Overview of Methods for Assessing Antimicrobial Use in Outpatient Settings in High-Income Countries: A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Study Design and PRISMA Adaptation
2.2. Study Aims and Type
2.3. Definitions
2.4. Search Strategy
2.5. Inclusion Criteria
2.6. Data Collection and Extraction
2.7. Method Characterization
2.8. Study Characterization
2.9. Risk of Bias Assessment
2.10. Data Analysis
3. Results
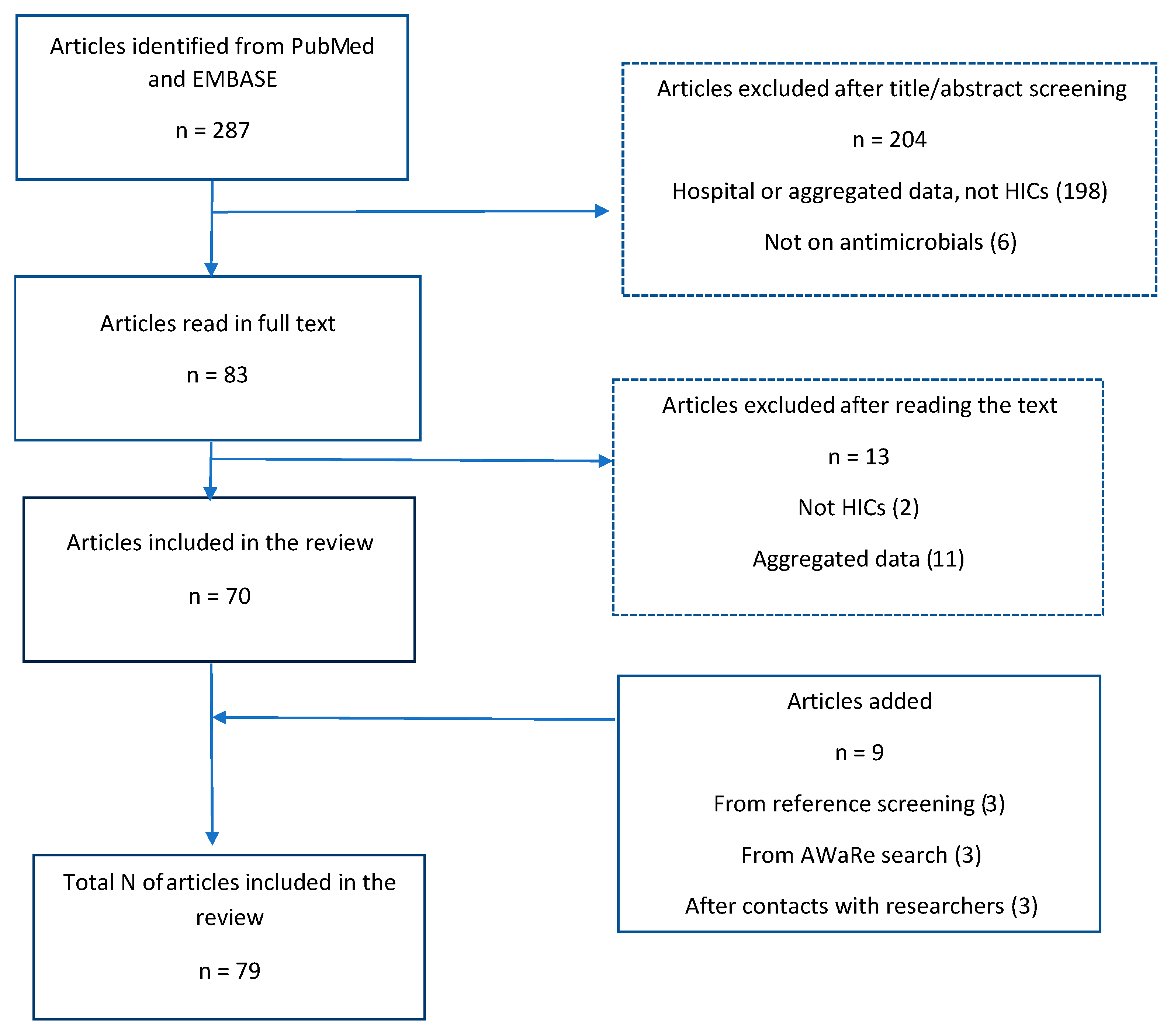
3.1. Search Results
3.2. Study Characteristics
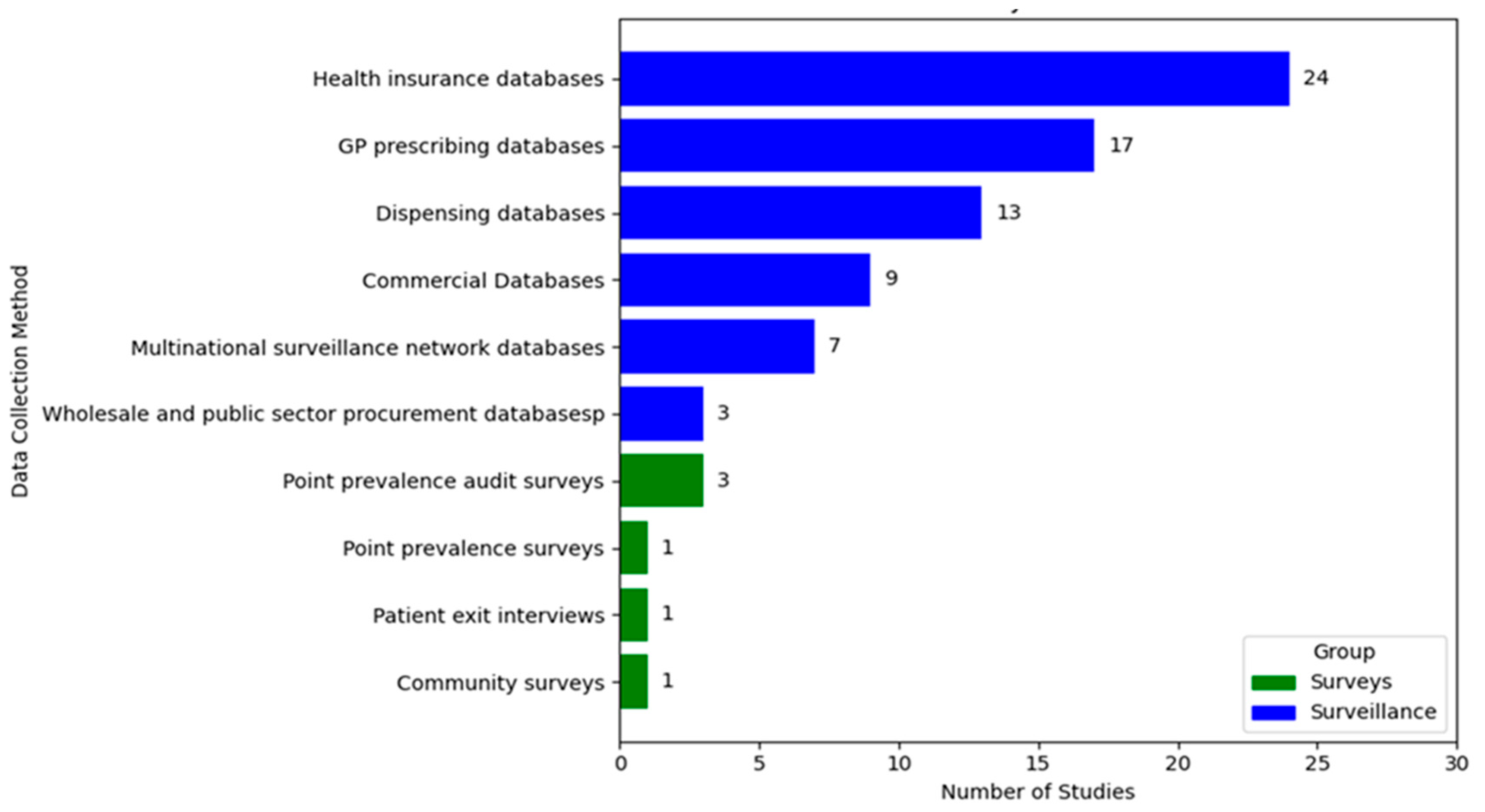
3.3. Methods and Data Sources
3.4. Studies Utilizing Routine Healthcare Databases
3.4.1. Studies Utilizing Health Insurance Databases
3.4.2. Studies Utilizing GP Prescribing Databases
3.4.3. Studies Using Commercial Databases
3.4.4. Wholesale and Public Sector Distribution Databases
3.4.5. Multinational Surveillance Network Database
3.5. Patient Surveys
3.5.1. Point Prevalence Surveys and Point Prevalence Audits Surveys
3.5.2. Patient Interview and Prescription Review
3.5.3. Community Surveys
3.6. Outcome Measures
- -
- AWaRe classification: stratified by Access, Watch, and Reserve categories
- -
- Antimicrobial classes: specific classes of antimicrobials
- -
- Spectrum of action: narrow-spectrum vs. broad-spectrum antibiotics
- -
- Age-specific prescribing
- -
- Regional (subnational) prescribing
- -
- Percentage of all patients prescribed antibiotics
- -
- Age-specific prescribing
- -
- Diagnosis-specific prescribing patterns: percentage of patients with specific syndromes or indications treated with antibiotics, including by AWaRe classification
- -
- Antibiotic use with or without prescription
Outcome Measure by AWaRe Classification
3.7. Registries
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| AMR | Antimicrobial Resistance |
| AMU | Antimicrobial Use |
| AWaRe | Access, Watch, Reserve (WHO Classification Of Antibiotics) |
| WHO | World Health Organization |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| GLASS-AMU | GLASS Module For Antimicrobial Use |
| HICs | High-Income Countries |
| DID | Defined Daily Doses Per 1000 Inhabitants Per Day |
| DOT | Days of Therapy |
| PID | Prescriptions Per 1000 Inhabitants Per Day |
| RTI | Respiratory Tract Infections |
| UTI | Urinary Tract Infections |
| PBS | Pharmaceutical Benefits Scheme (Australia) |
| NDB | National Database of Health Insurance Claims and Specific Health Checkups (Japan) |
| NorPD | Norwegian Prescription Database |
| SFK | Foundation for Pharmaceutical Statistics (Netherlands) |
| INFARMED | Portuguese National Authority of Medicines and Health Products |
| CPRD | Clinical Practice Research Datalink (UK) |
| EMRALD | Electronic Medical Record Administrative Data Linked Database (Canada) |
| ODB | Ontario Drug Benefit (Canada) |
| IPCRN | Irish Primary Care Research Network |
| ESAC-Net | European Surveillance of Antimicrobial Consumption Network |
| ECDC | European Centre for Disease Prevention and Control |
| AGAR | Australian Group on Antimicrobial Resistance |
| APAS | Australian Passive AMR Surveillance |
| AURA | Antimicrobial Use and Resistance in Australia |
| THIN | The Health Improvement Network (UK) |
| EMRPC | Electronic Medical Records Primary Care (Ontario, Canada) |
| SID | Standard Units Per 1000 Inhabitants Per Day |
| PrID | Prescriptions Per 1000 Inhabitants Per Day |
| PPS | Point Prevalence Survey |
| PPAS | Point Prevalence Audit Survey |
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Byrne, M.K.; Miellet, S.; McGlinn, A.; Fish, J.; Meedya, S.; Reynolds, N.; van Oijen, A.M. The drivers of antibiotic use and misuse: The development and investigation of a theory driven community measure. BMC Public Health 2019, 19, 1425. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: Antibiotic Use Data for 2022. Available online: https://iris.who.int/server/api/core/bitstreams/eede4321-72b5-4185-b422-c2ce07f185df/content (accessed on 1 June 2025).
- Sharland, M.; Zanichelli, V.; Ombajo, L.A.; Bazira, J.; Cappello, B.; Chitatanga, R.; Chuki, P.; Gandra, S.; Getahun, H.; Harbarth, S.; et al. The WHO essential medicines list AWaRe book: From a list to a quality improvement system. Clin. Microbiol. Infect. 2022, 28, 1533–1535. [Google Scholar] [CrossRef]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Michalsen, B.O.; Xu, A.X.T.; Alderson, S.L.; Bjerrum, L.; Brehaut, J.; Bucher, H.C.; Clarkson, J.; Duncan, E.; Grimshaw, J.; Gunnarsson, R.; et al. Regional and national antimicrobial stewardship activities: A survey from the Joint Programming Initiative on Antimicrobial Resistance—Primary Care Antibiotic Audit and Feedback Network (JPIAMR-PAAN). JAC-Antimicrob. Resist. 2023, 5, dlad048. [Google Scholar] [CrossRef]
- Ebell, M.H.; Radke, T. Antibiotic use for viral acute respiratory tract infections remains common. Am. J. Manag. Care 2015, 21, e567–e575. [Google Scholar] [PubMed]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- McGavock, H. Handbook of Drug Use Research Methodology, 1st ed.; United Kingdom Drug Utilisation Research Group: Newcastle upon Tyne, UK, 2000. [Google Scholar]
- Wettermark, B.; Zoëga, H.; Furu, K.; Korhonen, M.; Hallas, J.; Nørgaard, M.; Almarsdottir, A.; Andersen, M.; Sundell, K.A.; Bergman, U.; et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—A literature review. Pharmacoepidemiol. Drug Saf. 2013, 22, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Boethius, G.; Wiman, F. Recording of drug prescriptions in the county of Jämtland, Sweden: I. Methodological aspects. Eur. J. Clin. Pharmacol. 1977, 12, 31–35. [Google Scholar] [CrossRef]
- Isacson, D. Heavy Use of Prescription Drugs: Pharmacoepidemiological Studies in a Swedish Community; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1987. [Google Scholar]
- Quinn, K.; Baker, M.J.; Evans, B. A population-wide profile of prescription drug use in Saskatchewan, 1989. Can. Med. Assoc. J. 1992, 146, 2177–2186. [Google Scholar]
- Furu, K.; Wettermark, B.; Andersen, M.; Martikainen, J.E.; Almarsdottir, A.B.; Sørensen, H.T. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin. Pharmacol. Toxicol. 2010, 106, 86–94. [Google Scholar] [CrossRef]
- United Nations General Assembly. Political Declaration of the High-Level Meeting on Antimicrobial Resistance; Resolution A/RES/79/2; United Nations: New York, NY, USA, 2024; Available online: https://documents.un.org/doc/undoc/ltd/n24/278/35/pdf/n2427835.pdf (accessed on 7 October 2024).
- World Bank Group Country Classifications by Income Level for FY24 (1 July 2023–30 June 2024). World Bank Blogs. n.d. Available online: https://blogs.worldbank.org/en/opendata/new-world-bank-group-country-classifications-income-level-fy24 (accessed on 21 April 2025).
- Mölstad, S.; Cars, O. Major change in the use of antibiotics following a national programme: Swedish Strategic Programme for the Rational Use of Antimicrobial Agents and Surveillance of Resistance (STRAMA). Scand. J. Infect. Dis. 1999, 31, 191–195. [Google Scholar] [CrossRef]
- Campos, J.; Ferech, M.; Lázaro, E.; de Abajo, F.; Oteo, J.; Stephens, P.; Goossens, H. Surveillance of outpatient antibiotic consumption in Spain according to sales data and reimbursement data. J. Antimicrob. Chemother. 2007, 60, 698–701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwartz, K.L.; Wilton, A.S.; Langford, B.J.; A Brown, K.; Daneman, N.; Garber, G.; Johnstone, J.; Adomako, K.; Achonu, C.; Tu, K. Comparing prescribing and dispensing databases to study antibiotic use: A validation study of the Electronic Medical Record Administrative data Linked Database (EMRALD). J. Antimicrob. Chemother. 2019, 74, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Glass-Kaastra, S.K.; Finley, R.; Hutchinson, J.; Patrick, D.M.; Weiss, K.; Conly, J. Variation in outpatient oral antimicrobial use patterns among Canadian provinces, 2000 to 2010. Can. J. Infect. Dis. Med Microbiol. 2014, 25, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Aabenhus, R.; Siersma, V.; Hansen, M.P.; Bjerrum, L. Antibiotic prescribing in Danish general practice 2004–2013. J. Antimicrob. Chemother. 2016, 71, 2286–2294. [Google Scholar] [CrossRef][Green Version]
- Jensen, M.L.V.; Aabenhus, R.M.; Holzknecht, B.J.; Bjerrum, L.; Jensen, J.N.; Siersma, V.; Córdoba, G. Antibiotic prescribing in Danish general practice in the elderly population from 2010 to 2017. Scand. J. Prim. Health Care 2021, 39, 498–505. [Google Scholar] [CrossRef]
- Blix, H.S.; Høye, S. Use of antibiotics during the COVID-19 pandemic. Tidsskr. Nor. Laegeforen. 2021, 141, 353–357. [Google Scholar] [CrossRef]
- Haugom, L.E.A.; Ruths, S.; Emberland, K.E.; Eliassen, K.E.R.; Rortveit, G.; Wensaas, K.-A. Consultations and antibiotic treatment for urinary tract infections in Norwegian primary care 2006–2015, a registry-based study. BMC Fam. Pract. 2021, 22, 127. [Google Scholar] [CrossRef]
- Dillen, H.; Burvenich, R.R.; De Burghgraeve, T.; Verbakel, J. Using Belgian Pharmacy Dispensing Data to Assess Antibiotic Use for Children in Ambulatory Care—PubMed. n.d. Available online: https://pubmed.ncbi.nlm.nih.gov/34980037/ (accessed on 21 April 2025).
- de Jong, L.A.W.; van der Linden, P.D.; Roukens, M.M.B.; van de Garde, E.M.W.; van der Velden, A.W.; Natsch, S.; on behalf of SWAB’s Working Group on Surveillance of Antimicrobial Use. Consecutive antibiotic use in the outpatient setting: An extensive, longitudinal descriptive analysis of antibiotic dispensing data in the Netherlands. BMC Infect. Dis. 2019, 19, 84. [Google Scholar] [CrossRef]
- Gomes, M.; Torre, C.; Guerreiro, J.; Nogueira, P.; Furtado, C. 11 years of outpatient antibiotic utilization in Portugal—Utilization pattern and regional comparison between 2004 and 2014. Pharmacoepidemiol. Drug 2015, 24, 1–587. [Google Scholar] [CrossRef]
- Calle-Miguel, L.; Pérez-Méndez, C.; García-García, E.; Moreno-Pavón, B.; Solís-Sánchez, G. Trends and Pattern of Antibiotic Use in Children in Northern Spain, Interpreting Data about Antibiotic Consumption in Pediatric Outpatients. Children 2022, 9, 442. [Google Scholar] [CrossRef]
- King, L.M.; Lovegrove, M.C.; Shehab, N.; Tsay, S.; Budnitz, D.S.; Geller, A.I.; Lind, J.N.; Roberts, R.M.; A Hicks, L.; Kabbani, S. Trends in US Outpatient Antibiotic Prescriptions During the Coronavirus Disease 2019 Pandemic. Clin. Infect. Dis. 2021, 73, e652–e660. [Google Scholar] [CrossRef]
- Birkett, D.J.; Mitchell, A.S.; Godeck, A.; Grigson, T.; Cully, R.; Lee, C. Profiles of antibacterial drug use in Australia and trends from 1987 to 1989: A report from the Drug Utilization Subcommittee of the Pharmaceutical Benefits Advisory Committee. Med. J. Aust. 1991, 155, 410–415. [Google Scholar] [CrossRef]
- Davey, P.; Ferech, M.; Ansari, F.; Muller, A.; Goossens, H.; ESAC Project Group. Outpatient antibiotic use in the four administrations of the UK: Cross-sectional and longitudinal analysis. J. Antimicrob. Chemother. 2008, 62, 1441–1447. [Google Scholar] [CrossRef]
- Gagliotti, C.; Mazzetti, I.; Moro, M.L. Comparison of sales and reimbursement data regarding outpatient antibiotic use in a northern Italian Region. Pharmacoepidemiol. Drug Saf. 2009, 18, 1115–1118. [Google Scholar] [CrossRef]
- Kinoshita, N.; Morisaki, N.; Uda, K.; Kasai, M.; Horikoshi, Y.; Miyairi, I. Nationwide study of outpatient oral antimicrobial utilization patterns for children in Japan (2013–2016). J. Infect. Chemother. 2019, 25, 22–27. [Google Scholar] [CrossRef]
- Okubo, Y.; Michihata, N.; Uda, K.; Kinoshita, N.; Horikoshi, Y.; Miyairi, I. Impacts of Primary Care Physician System on Healthcare Utilization and Antibiotic Prescription: Difference-in-Differences and Causal Mediation Analyses. Pediatr. Infect. Dis. J. 2020, 39, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Nariai, H.; Michels, K.B.; Kim-Farley, R.J.; Nishi, A.; Arah, O.A.; Kinoshita, N.; Uda, K.; Miyairi, I. Change in clinical practice variations for antibiotic prescriptions across different pediatric clinics: A Japan’s nationwide observational study. J. Infect. Chemother. 2021, 27, 1621–1625. [Google Scholar] [CrossRef]
- Ono, A.; Ishikane, M.; Kusama, Y.; Tanaka, C.; Ono, S.; Tsuzuki, S.; Muraki, Y.; Yamasaki, D.; Tanabe, M.; Ohmagari, N. The first national survey of antimicrobial use among dentists in Japan from 2015 to 2017 based on the national database of health insurance claims and specific health checkups of Japan. PLoS ONE 2020, 15, e0244521. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Park, S.J.; Byun, S.J.; Choe, Y.-J.; Shin, J.-Y. Increased use of third-generation cephalosporin antibiotics in the outpatient setting in Korean children and adolescents. Pediatr. Int. 2018, 60, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, E.; Lee, S.O.; Kim, D.-S. Antibiotic use in South Korea from 2007 to 2014: A health insurance database-generated time series analysis. PLoS ONE 2017, 12, e0177435. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Park, G.C.; An, H.; Chun, B.C.; Sohn, J.W.; Kim, M.J. Trends of Antibiotic Consumption in Korea According to National Reimbursement Data (2008–2012): A Population-Based Epidemiologic Study. Medicine 2015, 94, e2100. [Google Scholar] [CrossRef]
- Gadzhanova, S.; Roughead, E. Prescribed antibiotic use in Australian children aged 0–12 years. Aust. Fam. Physician 2020, 45, 134–138. Available online: https://search.informit.org/doi/10.3316/informit.926789487361680 (accessed on 7 November 2025). [CrossRef]
- Contreras, J.; Oguoma, V.; Todd, L.; Naunton, M.; Collignon, P.; Bushell, M. Restricting access to antibiotics: The effectiveness of a ‘no repeats’ government policy intervention. Res. Soc. Adm. Pharm. 2023, 19, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Gielen, B.; Blommaert, A.; Beutels, P.; Hens, N.; Goossens, H. Appropriate international measures for outpatient antibiotic prescribing and consumption: Recommendations from a national data comparison of different measures. J. Antimicrob. Chemother. 2014, 69, 529–534. [Google Scholar] [CrossRef]
- Struyf, T.; Vandael, E.; Leroy, R.; Mertens, K.; Catry, B. Antimicrobial prescribing by Belgian dentists in ambulatory care, from 2010 to 2016. Int. Dent. J. 2020, 69, 480–487. [Google Scholar] [CrossRef]
- Russo, V.; Monetti, V.M.; Guerriero, F.; Trama, U.; Guida, A.; Menditto, E.; Orlando, V. Prevalence of antibiotic prescription in southern Italian outpatients: Real-world data analysis of socioeconomic and sociodemographic variables at a municipality level. Clin. Outcomes Res. 2018, 10, 251–258. [Google Scholar] [CrossRef]
- Cangini, A.; Fortinguerra, F.; Di Filippo, A.; Pierantozzi, A.; Da Cas, R.; Villa, F.; Trotta, F.; Moro, M.L.; Gagliotti, C. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br. J. Clin. Pharmacol. 2021, 87, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Bara, W.; Brun-Buisson, C.; Coignard, B.; Watier, L. Outpatient Antibiotic Prescriptions in France: Patients and Providers Characteristics and Impact of the COVID-19 Pandemic. Antibiotics 2022, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Bernier, A.; Delarocque-Astagneau, E.; Ligier, C.; Vibet, M.-A.; Guillemot, D.; Watier, L. Outpatient Antibiotic Use in France between 2000 and 2010, After the Nationwide Campaign, It Is Time To Focus on the Elderly. Antimicrob. Agents Chemother. 2014, 58, 71–77. [Google Scholar] [CrossRef]
- Saatchi, A.; Morris, A.M.; Patrick, D.M.; Mccormack, J.; Reyes, R.C.; Morehouse, P.; Reid, J.; Shariff, S.; Povitz, M.; Silverman, M.; et al. Outpatient antibiotic use in British Columbia, Canada: Reviewing major trends since 2000. JAC Antimicrob. Resist. 2021, 3, dlab116. [Google Scholar] [CrossRef]
- Vojvodić, Ž.; Daus Šebeđak, D. Outpatient antibiotic consumption for urinary infections in Croatia 2005–2014: What can be learned from utilization trends. Slov. J. Public Health 2018, 57, 183–191. [Google Scholar] [CrossRef]
- Scholle, O.; Rasmussen, L.; Reilev, M.; Viebrock, J.; Haug, U. Comparative Analysis of Outpatient Antibiotic Prescribing in Early Life: A Population-Based Study Across Birth Cohorts in Denmark and Germany. Infect. Dis. Ther. 2024, 13, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, E.; Sepponen, K.; Lauhio, A.; Saastamoinen, L. Outpatient Antibiotic Use and Costs in Adults: A Nationwide Register-Based Study in Finland 2008–2019. Antibiotics 2022, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Roos, R.; Verrall, A.; Smith, A.; Thomas, M.G. Trends, demographics and disparities in outpatient antibiotic consumption in New Zealand: A national study. J. Antimicrob. Chemother. 2016, 71, 3593–3598. [Google Scholar] [CrossRef]
- Olesen, S.W.; Barnett, M.L.; MacFadden, D.R.; Lipsitch, M.; Grad, Y.H. Trends in outpatient antibiotic use and prescribing practice among US older adults, 2011–2015: Observational study. BMJ 2018, 362, k3155. [Google Scholar] [CrossRef] [PubMed]
- van den Broek d’Obrenan, J.; Verheij, T.J.M.; Numans, M.E.; van der Velden, A.W. Antibiotic use in Dutch primary care: Relation between diagnosis, consultation and treatment. J. Antimicrob. Chemother. 2014, 69, 1701–1707. [Google Scholar] [CrossRef]
- van der Velden, A.W.; van Triest, M.I.; Schoffelen, A.F.; Verheij, T.J.M. Structural Antibiotic Surveillance and Stewardship via Indication-Linked Quality Indicators: Pilot in Dutch Primary Care. Antibiotics 2020, 9, 670. [Google Scholar] [CrossRef]
- Haeseker, M.B.; Dukers-Muijrers, N.H.T.M.; Hoebe, C.J.P.A.; Bruggeman, C.A.; Cals, J.W.L.; Verbon, A. Trends in Antibiotic Prescribing in Adults in Dutch General Practice. PLoS ONE 2012, 7, e51860. [Google Scholar] [CrossRef]
- Smith, S.; I Hawker, J.; E Smith, G.; Morbey, R.; Johnson, A.P.; Fleming, D.M.; Shallcross, L.; Hayward, A.C. A standardized methodology for the surveillance of antimicrobial prescribing linked to clinical indications in primary care. J. Public Health 2018, 40, 630–638. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Sun, X.; Charlton, J.; Winter, J.R.; Bunce, C.; Boiko, O.; Fox, R.; Little, P.; Moore, M.; Hay, A.D.; et al. Serious bacterial infections and antibiotic prescribing in primary care: Cohort study using electronic health records in the UK. BMJ Open 2020, 10, e036975. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gulliford, M.C. Reducing antibiotic prescribing in primary care in England from 2014 to 2017: Population-based cohort study. BMJ Open 2019, 9, e023989. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Langford, B.J.; Daneman, N.; Chen, B.; Brown, K.A.; McIsaac, W.; Tu, K.; Candido, E.; Johnstone, J.; Leung, V.; et al. Unnecessary antibiotic prescribing in a Canadian primary care setting: A descriptive analysis using routinely collected electronic medical record data. CMAJ Open 2020, 8, E360–E369. [Google Scholar] [CrossRef]
- Edwards, B.; Wilson, R.; McDonald, G.; Daley, P. Population-based outpatient antimicrobial use in Newfoundland and Labrador: A retrospective descriptive study. Can. Med. Assoc. Open Access J. 2023, 11, E1109–E1117. [Google Scholar] [CrossRef]
- Soudais, B.; Lacroix-Hugues, V.; Meunier, F.; Gillibert, A.; Darmon, D.; Schuers, M. Diagnosis and management of male urinary tract infections: A need for new guidelines. Study from a French general practice electronic database. Fam. Pract. 2021, 38, 432–440. [Google Scholar] [CrossRef]
- Trinh, N.T.H.; Cohen, R.; Lemaitre, M.; Chahwakilian, P.; Coulthard, G.; Bruckner, T.A.; Milic, D.; Levy, C.; Chalumeau, M.; Cohen, J.F. Community antibiotic prescribing for children in France from 2015 to 2017: A cross-sectional national study. J. Antimicrob. Chemother. 2020, 75, 2344–2352. [Google Scholar] [CrossRef]
- Bernardo, C.D.O.; Gonzalez-Chica, D.; Stocks, N. Influenza-like illness and antimicrobial prescribing in Australian general practice from 2015 to 2017: A national longitudinal study using the MedicineInsight dataset. BMJ Open 2019, 9, e026396. [Google Scholar] [CrossRef]
- Galvin, S.; Callan, A.; Cormican, M.; Duane, S.; Bennett, K.; Murphy, A.W.; Vellinga, A. Improving antimicrobial prescribing in Irish primary care through electronic data collection and surveillance: A feasibility study. BMC Fam. Pract. 2015, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Liberati, C.; Cantarutti, A.; Di Chiara, C.; Lupattelli, A.; Sharland, M.; Giaquinto, C.; Hsia, Y.; Doná, D. Antibiotic Prescription Patterns in the Paediatric Primary Care Setting before and after the COVID-19 Pandemic in Italy: An Analysis Using the AWaRe Metrics. Antibiotics 2022, 11, 457. [Google Scholar] [CrossRef]
- Ramalhinho, I.; Ribeirinho, M.; Vieira, I.; Cabrita, J. Evolution of outpatient antibiotic use in Portugal mainland 2000–2009. Acta Med. Port. 2012, 25, 20–28. [Google Scholar]
- Alshareef, H.; Alanazi, A.; Alatawi, N.; Eleshmawy, N.; Ali, M. Assessment of antibiotic prescribing patterns at dental and primary health care clinics according to WHO Access, Watch, Reserve (AWaRe) classification. Am. J. Infect. Control. 2023, 51, 289–294. [Google Scholar] [CrossRef]
- Cronberg, O.; Tyrstrup, M.; Ekblom, K.; Hedin, K. Diagnosis-linked antibiotic prescribing in Swedish primary care—A comparison between in-hours and out-of-hours. BMC Infect. Dis. 2020, 20, 616. [Google Scholar] [CrossRef]
- Martínez-González, N.A.; Di Gangi, S.; Pichierri, G.; Neuner-Jehle, S.; Senn, O.; Plate, A. Time Trends and Factors Associated with Antibiotic Prescribing in Swiss Primary Care (2008 to 2020). Antibiotics 2020, 9, 837. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Chen, C.; Langford, B.J.; Brown, K.A.; Daneman, N.; Johnstone, J.; Wu, J.H.; Leung, V.; Garber, G. Validating a popular outpatient antibiotic database to reliably identify high prescribing physicians for patients 65 years of age and older. PLoS ONE 2019, 14, e0223097. [Google Scholar] [CrossRef]
- Kitano, T.; Langford, B.J.; A Brown, K.; Pang, A.; Chen, B.; Garber, G.; Daneman, N.; Tu, K.; Leung, V.; Candido, E.; et al. The Association Between High and Unnecessary Antibiotic Prescribing: A Cohort Study Using Family Physician Electronic Medical Records. Clin. Infect. Dis. 2021, 72, e345–e351. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.D.; Shurgold, J.; Smith, G.; MacFadden, D.R.; Schwartz, K.L.; Daneman, N.; Tropper, D.G.; Brooks, J. The impact of COVID-19 on community antibiotic use in Canada: An ecological study. Clin. Microbiol. Infect. 2022, 28, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bizune, D.; Gouin, K.; Powell, L.; Hersh, A.L.; Hicks, L.A.; Kabbani, S. Update on outpatient antibiotic prescribing during the COVID-19 pandemic: United States, 2020–2022. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e193. [Google Scholar] [CrossRef]
- Trinh, N.T.H.; Chahwakilian, P.; A Bruckner, T.; Sclison, S.; Levy, C.; Chalumeau, M.; Milic, D.; Cohen, R.; Cohen, J.F. Discrepancies in national time trends of outpatient antibiotic utilization using different measures: A population-based study in France. J. Antimicrob. Chemother. 2018, 73, 1395–1401. [Google Scholar] [CrossRef]
- Kern, W.V.; Kostev, K. Prevalence of and Factors Associated with Antibiotic Prescriptions in Patients with Acute Lower and Upper Respiratory Tract Infections—A Case-Control Study. Antibiotics 2021, 10, 455. [Google Scholar] [CrossRef]
- Popescu, G.A.; Mathyas, L.; Ciolan, C.; Șerban, R.; Pistol, A. Antibacterial consumption in Romania in 2012: Specific features and quality indicators for community usage. BMC Infect. Dis. 2013, 13, O17. [Google Scholar] [CrossRef][Green Version]
- Plüss-Suard, C.; Friedli, O.; Labutin, A.; Gasser, M.; Mueller, Y.; Kronenberg, A. Post-pandemic consumption of outpatient antibiotics in Switzerland up to pre-pandemic levels, 2018–2023: An interrupted time series analysis. CMI Commun. 2024, 1, 105037. [Google Scholar] [CrossRef]
- Zarb, P.; Borg, M. Consumption of antibiotics within ambulatory care in Malta. Malta Med. J. 2011, 23, 13–18. [Google Scholar]
- Khan, R.; Gangar, M.; Gangar, M.; Motilal, S. Eight years of antibiotic consumption at a primary care outpatient facility in Trinidad and Tobago 2011-18: A synopsis of consumption trends. JAC Antimicrob. Resist. 2021, 3, dlab162. [Google Scholar] [CrossRef]
- Lass, J.; Mitt, P.; Telling, K.; Linask, E.; Laius, O.; Sepp, E. Outpatient antibiotic use in Estonia—Eesti Arst—Eesti Arstide Liidu ajakiri. Eesti. Arst. 2020, 99, 604–613. [Google Scholar]
- European Centre for Disease Prevention and Control. Data Source Overview for Reporting Antimicrobial Consumption, EU/EEA Countries. Available online: https://qap.ecdc.europa.eu/public/extensions/AMC2_Dashboard/AMC2_Dashboard.html#data-source-tab (accessed on 4 April 2025).
- Ferech, M.; Coenen, S.; Malhotra-Kumar, S.; Dvorakova, K.; Hendrickx, E.; Suetens, C.; Goossens, H.; ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient antibiotic use in Europe. J. Antimicrob. Chemother. 2006, 58, 401–407. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, L.; Armstrong, D.; Ashworth, M.; Dregan, A.; Malik, U.; White, P. National disparities in the relationship between antimicrobial resistance and antimicrobial consumption in Europe: An observational study in 29 countries. J. Antimicrob. Chemother. 2017, 72, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; on behalf of the ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient antibiotic use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66 (Suppl. S6), vi3–vi12. [Google Scholar] [CrossRef] [PubMed]
- Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Versporten, A.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S. Consumption of penicillins in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii14–ii21. [Google Scholar] [CrossRef]
- Versporten, A.; Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; Strauss, R. Consumption of tetracyclines, sulphonamides and trimethoprim, and other antibacterials in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii45–ii59. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Adriaenssens, N.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S. Consumption of antibiotics in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii7–ii13. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Hoxha, A.; Quinten, C.; Ayele, G.M.; Coenen, S.; Versporten, A.; Adriaenssens, N.; Muller, A.; Heuer, O.; Monnet, D.L.; et al. Change-points in antibiotic consumption in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii68–ii78. [Google Scholar] [CrossRef]
- March-López, P.; Madridejos, R.; Tomas, R.; Boix-Palop, L.; Arcenillas, P.; Gómez, L.; Padilla, E.; Xercavins, M.; Martinez, L.; Massats, Ú.; et al. Applicability of Outpatient Quality Indicators for Appropriate Antibiotic Use in a Primary Health Care Area: A Point Prevalence Survey. Antimicrob. Agents Chemother. 2020, 64, e01266-20. [Google Scholar] [CrossRef]
- van der Velden, A.W.; A Bax, E.; Bongard, E.; Aabenhus, R.M.; Anastasaki, M.; Anthierens, S.; Balan, A.; Böhmer, F.; Bruno, P.; Chlabicz, S.; et al. Primary care for patients with respiratory tract infection before and early on in the COVID-19 pandemic: An observational study in 16 European countries. BMJ Open 2021, 11, e049257. [Google Scholar] [CrossRef]
- van der Velden, A.W.; van de Pol, A.C.; Bongard, E.; Cianci, D.; Aabenhus, R.; Balan, A.; Böhmer, F.; Lang, V.B.; Bruno, P.; Chlabicz, S.; et al. Point-of-care testing, antibiotic prescribing, and prescribing confidence for respiratory tract infections in primary care: A prospective audit in 18 European countries. BJGP Open 2022, 6, 1–1098. [Google Scholar] [CrossRef]
- Vellinga, A.; Luke-Currier, A.; Garzón-Orjuela, N.; Aabenhus, R.; Anastasaki, M.; Balan, A.; Böhmer, F.; Lang, V.B.; Chlabicz, S.; Coenen, S.; et al. Disease-Specific Quality Indicators for Outpatient Antibiotic Prescribing for Respiratory Infections (ESAC Quality Indicators) Applied to Point Prevalence Audit Surveys in General Practices in 13 European Countries. Antibiotics 2023, 12, 572. [Google Scholar] [CrossRef]
- Tzimis, L.; Katsantonis, N.; Leledaki, A.; Vasilomanolakis, K.; Kafatos, A. Antibiotics prescription for indigent patients in primary care. J. Clin. Pharm. Ther. 1997, 22, 227–235. [Google Scholar] [CrossRef]
- Surveys—Eurobarometer. n.d. Available online: https://europa.eu/eurobarometer/surveys/browse/all/series/29716 (accessed on 15 October 2023).
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2022 (accessed on 7 November 2025).
- Haugen, P.; Simonsen, G.S.; Primicerio, R.; Furberg, A.-S.; Småbrekke, L. Antibiotics to outpatients in Norway-Assessing effect of latitude and municipality population size using quantile regression in a cross-sectional study. Pharm. Stat. 2018, 17, 4–11. [Google Scholar] [CrossRef]
- Chae, J.; Kim, B.; Kim, D.-S. Changes in antibiotic consumption patterns after the implementation of the National Action Plan according to the Access, Watch, Reserve (AWaRe) classification system. Int. J. Infect. Dis. 2022, 122, 345–351. [Google Scholar] [CrossRef]
- WHO News: World Leaders Commit to Decisive Action on Antimicrobial Resistance. n.d. Available online: https://www.who.int/news/item/26-09-2024-world-leaders-commit-to-decisive-action-on-antimicrobial-resistance (accessed on 22 April 2025).
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2022. 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-consumption-eueea-esac-net-annual-epidemiological-report-2022 (accessed on 7 November 2025).
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2023. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-consumption-eueea-esac-net-annual-epidemiological-report-2023 (accessed on 20 January 2025).
- Plüss-Suard, C.; Niquille, A.; Héquet, D.; Krähenbühl, S.; Pichon, R.; Zanetti, G.; Bugnon, O.; Petignat, C. Decrease in Antibacterial Use and Facility-Level Variability After the Introduction of Guidelines and Implementation of Physician-Pharmacist-Nurse Quality Circles in Swiss Long-term Care Facilities. J. Am. Med Dir. Assoc. 2020, 21, 78–83. [Google Scholar] [CrossRef]
- Funiciello, E.; Lorenzetti, G.; Cook, A.; Goelen, J.; Moore, C.E.; Campbell, S.M.; Godman, B.; Tong, D.; Huttner, B.; Chuki, P.; et al. Identifying AWaRe indicators for appropriate antibiotic use: A narrative review. J. Antimicrob. Chemother. 2024, 79, 3063–3077. [Google Scholar] [CrossRef]
- TARGET Antibiotics Toolkit Hub|RCGP Learning. n.d. Available online: https://elearning.rcgp.org.uk/course/view.php?id=553 (accessed on 20 January 2025).
- McNulty, C.; Hawking, M.; Lecky, D.; Jones, L.; Owens, R.; Charlett, A.; Butler, C.; Moore, P.; Francis, N. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: Pragmatic randomized controlled trial of the TARGET antibiotics workshop. J. Antimicrob. Chemother. 2018, 73, 1423–1432. [Google Scholar] [CrossRef]
- Government of Australia. Surveillance of Antimicrobial Use and Resistance in Human Health. Antimicrobial Resistance 2023. Available online: https://www.amr.gov.au/australias-response/objective-5-integrated-surveillance-and-response-resistance-and-usage/surveillance-antimicrobial-use-and-resistance-human-health (accessed on 22 December 2023).
- Turnidge, J.D.; Meleady, K.T. Antimicrobial Use and Resistance in Australia (AURA) surveillance system: Coordinating national data on antimicrobial use and resistance for Australia. Aust. Health Rev. 2018, 42, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Dalwai, A.; Hillock, N. Antimicrobial surveillance in South Australian prisons: A pilot study. Aust. Health Rev. 2024, 48, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A. Systematic and Nonsystematic Reviews: Choosing an Approach. In Healthcare Simulation Research: A Practical Guide; Nestel, D., Hui, J., Kunkler, K., Scerbo, M.W., Calhoun, A.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 55–60. [Google Scholar] [CrossRef]
- Giamarellou, H.; Galani, L.; Karavasilis, T.; Ioannidis, K.; Karaiskos, I. Antimicrobial Stewardship in the Hospital Setting: A Narrative Review. Antibiotics 2023, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
| Category | Subcategory | No. Studies (%) |
|---|---|---|
| by study year | 1991–2000 | 3 (3.8) |
| 2000–2009 | 4 (5.1) | |
| 2010–2023 | 72 (91.1) | |
| by WHO Region | WHO European Region (34 of 53 Member States are HICs) | 53 (67.1) |
| WHO Region of the Americas (13 of 35 Member States are HICs) | 13 (16.5) | |
| WHO Western Pacific Region (10 of 38 Member States are HICs) | 12 (15.2) | |
| WHO Eastern Mediterranean Region (6 of 22 Member States are HICs) | 1 (1.3) | |
| WHO African Region (0 of 47 Member States are HICs) | 0 (0) | |
| WHO South-East Asia Region (0 of 11 Member States are HICs) | 0 (0) | |
| by data collection method | Data retrieved from routine medicine monitoring databases | 73 (92.4) |
| Data collected through surveys | 6 (7.6) |
| Metric | Definition | Denominator | Suggested Use-Cases |
|---|---|---|---|
| DID | Number of DDDs dispensed or prescribed per 1000 inhabitants per day | Population size | Best for cross-country comparisons; limited for pediatrics due to adult-based WHO DDD standards |
| DOT | Number of days a patient receives an antimicrobial, regardless of dose | Patient-days or encounters | Recommended by CDC for stewardship; reflects treatment duration independent of dose |
| PID/PrID | Number of prescriptions issued per 1000 inhabitants per day | Population size | Useful for assessing prescriber behavior and appropriateness audits |
| SID | Number of standard units (e.g., tablets, capsules) dispensed | Population size or pharmacy data | Helpful when DDD calculation is not feasible; often used in procurement or wholesale data |
| AWaRe Stratified Use | Proportion of total antimicrobial consumption classified by WHO AWaRe categories (Access, Watch, Reserve) | Total antimicrobial use (DDD, DOT, or prescriptions) | Essential for monitoring alignment with WHO stewardship targets; supports policy benchmarking and prioritization |
| Methods | Description | Limitations |
|---|---|---|
| Routine Surveillance | ||
| Dispensing databases | Offers a closer picture of patient antimicrobial use compared to prescribing, procurement, or wholesale data. | Difficult to obtain from the private sector; does not provide information on actual patient use or prescriber behavior. |
| Health insurance databases | Provides patient-level data on antimicrobial use disaggregated by patient demographics, geographic characteristics, and indications for use. | Coverage of only insured populations and reimbursed antimicrobials; potential gaps in administrative data. |
| GP prescribing databases | Contains patient characteristics, diagnosis, prescriptions, dose duration, indications, and co-prescribed medicines. | Limited by the number of participating GPs; may not represent the entire region or nation; prescribed antibiotics may not be dispensed. |
| Wholesale and public sector distribution databases | Provides aggregated data on the distribution and procurement of antibiotics from wholesalers and public health sectors. | May not capture patient-level details or actual usage; limits insights into prescribing behaviors and patient adherence. |
| Surveillance networks databases | Uses national sales or reimbursement data to monitor antibiotic consumption across countries. | Heterogeneity in healthcare systems among different countries may hinder direct comparisons. |
| Surveys | ||
| Point Prevalence Surveys (PPS) | Customizable data collection tools tailored to specific study parameters. | Resource-intensive; may fail to capture dynamic trends unless conducted repeatedly. |
| Point Prevalence Audit Surveys (PPAS) | Examines consultation and management characteristics of patients with specific conditions. | Resource-intensive; may fail to capture dynamic trends unless conducted repeatedly. |
| Patient exit interviews | Capture real-time information on prescribing, dispensing (with or without a prescription), and patient understanding after consultations. | Resource-intensive; may suffer from recall and social desirability bias and may not reflect actual antibiotic use or adherence. |
| Community Surveys | Provides consumer-level data closely reflecting actual antimicrobial use in outpatients. | Resource-intensive, may suffer from representativeness and bias issues. |
| Country and Year of Publication | Reference | Methodology (Type of Database/Survey) | AWaRe Indicators |
|---|---|---|---|
| Spain, 2021 | [29] | National pharmacy dispensing database | AWaRe proportions (Access, Watch, Reserve) |
| Japan, 2020 | [36] | National health insurance claims database | AWaRe proportions |
| Denmark and Germany, 2021 | [51] | GP prescribing database (electronic medical records) | AWaRe proportions |
| Canada, 2021 | [62] | National claims database | AWaRe proportions |
| France, 2021 | [64] | National claims database (IQVIA) | AWaRe proportions |
| Italy, 2022 | [67] | Pedianet pediatric EMR database | Antibiotic index, Access-to-Watch index, Amoxicillin-to-Co-amoxiclav index |
| Saudi Arabia | [69] | Hospital outpatient pharmacy dispensing database | AWaRe proportions |
| Switzerland | [71] | Survey of outpatient prescribing practices | AWaRe proportions |
| Switzerland | [80] | Wholesale distribution database | AWaRe proportions |
| No. | Country and Reference | Registries/Database | Data Granularity | Data Access |
|---|---|---|---|---|
| 1 | Australia, [31,42] | Pharmaceutical Benefits Scheme (PBS) [https://www.pbs.gov.au/pbs/home] https://www.pbs.gov.au/pbs/home (accessed on 7 November 2025 Medicine Insight [https://www.nps.org.au/medicine-insight/using-medicineinsight-data] (accessed on 7 November 2025) | ATC Level: 5 Age/sex stratification available Sector stratification available | PBS—Open dashboard Medicine Insight—Proprietary |
| 2 | Belgium [26,43,44] | Farmanet—community pharmacy reimbursed dispensations [https://www.riziv.fgov.be/nl/statistieken/geneesmiddel/Paginas/Statistieken-geneesmiddelen-apotheken-farmanet.aspx] (accessed on 7 November 2025) | ATC Level: 3 and 5 Age/sex stratification available Sector stratification available | Restricted access—data request needed, not fully open dashboard |
| 3 | Croatia [50] | Agency for Medicinal Products and Medical Devices (HALMED) [https://www.halmed.hr/en/O-HALMED-u/Osnovni-podaci-i-dokumenti/HALMED-i-korisnici/] (accessed on 7 November 2025) | ATC Level: 5 Age/sex stratification not available Sector stratification not available | Reports only, no interactive dashboard; proprietary for detailed data [halmed.hr] |
| 4 | Denmark [22,23] | National Prescription Registry [https://sundhedsdatastyrelsen.dk/borger/om-sundhedsdata/sundhedsdatastyrelsens-registre] (accessed on 7 November 2025) | ATC Level: 4 and 5 Age/sex stratification available Sector stratification available | Aggregated data via eSundhed (open); individual-level requires application |
| 5 | Finland [52] | Finnish Prescription Registry/Kelasto [https://raportit.kela.fi/ibi_apps/WFServlet?IBIF_ex=NIT137AL&YKIELI=E] (accessed on 7 November 2025) | ATC Level: 5 Age/sex stratification available Sector stratification available | Open dashboard (Kelasto statistical reports) |
| 6 | France [47,48] | National Health Insurance (SNDS) [https://www.snds.gouv.fr/SNDS/Accueil] (accessed on 7 November 2025) | ATC Level: 3 and 4 Age/sex stratification available Sector stratification available | Mixed: Open data (Open Medic) + proprietary for detailed SNDS access |
| 7 | Japan [37] | National Database of Health Insurance Claims and Specific Health Checkups (NDB) [https://ndb6nc.ncgm.go.jp/eng/outline/index.html] (accessed on 7 November 2025) | ATC Level: 3 Age/sex stratification available Sector stratification available | Open data portal available (NDB Open Data) |
| 8 | Norway [25] | Norwegian Prescription Database (NorPD) [https://www.norpd.no/] (accessed on 7 November 2025) | ATC Level: 5 Age/sex stratification available Sector stratification available | Open aggregated reports; detailed data requires application |
| 9 | Netherlands [27] | Foundation for Pharmaceutical Statistics (SFK) [https://www.hiv-monitoring.nl/en/research-using-our-data/datakoppelingen/sfk] (accessed on 7 November 2025) | ATC Level: 5 Age/sex stratification available Sector stratification available | Proprietary (data sold via SFK or partners like SpotOnInsights) |
| 10 | New Zealand [53] | Pharmaceutical Collection https://www.tewhatuora.govt.nz/for-health-professionals/data-and-statistics/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection (access on 7 November 2025) | ATC Level: 5 Age/sex stratification available Sector stratification available | Open summary stats via Ministry of Health; detailed data restricted |
| 11 | Portugal [68] | INFARMED [https://www.infarmed.pt/web/infarmed-en/about-infarmed] (accessed on 7 November 2025) | ATC Level: 3 Age/sex stratification limited Sector stratification not available | Reports only, no open dashboard; proprietary for detailed data |
| 12 | Republic of Korea [38,39,40] | Health Insurance Review and Assessment Service (HIRA) [https://www.hira.or.kr/eng/main.do] | ATC Level: 4 Age/sex stratification available Sector stratification available | Open dashboard for claims data (HIRA public portal) |
| 13 | Sweden [15,18] | Swedish Prescribed Drug Register [https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-prescribed-drug-register/] (accessed on 7 November 2025) Swedish eHealth Agency [https://www.ehalsomyndigheten.se/languages/english/welcome-to-the-swedish-ehealth-agency/] (accessed on 7 November 2025) | ATC Level: 3 Age/sex stratification available Sector stratification available | Reports available, detailed data requires application |
| 14 | United Kingdom [59,60] | Clinical Practice Research Datalink (CPRD)—MHRA [https://cprd.com/] (accessed on 7 November 2025) | ATC Level: 3 Age/sex stratification available Sector stratification available | Proprietary (requires license and approval) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotwani, A.; Chauhan, M.; Roughead, E.; Muller, A.; Escher, M.; Huttner, B.; Ivanovska, V. Overview of Methods for Assessing Antimicrobial Use in Outpatient Settings in High-Income Countries: A Narrative Review. Antibiotics 2025, 14, 1161. https://doi.org/10.3390/antibiotics14111161
Kotwani A, Chauhan M, Roughead E, Muller A, Escher M, Huttner B, Ivanovska V. Overview of Methods for Assessing Antimicrobial Use in Outpatient Settings in High-Income Countries: A Narrative Review. Antibiotics. 2025; 14(11):1161. https://doi.org/10.3390/antibiotics14111161
Chicago/Turabian StyleKotwani, Anita, Mihir Chauhan, Elizabeth Roughead, Arno Muller, Martina Escher, Benedikt Huttner, and Verica Ivanovska. 2025. "Overview of Methods for Assessing Antimicrobial Use in Outpatient Settings in High-Income Countries: A Narrative Review" Antibiotics 14, no. 11: 1161. https://doi.org/10.3390/antibiotics14111161
APA StyleKotwani, A., Chauhan, M., Roughead, E., Muller, A., Escher, M., Huttner, B., & Ivanovska, V. (2025). Overview of Methods for Assessing Antimicrobial Use in Outpatient Settings in High-Income Countries: A Narrative Review. Antibiotics, 14(11), 1161. https://doi.org/10.3390/antibiotics14111161






