Dosing Evaluation of Ceftazidime–Avibactam in Intensive Care Unit Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation
Abstract
1. Introduction
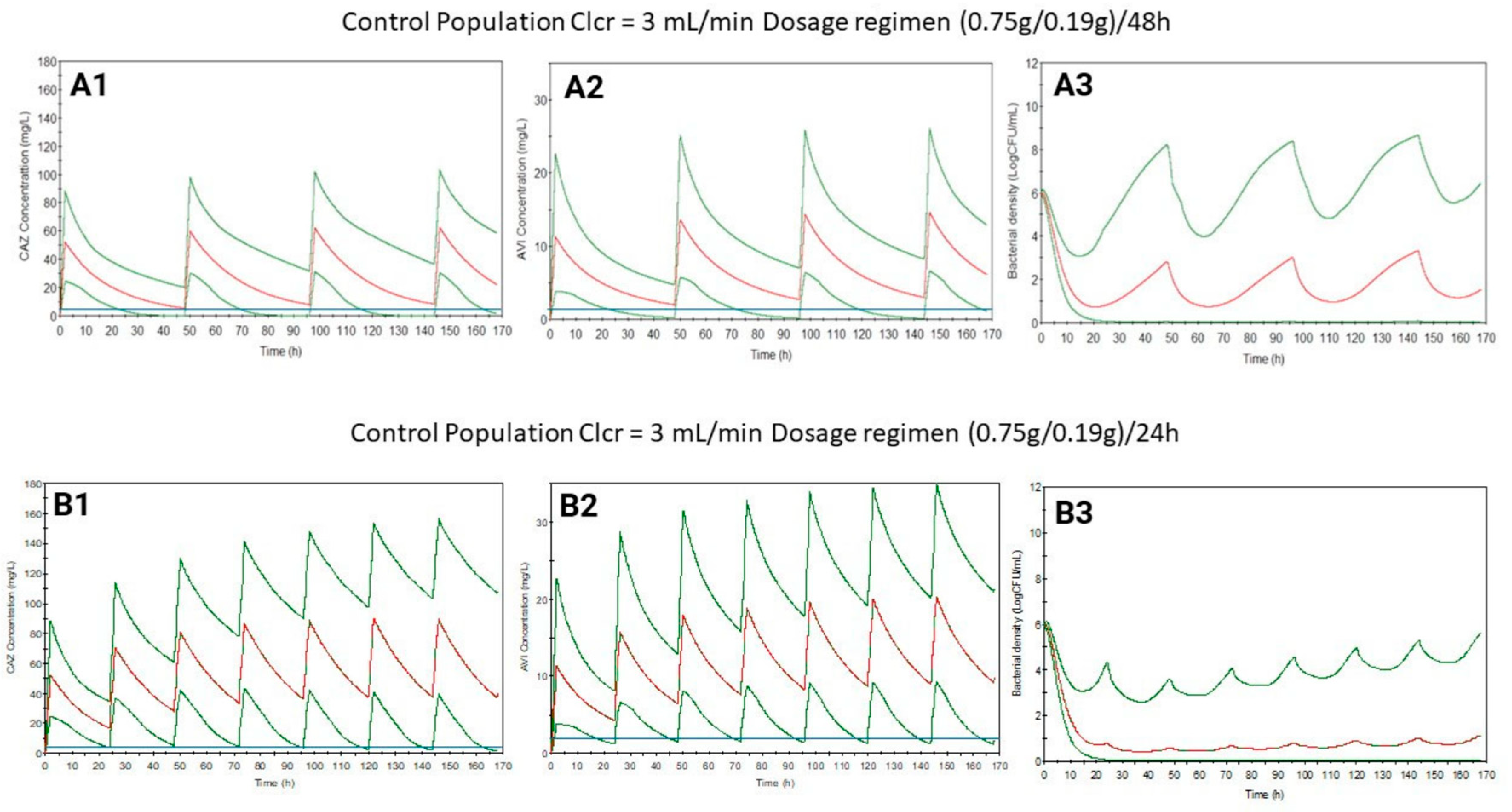
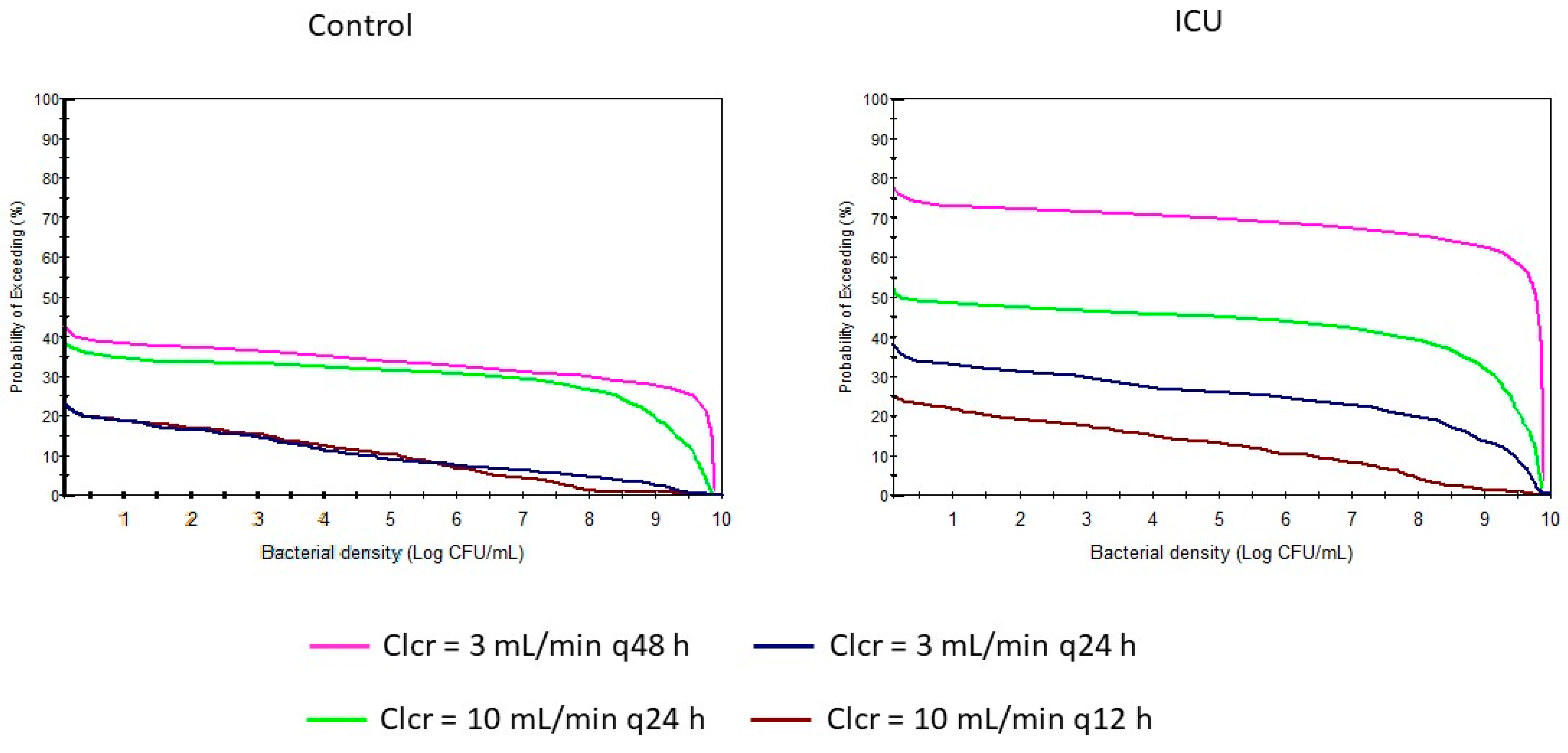
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Pharmacokinetic–Pharmacodynamic (PK/PD) Modeling
- Pharmacokinetic model
- Pharmacodynamic model
- -
- Population 1 (P₁) with active microbial growth and an initial inoculum of 10⁶ CFU/mL.
- -
- Population 2 (P₂) in the resting phase with an initial inoculum of 1/10⁷ CFU/mL.
- Model validation
- Monte Carlo simulations
4.3. PK/PD Analysis
- -
- Time during the serum drug concentration remains above the minimum inhibitory concentration (MIC) (T > MIC)
- -
- Ratio of the trough serum concentration to the MIC (Cmin/MIC).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wicha, S.G.; Märtson, A.-G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.-W.; Minichmayr, I.K.; the International Society of Anti-Infective Pharmacology (ISAP), I.D. (EPASG), the PK/PD study group of the European Society of Clinical Microbiology. From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Gatta, M.M.; Martin-Suarez, A.; Lanao, J.M. Approaches for Dosage Individualisation in Critically Ill Patients. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas Aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Zhou, D.; Nichols, W.W.; Townsend, A.; Newell, P.; Li, J. Selecting the Dosage of Ceftazidime–Avibactam in the Perfect Storm of Nosocomial Pneumonia. Eur. J. Clin. Pharmacol. 2020, 76, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Rodríguez-Gascón, A.; Isla, A.; Barrasa, H.; Del Barrio-Tofiño, E.; Oliver, A.; Canut, A.; Solinís, M.Á. Pseudomonas Aeruginosa Susceptibility in Spain: Antimicrobial Activity and Resistance Suppression Evaluation by PK/PD Analysis. Pharmaceutics 2021, 13, 1899. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Falcone, M.; Viale, P.; Tiseo, G.; Pai, M. Pharmacokinetic Drug Evaluation of Avibactam + Ceftazidime for the Treatment of Hospital-Acquired Pneumonia. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 331–340. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, J.; Mi, W.; Zhang, X.; Ren, X.; Shen, C.; Lu, C. Ceftazidime-Avibactam Induced Renal Disorders: Past and Present. Front. Pharmacol. 2024, 15, 1329307. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Zhao, C.; Zhao, S.; Zhao, L.; Yang, Y.; Wang, Y.; Meng, H.; Sun, J. Clinical Outcomes and Risk Factors for Death in Critically Ill Patients with Carbapenem-Resistant Klebsiella Pneumoniae Treated with Ceftazidime-Avibactam: A Retrospective Study. Infect. Drug Resist. 2024, 17, 239–248. [Google Scholar] [CrossRef]
- Pfizer Limited Zavicefta 2 g/0.5g Powder for Concentrate for Solution for Infusion Summary of Product Characteristics. Available online: https://cima.aemps.es/cima/dochtml/ft/1161109001/ft_1161109001.html (accessed on 22 July 2024).
- Fresan, D.; Luque, S.; Benítez-Cano, A.; Sorlí, L.; Milagro Montero, M.; De-Antonio, M.; Prim, N.; Vega, V.; Horcajada, J.P.; Grau, S. Pharmacokinetics/Pharmacodynamics and Therapeutic Drug Monitoring of Ceftazidime/Avibactam Administered by Continuous Infusion in Patients with MDR Gram-Negative Bacterial Infections. J. Antimicrob. Chemother. 2023, 78, 678–683. [Google Scholar] [CrossRef]
- Sy, S.K.B.; Zhuang, L.; Xia, H.; Beaudoin, M.-E.; Schuck, V.J.; Nichols, W.W.; Derendorf, H. A Mathematical Model-Based Analysis of the Time-Kill Kinetics of Ceftazidime/Avibactam against Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 2018, 73, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Rico-Fontalvo, J.; Correa-Guerrero, J.; Martínez-Ávila, M.C.; Daza-Arnedo, R.; Rodriguez-Yanez, T.; Almanza-Hurtado, A.; Cabrales, J.; Mendoza-Paternina, C.J.; Frías-Salazar, A.; Morales-Fernández, J. Critically Ill Patients with Renal Hyperfiltration: Optimizing Antibiotic Dose. Int. J. Nephrol. 2023, 2023, 6059079. [Google Scholar] [CrossRef] [PubMed]
- Georges, B.; Conil, J.-M.; Ruiz, S.; Seguin, T.; Cougot, P.; Fourcade, O.; Houin, G.; Saivin, S. Ceftazidime Dosage Regimen in Intensive Care Unit Patients: From a Population Pharmacokinetic Approach to Clinical Practice via Monte Carlo Simulations. Br. J. Clin. Pharmacol. 2012, 73, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Rinaldi, M.; Gaibani, P.; Siniscalchi, A.; Tonetti, T.; Giannella, M.; Viale, P.; Pea, F. A Descriptive Pharmacokinetic/Pharmacodynamic Analysis of Continuous Infusion Ceftazidime-Avibactam for Treating DTR Gram-Negative Infections in a Case Series of Critically Ill Patients Undergoing Continuous Veno-Venous Haemodiafiltration (CVVHDF). J. Crit. Care 2023, 76, 154301. [Google Scholar] [CrossRef]
- Gatti, M.; Viale, P.; Pea, F. Therapeutic Drug Monitoring of Ceftazidime/Avibactam: Why One Leg Is Not Enough to Run. J. Antimicrob. Chemother. 2024, 79, 195–199. [Google Scholar] [CrossRef]
- Stein, G.E.; Smith, C.L.; Scharmen, A.; Kidd, J.M.; Cooper, C.; Kuti, J.; Mitra, S.; Nicolau, D.P.; Havlichek, D.H. Pharmacokinetic and Pharmacodynamic Analysis of Ceftazidime/Avibactam in Critically Ill Patients. Surg. Infect. (Larchmt) 2019, 20, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Merdjan, H.; Tarral, A.; Das, S.; Li, J. Phase 1 Study Assessing the Pharmacokinetic Profile and Safety of Avibactam in Patients with Renal Impairment. J. Clin. Pharmacol. 2017, 57, 211–218. [Google Scholar] [CrossRef]
- Welage, L.S.; Schultz, R.W.; Schentag, J.J. Pharmacokinetics of Ceftazidime in Patients with Renal Insufficiency. Antimicrob. Agents Chemother. 1984, 25, 201–204. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef]
- Rubinstein, R.Y.; Kroese, D.P. Simulation and the Monte Carlo Method; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-63216-1. [Google Scholar]
- Mahmood, I.; Tegenge, M.A. A Comparative Study Between Allometric Scaling and Physiologically Based Pharmacokinetic Modeling for the Prediction of Drug Clearance From Neonates to Adolescents. J. Clin. Pharmacol. 2019, 59, 189–197. [Google Scholar] [CrossRef]
- Flamm, R.K.; Nichols, W.W.; Sader, H.S.; Farrell, D.J.; Jones, R.N. In Vitro Activity of Ceftazidime/Avibactam against Gram-Negative Pathogens Isolated from Pneumonia in Hospitalised Patients, Including Ventilated Patients. Int. J. Antimicrob. Agents 2016, 47, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Taccone, F.; Villois, P.; Scheetz, M.H.; Rhodes, N.J.; Briscoe, S.; McWhinney, B.; Nunez-Nunez, M.; Ungerer, J.; Lipman, J.; et al. β-Lactam Pharmacodynamics in Gram-Negative Bloodstream Infections in the Critically Ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]



| Mean Value Predicted | Mean Value Observed | AFE | |
|---|---|---|---|
| a (mg/L) | 95.9 | 90.1 [7] | 1.06 |
| b (mg/L) | 67.8 | 53.8 [11] | 1.27 |
| c (mg/L) | 15.9 | 14.5 [7] | 1.10 |
| Bacterial density d (LogCFU/mL) | 9.23 | 9.1 [12] | 1.01 |
| Bacterial density e (LogCFU/mL) | 8.78 | 8.0 [12] | 1.10 |
| Bacterial density f (LogCFU/mL) | 6.57 | 4.7 [12] | 1.40 |
| Population | Clcr (mL/min) | Ceftazidime PK Parameters | Avibactam PK Parameters | ||||
|---|---|---|---|---|---|---|---|
| Cl (L/h) [CV (%)] | V (L) [CV (%)] | t ½ (h) [CV (%)] | Cl (L/h) [CV (%)] | V (L) [CV (%)] | t ½ (h) [CV (%)] | ||
| Control | 100 | 6.08 [35.2] | 15.7 [40.8] | 1.99 [53.3] | 12.2 [26.5] | 20.2 [53.6] | 1.22 [59.0] |
| 60 | 3.82 [34.5] | 3.17 [53.0] | 7.57 [26.6] | 1.96 [59.2] | |||
| 40 | 2.26 [40.3] | 4.55 [52.7] | 5.19 [26.8] | 2.87 [59.2] | |||
| 20 | 1.59 [34.0] | 7.61 [52.7] | 2.99 [28.1] | 5.00 [59.8] | |||
| 10 | 0.93 [35.5] | 13.1 [54.2] | 1.65 [32.1] | 9.24 [62.6] | |||
| 3 | 0.48 [47.9] | 27.7 [65.3] | 0.73 [53.4] | 24.4 [80.1] | |||
| ICU | 100 | 5.44 [31.8] | 34.5 [33.9] | 4.84 [47.7] | 10.9 [28.0] | 51.0 [31.7] | 3.49 [41.5] |
| 60 | 3.73 [31.4] | 7.04 [47.3] | 6.90 [27.7] | 5.49 [41.02] | |||
| 40 | 2.86 [31.8] | 9.23 [47.7] | 4.86 [28.0] | 7.80 [41.3] | |||
| 20 | 2.05 [35.1] | 13.1 [49.3] | 2.99 [32.8] | 12.8 [42] | |||
| 10 | 1.56 [41.7] | 17.9 [53.4] | 1.85 [36.2] | 21.4 [45.3] | |||
| 3 | 1.22 [50.8] | 24.6 [61.7] | 1.06 [55.7] | 43.2 [60.5] | |||
| Dosage Regimen | Population | Clcr (mL/min) | Dosage Regimen ((CAZg/AVIg)/h) | Bacterial Density Change a | T > MIC | Cmin/MIC |
|---|---|---|---|---|---|---|
| (%) (5/95%) | (%) (5/95%) | Mean (5/95%) | ||||
| Summary of Product Characteristics | Control | 100 | (2.0/0.5)/8 | −63.7 (−99.3/24.5) | 95.4 (62.9/100) | 0.81 (0.00/4.75) |
| 60 | (2.0/0.5)/8 | −78.3 (−99.3/−1.50) | 100 (75.9/100) | 4.42 (0.03/15.4) | ||
| 40 | (1.0/0.25)/8 | −76.0 (−99.3/3.83) | 100 (74.5/100) | 4.25 (0.12/12.9) | ||
| 20 | (0.75/0.19)/12 | −69.5 (−99.3/16.0) | 100 (66.8/100) | 3.40 (0.06/10.7) | ||
| 10 | (0.75/0.19)/24 | −51.2 (−99.0/38.5) | 100 (53.1/100) | 2.04 (0.01/7.62) | ||
| 3 | (0.75/0.19)/48 | −44.7 (−98.5/44.0) | 100 (42.5/100) | 2.08 (0.00/9.13) | ||
| ICU | 100 | (2.0/0.5)/8 | −77.5 (−99.3/0.67) | 100 (77.6/100) | 4.47 (0.20/12.7) | |
| 60 | (2.0/0.5)/8 | −84.8 (−99.3/−19) | 100 (100/100) | 9.76 (1.70/21.6) | ||
| 40 | (1.0/0.25)/8 | −80.0 (−99.3/−5.33) | 100 (98.4/100) | 6.43 (1.06/14.3) | ||
| 20 | (0.75/0.19)/12 | −65.7 (−99.3/25.5) | 100 (64.6/100) | 3.50 (0.00/19.0) | ||
| 10 | (0.75/0.19)/24 | −30.2 (−96.83/57.83) | 100 (38.2/100) | 1.18 (0.00/19.0) | ||
| 3 | (0.75/0.19)/48 | 13.0 (−78.8/70.5) | 59.6 (23.5/100) | 0.35 (0.00/19.0) | ||
| Suggested | Control | 10 | (0.75/0.19)/12 | −83.7 (−99.3/−15.3) | 100 (100/100) | 10.1 (1.88/22.0) |
| 3 | (0.75/0.19)/24 | −80.8 (−99.3/−6.5) | 100 (75.0/100) | 9.37 (0.53/25.7) | ||
| ICU | 10 | (0.75/0.19)/12 | −79.5 (−99.3/−0.83) | 100 (94.1/100) | 6.00 (0.97/13.2) | |
| 3 | (0.75/0.19)/24 | −56.5 (−99.2/37) | 100 (44.8/100) | 2.63 (0.01/9.35) |
| Clcr (mL/min) | CAZ Dose (g) | AVI Dose (g) | Interval (h) | |
|---|---|---|---|---|
| SmPC | 100 | 2.0 | 0.5 | 8 |
| 60 | 2.0 | 0.5 | 8 | |
| 40 | 1.0 | 0.25 | 8 | |
| 20 | 0.75 | 0.19 | 12 | |
| 10 | 0.75 | 0.19 | 24 | |
| 3 | 0.75 | 0.19 | 48 |
| Parameter | Control Population | ICU Patient Population | ||
|---|---|---|---|---|
| CAZ | AVI | CAZ | AVI | |
| Vd (L/kg) | 0.21 (38.1) | 0.27 (51.8) | 0.46 (30.4) | 0.68 (27.9) |
| Cli (L/h) | 0.39 (55.6) | 0.53 (65.6) | 1.15 (54.8) | 0.89 (65.2) |
| CLs | 0.06 (36.9) | 0.12 (27.3) | 0.04 (37.2) | 0.10 (30.0) |
| Parameter | Description | Value [CV (%)] | Units |
|---|---|---|---|
| Nmax | Maximum load capacity achievable in the system. | 9.89 [2.06] | CFU/mL |
| kgrowth,1 | Bacterial growth associated with the log10 of the active population P1. | 0.346 [20.9] | h−1 |
| kgrowth,2 | Bacterial growth rate constant associated with the log10 of the active population P₂. | (1/107) × Kgrowth,1 | h−1 |
| Emax | Maximum kill rate constant due to CAZ. | 0.240 [16.0] | h−1 |
| A | First coefficient of the biexponential function to characterize the EC50 of CAZ. | 52.3 [17.2] | mg/L |
| B | Second coefficient of the biexponential function to characterize the EC50 of CAZ. | 12.6 [26.0] | mg/L |
| α | Exponential constant associated with parameter A that describes the relationship between the concentration of AVI and the potency of CAZ. | 2.38 [119] | L/mg |
| β | Exponential constant associated with parameter B that describes the relationship between the concentration of AVI and the potency of CAZ. | 9.67 E2 [7.01] | L/mg |
| ϒ | Hill coefficient characterizing the steepness of the slope of the sigmoidal Emax curve associated with the increase in potency of CAZ by AVI. | 2.60 [34.23] | - |
| δ1 | Exponential constant of the delay function to retard the active population, P₁. | 4.23 E2 (fixed) | h−1 |
| δ2 | Exponential constant of the delay function to slow the initial death of the active population, P₁. | 0.213 [17.46] | h−1 |
| k1–2 | Rate constant for the conversion of bacterial cells from active to resting states. | 5.0 E3 (fixed) | CFU/mL/h |
| Degmax | Maximum degradation rate constant of CAZ. | 7.71 E2 [51.9] | h−1 |
| Km | CFU density that yielded 50% of the maximum degradation rate. | 8.5 (fixed) | CFU/mL |
| Hill coefficient that characterizes the slope of the sigmoid Emax model for CAZ degradation. | 1.46 [82.2] | - | |
| IC₅₀ | AVI concentration that yielded a 50% decrease in the degradation rate. | 1.96 [58.2] | mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazo, H.; Aguazul, Y.; Lanao, J.M. Dosing Evaluation of Ceftazidime–Avibactam in Intensive Care Unit Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation. Antibiotics 2024, 13, 861. https://doi.org/10.3390/antibiotics13090861
Zazo H, Aguazul Y, Lanao JM. Dosing Evaluation of Ceftazidime–Avibactam in Intensive Care Unit Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation. Antibiotics. 2024; 13(9):861. https://doi.org/10.3390/antibiotics13090861
Chicago/Turabian StyleZazo, Hinojal, Yuridia Aguazul, and José M. Lanao. 2024. "Dosing Evaluation of Ceftazidime–Avibactam in Intensive Care Unit Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation" Antibiotics 13, no. 9: 861. https://doi.org/10.3390/antibiotics13090861
APA StyleZazo, H., Aguazul, Y., & Lanao, J. M. (2024). Dosing Evaluation of Ceftazidime–Avibactam in Intensive Care Unit Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation. Antibiotics, 13(9), 861. https://doi.org/10.3390/antibiotics13090861









