Comparative Analysis of Phenotypic and Genotypic Antibiotic Susceptibility of Pasteurella multocida Isolated from Various Host Species in France and Hungary
Abstract
1. Introduction
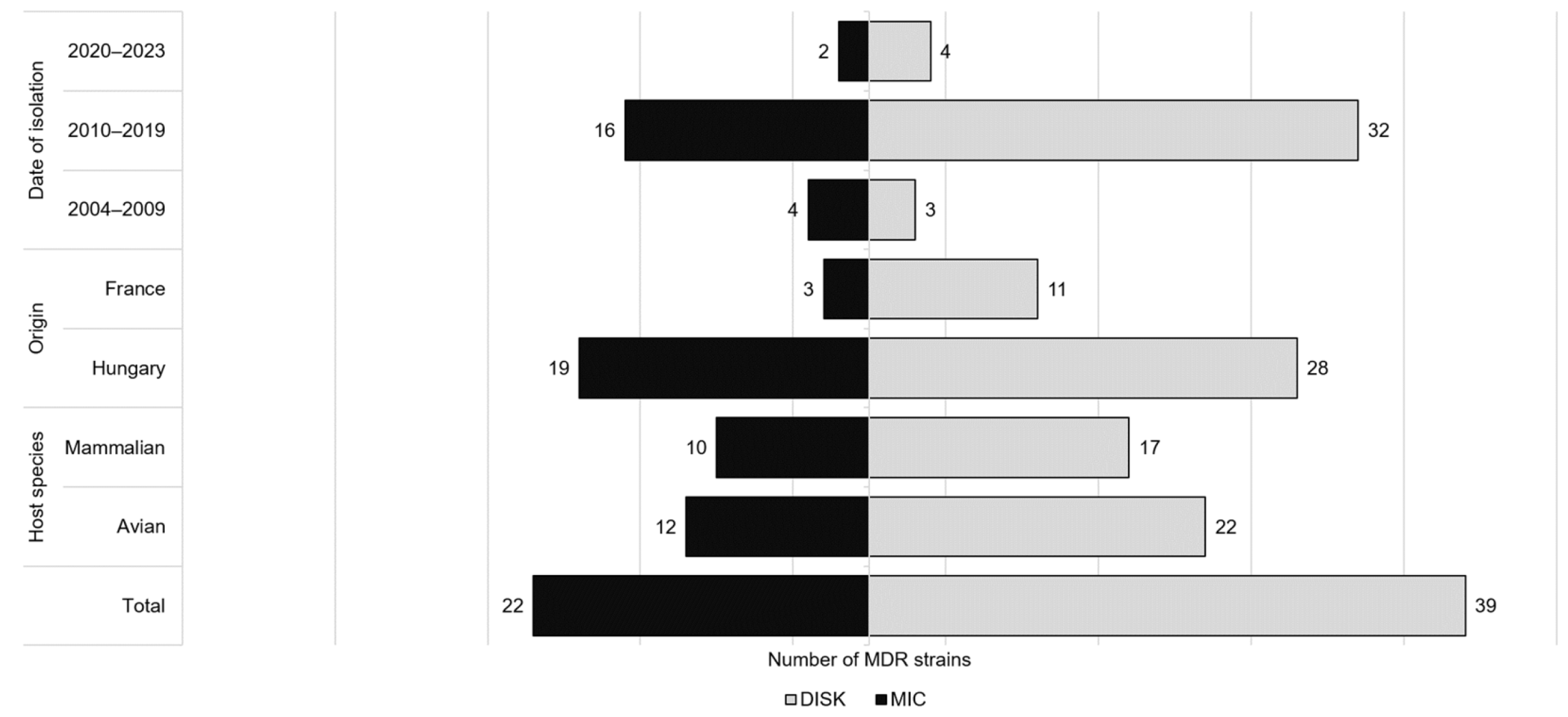
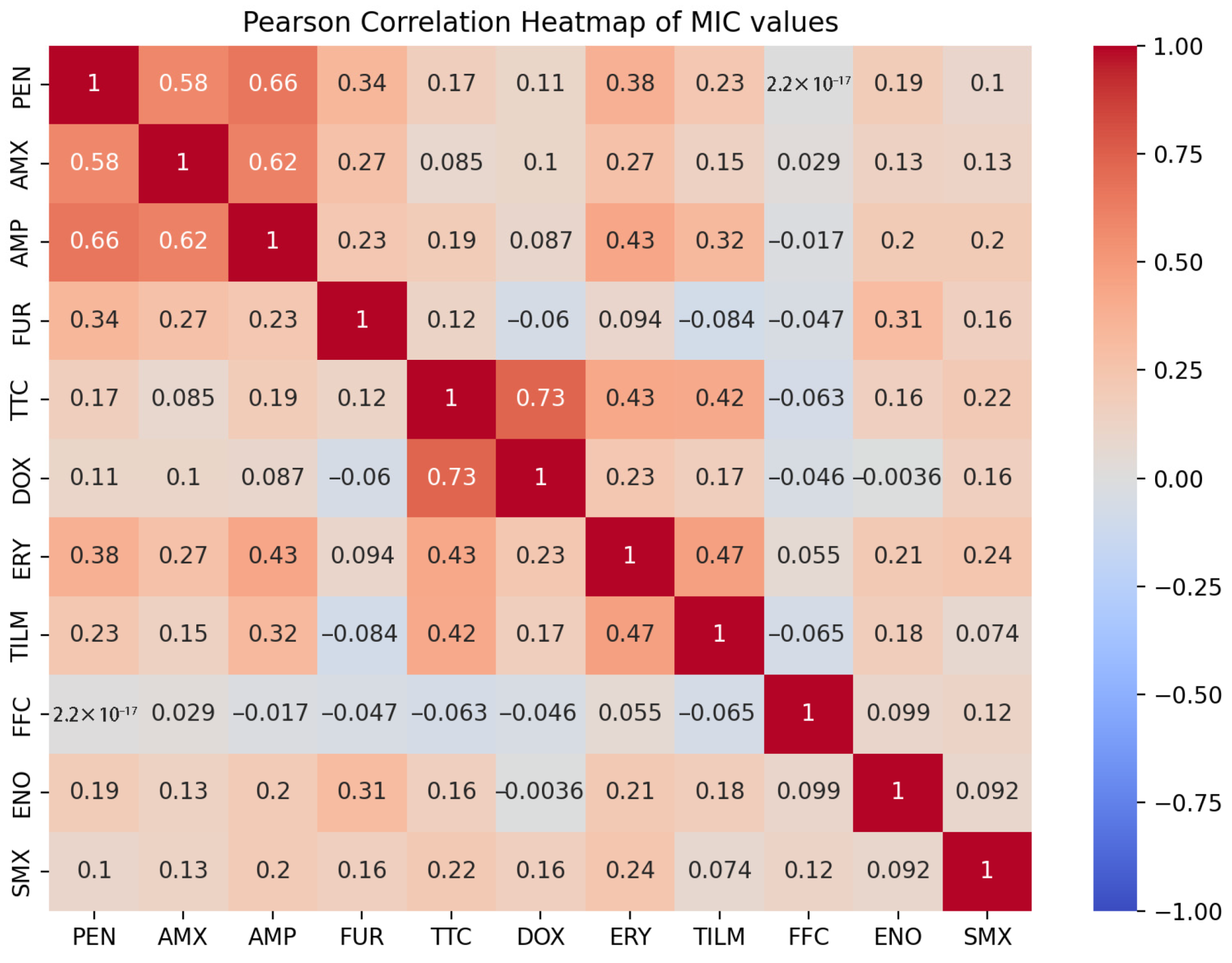
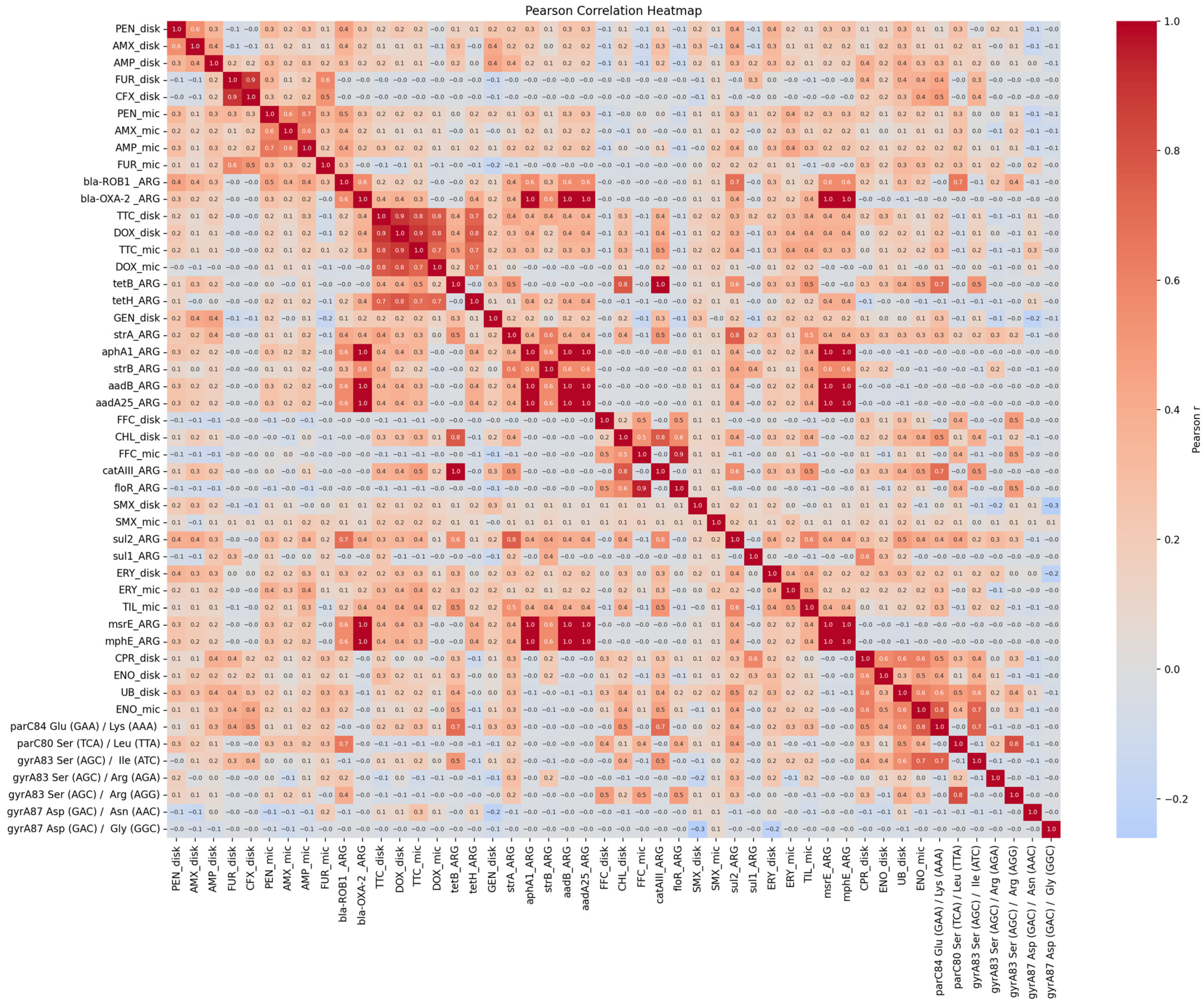
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antibiotic Susceptibility Testing
4.3. Whole Genome Sequencing and Bioinformatical Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARG | antimicrobial resistance gene |
| BHI | Brain Heart Infusion |
| BV-BRC- | Bacterial and Viral Bioinformatics Resource Center |
| CARD | Comprehensive Antibiotic Resistance Database |
| CLSI | Clinical and Laboratory Standards Institute |
| ECOFF | epidemiological cut-off value |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MDR | multidrug-resistant |
| MIC | minimal inhibitory concentration |
| PCR | polymerase chain reaction |
| RGI | Resistance Gene Identifier |
| SNP | single-nucleotide polymorphism |
References
- Rhoades, K.R.; Rimler, R.B. Fowl Cholera. In Pasteurella and Pasteurellosis; Adam, C., Rutter, J.M., Eds.; Academic Press Limited: London, UK, 1989; pp. 95–113. ISBN 978-0-12-044274-4. [Google Scholar]
- Magyar, T.; Lax, A.J. Atrophic Rhinitis. In Polymicrobial Diseases; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- De Alwis, M.C.L. Haemorrhagic Septicaemia; ACIAR Monograph Series; Australian Centre for International Agricultural Research: Canberra, Australia, 1999; ISBN 978-1-86320-269-5. [Google Scholar]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Freshwater, A. Why Your Housecat’s Trite Little Bite Could Cause You Quite a Fright: A Study of Domestic Felines on the Occurrence and Antibiotic Susceptibility of Pasteurella multocida. Zoonoses Public Health 2008, 55, 507–513. [Google Scholar] [CrossRef]
- Bhargav, A.; Gupta, S.; Seth, S.; James, S.; Fatima, F.; Chaurasia, P.; Ramachandran, S. Knowledgebase of Potential Multifaceted Solutions to Antimicrobial Resistance. Comput. Biol. Chem. 2022, 101, 107772. [Google Scholar] [CrossRef] [PubMed]
- Sy, C.L.; Chen, P.-Y.; Cheng, C.-W.; Huang, L.-J.; Wang, C.-H.; Chang, T.-H.; Chang, Y.-C.; Chang, C.-J.; Hii, I.-M.; Hsu, Y.-L.; et al. Recommendations and Guidelines for the Treatment of Infections Due to Multidrug Resistant Organisms. J. Microbiol. Immunol. Infect. 2022, 55, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rubin, J.E. Antimicrobial Susceptibility Testing Methods and Interpretation of Results. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; pp. 11–20. ISBN 978-1-118-67501-4. [Google Scholar]
- Enne, V.I.; Bennett, P.M. Methods to Determine Antibiotic Resistance Gene Silencing. In Antibiotic Resistance Protocols, 2nd ed.; Methods in Molecular Biology; Gillespie, S.H., McHugh, T.D., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 29–44. ISBN 978-1-60327-279-7. [Google Scholar]
- Zankari, E.; Hasman, H.; Kaas, R.S.; Seyfarth, A.M.; Agersø, Y.; Lund, O.; Larsen, M.V.; Aarestrup, F.M. Genotyping Using Whole-Genome Sequencing Is a Realistic Alternative to Surveillance Based on Phenotypic Antimicrobial Susceptibility Testing. J. Antimicrob. Chemother. 2013, 68, 771–777. [Google Scholar] [CrossRef]
- Oniciuc, E.A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; Santos, J.A.; Alvarez-Ordóñez, A. The Present and Future of Whole Genome Sequencing (WGS) and Whole Metagenome Sequencing (WMS) for Surveillance of Antimicrobial Resistant Microorganisms and Antimicrobial Resistance Genes across the Food Chain. Genes 2018, 9, 268. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Lansac, N.; Ménard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Correlation between the Resistance Genotype Determined by Multiplex PCR Assays and the Antibiotic Susceptibility Patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000, 44, 231–238. [Google Scholar] [CrossRef]
- Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023. Vet. Sci. 2024, 11, 194. [Google Scholar] [CrossRef]
- Kumakawa, M.; Akama, R.; Hosoi, Y.; Hiraoka, Y.; Harada, S.; Matsuda, M.; Kawanishi, M.; Sekiguchi, H. Establishing Tentative Cut-off Values for Disk Diffusion Method for Pasteurella multocida and Mannheimia haemolytica from Livestock Animals in Japan. J. Vet. Med. Sci. 2025, 87, 349–355. [Google Scholar] [CrossRef]
- Sellyei, B.; Varga, Z.; Szentesi-Samu, K.; Kaszanyitzky, É.; Magyar, T. Antimicrobial Susceptibility of Pasteurella multocida Isolated from Swine and Poultry. Acta Vet. Hung. 2009, 57, 357–367. [Google Scholar] [CrossRef]
- Cid, D.; Fernández-Garayzábal, J.F.; Pinto, C.; Domínguez, L.; Vela, A.I. Antimicrobial Susceptibility of Pasteurella multocida Isolated from Sheep and Pigs in Spain—Short Communication. Acta Vet. Hung. 2019, 67, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Petrocchi-Rilo, M.; Gutiérrez-Martín, C.-B.; Pérez-Fernández, E.; Vilaró, A.; Fraile, L.; Martínez-Martínez, S. Antimicrobial Resistance Genes in Porcine Pasteurella multocida Are Not Associated with Its Antimicrobial Susceptibility Pattern. Antibiotics 2020, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Schönecker, L.; Schnyder, P.; Schüpbach-Regula, G.; Meylan, M.; Overesch, G. Prevalence and Antimicrobial Resistance of Opportunistic Pathogens Associated with Bovine Respiratory Disease Isolated from Nasopharyngeal Swabs of Veal Calves in Switzerland. Prev. Vet. Med. 2020, 185, 105182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yuan, D.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; Zhang, S.; et al. Emergence of a Multidrug-Resistant Hypervirulent Pasteurella multocida ST342 Strain with a floR-Carrying Plasmid. J. Glob. Antimicrob. Resist. 2020, 20, 348–350. [Google Scholar] [CrossRef]
- Jeong, J.; Kang, M.S.; Jeong, O.M.; Lee, H.J.; Lee, J.Y.; Kwon, Y.K.; Park, J.W.; Kim, J.H. Investigation of Genetic Diversity of Pasteurella multocida Isolated from Diseased Poultry in Korea. Braz. J. Poult. Sci. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Sabsabi, M.A.; Zakaria, Z.; Abu, J.; Faiz, N.M. Molecular Characterisation and Antibiotic Sensitivity Profile of Pasteurella multocida Isolated from Poultry Farms in Malaysia. Austral J. Vet. Sci. 2021, 53, 121–126. [Google Scholar] [CrossRef]
- Vilaró, A.; Novell, E.; Enrique-Tarancón, V.; Balielles, J.; Vilalta, C.; Martinez, S.; Fraile Sauce, L.J. Antimicrobial Susceptibility Pattern of Porcine Respiratory Bacteria in Spain. Antibiotics 2020, 9, 402. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Moon, D.-C.; Lee, Y.J.; Hyun, B.-H.; Lim, S.-K. Antimicrobial Resistance of Pasteurella multocida Strains Isolated from Pigs between 2010 and 2016. Vet. Rec. Open 2018, 5, e000293. [Google Scholar] [CrossRef]
- Jones, K.H.; Thornton, J.K.; Zhang, Y.; Mauel, M.J. A 5-Year Retrospective Report of Gallibacterium anatis and Pasteurella multocida Isolates from Chickens in Mississippi. Poult. Sci. 2013, 92, 3166–3171. [Google Scholar] [CrossRef]
- Khamesipour, F.; Momtaz, H.; Azhdary Mamoreh, M. Occurrence of Virulence Factors and Antimicrobial Resistance in Pasteurella multocida Strains Isolated from Slaughter Cattle in Iran. Front. Microbiol. 2014, 5, 536. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Z.; Hu, J.; Wu, B.; Cai, X.; He, Q.; Chen, H. Isolation, Antimicrobial Resistance, and Virulence Genes of Pasteurella multocida Strains from Swine in China. J. Clin. Microbiol. 2009, 47, 951–958. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Hu, Z.; Zhang, Y.; Chang, Y.-F.; Zhou, Q.; Yan, Z.; Zhang, X.; Chen, L.; Li, W.; et al. Characterization of Pasteurella multocida Isolated from Ducks in China from 2017 to 2019. Microb. Pathog. 2021, 160, 105196. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Ruiz, J.; Goñi, P.; De Anta, M.T. Detection of Mutations in parC in Quinolone-Resistant Clinical Isolates of Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Qi, Y.; Xue, J.-Z.; Xu, G.-Y.; Xu, Y.-X.; Li, X.-Y.; Muhammad, I.; Kong, L.-C.; Ma, H.-X. Transcriptomic Changes and satP Gene Function Analysis in Pasteurella multocida with Different Levels of Resistance to Enrofloxacin. Vet. Sci. 2023, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-C.; Gao, D.; Gao, Y.-H.; Liu, S.-M.; Ma, H.-X. Fluoroquinolone Resistance Mechanism of Clinical Isolates and Selected Mutants of Pasteurella multocida from Bovine Respiratory Disease in China. J. Vet. Med. Sci. 2014, 76, 1655–1657. [Google Scholar] [CrossRef]
- Cárdenas, M.; Barbé, J.; Llagostera, M.; Miró, E.; Navarro, F.; Mirelis, B.; Prats, G.; Badiola, I. Quinolone Resistance-Determining Regions of gyrA and parC in Pasteurella multocida Strains with Different Levels of Nalidixic Acid Resistance. Antimicrob. Agents Chemother. 2001, 45, 990–991. [Google Scholar] [CrossRef]
- Dasgupta, N.; Paul, D.; Chanda, D.D.; Chetri, S.; Chakravarty, A.; Bhattacharjee, A. Observation of a New Pattern of Mutations in gyrA and parC within Escherichia coli Exhibiting Fluroquinolone Resistance. Indian J. Med. Microbiol. 2018, 36, 131–135. [Google Scholar] [CrossRef]
- Dayao, D.A.E.; Gibson, J.S.; Blackall, P.J.; Turni, C. Antimicrobial Resistance in Bacteria Associated with Porcine Respiratory Disease in Australia. Vet. Microbiol. 2014, 171, 232–235. [Google Scholar] [CrossRef]
- Nor Amdan, N.A.; Shahrulzamri, N.A.; Hashim, R.; Mohamad Jamil, N. Understanding the Evolution of Macrolides Resistance: A Mini Review. J. Glob. Antimicrob. Resist. 2024, 38, 368–375. [Google Scholar] [CrossRef]
- Sahay, S.; Natesan, K.; Prajapati, A.; Kalleshmurthy, T.; Shome, B.R.; Rahman, H.; Shome, R. Prevalence and Antibiotic Susceptibility of Mannheimia haemolytica and Pasteurella multocida Isolated from Ovine Respiratory Infection: A Study from Karnataka, Southern India. Vet. World 2020, 13, 1947–1954. [Google Scholar] [CrossRef]
- Timsit, E.; Hallewell, J.; Booker, C.; Tison, N.; Amat, S.; Alexander, T.W. Prevalence and Antimicrobial Susceptibility of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni Isolated from the Lower Respiratory Tract of Healthy Feedlot Cattle and Those Diagnosed with Bovine Respiratory Disease. Vet. Microbiol. 2017, 208, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Tham, N.T.T.; Schwarz, S. New Plasmid-Borne Antibiotic Resistance Gene Cluster in Pasteurella multocida. Antimicrob. Agents Chemother. 2003, 47, 2978–2980. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.B.; Kadlec, K.; Sweeney, M.T.; Brzuszkiewicz, E.; Liesegang, H.; Daniel, R.; Murray, R.W.; Watts, J.L.; Schwarz, S. ICEPmu1, an Integrative Conjugative Element (ICE) of Pasteurella multocida: Structure and Transfer. J. Antimicrob. Chemother. 2012, 67, 91–100. [Google Scholar] [CrossRef]
- Giguère, S. Antimicrobial Drug Action and Interaction. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; pp. 1–10. ISBN 978-1-118-67501-4. [Google Scholar]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Medchemcomm 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Bedier, E.T.; Labib, S.R.; Ahmed, A.M. Characterization of Antimicrobial Resistance Genes of Pasteurella multocida Isolated from Diseased Chickens in Egypt. J. Hell. Vet. Med. Soc. 2022, 73, 4165–4172. [Google Scholar] [CrossRef]
- San Millan, A.; Escudero, J.A.; Gutierrez, B.; Hidalgo, L.; Garcia, N.; Llagostera, M.; Dominguez, L.; Gonzalez-Zorn, B. Multiresistance in Pasteurella multocida Is Mediated by Coexistence of Small Plasmids. Antimicrob. Agents Chemother. 2009, 53, 3399–3404. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Huovinen, P. Resistance to Trimethoprim-Sulfamethoxazole. Clin. Infect. Dis. 2001, 32, 1608–1614. [Google Scholar] [CrossRef]
- Kulczycka-Mierzejewska, K.; Sadlej, J.; Trylska, J. Molecular Dynamics Simulations Suggest Why the A2058G Mutation in 23S RNA Results in Bacterial Resistance against Clindamycin. J. Mol. Model. 2018, 24, 191. [Google Scholar] [CrossRef]
- Cross, S.L.; Gelfand, M. Pasteurella multocida Infection Treatment & Management: Approach Considerations, Medical Care, Surgical Care. In Pasteurella multocida Infection; Medscape: New York, NY, USA, 2025. [Google Scholar]
- Townsend, K.M.; Frost, A.J.; Lee, C.W.; Papadimitriou, J.M.; Dawkins, H.J. Development of PCR Assays for Species- and Type-Specific Identification of Pasteurella multocida Isolates. J. Clin. Microbiol. 1998, 36, 1096–1100. [Google Scholar] [CrossRef]
- Winkler, M.A.; Cockerill, F.R.; Craig, W.A.; Dudley, M.N.; Eliopoulos, G.M.; Hecht, D.W.; Hindler, J.A.; Lowery, D.E.; Sheehan, D.J.; Tenover, F.C.; et al. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard: M2-A9, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; ISBN 1-56238-586-0. [Google Scholar]
- Shryock, T.R.; Apley, M.; Jones, R.N.; Lein, D.H.; Thornsberry, C.; Walker, R.D.; Watts, J.L.; White, D.G.; Wu, C.C. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Approved Standard, 2nd ed.; NCCLS Document; NCCLS: Wayne, PA, USA, 2002; ISBN 978-1-56238-461-6. [Google Scholar]
- Papich, M.G.; Simjee, S.; Apley, M.; Frana, T.S.; Knapp, C.C.; Lubbers, B.V.; Rose, M.; Schwarz, S.; Silley, P.; Sweeney, M.T.; et al. Performance Standards for Antimicrobial Disk Susceptibility Tests for Bacteria Isolated from Animals: CLSI Supplement VET01S; Replaces VET01-S2, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 978-1-56238-908-6. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]



| Disk Diffusion | ||||
|---|---|---|---|---|
| No. of Antibiotic Classes with Resistant Strains | No. of the Resistant Strains | Antibiotic Classes | Strain ID | Host Species (n) |
| 3 | 3 | AG-LA-SA | 4373, 4380, 4389 | cattle |
| 3 | 1 | LA-SA-FC | 4480 | goose |
| 3 | 1 | LA-SA-FQ | 4502 | goose |
| 3 | 1 | MAC-LA-SA | 4489 | turkey |
| 3 | 8 | PN-LA-SA | 4376, 4385, 4400, 4410, 4541, 4576, 4926, 5050 | cattle (3) goose (2) turkey (2) duck (1) |
| 3 | 1 | PN-MAC-LA | 4488 | turkey |
| 3 | 1 | PN-TET-LA | 4483 | duck |
| 3 | 2 | TET-LA-SA | 4319, 4573 | duck, goose |
| 4 | 6 | PN-AG-LA-SA | 4149, 4174, 4190, 4193, 4199, 4251 | cattle (5) albatross (1) |
| 4 | 1 | PN-CEP-LA-FQ | 4148 | cattle |
| 4 | 4 | PN-LA-SA-FQ | 4147, 4217, 4231, 4253 | goose (3) duck (1) |
| 4 | 2 | PN-MAC-LA-SA | 4216, 4221 | cattle (1) turkey (1) |
| 4 | 2 | PN-TET-LA-SA | 4138, 4218 | duck, goose |
| 5 | 1 | PN-AG-LA-SA-FQ | 3770 | cattle |
| 5 | 1 | PN-MAC-LA-SA-FQ | 4122 | duck |
| 5 | 1 | PN-TET-LA-SA-FQ | 4082 | duck |
| 6 | 1 | PN-AG-TET-MAC-LA-SA | 3378 | cattle |
| 7 | 1 | PN-AG-MAC-LA-SA-FC-FQ | 3036 | cattle |
| 8 | 1 | PN-AG-TET-MAC-LA-SA-FC-FQ | 3029 | cattle |
| Minimum Inhibitory Concentration (µg/mL) | MIC50 | MIC90 | R (%) | ECOFF (µg/mL) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 512< | 512 | 256< | 256 | 128< | 128 | 64< | 64 | 32< | 32 | 16< | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.25> | 0.125 | 0.125> | 0.06 | 0.06> | 0.03 | 0.03> | 0.015 | 0.015> | |||||
| PEN | 8 | 0 | 1 | 0 | 0 | 1 | 13 | 19 | 0 | 24 | 0 | 11 | 0 | 3 | 0 | 0 | 0 | 0.25 S | 8 R | 12.5 | - | ||||||||||
| AMX | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 5 | 29 | 31 | 6 | 0 | 1 | 0 | 0 | 0 | 1 I | 2 R | 16.3 | 0.5 | ||||||||||
| AMP | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 3 | 1 | 16 | 32 | 9 | 0 | 13 | 0 | 0 | 0 | 0.5 S | 4 R | 12.5 | 0.5 | ||||||||||
| FUR | 1 | 0 | 2 | 1 | 4 | 9 | 13 | 13 | 0 | 6 | 0 | 1 | 0 | 19 | 0 | 1 | 10 | 0.25 S | 1 S | 3.8 | 0.06 | ||||||||||
| TTC | 0 | 4 | 0 | 2 | 2 | 0 | 0 | 2 | 13 | 38 | 0 | 11 | 0 | 7 | 0 | 1 | 0 | 0.25 S | 1 S | 10.0 | 2 | ||||||||||
| DOX | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 2 | 1 | 2 | 15 | 0 | 28 | 0 | 11 | 16 | 0.125 S | 0.25 S | 2.5 | 1 | ||||||||||
| ERY | 2 | 2 | 0 | 2 | 0 | 3 | 5 | 8 | 25 | 22 | 6 | 4 | 0 | 1 | 0 | 0 | 0 | 2 I | 16 R | 17.5 | 16 | ||||||||||
| TIL | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 3 | 7 | 22 | 23 | 12 | 5 | 0 | 3 | 2 S | 8 S | 7.5 | 32 | ||||||||||
| CD | 6 | 1 | 0 | 12 | 0 | 45 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 R | 64 R | 100.0 | - | ||||||||||
| FFC | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 4 | 2 | 45 | 14 | 0 | 12 | 0 | 0 | 0 | 0.5 S | 1 S | 2.5 | 1 | ||||||||||
| ENO | 0 | 0 | 0 | 0 | 4 | 1 | 3 | 3 | 0 | 3 | 0 | 9 | 0 | 14 | 0 | 1 | 42 | 0.015> S | 0.25 S | 5.0 | 0.06 | ||||||||||
| SMX | 51 | 5 | 0 | 13 | 0 | 8 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 512< R | 512< R | 70.0 | - | ||||||||||
| UB | 1 | 3 | 0 | 2 | 0 | 1 | 0 | 4 | 2 | 5 | 3 | 9 | 0 | 23 | 0 | 0 | 27 | 0.125 | 4 | - | 0.5 | ||||||||||
| APR | 1 | 12 | 0 | 33 | 0 | 28 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 64 | - | - | ||||||||||
| MIC | ||||
|---|---|---|---|---|
| No. of Antibiotic Classes with Resistant Strains | No. of the Resistant Strains | Antibiotic Classes | Strain ID | Host Species (n) |
| 3 | 2 | LA-SA-FC | 4319, 4480 | goose |
| 3 | 3 | MAC-LA-SA | 4217, 4318, 4924 | cattle (1) goose (1) turkey (1) |
| 3 | 4 | PN-LA-SA | 4253, 4340, 4376, 4483 | cattle (1) duck (1) goose (1) turkey (1) |
| 3 | 1 | TET-LA-SA | 4380 | duck |
| 4 | 1 | TET-MAC-LA-SA | 4251 | cattle |
| 4 | 3 | PN-MAC-LA-SA | 3509, 3699, 3700 | cattle (1) goat (2) |
| 4 | 1 | PN-CEP-LA-SA | 4576 | duck |
| 4 | 1 | MAC-LA-FQ-SA | 4147 | cattle |
| 5 | 1 | CEP-TET-MAC-LA-SA | 4539 | goose |
| 5 | 1 | PN-CEP-LA-FQ-SA | 4149 | cattle |
| 5 | 3 | PN-TET-MAC-LA-SA | 3029, 3036, 4221 | cattle (1) goose (2) |
| 5 | 1 | TET-MAC-LA-FQ-SA | 4231 | cattle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintér, K.; Domán, M.; Wehmann, E.; Gantelet, H.; Magyar, T. Comparative Analysis of Phenotypic and Genotypic Antibiotic Susceptibility of Pasteurella multocida Isolated from Various Host Species in France and Hungary. Antibiotics 2025, 14, 906. https://doi.org/10.3390/antibiotics14090906
Pintér K, Domán M, Wehmann E, Gantelet H, Magyar T. Comparative Analysis of Phenotypic and Genotypic Antibiotic Susceptibility of Pasteurella multocida Isolated from Various Host Species in France and Hungary. Antibiotics. 2025; 14(9):906. https://doi.org/10.3390/antibiotics14090906
Chicago/Turabian StylePintér, Krisztina, Marianna Domán, Enikő Wehmann, Hubert Gantelet, and Tibor Magyar. 2025. "Comparative Analysis of Phenotypic and Genotypic Antibiotic Susceptibility of Pasteurella multocida Isolated from Various Host Species in France and Hungary" Antibiotics 14, no. 9: 906. https://doi.org/10.3390/antibiotics14090906
APA StylePintér, K., Domán, M., Wehmann, E., Gantelet, H., & Magyar, T. (2025). Comparative Analysis of Phenotypic and Genotypic Antibiotic Susceptibility of Pasteurella multocida Isolated from Various Host Species in France and Hungary. Antibiotics, 14(9), 906. https://doi.org/10.3390/antibiotics14090906






