Abstract
Objectives: To describe annual trends in the susceptibility of clinical isolates of Pseudomonas aeruginosa from Latin America to ceftolozane/tazobactam. Methods: The Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program collected 10,188 P. aeruginosa isolates from 57 unique clinical sites in 12 Latin American countries from 2016 to 2024. MICs were determined by reference broth microdilution testing and interpreted using 2025 CLSI M100 breakpoints. Results: Overall, 86.3% of clinical isolates of P. aeruginosa collected in Latin America were susceptible to ceftolozane/tazobactam, including 45.5% of multidrug-resistant (MDR) isolates. From 2016 to 2024, annual percent susceptible values for ceftolozane/tazobactam ranged from 84.9% (2016, n = 779) to 89.2% (2023, n = 1144), with a statistically significant linear trend for increasing susceptibility (p = 0.024; Cochran–Armitage test for trend). However, limiting analysis solely to the 14 clinical sites, from six countries, that participated in each of the nine years (n = 4565) indicated that the annual percent susceptible values for ceftolozane/tazobactam remained unchanged from 2016 (82.6%) to 2024 (83.9%) (p = 0.367; percent susceptible value range, 82.6 to 89.1%). Every year, from 2016 to 2024, all P. aeruginosa isolates from pediatric patients (<18 years of age) were consistently more susceptible to ceftolozane/tazobactam than those from adult patients (90.3 to 95.0%/year versus 83.3 to 88.6%/year, respectively). Significant variation (p < 0.05) in annual ceftolozane/tazobactam percent susceptible values was not observed for isolates from blood, intra-abdominal, and respiratory tract sources, while isolates from urine showed a trend of increasing ceftolozane/tazobactam susceptibility from 73.1% (2018, n = 145) to 90.6% (2023, n = 117) (p < 0.0001). Among individual countries that participated each year, P. aeruginosa isolates from all except Guatemala displayed stable or increasing rates of susceptibility to ceftolozane/tazobactam. Conclusions: Since it was first tested by the SMART program in 2016, and for 8 years thereafter, the in vitro activity of ceftolozane/tazobactam has remained consistent against clinical isolates of P. aeruginosa from the Latin American region (overall, 86.3% susceptible), with limited resistance development restricted to specific clinical sites.
1. Introduction
Pseudomonas aeruginosa is a leading cause of healthcare-associated infections, particularly in patients with compromised immune systems, such as those in intensive care units, with burns, or suffering from chronic respiratory conditions like cystic fibrosis []. This organism possesses intrinsic antimicrobial resistance mechanisms, including efflux pumps, low outer membrane permeability, and a chromosomal AmpC-type β-lactamase, as well as the ability to acquire additional resistance determinants and develop chromosomal mutations that can lead to antimicrobial treatment failure []. In Latin America, the prevalence of multidrug-resistant (MDR) P. aeruginosa isolates has become a major clinical and public health concern, complicating empirical treatment and resulting in increased patient morbidity and mortality [].
Ceftolozane was developed to address the growing resistance of P. aeruginosa to marketed β-lactams and was combined with tazobactam, an established β-lactamase inhibitor []. The combination ceftolozane/tazobactam has shown potent in vitro activity against MDR and carbapenem-resistant P. aeruginosa [], including isolates with AmpC overexpression or efflux pump activity, although it is inactive versus strains harboring metallo-β-lactamases (MBLs) []. Since its approval for use in the United States in 2014, and European and Latin American countries in 2015, ceftolozane/tazobactam has become an important therapeutic option for treating complicated intra-abdominal infections, complicated urinary tract infections, and hospital-acquired pneumonia caused by P. aeruginosa [].
Latin America has been recognized as a hotspot for high rates of resistance among Gram-negative bacilli, including P. aeruginosa []. The Study for Monitoring Antimicrobial Resistance Trends (SMART) global surveillance program is an ongoing international initiative that monitors trends in antimicrobial resistance among clinically important Gram-negative bacterial pathogens. The purpose of this study was to perform longitudinal analyses (i.e., investigate annual trends) of ceftolozane/tazobactam susceptibility among clinical isolates of P. aeruginosa collected in Latin American countries by SMART from 2016 to 2024, including comparisons of ceftolozane/tazobactam susceptibility for isolates from adult and pediatric patients, from different infection sources, and from different countries.
2. Results
From 2016 to 2024, Latin American clinical laboratories participating in the SMART global surveillance program collected a total of 65,527 isolates of Gram-negative bacilli, of which 10,188 (15.5%) were P. aeruginosa. The percent susceptible value for ceftolozane/tazobactam tested against all isolates of P. aeruginosa collected in Latin America from 2016 to 2024 was 86.3%, approximately 12–14 percentage points higher than for the antipseudomonal cephalosporins ceftazidime and cefepime and approximately 19 percentage points higher than for meropenem (Table 1). Overall, 23.1%, 23.0%, and 17.1% of the P. aeruginosa collected in Latin America were found to be resistant to piperacillin/tazobactam, ceftazidime, and cefepime, respectively. Ceftolozane/tazobactam inhibited 53.3% of piperacillin/tazobactam-resistant, 43.8% of ceftazidime-resistant, and 35.6% of cefepime-resistant isolates. In total, 26.6% of isolates were resistant to meropenem, and over half of these (53.2%) were susceptible to ceftolozane/tazobactam. In total, 2292 (22.5%) and 1143 (14.2%) isolates were identified as multidrug-resistant (MDR) and difficult-to-treat resistant (DTR), respectively. Against these more resistant isolate subsets, ceftolozane/tazobactam inhibited 45.5% of MDR and 31.9% of DTR phenotypes.

Table 1.
Percent susceptible values for ceftolozane/tazobactam and comparator agents against clinical isolates of P. aeruginosa collected in Latin American countries from 2016 to 2024.
Trends in annual MDR, DTR, and ceftolozane/tazobactam-susceptible percentages over the 9-year period from 2016 to 2024 are shown in Figure 1. As considerable variability regarding country/site participation in the SMART program over this time period existed (see Supplementary Data, Table S1), leading to potential bias, longitudinal analysis was performed twice—once including the totality of isolates collected and a second time including solely the isolates from the clinical sites that contributed each year. Evaluating all isolates (Figure 1A), the percent susceptible value for ceftolozane/tazobactam ranged from 84.9% in 2016 to 89.2% in 2023, with a marginal decrease in susceptibility in 2024 to 87.1%, demonstrating a statistically significant increasing trend in susceptibility over time (p = 0.024). MDR percentages ranged from 22.1% (2022) to 16.6% (2023), with no increasing or decreasing trend evident. DTR percentages were highest in 2016 (15.8%) and lowest in 2023 (12.2%), with a decreasing trend (p = 0.039). Evaluating isolates from the consistently contributing sites yielded similar results (Figure 1B); the percent susceptible value for ceftolozane/tazobactam ranged from 82.6% in 2016 to 89.1% in 2021, with 83.9% susceptible in 2024 and with no significant trend of increasing or decreasing susceptibility observed. MDR percentages were highest in 2016 (27.8%) and lowest in 2019, 2021, and 2023 (16.9% in each of those years), with no significant trend. In contrast, DTR percentages showed a decreasing trend (p = 0.009), ranging from 17.9% in 2016 to 10.1% in 2023. However, the MDR and DTR rates were higher, both in the full data set and the set from the continuously contributing sites, in 2024 compared to the previous year.
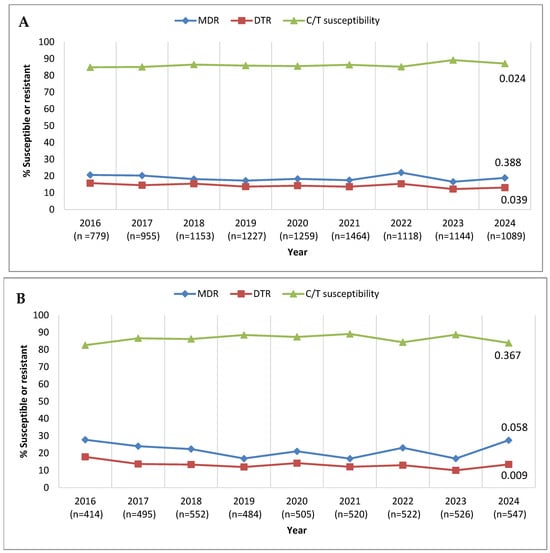
Figure 1.
Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates identified as multidrug-resistant (MDR), difficult-to-treat resistant (DTR), and ceftolozane/tazobactam-susceptible (C/T-susceptible) among (A) all isolates collected in Latin America and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Significance in trends over time was determined by the Cochran–Armitage test: two-tailed p-values are shown in the figure (p < 0.05 was considered statistically significant). Percentages presented in this figure are provided in Supplementary Table S2.
Although ceftolozane/tazobactam is approved in the United States and Europe for treating complicated intra-abdominal and complicated urinary tract infections in pediatric patients, it is not yet licensed for pediatric use in some Latin American countries; thus, there is interest in ceftolozane/tazobactam activity against pathogens isolated from younger patients in the region. Figure 2 presents annual trends in ceftolozane/tazobactam susceptibility among P. aeruginosa from adult and pediatric (birth to <18 years old) patients, considering all isolates collected in Latin America from 2016 to 2024 (Figure 2A) and only those from the consistently contributing sites (Figure 2B). In both cases, isolates from pediatric patients were more susceptible to ceftolozane/tazobactam than those from adult patients each year. Among the totality collected each year, the difference in susceptibility between isolates from pediatric and adult patient populations ranged from 11.7% in 2016 to 4.2% in 2023. The pediatric/adult gap in susceptibility rates was even more pronounced among the isolates from the consistently contributing sites, ranging from a high of 16.7% in 2016 to 7.2% in 2021.
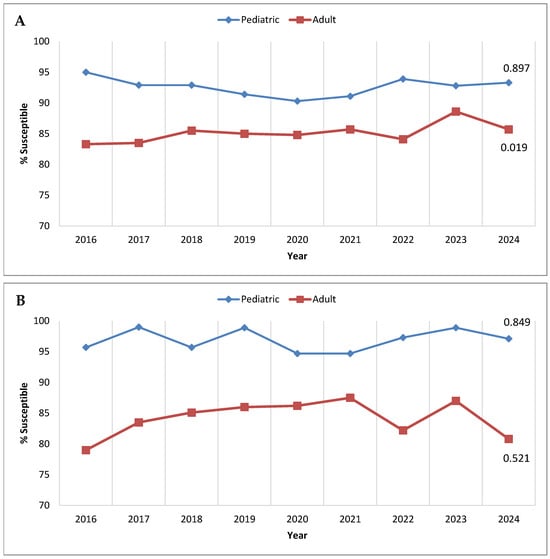
Figure 2.
Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates testing as ceftolozane/tazobactam-susceptible, stratified by patient age, among (A) all isolates collected in Latin America and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Significance in trends over time was determined by the Cochran–Armitage test: two-tailed p-values are shown in the figure (p < 0.05 was considered statistically significant). Percentages presented in this figure are provided in Supplementary Table S3.
Figure 3 examines P. aeruginosa percent susceptible values for ceftolozane/tazobactam by infection source. Considering all isolates, the percent susceptible values for isolates from intra-abdominal and respiratory tract infections were consistently ≥83% each year, with no statistically significant trends (Figure 3A). Isolates from bloodstream infections were first collected for the SMART program in 2018, and 91.0% of the P. aeruginosa isolates that year were susceptible to ceftolozane/tazobactam; the percent susceptible values for bloodstream isolates decreased to 78.4% in 2020 but rose to 89.0% in 2024 (no significant trend noted). By contrast, urinary tract infection isolates demonstrated a strong linear trend of increasing susceptibility to ceftolozane/tazobactam, from 73.8% susceptible in 2016 to 88.1% susceptible in 2024 (p < 0.0001). Similar results were observed when considering the isolates from the consistently contributing sites (Figure 3B); no significant trends in ceftolozane/tazobactam susceptibility were observed for intra-abdominal, respiratory tract, or bloodstream infection isolates, while susceptibility among urinary tract infection isolates displayed a significant increasing trend (p = 0.011), from 70.0% susceptible in 2016 to 86.9% susceptible in 2024.
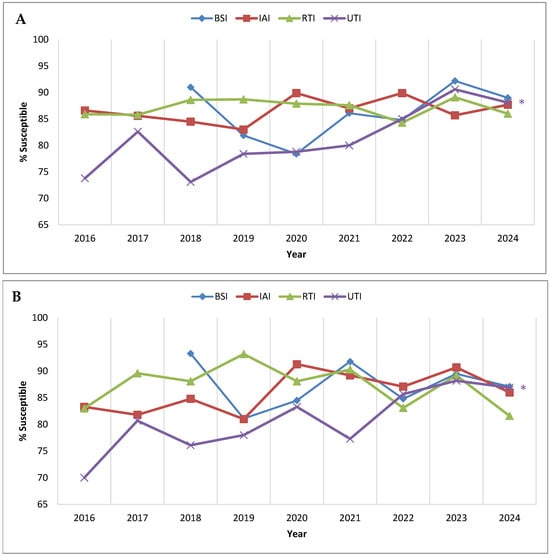
Figure 3.
Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates testing as ceftolozane/tazobactam-susceptible, stratified by infection source, among (A) all isolates collected in Latin America and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Significance in trends over time was determined by the Cochran–Armitage test: two-tailed p-values are shown in the figure (p < 0.05 was considered statistically significant). * indicates increasing trend (p < 0.05). Percentages, numbers of isolates, and p-values presented in this figure are provided in Supplementary Table S4. Abbreviations: BSI, bloodstream infection; IAI, intra-abdominal infection; RTI, respiratory tract infection; UTI, urinary tract infection. BSI isolates were not tested in 2016 and 2017.
Among the countries that participated in SMART each year from 2016 to 2024, three (Argentina, Chile, and Panama) exhibited a significant trend of increasing susceptibility to ceftolozane/tazobactam (Figure 4A). No trend was observed for Colombia and Puerto Rico; however, the P. aeruginosa isolates collected in Guatemala displayed a significant trend of decreasing susceptibility to ceftolozane/tazobactam. Two hospitals in Guatemala participated in the SMART program—one contributing isolates each year from 2016 to 2024 and a second participating only from 2021 to 2024 (Table S1). The decrease in P. aeruginosa susceptibility to ceftolozane/tazobactam in Guatemala was largely driven by the former, as evident in Figure 4B. The percent susceptible value for ceftolozane/tazobactam was ≥82.8% for the consistently participating site in Guatemala from 2016 to 2021 but exhibited a precipitous decrease in 2022 (72.2% susceptible), and, in 2024, just 25.5% of P. aeruginosa isolates collected by this site were susceptible to ceftolozane/tazobactam.
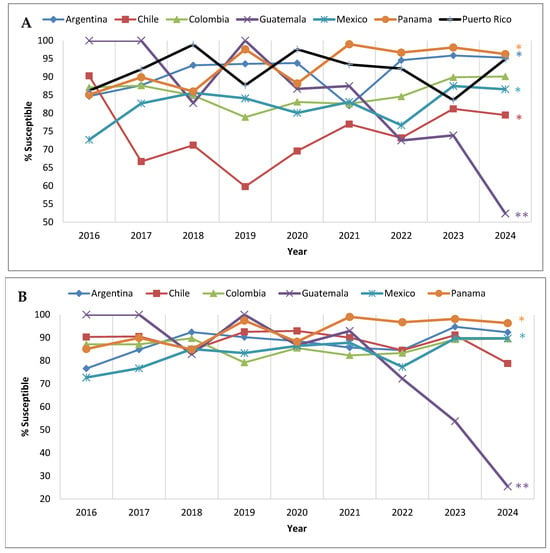
Figure 4.
Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates that tested as ceftolozane/tazobactam-susceptible, by country, among (A) all isolates collected in the Latin American region from countries that participated in the SMART program each year and (B) isolates collected from clinical sites that participated each year from 2016 to 2024. Significance in trends over time determined by Cochran–Armitage test. * indicates significant increasing trend and ** indicates significant decreasing trend (p < 0.05). Percentages, numbers of isolates, and p-values presented in this figure are provided in Supplementary Table S5.
3. Discussion
Over the last decade in Latin America, ceftolozane/tazobactam has played a significant role as a treatment for infections caused by P. aeruginosa, a challenging, often MDR pathogen. During this time, the Latin American region has observed changes in susceptibility patterns associated with antibiotic pressure, emerging resistance mechanisms, and the introduction of new antimicrobial agents, including ceftolozane/tazobactam and ceftazidime/avibactam. An earlier study, conducted in Latin America prior to ceftolozane/tazobactam’s licensing, reported that, among P. aeruginosa collected from 2013 to 2015, 83.2% of isolates from Argentina, 90.6% of isolates from Brazil, 77.5% of isolates from Chile, and 90.5% of isolates from Mexico were ceftolozane/tazobactam-susceptible. The same study reported that 86.8% of all P. aeruginosa isolates tested were ceftolozane/tazobactam-susceptible [], a value remarkably close to our finding of 86.3% susceptible in our combined 2016 to 2024 data set (Table 1). The Program to Assess Ceftolozane/Tazobactam Susceptibility (PACTS) global surveillance study tested 622 P. aeruginosa isolates from Latin American countries collected in 2015–2017 and reported a similarly high susceptibility rate to ceftolozane/tazobactam—90.8% []. On the other hand, in another study of 508 clinical isolates of P. aeruginosa collected from 2016 to 2017 at 20 hospitals in five Latin American countries, just 68.1% were ceftolozane/tazobactam-susceptible. This included percent susceptible values of 80.6% for isolates from Chile, 70% for isolates from Argentina, 68.3% for isolates from Brazil, 66.1% for isolates from Colombia, and 64.4% for isolates from Mexico []. While the reported value for isolates from Chile is consistent with our finding in the present study, the precent susceptible values for Argentina, Colombia, and Mexico were lower than we observed in each year of the SMART program. Tuon et al. examined a set of 132 P. aeruginosa isolates collected in Brazil in 2016–2017 and reported that 84.9% of isolates were ceftolozane/tazobactam-susceptible []. Brazil did not participate in the SMART program in 2024; however, from 2016 to 2023, 92.0% of the 1400 P. aeruginosa collected for the program were ceftolozane/tazobactam-susceptible.
In the current study, country-specific data revealed that, in most Latin American countries, P. aeruginosa susceptibility to ceftolozane/tazobactam remained highly stable from 2016 to 2024 when only clinical sites that participated each year were considered, with the notable exception of one hospital in Guatemala (Figure 4B). All P. aeruginosa isolates collected by this clinical site in 2016, 2017, and 2019 were ceftolozane/tazobactam-susceptible; however, in 2023, only 53.8% of submitted isolates were ceftolozane/tazobactam-susceptible, and, in 2024, only 25.5% of submitted isolates were ceftolozane/tazobactam-susceptible. Although the molecular characterization of ceftolozane/tazobactam-non-susceptible isolates is beyond the scope of our current report, we did note that, in 2023, among the 22 meropenem-non-susceptible isolates that were examined for β-lactamase carriage from this clinical site, 18 harbored an MBL (nine carried IMP-1 and nine carried VIM-2) (unpublished internal data on file). Previous work by the Antimicrobial Testing Leadership and Surveillance (ATLAS) global surveillance program reported that, among meropenem-non-susceptible P. aeruginosa collected in Guatemala from 2017 to 2019, approximately 38% (n = 42) carried a VIM MBL []. Despite regulatory reform in Guatemala, antibiotics remain widely available in convenience stores and are frequently dispensed without prescription []. This challenge to antibiotic stewardship in the country may contribute to increased resistance rates.
Limitations of the present study include its narrow scope (one antimicrobial agent against one bacterial species) and the fact that there were changes in participation by individual medical centers and countries over the years surveyed. In fact, all in vitro antimicrobial susceptibility testing surveillance programs can be challenged by varying participation among individual medical centers and countries when evaluating annual trends. For the 9-year trends presented here, we would have had to exclude the majority of sites (>75%) and isolates (>55% of the total collected) if we had focused solely on continuously participating sites. Given this data limitation, we opted to present trends for both the continuously participating clinical sites and all clinical sites. On a regional level, both sets of data lead to a similar conclusion—that is, that there is no evidence that the P. aeruginosa percent susceptible values for ceftolozane/tazobactam in Latin American countries changed significantly over time from 2016 to 2024, a time period over which the agent was used by care providers to treat their patients in these countries. Finer analysis, such as country-specific data, is more likely to show disparities, as can be seen in Figure 4. For example, in Chile, two clinical sites were consistent contributors to the program each year, while a third clinical site participated in all years except 2016. The inclusion of the third clinical site reduced the overall P. aeruginosa percent susceptible value for ceftolozane/tazobactam by >20 percentage points in 2017 and 2020 and by >30 percentage points in 2019. Clearly, outbreaks of β-lactam-resistant strains of P. aeruginosa, including MBL-carrying isolates, at a single institution can have a major impact on the overall percent susceptible values reported for a country when there are, overall, only a limited number of participating clinical sites.
4. Methods
4.1. Bacterial Isolates
Each clinical laboratory site that participated in the SMART global surveillance program was asked to collect consecutive, clinically significant isolates of aerobic or facultatively anaerobic Gram-negative bacilli from intra-abdominal infections (IAI), respiratory tract infections (RTI), urinary tract infections (UTI), and, starting in 2018, bloodstream infections (BSI). Isolates were restricted to one isolate per patient per Gram-negative species per year. All isolates were transported to a central laboratory (IHMA, Schaumburg, IL, USA), where they were re-identified using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA) prior to antimicrobial susceptibility testing.
From 2016 to 2024, the SMART surveillance program collected a total of 10,188 P. aeruginosa isolates from 57 unique clinical sites in 12 countries in Latin America (Argentina, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Panama, Peru, Puerto Rico, and Venezuela); however, not all countries/sites participated each year. Supplementary Table S1 provides the number of P. aeruginosa isolates collected over this time period by country and clinical site. In total, 14 clinical sites in 6 countries were consistent annual contributors to the program from 2016 to 2024.
4.2. Antimicrobial Susceptibility Testing
The Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution method was used to determine isolate MICs []. MICs were interpreted as susceptible, intermediate, or resistant using 2025 CLSI M100 breakpoints []. P. aeruginosa isolates were classified as MDR based on resistance to ≥3 sentinel agents in differing drug classes (amikacin [aminoglycosides], aztreonam [monobactams], cefepime [cephalosporins], colistin [polymyxins], meropenem [carbapenems], levofloxacin [fluoroquinolones], and piperacillin/tazobactam [penicillin/β-lactamase inhibitor combinations]) []. Difficult-to-treat resistant (DTR) phenotypes were categorized using the criteria published by Kadri et al. []. Specifically, DTR phenotypes were defined by isolates not susceptible (intermediate or resistant) to all tested β-lactams (aztreonam, ceftazidime, cefepime, imipenem, meropenem, piperacillin-tazobactam), as well as fluoroquinolones (levofloxacin). The DTR definition excludes newer β-lactam/β-lactamase inhibitor combinations like ceftolozane/tazobactam.
4.3. Statistical Analysis
The Cochran–Armitage test was used to assess linear trends in percentage susceptible values from 2016 to 2024 using XLSTAT v2024.2.2 (Lumivero, Denver, CO, USA). A two-tailed p-value < 0.05 was considered statistically significant.
5. Conclusions
The current study demonstrates that, since 2016, when ceftolozane/tazobactam was added to the SMART global surveillance program, it has retained potent antimicrobial activity against P. aeruginosa in the Latin American region, and, to date, only localized decreases in percent susceptible values have been detected, likely the result of the spread of MBLs, which severely limit all therapeutic options for affected patients. However, the potential for increases in MDR and DTR isolates, and outbreaks with isolates carrying MBLs and other mechanisms of β-lactam resistance, warrants continued monitoring, both locally and by international surveillance programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14101018/s1. Table S1. Number of P. aeruginosa isolates collected for the SMART program by Latin American country and clinical site, 2016–2024. Table S2. Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates identified as multidrug resistant (MDR), difficult-to-treat resistant (DTR), and ceftolozane/tazobactam-susceptible (C/T-susceptible) among (A) all isolates collected in Latin America, and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Table S3. Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates testing as ceftolozane/tazobactam susceptible stratified by patient age, among (A) all isolates collected in Latin America, and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Table S4. Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates testing as ceftolozane/tazobactam susceptible stratified by infection source, among (A) all isolates collected in Latin America, and (B) isolates collected from clinical sites that participated in the SMART program each year from 2016 to 2024. Table S5. Longitudinal trends from 2016 to 2024 in the percentage of P. aeruginosa isolates that tested as ceftolozane/tazobactam susceptible, by country among (A) all isolates collected in the Latin American region from countries that participated in the SMART program each year and (B) isolates collected from clinical sites that participated each year from 2016 to 2024.
Author Contributions
M.G.W.: conceptualization, methodology, analysis, writing, editing; J.A.K.: formal analysis, writing, editing, and review; T.J.P., F.S., M.R.M., K.Y. and D.F.S.: conceptualization, editing, and review. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research, which included compensation for services related to preparing this manuscript, was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Institutional Review Board Statement
Not applicable.
Institutional Consent Statement
Not applicable.
Data Availability Statement
Data presented in this manuscript are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all laboratory participants for their contributions to the SMART global surveillance program.
Conflicts of Interest
M.G.W. and D.F.S. are employees of IHMA, which receives funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, for the SMART global surveillance program. J.A.K. is a consultant to IHMA. T.J.P., F.S., K.Y. and M.R.M. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and own stock and options in Merck & Co., Inc., Rahway, NJ, USA. The IHMA authors and J.A.K. do not have personal financial interests in the sponsor of this work (Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA).
References
- Gellatly, S.L.; Hancock, R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [PubMed]
- López-Causapé, C.; Cabot, G.; Del Barrio-Tofiño, E.; Oliver, A. The Versatile Mutational Resistome of Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Gales, A.C.; Streit, J.M.; Huband, M.D.; Tsakris, A.; Jones, R.N. Geographic and Temporal Patterns of Antimicrobial Resistance in Pseudomonas aeruginosa over 20 Years from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Chung, P.; Adam, H.; Zelenitsky, S.; Denisuik, A.; Schweizer, F.; Lagace-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Walkty, A.; et al. Ceftolozane/tazobactam: A novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014, 74, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2018, 62, e01970-17. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Bruno, C.J.; DeRyke, C.A. Perspectives on the use of ceftolozane/tazobactam: A review of clinical trial data and real-world evidence. Future Microbiol. 2024, 19, 465–480. [Google Scholar] [CrossRef] [PubMed]
- G.B.D 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Shortridge, D.; Sader, H.S.; Gales, A.; Castanheira, M.; Flamm, R.K. Ceftolozane-tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing healthcare-associated infections in Latin America: Report from an antimicrobial surveillance program (2013–2015). Braz. J. Infect. Dis. 2017, 21, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Pfaller, M.A.; Streit, J.M.; Flamm, R.K. Antimicrobial Activity of Ceftolozane-Tazobactam Tested against Contemporary (2015–2017) P. aeruginosa Isolates from a Global Surveillance Program. J. Glob. Antimicrob. Resist. 2020, 21, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Betancur, J.C.; De La Cadena, E.; Mojica, M.F.; Hernandez-Gomez, C.; Correa, A.; Radice, M.A.; Castaneda-Mendez, P.; Jaime-Villalon, D.A.; Gales, A.C.; Munita, J.M.; et al. Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries. Antibiotics 2022, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Cieslinski, J.; Rodrigues, S.D.S.; Serra, F.B.; Paula, M.D. Evaluation of in vitro activity of ceftolozane-tazobactam against recent clinical bacterial isolates from Brazil—The EM200 study. Braz. J. Infect. Dis. 2020, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Kazmierczak, K.M.; Valente, M.; Luengas, E.L.; Baudrit, M.; Quintana, A.; Irani, P.; Stone, G.G.; Sahm, D.F. In vitro activity of ceftazidime-avibactam against Enterobacterales and Pseudomonas aeruginosa isolates collected in Latin America as part of the ATLAS global surveillance program, 2017–2019. Braz. J. Infect. Dis. 2021, 25, 101647. [Google Scholar] [CrossRef] [PubMed]
- Rojop, N.; Moreno, P.; Grajeda, L.; Romero, J.; Reynoso, L.; Munoz, E.; Palmer, G.H.; Cordon-Rosales, C.; Call, D.R.; Ramay, B.M. Informal sale of antibiotics in Guatemalan convenience stores before and after implementation of federal antibiotic dispensing legislation. BMC Pharmacol. Toxicol. 2024, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Approved Standard M7-Ed12; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 12th ed. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024.
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. 35th ed. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2025.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).