High Prevalence of Antimicrobial Resistance Genes in Rabbit Farms from Sumy Region, Ukraine
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. Molecular Detection of AMR Genes
2.4. Statistical Analysis
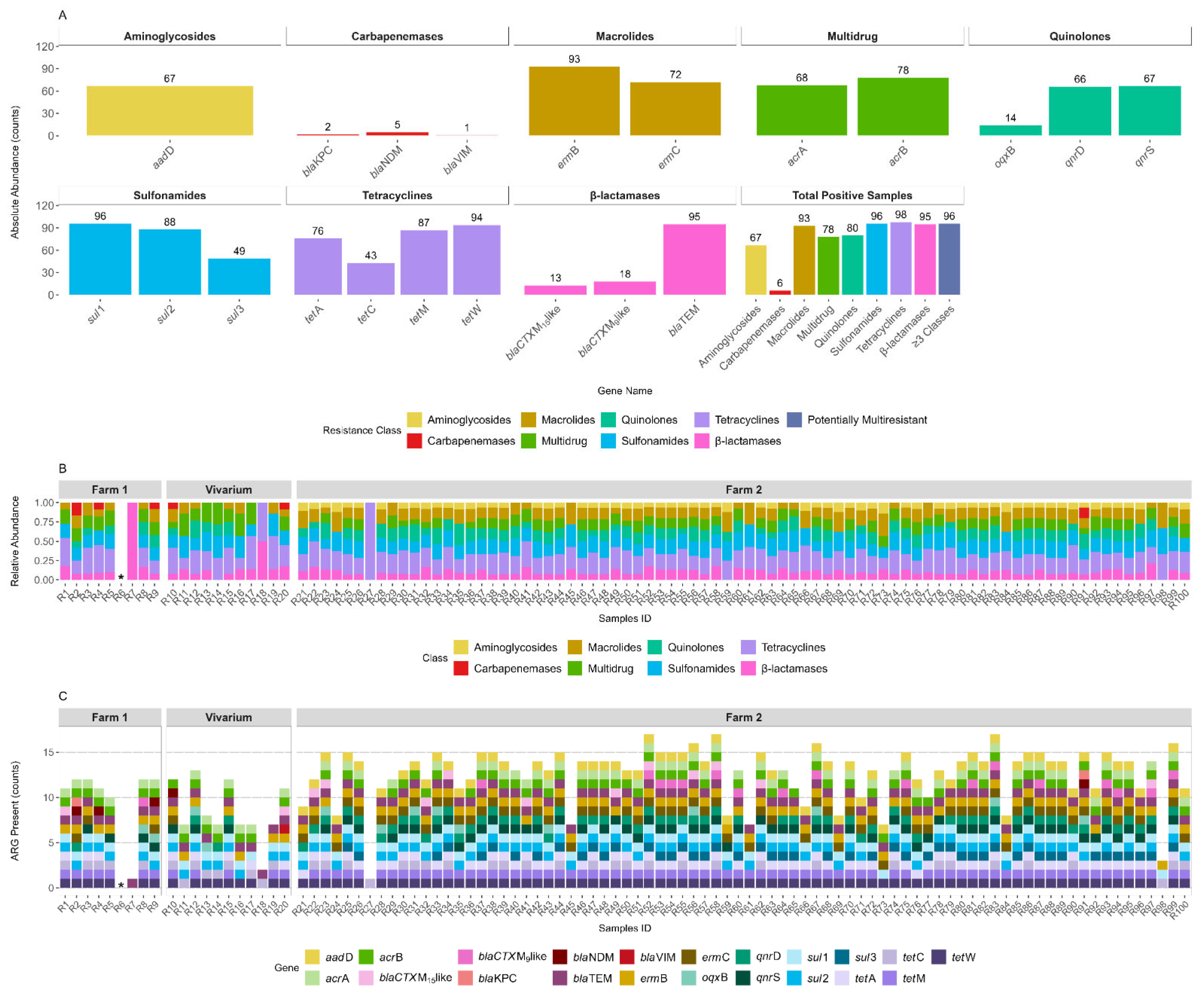
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flynn, C.E.; Guarner, J. Emerging Antimicrobial Resistance. Mod. Pathol. 2023, 36, 100249. [Google Scholar] [CrossRef]
- Rizzo, A.; Piccinno, M.; Lillo, E.; Carbonari, A.; Jirillo, F.; Sciorsci, R.L. Antimicrobial Resistance and Current Alternatives in Veterinary Practice: A Review. Curr. Pharm. Des. 2023, 29, 312–322. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Scott, L.C.; Menzies, P.I. Antimicrobial Resistance and Small Ruminant Veterinary Practice. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 23–32. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food-Producing Animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Chen, B.; Hao, L.; Guo, X.; Wang, N.; Ye, B. Prevalence of Antibiotic Resistance Genes of Wastewater and Surface Water in Livestock Farms of Jiangsu Province, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 13950–13959. [Google Scholar] [CrossRef] [PubMed]
- Luiken, R.E.; Heederik, D.J.; Scherpenisse, P.; Van Gompel, L.; van Heijnsbergen, E.; Greve, G.D.; Jongerius-Gortemaker, B.G.; Tersteeg-Zijderveld, M.H.; Fischer, J.; Juraschek, K.; et al. Determinants for Antimicrobial Resistance Genes in Farm Dust on 333 Poultry and Pig Farms in Nine European Countries. Environ. Res. 2022, 208, 112715. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Bougouffa, S.; Park, T.-J.; Lau, A.; Tong, M.-K.; Chow, K.-H.; Ho, P.-L. Sharing of Antimicrobial Resistance Genes between Humans and Food Animals. mSystems 2022, 7, e0077522. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Havrysh, O.; Korzeniowska, M.; Getya, A. Potential and Limitations of Rabbit Meat in Maintaining Food Security in Ukraine. Meat Sci. 2023, 204, 109293. [Google Scholar] [CrossRef]
- Szendrő, K.; Szabó-Szentgróti, E.; Szigeti, O. Consumers’ Attitude to Consumption of Rabbit Meat in Eight Countries Depending on the Production Method and Its Purchase Form. Foods 2020, 9, 654. [Google Scholar] [CrossRef]
- Kotelevych, V.A. Veterinary and Sanitary Evaluation of Rabbit as an Important Reserve of Dietary Products. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2019, 21, 58–64. [Google Scholar] [CrossRef]
- Falcão-e-Cunha, L.; Solla, L.C.; Maertens, L.; Marounek, M.; Pinheiro, V.; Freire, J.; Mourão, J.L. Alternatives to Antibiotic Growth Promoters in Rabbit Feeding: A Review. World Rabbit. Sci. 2007, 15, 127–140. [Google Scholar] [CrossRef]
- Agnoletti, F.; Brunetta, R.; Bano, L.; Drigo, I.; Mazzolini, E. Longitudinal Study on Antimicrobial Consumption and Resistance in Rabbit Farming. Int. J. Antimicrob. Agents 2018, 51, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Crovato, S.; Menegon, F.; Mascarello, G.; Pinto, A.; Nadin, A.; Piovan, G.; Ricaldi, G.; Di Martino, G.; Pozza, G. Development of a Training Strategy Aimed at Increasing Veterinarians’ Awareness of the Proper Use of Antibiotics on Rabbit Farms. Animals 2023, 13, 2411. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. Assessment of Animal Diseases Caused by Bacteria Resistant to Antimicrobials: Rabbits. EFSA J. 2021, 19, e06999. [Google Scholar] [CrossRef] [PubMed]
- Drigo, I.; Mazzolini, E.; Bacchin, C.; Tonon, E.; Puiatti, C.; Bano, L.; Spigaglia, P.; Barbanti, F.; Agnoletti, F. Molecular Characterization and Antimicrobial Susceptibility of Clostridium difficile Isolated from Rabbits Raised for Meat Production. Vet. Microbiol. 2015, 181, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, F.; Mazzolini, E.; Bacchin, C.; Bano, L.; Berto, G.; Rigoli, R.; Muffato, G.; Coato, P.; Tonon, E.; Drigo, I. First Reporting of Methicillin-Resistant Staphylococcus aureus (MRSA) ST398 in an Industrial Rabbit Holding and in Farm-Related People. Vet. Microbiol. 2014, 170, 172–177. [Google Scholar] [CrossRef]
- Jeżak, K.; Kozajda, A. Occurrence and Spread of Antibiotic-Resistant Bacteria on Animal Farms and in Their Vicinity in Poland and Ukraine—Review. Environ. Sci. Pollut. Res. 2022, 29, 9533–9559. [Google Scholar] [CrossRef]
- Bento, J.T.; Gomes-Gonçalves, S.; Cruz, R.; Esteves, F.; Baptista, A.L.; Aires Pereira, M.; Caseiro, P.; Carreira, P.; Figueira, L.; Mesquita, J.R.; et al. The Prevalence of Antimicrobial Resistance Genes in the Environments of Small Ruminant Farms from Central Portugal. Antibiotics 2025, 14, 576. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Yang, Y.; Shen, Z.; Cai, C.; Wang, Y.; Walsh, T.R.; Shen, J.; Wu, Y.; Wang, S. High Prevalence and Persistence of Carbapenem and Colistin Resistance in Livestock Farm Environments in China. J. Hazard. Mater. 2021, 406, 124298. [Google Scholar] [CrossRef]
- Poulin-Laprade, D.; Brouard, J.-S.; Gagnon, N.; Turcotte, A.; Langlois, A.; Matte, J.J.; Carrillo, C.D.; Zaheer, R.; McAllister, T.A.; Topp, E.; et al. Resistance Determinants and Their Genetic Context in Enterobacteria from a Longitudinal Study of Pigs Reared under Various Husbandry Conditions. Appl. Environ. Microbiol. 2021, 87, e02612-20. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Y.; Cui, L.; Cao, Z.; Zhang, F.; Wang, X.; Feng, J.; Dai, M. The Influencing Factors of Bacterial Resistance Related to Livestock Farm: Sources and Mechanisms. Front. Anim. Sci. 2021, 2, 650347. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic Resistance Genes from Livestock Waste: Occurrence, Dissemination, and Treatment. npj Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Tavares, T.; López, M.; Rojo-Bezares, B.; Pereira, J.E.; Falco, V.; Valentão, P.; Igrejas, G.; Sáenz, Y.; et al. Rabbits as a Reservoir of Multidrug-Resistant Escherichia Coli: Clonal Lineages and Public Health Impact. Antibiotics 2024, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, A.; Wafaa, A. Pasteurellosis: A Significant Bacterial Disease in Rabbit Production. Vet. Stanica 2023, 54, 569–580. [Google Scholar] [CrossRef]
- Solans, L.; Arnal, J.L.; Sanz, C.; Benito, A.; Chacón, G.; Alzuguren, O.; Fernández, A.B. Rabbit Enteropathies on Commercial Farms in the Iberian Peninsula: Etiological Agents Identified in 2018–2019. Animals 2019, 9, 1142. [Google Scholar] [CrossRef]
- Deeb, B.J. Respiratory Disease and Pasteurellosis. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2004; pp. 172–182. [Google Scholar] [CrossRef]
- Prescott, J.F. Escherichia coli and Diarrhoea in the Rabbit. Vet. Pathol. 1978, 15, 237–248. [Google Scholar] [CrossRef]
- Vancraeynest, D.; Hermans, K.; Martel, A.; Vaneechoutte, M.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Resistance and Resistance Genes in Staphylococcus aureus Strains from Rabbits. Vet. Microbiol. 2004, 101, 245–251. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Li, Y.; Huang, J. Antibiotic Resistance Spectrums of Escherichia coli and Enterococcus spp. Strains against Commonly Used Antimicrobials from Commercial Meat-Rabbit Farms in Chengdu City, Southwest China. Front. Vet. Sci. 2024, 11, 1369655. [Google Scholar] [CrossRef]
- Kosenko, Y.M.; Ostapiv, N.V.; Zaruma, L.E. Safety of Tetracyclines for Public Health and the Environment. Sci. Tech. Bull. State Sci. Res. Control Inst. Vet. Med. Prod. Fodd. Addit. Inst. Anim. Biol. 2024, 25, 62–73. [Google Scholar] [CrossRef]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Mroczkowska, J.E.; Barlow, M. Fitness Trade-Offs in blaTEM Evolution. Antimicrob. Agents Chemother. 2008, 52, 2340–2345. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Castillo, F.Y.; Guerrero-Barrera, A.L.; Avelar-González, F.J. An Overview of Carbapenem-Resistant Organisms from Food-Producing Animals, Seafood, Aquaculture, Companion Animals, and Wildlife. Front. Vet. Sci. 2023, 10, 1158588. [Google Scholar] [CrossRef] [PubMed]
- Ramsamy, Y.; Mlisana, K.P.; Amoako, D.G.; Abia, A.L.K.; Ismail, A.; Allam, M.; Mbanga, J.; Singh, R.; Essack, S.Y. Mobile Genetic Elements-Mediated Enterobacterales-Associated Carbapenemase Antibiotic Resistance Genes Propagation between the Environment and Humans: A One Health South African Study. Sci. Total Environ. 2022, 806, 150641. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Lee, J. The Threat of Carbapenem-Resistant Bacteria in the Environment: Evidence of Widespread Contamination of Reservoirs at a Global Scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef]
- Aldali, H.J.; Khan, A.; Alshehri, A.A.; Aldali, J.A.; Meo, S.A.; Hindi, A.; Elsokkary, E.M. Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study. Microorganisms 2023, 11, 1595. [Google Scholar] [CrossRef]
- Mu, Q.; Li, J.; Sun, Y.; Mao, D.; Wang, Q.; Luo, Y. Occurrence of Sulfonamide-, Tetracycline-, Plasmid-Mediated Quinolone- and Macrolide-Resistance Genes in Livestock Feedlots in Northern China. Environ. Sci. Pollut. Res. 2015, 22, 6932–6940. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 July 2025).
- Zhou, Z.; Chen, H. Evaluating Human Exposure to Antibiotic Resistance Genes. Biosaf. Health 2024, 6, 98–100. [Google Scholar] [CrossRef]
- Farrukh, M.; Munawar, A.; Nawaz, Z.; Hussain, N.; Hafeez, A.B.; Szweda, P. Antibiotic Resistance and Preventive Strategies in Foodborne Pathogenic Bacteria: A Comprehensive Review. Food Sci. Biotechnol. 2025, 34, 2101–2129. [Google Scholar] [CrossRef] [PubMed]
- Castillo Neyra, R.; Vegosen, L.; Davis, M.F.; Price, L.; Silbergeld, E.K. Antimicrobial-Resistant Bacteria: An Unrecognized Work-Related Risk in Food Animal Production. Saf. Health Work. 2012, 3, 85–91. [Google Scholar] [CrossRef]
- Conceição, S.; Queiroga, M.C.; Laranjo, M. Antimicrobial Resistance in Bacteria from Meat and Meat Products: A One Health Perspective. Microorganisms 2023, 11, 2581. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Fu, Y.; Mei, Z.; Schäffer, A.; Dou, Q.; Amelung, W.; Elsner, M.; Adu-Gyamfi, J.; Heng, L.; Virta, M.; et al. Antibiotic Resistance Genes in Food Production Systems Support One Health Opinions. Curr. Opin. Environ. Sci. Health 2023, 34, 100492. [Google Scholar] [CrossRef]
- Seyoum, M.M.; Ashworth, A.J.; Feye, K.M.; Ricke, S.C.; Owens, P.R.; Moore, P.A.; Savin, M. Long-Term Impacts of Conservation Pasture Management in Manuresheds on System-Level Microbiome and Antibiotic Resistance Genes. Front. Microbiol. 2023, 14, 1227006. [Google Scholar] [CrossRef]
- Karwowska, E. Antibiotic Resistance in the Farming Environment. Appl. Sci. 2024, 14, 5776. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.-R.; Bellato, A.; Robino, P.; Galosi, L.; Papeschi, C.; Rossi, G.; Fileni, E.; Linardi, M.; Cuteri, V.; Chiesa, F.; et al. Analysis of the Antibiotic Resistance Profiles in Methicillin-Sensitive S. Aureus Pathotypes Isolated on a Commercial Rabbit Farm in Italy. Antibiotics 2020, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.; Aseem, A.; Banjara, S.K.; Veleri, S. The Role of Food Chain in Antimicrobial Resistance Spread and One Health Approach to Reduce Risks. Int. J. Food Microbiol. 2023, 391–393, 110148. [Google Scholar] [CrossRef]
| Class | Gene | Sequence (5′ > 3′) | Annealing Temperature (°C) | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| Tetracyclines | tetA | F:GCTACATCCTGCTTGCCTTC R:CATAGATCGCCGTGAAGAGG | 60 | 210 | [6] |
| tetC | F:TGCAACTCGTAGGACAGGTG R:ACCAGTGACGAAGGCTTGAG | 60 | 139 | ||
| tetM | F:ACAGAAAGCTTATTATATAAC R:GGCGTGTCTATGATGTTCAC | 51 | 171 | ||
| tetW | F:GAGAGCCTGCTATATGCCAGC R:GGGCGTATCCACAATGTTAAC | 60 | 168 | ||
| Carbapenemases | blaNDM | F:CAGTCGCTTCCAACGGTTTG R: AACGCATTGGCATAAGTCGC | 60 | 217 | [20] |
| blaVIM | F:AAAACACAGCGGCACTTCTC R: AATCTCGTTCCCCTCTACCTC | 60 | 182 | ||
| blaKPC | F:CATTCGCTAAACTCGAACAGG R: TTTTGCCGTAACGGATGG | 60 | 201 | ||
| Extended-spectrum β-lactamases (ESBL) | blaCTX-M-15like | F:GCTGGTGACATGGATGAAAG R: TAGGTTGAGGCTGGGTGAAG | 60 | 87 | [20] |
| blaCTX-M-9like | F:GTTGGTGACGTGGCTCAAAG R: GTTGCGGCTGGGTAAAATAG | 60 | 89 | ||
| blaTEM | F:TCTGACAACGATCGGAGGAC R: TGCCGGGAAGCTAGAGTAAG | 60 | 86 | ||
| Macrolides | ermB | F:AGGGTTGCTCTTGCACACTC R: CTGTGGTATGGCGGGTAAGT | 58 | 119 | [6] |
| ermC | F:GAAATCGGCTCAGGAAAAGG R: TAGCAAACCCGTATTCCACG | 56 | 292 | ||
| Quinolones | qnrD | F:GCTGGTGACATGGATGAAAG R: TAGGTTGAGGCTGGGTGAAG | 57 | 373 | |
| qnrS | F:GTTGGTGACGTGGCTCAAAG R: GTTGCGGCTGGGTAAAATAG | 55.3 | 134 | ||
| oqxB | F:TCTGACAACGATCGGAGGAC R: TGCCGGGAAGCTAGAGTAAG | 60 | 131 | ||
| Sulfonamides | sul1 | F:TGTCGAACCTTCAAAAGCTG R: TGGACCCAGATCCTTTACAG | 60 | 113 | |
| sul2 | F:ATCTGCCAAACTCGTCGTTA R: CAATGTGATCCATGATGTCG | 60 | 89 | ||
| sul3 | F:AGGCTTGGCAAAGTCAGATT R: CACCAGCCTCAACTAAAGCA | 57 | 152 | ||
| Aminoglycosides | aadD | F:ATGGGGATGATGTTAAGGCT R: TCACTTCCACCTTCCACTCA | 55 | 153 | |
| Multi-drug | acrA | F:CTCTCAGGCAGCTTAGCCCTAA R: TGCAGAGGTTCAGTTTTGACTGTT | 60 | 107 | |
| acrB | F:GGTCGATTCCGTTCTCCGTTA R:CTACCTGGAAGTAAACGTCATTGGT | 60 | 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes-Gonçalves, S.; Bento, J.T.; Machado, A.; Dudnyk, Y.; Shkromada, O.; Rebenko, H.; Bordalo, A.A.; Mesquita, J.R. High Prevalence of Antimicrobial Resistance Genes in Rabbit Farms from Sumy Region, Ukraine. Antibiotics 2025, 14, 907. https://doi.org/10.3390/antibiotics14090907
Gomes-Gonçalves S, Bento JT, Machado A, Dudnyk Y, Shkromada O, Rebenko H, Bordalo AA, Mesquita JR. High Prevalence of Antimicrobial Resistance Genes in Rabbit Farms from Sumy Region, Ukraine. Antibiotics. 2025; 14(9):907. https://doi.org/10.3390/antibiotics14090907
Chicago/Turabian StyleGomes-Gonçalves, Sara, Jaqueline T. Bento, Ana Machado, Yevheniia Dudnyk, Oksana Shkromada, Halyna Rebenko, Adriano A. Bordalo, and João R. Mesquita. 2025. "High Prevalence of Antimicrobial Resistance Genes in Rabbit Farms from Sumy Region, Ukraine" Antibiotics 14, no. 9: 907. https://doi.org/10.3390/antibiotics14090907
APA StyleGomes-Gonçalves, S., Bento, J. T., Machado, A., Dudnyk, Y., Shkromada, O., Rebenko, H., Bordalo, A. A., & Mesquita, J. R. (2025). High Prevalence of Antimicrobial Resistance Genes in Rabbit Farms from Sumy Region, Ukraine. Antibiotics, 14(9), 907. https://doi.org/10.3390/antibiotics14090907









