Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Description of Studies
2.1.1. Results of the Search
2.1.2. Design
2.1.3. Patient Population
2.1.4. Interventions
2.1.5. Comparison
2.1.6. Outcomes
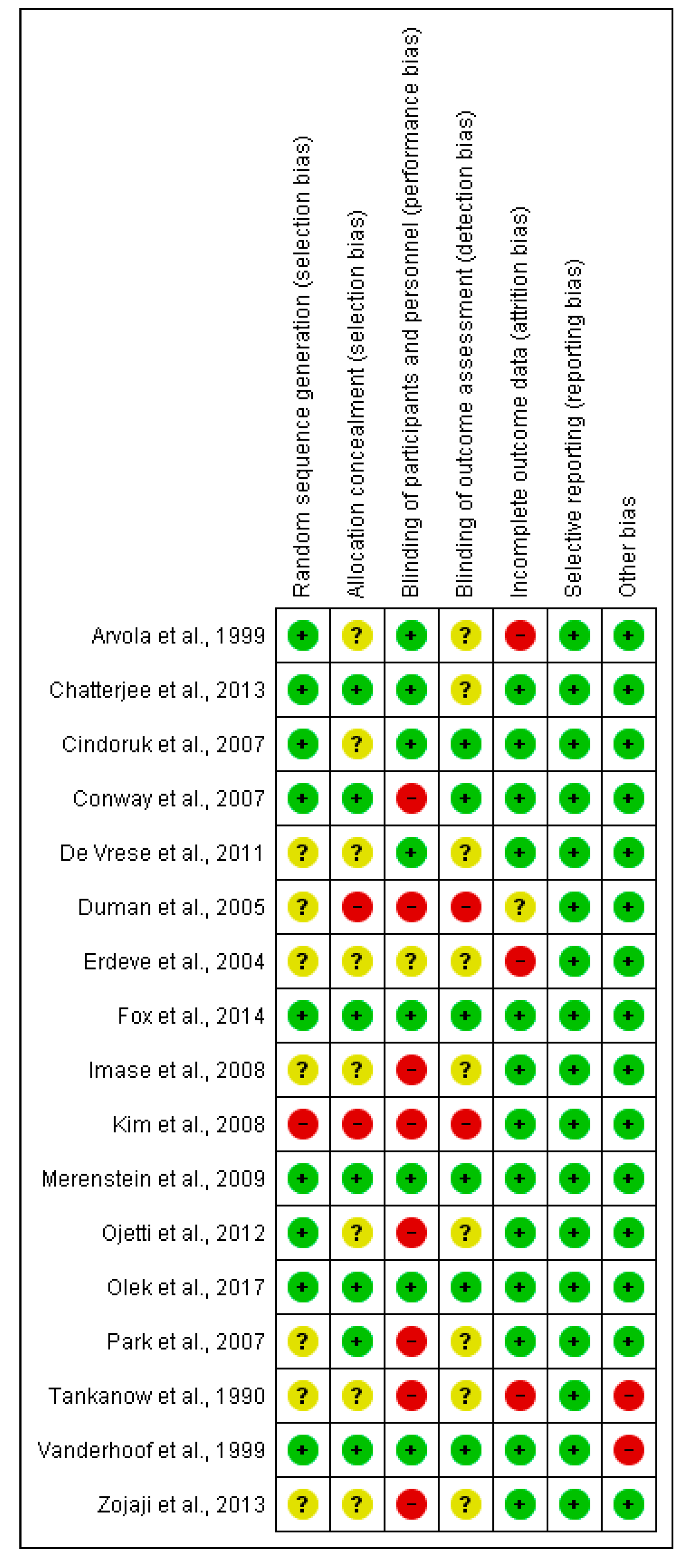
2.2. Risk of Bias in Included Studies
2.3. Effects of Interventions
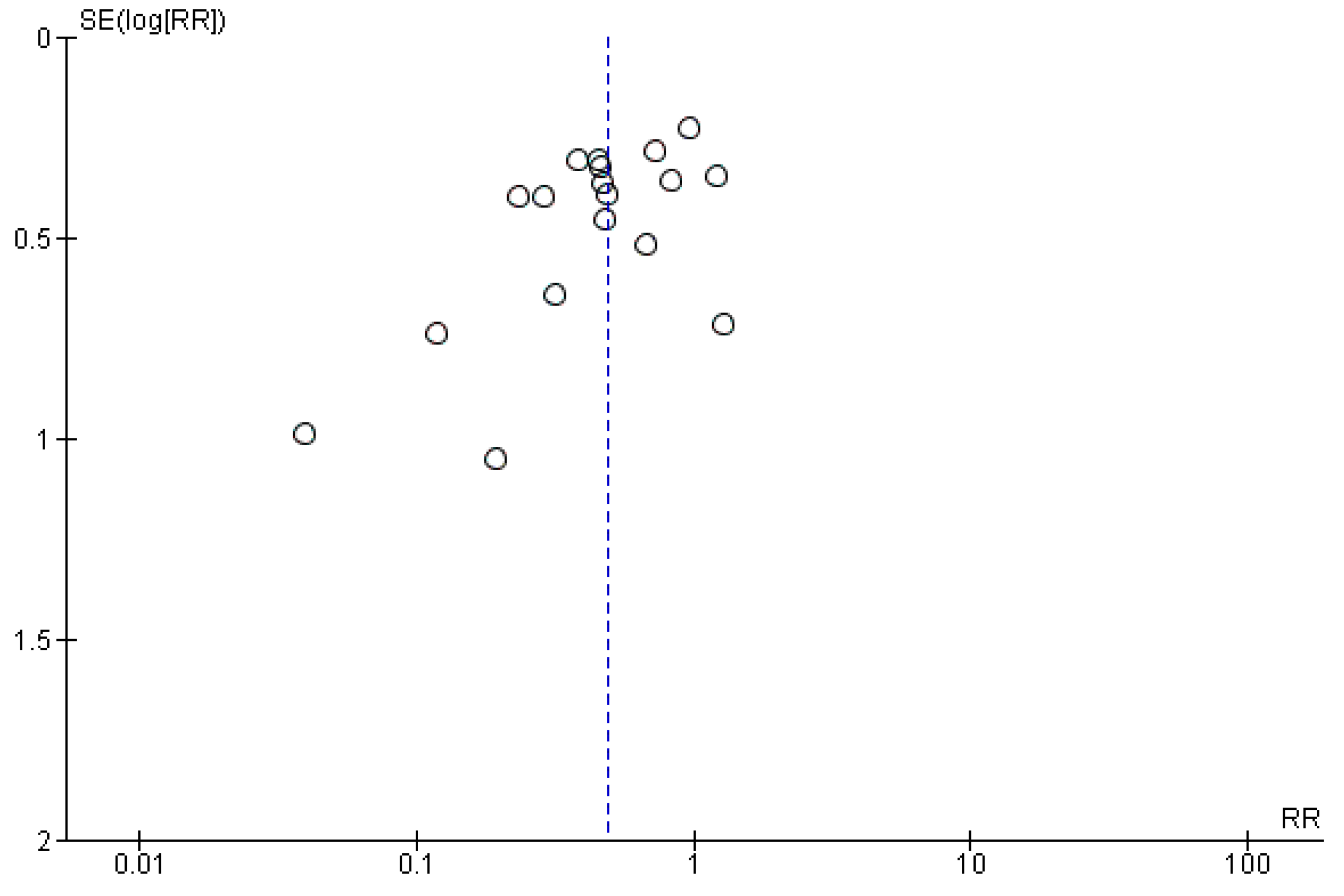
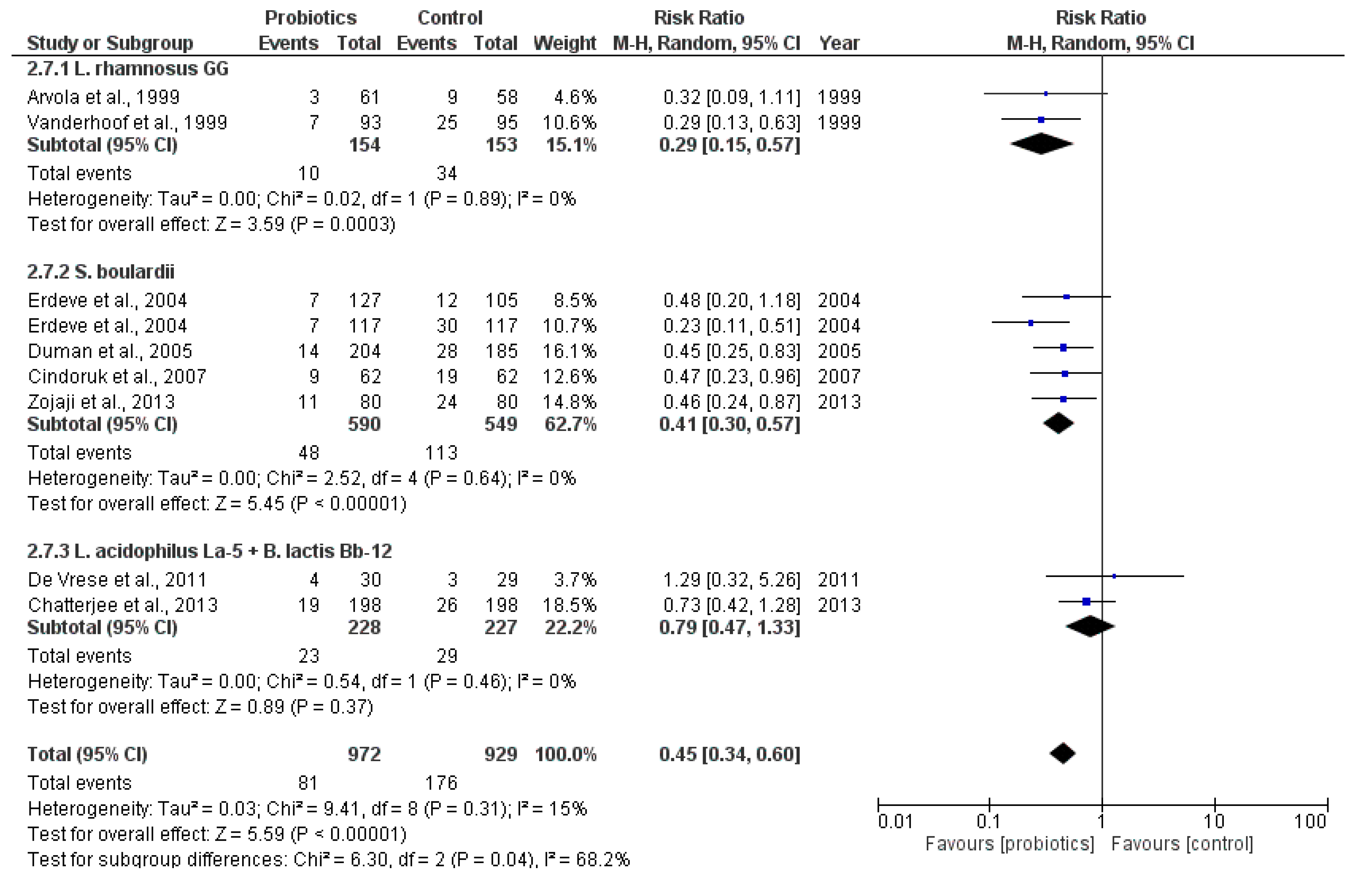
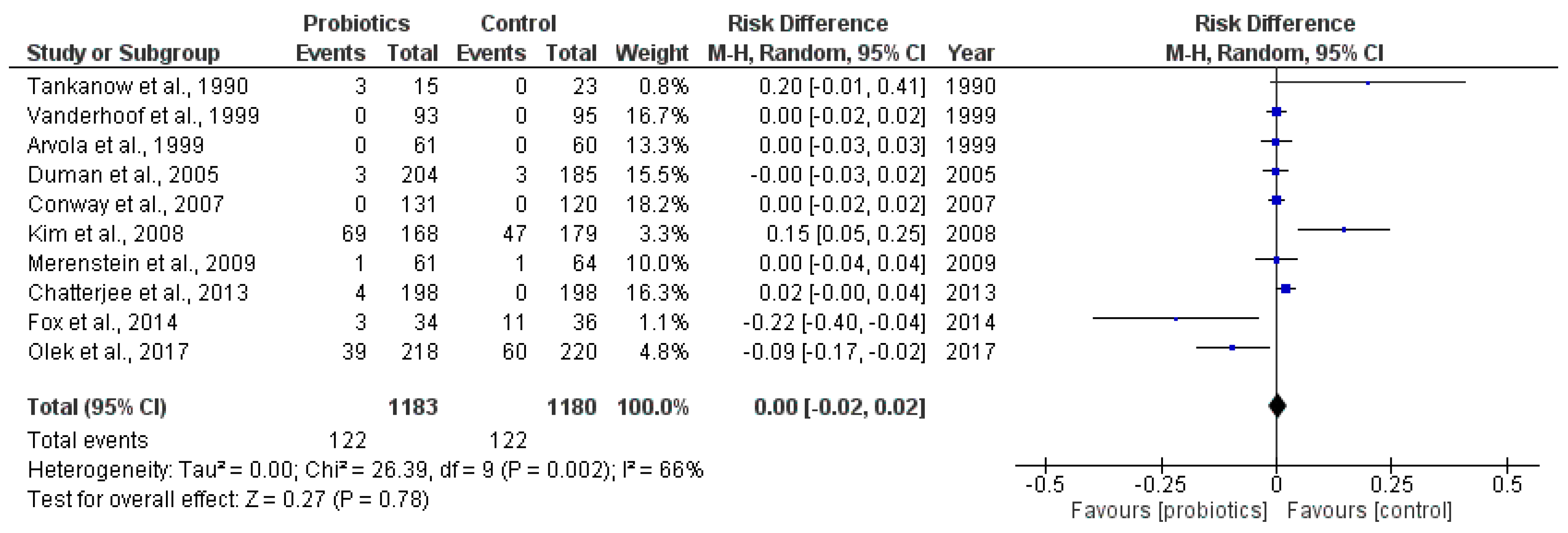
2.3.1. Main Outcome: Incidence of Antibiotic-Associated Diarrhea
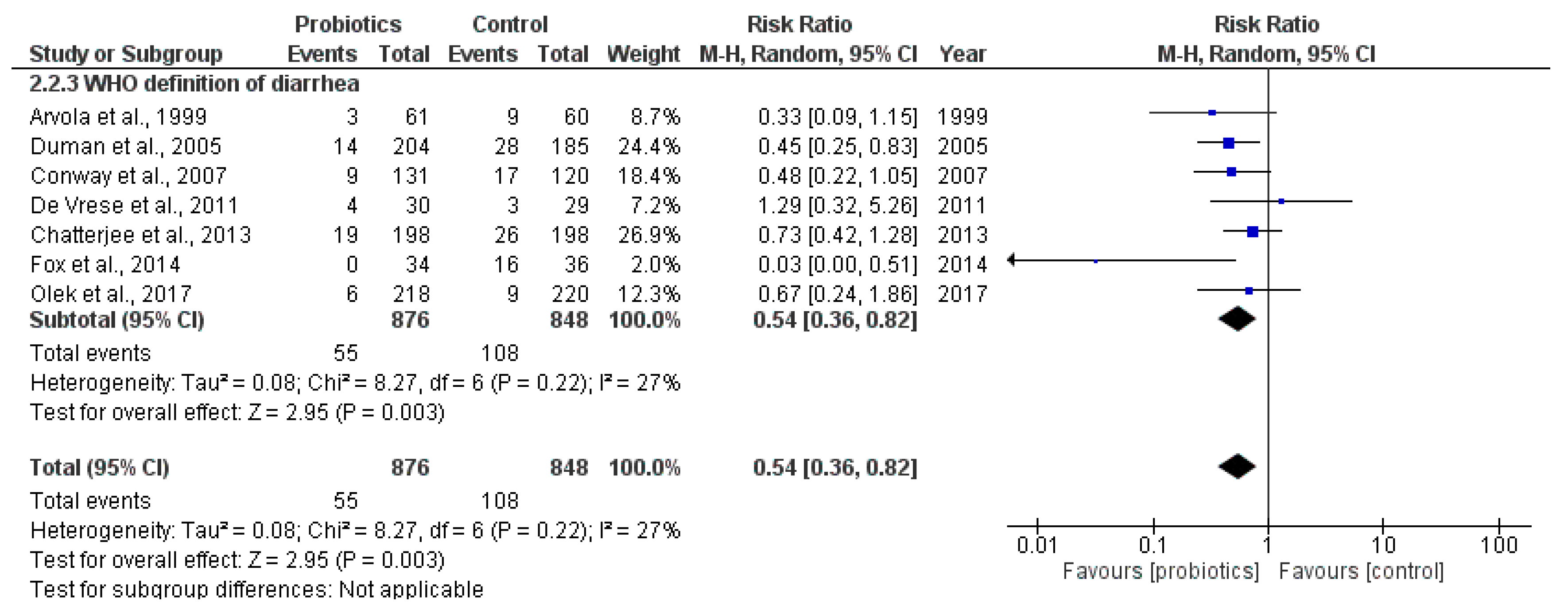
2.3.2. Secondary Outcome: Incidence of Antibiotic-Associated Diarrhea Using the Criteria Defined by WHO
2.3.3. Secondary Outcome: Mean Duration of Diarrhea
2.3.4. Secondary Outcome: Incidence of Adverse Events
2.3.5. Dose-Response Analysis
2.4. Subgroup Analyses
2.4.1. Age Groups
2.4.2. Trials with H. pylori Eradication Therapy
2.4.3. Low Risk of Bias
2.4.4. Intention-To-Treat Analyses
3. Discussion
4. Materials and Methods
4.1. Criteria for Selecting Studies for This Review
4.1.1. Types of Studies
4.1.2. Types of Participants
4.1.3. Types of Interventions
4.1.4. Types of Outcome Measures
- Incidence of antibiotic-associated diarrhea (AAD)
- Incidence of AAD using the criteria defined by WHO:
- Mean duration of diarrhea (MDD) in days
- Number and types of adverse events
4.2. Search Methods for Identification of Studies
4.3. Data Collection and Analysis
4.3.1. Study Selection
4.3.2. Data Extraction and Management
4.3.3. Quality Assessments
4.3.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McFarland, L.V. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig. Dis. (Basel Switz.) 1998, 16, 292–307. [Google Scholar] [CrossRef]
- Wistrom, J.; Norrby, S.R.; Myhre, E.B.; Eriksson, S.; Granstrom, G.; Lagergren, L.; Englund, G.; Nord, C.E.; Svenungsson, B. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J. Antimicrob. Chemother. 2001, 47, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet (Lond. Engl.) 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nation/World Health Organization. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396s–402s. [Google Scholar] [PubMed]
- Goldenberg, J.Z.; Lytvyn, L.; Steurich, J.; Parkin, P.; Mahant, S.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2015, Cd004827. [Google Scholar] [CrossRef]
- Tankanow, R.M.; Ross, M.B.; Ertel, I.J.; Dickinson, D.G.; McCormick, L.S.; Garfinkel, J.F. A double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhea. DICP 1990, 24, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.A.; Whitney, D.B.; Antonson, D.L.; Hanner, T.L.; Lupo, J.V.; Young, R.J. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J. Pediatr. 1999, 135, 564–568. [Google Scholar] [CrossRef]
- Arvola, T.; Laiho, K.; Torkkeli, S.; Mykkanen, H.; Salminen, S.; Maunula, L.; Isolauri, E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study. Pediatrics 1999, 104, e64. [Google Scholar] [CrossRef] [PubMed]
- Erdeve, O.; Tiras, U.; Dallar, Y. The probiotic effect of Saccharomyces boulardii in a pediatric age group. J. Trop. Pediatr. 2004, 50, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Duman, D.G.; Bor, S.; Ozutemiz, O.; Sahin, T.; Oguz, D.; Istan, F.; Vural, T.; Sandkci, M.; Işksal, F.; Simşek, I.; et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Park, D.I.; Choi, J.S.; Kang, M.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. The effect of probiotics on Helicobacter pylori eradication. Hepatogastroenterology 2007, 54, 2032–2036. [Google Scholar] [PubMed]
- Cindoruk, M.; Erkan, G.; Karakan, T.; Dursun, A.; Unal, S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: A prospective randomized placebo-controlled double-blind study. Helicobacter 2007, 12, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.; Hart, A.; Clark, A.; Harvey, I. Does eating yogurt prevent antibiotic-associated diarrhoea? A placebo-controlled randomised controlled trial in general practice. Br. J. Gen. Pract. 2007, 57, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Imase, K.; Takahashi, M.; Tanaka, A.; Tokunaga, K.; Sugano, H.; Tanaka, M.; Ishida, H.; Kamiya, S.; Takahashi, S. Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol. Immunol. 2008, 52, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Kim, N.; Lee, S.H.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jeong, S.H.; Lee, D.H.; Kim, J.S.; Jung, H.C.; et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.J.; Foster, J.; D’Amico, F. A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: The measuring the influence of Kefir (MILK) Study. Arch. Pediatr. Adolesc. Med. 2009, 163, 750–754. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Kristen, H.; Rautenberg, P.; Laue, C.; Schrezenmeir, J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J. Dairy Res. 2011, 78, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Bruno, G.; Ainora, M.E.; Gigante, G.; Rizzo, G.; Roccarina, D.; Gasbarrini, A. Impact of Lactobacillus reuteri Supplementation on Anti-Helicobacter pylori Levofloxacin-Based Second-Line Therapy. Gastroenterol. Res. Pract. 2012, 2012, 740381. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kar, P.; Das, T.; Ray, S.; Gangulyt, S.; Rajendiran, C.; Mitra, M. Randomised placebo-controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus LA-5 and Bifidobacterium BB-12 for prevention of antibiotic-associated diarrhoea. J. Assoc. Phys. India 2013, 61, 708–712. [Google Scholar]
- Zojaji, H.; Ghobakhlou, M.; Rajabalinia, H.; Ataei, E.; Jahani Sherafat, S.; Moghimi-Dehkordi, B.; Bahreiny, R. The efficacy and safety of adding the probiotic Saccharomyces boulardiito standard triple therapy for eradication of H.pylori: A randomized controlled trial. Gastroenterol. Hepatol. Bed. Bench 2013, 6, S99–S104. [Google Scholar] [PubMed]
- Fox, M.J.; Ahuja, K.D.; Robertson, I.K.; Ball, M.J.; Eri, R.D. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open 2015, 5, e006474. [Google Scholar] [CrossRef] [PubMed]
- Olek, A.; Woynarowski, M.; Ahren, I.L.; Kierkus, J.; Socha, P.; Larsson, N.; Önning, G. Efficacy and Safety of Lactobacillus plantarum DSM 9843 (LP299V) in the Prevention of Antibiotic-Associated Gastrointestinal Symptoms in Children-Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2017, 186, 82–86. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diarrhoeal Disease. Available online: http://www.who.int/mediacentre/factsheets/fs330/en/ (accessed on 1 June 2017).
- Ouwehand, A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes 2016, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed]
- Petersen, I.; Hayward, A.C. Antibacterial prescribing in primary care. J. Antimicrob. Chemother. 2007, 60, i43–i47. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.; Ruelaz, A.; Wang, Z.; Miles, J.N.; Suttorp, M.J.; Johnsen, B.; Shanman, R.; Slusser, W.; Fu, N.; et al. Safety of Probiotics Used to Reduce Risk and Prevent or Treat Disease; Evidence Report/Technology Assessment No. 200; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2011. [Google Scholar]
- WHO. Appendix 1, Definitions of health-care settings and other related terms. In Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2009; Available online: https://www.ncbi.nlm.nih.gov/books/NBK144006/ (accessed on 1 June 2017).
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8: Assessing Risk of Bias in Included Studies; Higgins, J.P.T., Green, S., Eds.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Schünemann, H., Brożek, J., Guyatt, G., Oxman, A., Eds.; Updated October 2013; The GRADE Working Group, 2013; Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 June 2017).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015. (Developed by Evidence Prime, Inc.). Available online: https://gradepro.org./ (accessed on 5 October 2017).
- Review Manager (RevMan) [Computer program]. Version 5.3; The Nordic Cochrane Centre; The Cochrane Collaboration: Copenhagen, Denmark, 2014.
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; Higgins, J.P.T.; Green, S. (Eds.) The Cochrane Collaboration: Copenhagen, Denmark, 2011; Available online: www.handbook.cochrane.org (accessed on 1 June 2017).
| RCT | Probiotic(s) Used (Genus and Strain) | Dosage | Duration of Treatment |
|---|---|---|---|
| Tankanow et al., 1990 [7] | Lactobacillus acidophilus Lactobacillus bulgaricus | 5.1 × 108 CFU, four times daily | 10 days |
| Vanderhoof et al., 1999 [8] | Lactobacillus rhamnosus GG | Children < 12 kg: 1 × 1010 CFU, once daily Children > 12 kg: 2 × 1010, once daily | 10 days |
| Arvola et al., 1999 [9] | Lactobacillus rhamnosus GG | 2 × 1010 CFU, twice daily | Seven to 10 days |
| Erdeve et al., 2004 [10] | Saccharomyces boulardii | Not mentioned | Not mentioned |
| Duman et al., 2005 [11] | Saccharomyces boulardii | 500 mg, twice daily | 14 days |
| Park et al., 2007 [12] | Bacillus subtilis Streptococcus faecium | two capsules three times a day: 2.5 × 109 CFU (Bacillus subtilis) 22.5 × 109 CFU (Streptococcus faecium) | Eight weeks |
| Cindoruk et al., 2007 [13] | Saccharomyces boulardii | 500 mg, twice daily | 14 days |
| Conway et al., 2007 [14] | Lactobacillus acidophilus | 109 CFU, once daily | 12 days |
| Streptococcus thermophilus | |||
| Bifidobacterium animalis lactis | |||
| Imase et al., 2008 [15] | Clostridium butyricum | 1 × 107 CFU per tablet Group B: two tablets, three times daily Group C: 4 tablets, three times daily | Seven days |
| Kim et al., 2008 [16] | Lactobacillus acidophilus Lactobacillus casei Bifidobacterium longum Streptococcus thermophilus | One bottle (150 mL) per day: >1 × 105 CFU/mL (L. acidophilus) >1 × 105 CFU/mL (L. casei) >1 × 106 CFU/mL (B. longum) >1 × 108 CFU/mL (S. themophilus) | At least three weeks |
| Merenstein et al., 2009 [17] | Lactococcus lactis Lactococcus plantarum Lactococcus rhamnosus Lactococcus casei Lactococcus lactis subspecies diacetylactis Leuconostoc cremoris Bifidobacterium longum Bifidobacterium breve Lactobacillus acidophilus Saccharomyces florentinus | One bottle (150 mL) per day, amount of CFU not mentioned | 10 days |
| De Vrese et al., 2011 [18] | Lactobacillus acidophilus LA-5 Bifidobacterium lactis BB-12 | >1 × 106 CFU/g, 125 g, twice daily | Five weeks |
| Ojetti et al., 2013 [19] | Lactobacillus reuteri | 1 × 108 CFU, three times daily | 14 days |
| Chatterjee et al., 2013 [20] | Lactobacillus acidophilus La-5, | 4 × 109 CFU | 14 days |
| Bifidobacterium Bb-12 | |||
| Zojaji et al., 2013 [21] | Saccharomyces boulardii | 250 mg twice daily, amount of CFU not mentioned | 14 days |
| Fox et al., 2014 [22] | Lactobacillus rhamnosus, G.G.; Lactobacillus acidophilus LA-5, Bifidobacterium Bb-12 | 5.2 × 109 CFU (L. rhamnosus) 5.9 × 109 CFU (B. Bb-12) 8.3 × 109 CFU (L. acidophilus LA-5) | Number of days not mentioned (“From the start to the end of their antibiotic treatment”) |
| Olek et al., 2017 [23] | Lactobacillus plantarum DSM9843 (LP299V) | 1 × 1010 CFU/capsule | Five to 10 days during antibiotic treatment and one week after (± two days) |
| RCT | Definition of Diarrhea |
|---|---|
| Tankanow et al., 1990 | One or more abnormally loose bowel movements/day throughout the study period of one to 10 days (parental reports) |
| Vanderhoof et al., 1999 | The presence of at least two liquid stools/day during at least two observation periods during the course of the study |
| Arvola et al., 1999 | At least three watery or loose stools/day for a minimum of two consecutive days |
| Erdeve et al., 2004 | Three or more watery stools/day during antibiotic treatment |
| Duman et al., 2005 | A change in bowel habits with at least three semi-solid or watery bowel movements/day for at least two consecutive days |
| Park et al., 2007 | Not specified (self-report) |
| Cindoruk et al., 2007 | Not specified (modified De Boer questionnaire categorizing diarrhea into “none”, “mild”, “moderate” and “severe”) |
| Conway et al., 2007 | Three or more loose stools/day over at least two consecutive days during the 12-day follow-up period |
| Imase et al., 2008 | “Loose or mostly loose stools”, not specified further |
| Kim et al., 2008 | Not specified other than categorized in groups (“none”, “mild”, “moderate”, “severe”) |
| Merenstein et al., 2009 | Not specified (parental reports) |
| De Vrese et al., 2011 | Three or more watery stools for at least one day (where at least one episode lay within the eradication week) |
| Ojetti et al., 2013 | Not specified other than categorized in groups (“none”, “mild”, “moderate”, “severe”) |
| Chatterjee et al., 2013 | Passage of at least three or more watery or loose stools/day for at least two consecutive days |
| Zojaji et al., 2013 | Not specified (self-report) |
| Fox et al., 2014 | Categories: |
| “A” (stool consistency ≥ 5, ≥2 stools/day for ≥2 days) | |
| “B” (stool consistency ≥ 5, ≥3 stools/day for ≥2 days) | |
| “C” (stool consistency ≥ 6, ≥2 stools/day for ≥2 days) | |
| “D” (stool consistency ≥ 6, ≥3 stools/day for ≥2 days) | |
| Olek et al., 2017 | ≥3 loose/watery stools/24 h starting after the initiation of antibiotic treatment |
| MDD (Days) | Range | Probiotic Group (N) | MDD (Days) | Range | Control Group (N) | |
|---|---|---|---|---|---|---|
| Vanderhoof et al., 1999 | 4.70 | N/A | 93 | 5.88 | N/A | 95 |
| Arvola et al., 1999 | 4.00 | 2–8 | 61 | 4.00 | 2–8 | 58 |
| De Vrese et al., 2011 | 1.00 | N/A | 30 | 4.70 | N/A | 29 |
| Chatterjee et al., 2013 | 2.00 | 1–3 | 198 | 4.00 | 3–5.5 | 198 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. https://doi.org/10.3390/antibiotics6040021
Blaabjerg S, Artzi DM, Aabenhus R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics. 2017; 6(4):21. https://doi.org/10.3390/antibiotics6040021
Chicago/Turabian StyleBlaabjerg, Sara, Daniel Maribo Artzi, and Rune Aabenhus. 2017. "Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis" Antibiotics 6, no. 4: 21. https://doi.org/10.3390/antibiotics6040021
APA StyleBlaabjerg, S., Artzi, D. M., & Aabenhus, R. (2017). Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics, 6(4), 21. https://doi.org/10.3390/antibiotics6040021





