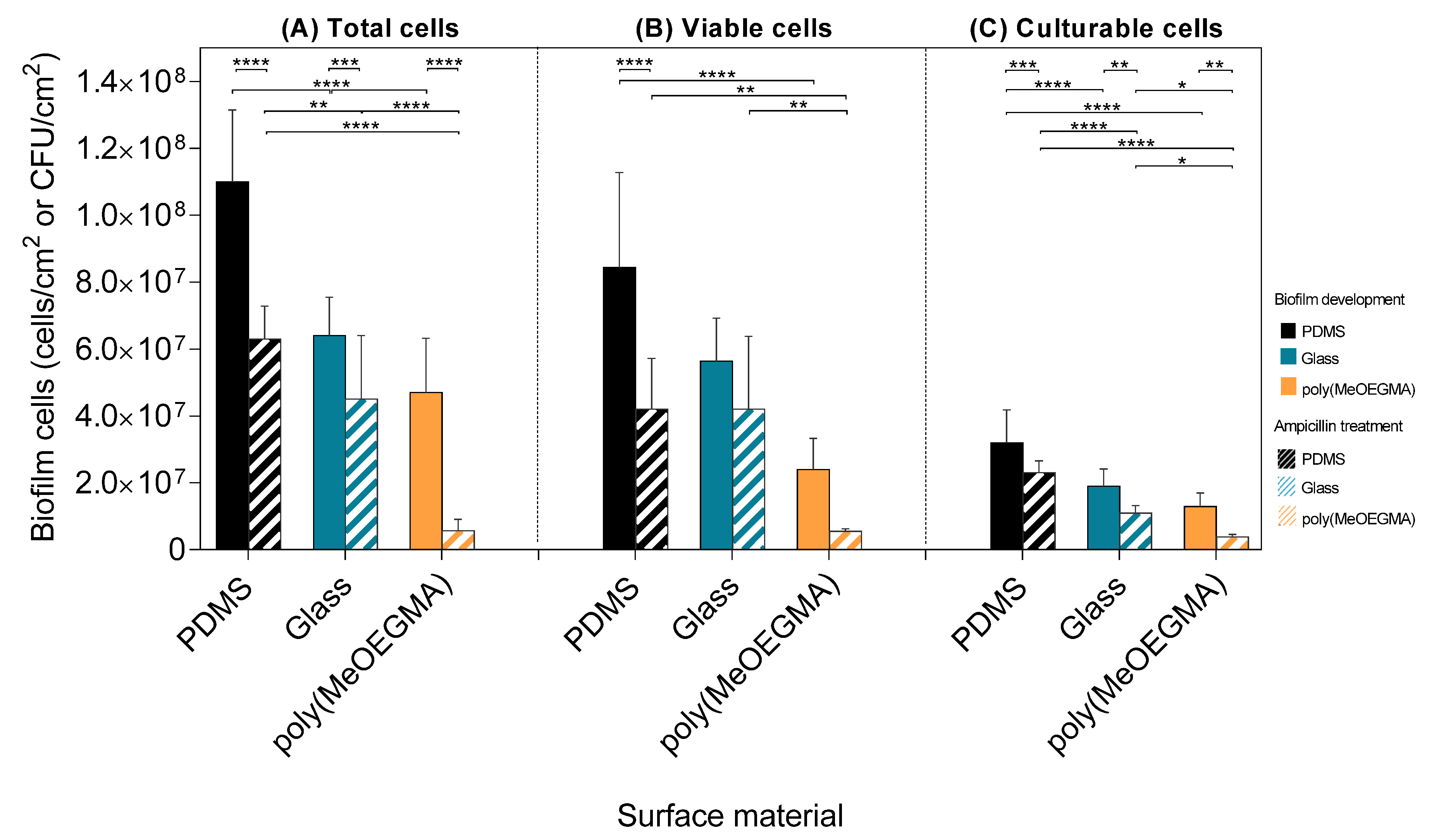
 , respectively), glass (■ and
, respectively), glass (■ and  , respectively) and poly(MeOEGMA) brush (■ and
, respectively) and poly(MeOEGMA) brush (■ and  , respectively). Standard deviations obtained from three replicates of each sample are represented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, and * for p < 0.05).
, respectively). Standard deviations obtained from three replicates of each sample are represented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, and * for p < 0.05).
 , respectively), glass (■ and
, respectively), glass (■ and  , respectively) and poly(MeOEGMA) brush (■ and
, respectively) and poly(MeOEGMA) brush (■ and  , respectively). Standard deviations obtained from three replicates of each sample are represented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, and * for p < 0.05).
, respectively). Standard deviations obtained from three replicates of each sample are represented. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison test (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, and * for p < 0.05).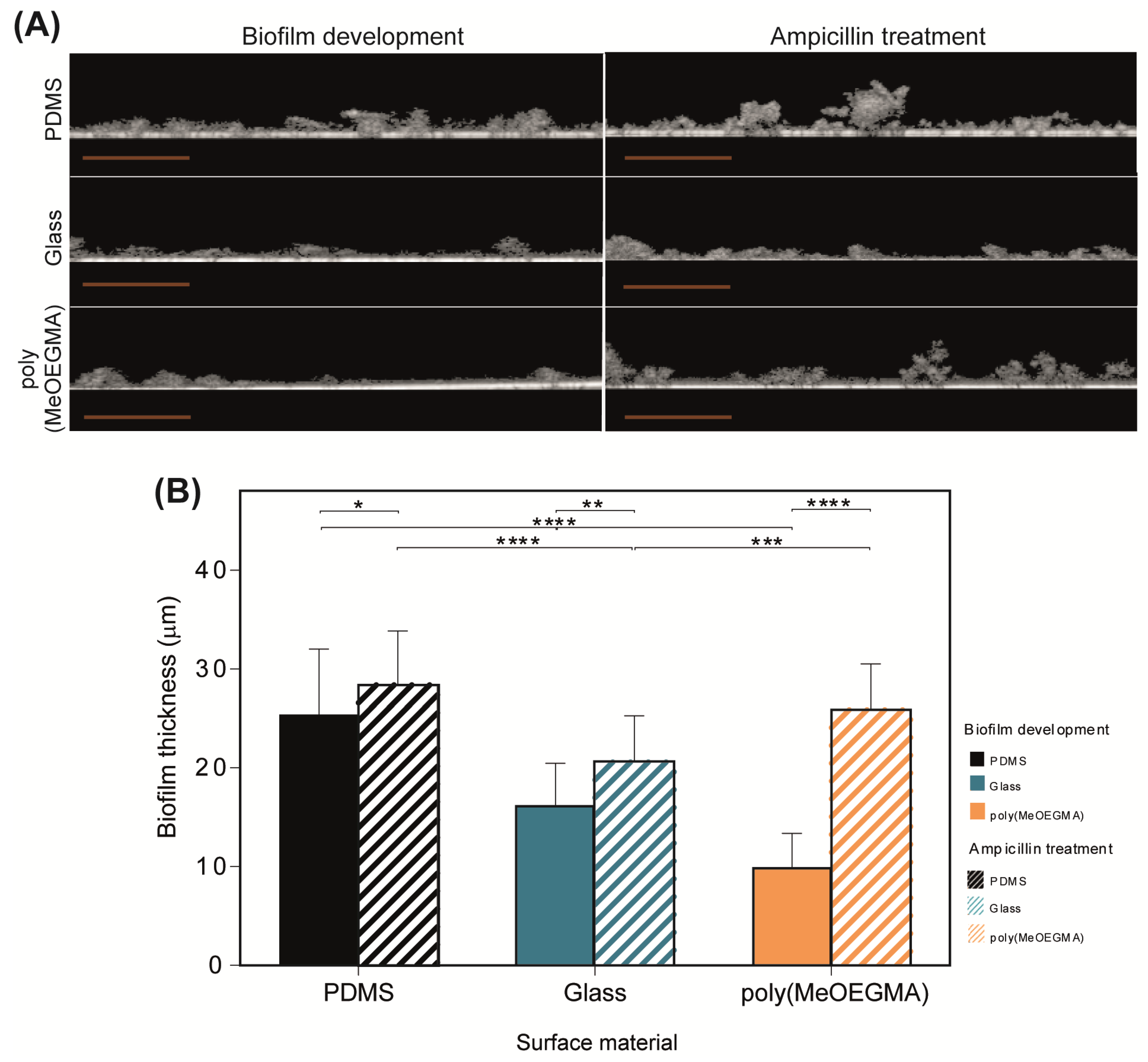
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.; Gomes, L.C.; Rodríguez-Emmenegger, C.; Mergulhão, F.J. Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility. Antibiotics 2020, 9, 216. https://doi.org/10.3390/antibiotics9050216
Alves P, Gomes LC, Rodríguez-Emmenegger C, Mergulhão FJ. Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility. Antibiotics. 2020; 9(5):216. https://doi.org/10.3390/antibiotics9050216
Chicago/Turabian StyleAlves, Patrícia, Luciana Calheiros Gomes, Cesar Rodríguez-Emmenegger, and Filipe José Mergulhão. 2020. "Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility" Antibiotics 9, no. 5: 216. https://doi.org/10.3390/antibiotics9050216
APA StyleAlves, P., Gomes, L. C., Rodríguez-Emmenegger, C., & Mergulhão, F. J. (2020). Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility. Antibiotics, 9(5), 216. https://doi.org/10.3390/antibiotics9050216




