Abstract
Background/Objectives: Urinary tract infection (UTI) is a prevalent microbial infection in medical practise, leading to significant patient morbidity and increased treatment costs, particularly in developing countries. This retrospective study, conducted at a tertiary care hospital in Dhaka, Bangladesh, aims to examine the antimicrobial resistance (AMR) patterns of uropathogens and evaluate whether these patterns are influenced by demographic factors such as gender, age, or patient status. Methods: Standard microbiological techniques were used to identify uropathogens, and AMR patterns were determined using the Kirby–Bauer disc diffusion method. Results: Out of 6549 urine samples, 1001 cultures were positive. The infection was more prevalent in females compared to males. The incidence of UTIs in children aged 0–10 years accounted for 12.59% of the total cases, with this age group also exhibiting the highest rate of polymicrobial infections. Among the bacterial uropathogens, 71.19% of isolates were multidrug resistant (MDR) and 84.27% were resistant to at least one antibiotic. Escherichia coli (n = 544, 73.90% MDR) and Klebsiella species (n = 143, 48.95% MDR) were the most common Gram-negative uropathogens, while Enterococcus species (n = 78, 94.87% MDR) was the predominant Gram-positive isolate in this study. Our results indicate that most uropathogens showed resistance against ceftazidime, followed by cefuroxime, trimethoprim-sulfamethoxazole, amoxicillin-clavulanate, and netilmicin. Moderate levels of resistance were observed against ciprofloxacin, levofloxacin, aztreonam, and cefpodoxime. Conclusions: Amikacin was observed to be effective against Gram-negative uropathogens, whereas cefixime was more active against Gram-positive microorganisms, such as Enterococcus species. Moreover, a principal coordinate analysis (PCoA) depicted no significant influence of gender, patient status, or age on AMR patterns. For the continued usefulness of most antibiotics, periodic analysis of the AMR patterns of uropathogens can help assess the rise of MDR bacteria, and therefore guide the selection of appropriate antibiotic treatment strategies.
1. Introduction
Urinary tract infections (UTIs) are caused by the invasion of microorganisms in the urinary tract, including the urinary bladder, prostate, kidney, or collecting system, and the condition may range from asymptomatic bacteriuria to perinephric abscess with sepsis [1,2,3]. They are considered to be the second-most prominent type of bacterial infections, affecting 150 million people annually around the globe, and account for up to 35% of all nosocomial infections [1,4,5]. UTI patients are effectively managed by identifying the causative pathogen of the infection first, and then selecting the effective antimicrobial agent against it [6].
Recently, the rising rates of resistance among bacterial uropathogens have become a major public health crisis worldwide. Escherichia coli is the principal uropathogen, accounting for 75 to 90% of bacterial isolates in uncomplicated UTIs [6,7,8,9]. Additionally, Klebsiella spp. are predominant pathogens in both UTIs and nosocomial infections, and easily tend to become multidrug resistant [6]. Several major factors associated with the increase in antimicrobial resistance (AMR) includes the misuse of antimicrobials [10], using broad-spectrum antimicrobials frequently that alter the intestinal flora, and the inappropriate dosage and duration of treatment [11,12]. This complicates the treatment of UTIs by increasing the rate of treatment failure and patient morbidity, costs of reassessment and retreatment, and the use of broad-spectrum antibiotics [9]. In a developing country like Bangladesh, a substantial number of patients with UTI obtain antibiotics straight from community pharmacies without a prescription or consulting with a medical professional [13]. Moreover, regional clinicians commonly diagnose microbial infections based on clinical judgement and prescribe broad-spectrum antibiotics on an empirical basis prior to the laboratory results of urine culture being available. These factors unfavourably affect the sensitivity pattern of microbes, potentially leading to the development and spread of AMR strains [14,15,16].
Yet, most AMR surveillance studies do not consider the situation of every geographical location in the world. Only extensive country-specific (and indeed, in some cases, regional) surveillance studies will provide information about the causative pathogens of UTIs and their AMR patterns, which will further aid clinicians in selecting the correct antimicrobial empirical treatment [13]. Therefore, this study was conducted to determine the AMR patterns of bacterial isolates from patient urine samples in 11 commonly prescribed antibiotics.
2. Results
2.1. Prevalence of UTI According to Age and Gender
In this study, a total of 6549 patients suspected of having a UTI were analyzed, comprising 2392 males and 4157 females, resulting in a male-to-female ratio of 1:1.74. Among them, 326 males and 675 females tested positive for a UTI. The overall infection rate was observed to be higher in females (16.25%) compared to males (13.62%), as shown in Table 1. Figure 1 presents the UTI rates for both genders across different age groups and polymicrobial UTI rates within these age brackets. A higher incidence of infection was noted among females aged 20 to 70 years, with the infection rate peaking in the age group of 50 to 60 years. In contrast, for males, the infection rate was high in the 50 to 80 years age range, with men aged 60 to 70 years showing the greatest prevalence. Regardless of gender, the elevated incidence of UTIs among children aged 0 to 10 years was noticeable. Furthermore, our study shows the polymicrobial infection rate was the highest in children, followed by people aged 60 to 70 years. In general, individuals aged 50 to 70 years predominantly tested positive for UTIs, with a marked decline in infection rates observed in those over 70 years of age.

Table 1.
Age and gender-specific infection rates of urinary tract infection (UTI).
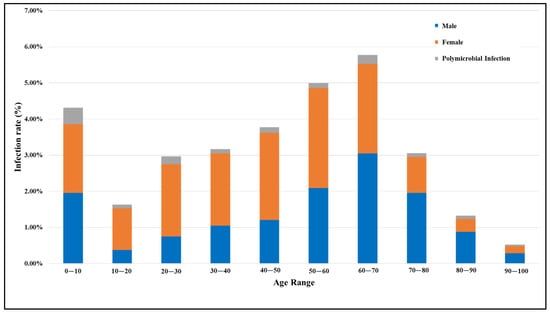
Figure 1.
Comparison of UTI rates in males and females across different age groups, including rates of polymicrobial UTIs (infections involving multiple different types of microorganisms).
2.2. Microbiological Profile of Uropathogens
Microbiological cultures of 6549 urine samples detected 1001 cases of UTI (Figure 2). Among these, 833 (83.22%) were bacterial infections, 58 (5.79%) were fungal infections (Candida spp.), and 110 (10.99%) were polymicrobial UTIs (Table S1). Among the Gram-negative bacterial isolates, the following were identified: E. coli (544 isolates; 54.35%), Klebsiella spp. (143 isolates; 14.29%), Enterobacter spp. (34 isolates; 3.40%), Pseudomonas spp. (nine isolates; 0.90%), Proteus spp. (eight isolates; 0.80%), Acinetobacter spp. (five isolates; 0.50%), Acetobacter spp. (two isolates; 0.20%), Citrobacter spp. (one isolate; 0.10%), and Morganella morganii (one isolate; 0.10%). In contrast, the Gram-positive bacterial isolates included Enterococcus spp. (78 isolates; 7.79%), Staphylococcus aureus (seven isolates; 0.70%), and Lactobacillus spp. (one isolate; 0.10%). Overall, the majority of the bacterial uropathogens were Gram-negative (89.68%), with E. coli being predominant, followed by Klebsiella spp. and Enterobacter spp. Among Gram-positive bacteria (10.32%), Enterococcus spp. was the most common uropathogen.
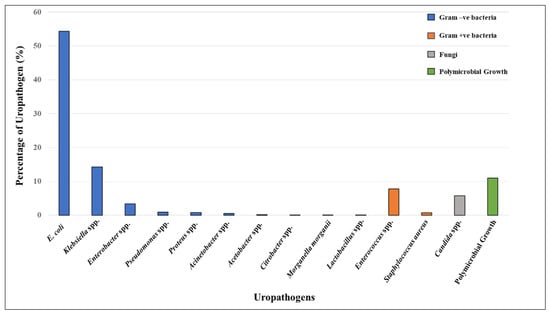
Figure 2.
Distribution of uropathogens isolated from patients positive for UTIs (n = 1001). The isolates include Gram-negative bacteria (blue bars)—E. coli, Klebsiella spp., Enterobacter, Pseudomonas, Proteus, Acinetobacter, Acetobacter, Citrobacter, and Morganella morganii; Gram-positive bacteria (orange bars)—Lactobacillus spp. *, Enterococcus, and Staphylococcus aureus; fungi (grey bar)—Candida spp.; and polymicrobial growth ** (green bar). * Although lactobacilli are part of the commensal human flora, it can also be a uropathogen in rare instances. ** Polymicrobial growth refers to multiple different types of microorganisms growing together; however, these were not identified in the clinical microbiology lab.
2.3. Antibiotic Resistance Patterns and Trends in Bacterial Uropathogens
Out of 833 unimicrobial bacterial uropathogens, 593 (71.19%) were identified as multidrug resistant (MDR) (Table 2). Among these, E. coli demonstrated an MDR prevalence of 73.90%, Klebsiella spp. 48.95%, Enterobacter spp. 70.59%, and Enterococcus spp. 94.85%. Figure 3 and Table S2 present the resistance profiles of these uropathogens to 11 antibiotics, while their resistance trends over time (months) are detailed in Table S3.

Table 2.
Prevalence of multidrug-resistant (MDR) pathogens causing UTIs.
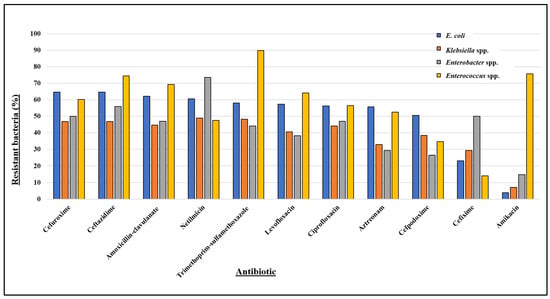
Figure 3.
Antibiotic resistance profile of most prevalent bacteria isolated from patients positive for UTIs, where E. coli (n = 544), Klebsiella spp. (n = 143), and Enterobacter spp. (n = 34) are Gram-negative and Enterococcus spp. (n = 78) is Gram-positive.
2.3.1. E. coli
E. coli shows over 50% resistance to several antibiotics, including cefuroxime (64.71%), ceftazidime (64.71%), amoxicillin–clavulanate (62.13%), netilmicin (60.48%), trimethoprim–sulfamethoxazole (58.09%), levofloxacin (57.35%), ciprofloxacin (56.25%), aztreonam (55.70%), and cefpodoxime (50.55%), and confers the least resistance towards cefixime (23.16%) and amikacin (3.86%) (Figure 3). The antibiotic resistance pattern of E. coli over time (Figure S1A) illustrates its constant elevated resistance towards trimethoprim–sulfamethoxazole, cefuroxime, and ceftazidime, while its overall resistance to cefixime mostly remains below 20%. However, it maintained low resistance levels against amikacin throughout the year.
2.3.2. Klebsiella spp.
Klebsiella spp. showed low resistance to amikacin (6.99%) (Figure 3), with resistance varying from 0% to 15.79% throughout our study period (Figure S1B). In contrast, the isolate exhibited nearly 50% resistance to netilmicin (48.95%), trimethoprim–sulfamethoxazole (48.25%), cefuroxime (46.85%), and ceftazidime (46.85%), along with noticeable resistance towards ampicillin, ciprofloxacin, and levofloxacin (Figure 3). Additionally, the resistance rate of Klebsiella spp. (Figure S1B) shows that it consistently maintained at least 40% resistance to trimethoprim–sulfamethoxazole, cefuroxime, and ceftazidime throughout most months of the year.
2.3.3. Enterobacter spp.
Enterobacter spp. projected high resistance against netilmicin (73.53%), and moderately high resistance against ceftazidime (55.88%) and cefuroxime (50.00%). In contrast, it showed comparatively lower resistance to aztreonam (29.41%), cefpodoxime (26.47%), and amikacin (14.71%) (Figure 3). Despite considerable fluctuations in the resistance rate of Enterobacter spp. (Figure S1C), it exhibited significant resistance to netilmicin, ceftazidime, and cefuroxime, and was generally susceptible to amikacin during most of the months.
2.3.4. Enterococcus spp.
Enterococcus spp. was the prevalent Gram-positive uropathogen in our study. Enterococcus spp. demonstrated more than 70% resistance to trimethoprim–sulfamethoxazole (89.74%), amikacin (75.64%), and ceftazidime (74.36%), while showing the least resistance to cefixime (14.10%) (Figure 3). Throughout most months, Enterococcus spp. exhibited high resistance (at least 60%) to trimethoprim–sulfamethoxazole, amoxicillin–clavulanate, ceftazidime, and amikacin (Figure S1D). An upwards trend in resistance to ciprofloxacin and levofloxacin was observed, increasing from 33% to 100% over the year, with a slight decrease in October. Except for a few of the months, Enterococcus spp. consistently exhibited less than 20% resistance to cefixime.
Overall, our results suggest that most isolates were significantly resistant against ceftazidime, cefuroxime, trimethoprim–sulfamethoxazole, amoxicillin–clavulanate, and netilmicin. Moderate levels of resistance were observed for levofloxacin, ciprofloxacin, aztreonam, and cefpodoxime. While the predominant Gram-negative uropathogens (E. coli, Klebsiella spp., and Enterobacter spp.) exhibited about 85% to 96% sensitivity to amikacin, the major Gram-positive uropathogen, Enterococcus spp., showed 85.90% susceptibility to cefixime.
2.4. Principal Coordinate Analysis (PCoA) of Antibiotic Resistance and Patient Factors
In order to assess any relationships between the antibiogram and patient clinical data, these antibiotic resistance/susceptibility data were then subjected to a principal coordinate analysis (PCoA) (Figure 4), where the Hamming distances between the points on the plot are close to original dissimilarities between the subjects. The figures here show the same PCoA scores based on the resistance profiles but have been encoded to show the differences in terms of UTI bacteria (Figure 4A), patients’ gender (Figure 4B), and age (Figure 4C), as well as whether they were an inpatient or outpatient (Figure 4D). It can be seen in these plots that the patient’s gender, age, and in- or outpatient status show no correlation with the antibiogram pattern. In addition, Figure 4A shows that there is also a lack of clustering according to antibiotic resistance and susceptibility with the causal UTI bacteria. This is interesting, as this shows that all antibiotics have variable levels of resistance/susceptibility which are not specific to a group of bacteria.
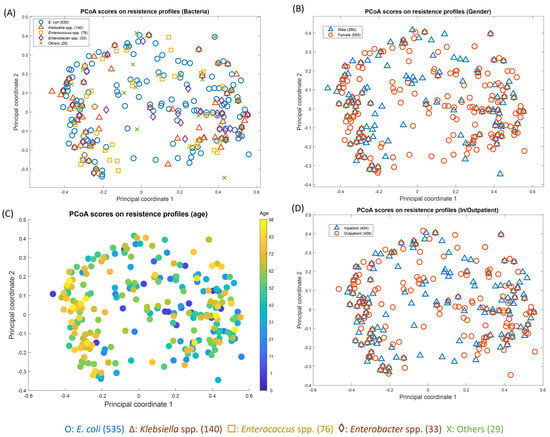
Figure 4.
Principal coordinate analysis (PCoA) scores on resistance profiles of E. coli (n = 544), Klebsiella spp. (n = 143), Enterococcus spp. (n = 78), Enterobacter spp. (n = 34), and others * in terms of (A) bacteria, (B) gender, (C) age, and (D) patient status (inpatient/outpatient). * Including: Pseudomonas spp. (n = 9), Proteus spp. (n = 8), Acinetobacter spp. (n = 5), Acetobacter spp. (n = 2), Citrobacter spp. (n = 1), Lactobacillus spp. (n = 1), and Morganella morganii (n = 1).
3. Discussion
UTIs are extensively encountered in clinical practise and have emerged as a global health problem, occurring as both community-acquired and nosocomial bacterial infections [1,3,7,17].
3.1. Higher Prevalence of UTIs among Females
Our study indicated that UTIs were more prevalent among females than males, which is in accordance with the findings of similar reports [6,7,13,17]. The anatomical differences between male and female genitourinary systems, such as the shorter urethra in women, combined with various host factors—including changes during sexual maturation, alterations in normal vaginal flora, pregnancy, and childbirth—likely contribute to the higher incidence of infections among females [17]. Furthermore, a study by Al-Badr and Al-Shaikh [18] states that approximately 50 to 60% of females experience a UTI at least once in their lifetime, especially the elderly.
3.2. Age-Related UTI Susceptibility in Men and Women
In our study, the incidence of UTI was highest in women aged 50 to 60 years, reflecting the impact of menopausal alterations on urinary tract health. Post-menopausal women are particularly susceptible to UTIs due to factors like pelvic prolapse, less lactobacilli in the vaginal flora, increased proliferation and colonization of E. coli in the periurethral area, decreased estrogen levels, and an elevated prevalence of medical conditions [18]. Men of 60 to 80 years were found to be more prone to UTIs, which may be linked to the elevated incidence of bladder outlet obstructions, like prostatitis and bladder stones, leading to less frequent urination [19]. Additionally, females aged 20 to 50 years exhibited higher infection rates than males, possibly since this age range encompasses the female reproductive years, during which pregnancy is known to increase the risk of UTI [20].
3.3. UTI Incidence and Polymicrobial Infection in Pediatric Populations
We also observed that 12.59% of UTI cases occurred in children aged 0–10 years. This finding aligns with other research highlighting significant UTI rates in children, for instance, studies in India [21], Nigeria [22], and Finland [23] reported 10.8%, 9%, and 1 to 5% of UTI cases in children, respectively. However, prolonged hospital stays might contribute to UTIs in children, particularly those caused by extended-spectrum β-lactamase (ESBL)-producing E. coli and Klebsiella spp. [24]. Furthermore, a significant proportion of patients with polymicrobial UTI were identified to be children compared to the other age groups. Robinson et al. [25] suggests that polymicrobial growth in pediatric UTI cases may result from contamination during urine sample collection. This is often due to the challenges of collecting uncontaminated samples from non-toilet-trained children, who may require methods such as suprapubic aspiration, urethral catheterization, pediatric urine collection bags, or the collection of clean-catch urine while the child is without a diaper. Additionally, contamination frequently occurs in the process of obtaining urine samples from uncircumcised male children due to the challenges associated with retracting the foreskin [25].
3.4. Fungal UTIs
In the present study, 5.79% of the total UTI cases were due to fungi, specifically Candida spp. Similar surveillance studies conducted in Iran recorded a 5% to 7% prevalence of UTIs associated with Candida spp., particularly Candida albicans [26,27]. These fungi are prominent opportunistic pathogens responsible for nosocomial UTIs [28]. A total of 51 out of 58 UTI cases caused by Candida spp. in our analysis were from hospitalized patients, the majority of whom were female. Candida albicans and non-Candida albicans Candida spp. are commonly present in the alimentary canal, oral cavity, and vagina of healthy individuals as part of the microflora. In premenopausal and healthy women, these microorganisms inhabit the external urethral opening. Immune deficiencies can disrupt the equilibrium between Candida albicans, non-Candida albicans Candida yeasts, and the host’s other normal flora. Such conditions trigger the transition of commensal Candida yeasts into opportunistic pathogens, leading to UTIs [29].
3.5. Gram-Positive Uropathogenic Bacteria
In terms of bacterial uropathogens, 10.32% of the isolates were Gram-positive, predominantly consisting of Enterococcus spp. and smaller numbers of Staphylococcus aureus and Lactobacillus spp. Although lactobacilli are part of the commensal human flora, the prior literature has identified Lactobacillus delbrueckii as a causative microorganism in UTIs affecting both genders [30,31]. A cross-sectional study by Malmartel et al. [32] in a French primary care reported Enterococcus spp. to be responsible for 7% of 1119 UTI cases, which is consistent with our findings. Enterococcus spp., which primarily causes complicated UTIs [1], contributes to over 30% of nosocomial infections due to its ability to survive on various surfaces for extended durations, rendering it the second most common pathogen responsible for catheter-associated UTIs [33]. While Enterococcus spp. showed high resistance to trimethoprim–sulfamethoxazole, amikacin, and ceftazidime in our study, cefixime emerged as the most effective antibiotic against it.
3.6. Gram-Negative Uropathogenic Bacteria
Of the bacterial isolates identified in our analysis, 89.68% were Gram-negative, consistent with findings from other studies [6,9,17]. The majority of these were Enterobacterales, including E. coli, Klebsiella spp., Enterobacter spp., Proteus spp., Citrobacter spp., and Morganella morganii. Enterobacterales possess specialized mechanisms for adhering to and colonizing the uroepithelium. These mechanisms include pili, adhesins, fimbriae, and receptors like the P-1 blood group phenotype, which facilitate their attachment to and persistence within the urogenital mucosa. These factors collectively strengthen Enterobacterales to cause UTIs, leading to their prominence among bacterial uropathogens [34].
3.7. Prevalence of E. coli and Klebsiella spp. as Predominant Uropathogens
In our study, E. coli was found to be the predominant etiological agent of UTIs, corroborating findings from previous studies that also identified E. coli as the principal uropathogen [6,7,9,13,16,17,20,35]. Furthermore, Klebsiella spp. was observed to be the second-most common uropathogen in this study, which also aligns with the findings reported by other researchers [6,14,17,36,37]. According to the WHO, E. coli and Klebsiella spp. are responsible for nearly 80% of UTI cases [38]. In the present analysis, they together accounted for 82.47% of the unimicrobial bacterial uropathogens. E. coli and Klebsiella spp. can cause both complicated and uncomplicated UTIs. In uncomplicated UTIs, the infection occurs in the lower urinary tract, where bacteria bind directly to the main protein components of the umbrella cell apical membrane in the bladder epithelium (uroepithelium) called uroplakins. These proteins protect mammalian bladder tissue from damaging agents in urine by forming a crystalline array. In contrast, complicated UTIs involve the upper urinary tract and occur when bacteria bind to kidney or bladder stones, urinary catheters, or when they are retained in the urinary tract due to a physical obstruction [1].
3.8. E. coli and Klebsiella spp.—Resistance towards Cephalosporins and Multidrug Resistance
Among the total bacterial uropathogens, 71.19% were MDR, and 84.27% of the isolates were resistant to at least one of the 11 antibiotics highlighted in this study. This is concerning because a similar study conducted in Bangladesh also reported 78.2% of uropathogens to be MDR [36], while studies in Nepal found multidrug resistance in 54% [35] and 41% [39] of UTI cases. In our study, 73.90% of E. coli and 48.95% of Klebsiella spp. possessed multidrug resistance. Our finding that E. coli has higher rates of MDR than Klebsiella spp. is consistent with studies reported in the literature [36,40,41,42,43,44]. Moreover, the antibiotic resistance profile shows 64.71% of E. coli and 46.85% of Klebsiella spp. confer resistance to cefuroxime and ceftazidime, which are second- and third-generation cephalosporins, respectively. This is particularly concerning because antibiotics of this class are frequently opted for as empiric therapy for patients with severe infections that necessitate hospitalization or intravenous administration [45]. The antibiotic resistance rate of these two uropathogens suggests a reduced efficacy of cefuroxime and ceftazidime in treating UTIs caused by E. coli and Klebsiella spp., regardless of seasonal or temporal variations. According to two other studies conducted in Bangladesh, E. coli and Klebsiella spp. exhibited 78.81% and 63.64% resistance to cefuroxime, respectively, in a teaching hospital [7], and 100% resistance to this antibiotic in a tertiary care hospital [14]. Several investigations also indicate E. coli and Klebsiella spp. show limited sensitivity to ceftazidime. For example, a study conducted in Nepal found E. coli and Klebsiella spp. to be 82.8% and 96.0% resistant, respectively [35]. Additionally, Sugianli et al. [38] reported in a systematic review (compiling laboratory-based surveillance reports from Australia, Bangladesh, India, and the Republic of Korea) that the prevalence of resistance of E. coli and Klebsiella spp. to ceftazidime ranges between 2.34% and 68.44%.
However, the elevated resistance against cefuroxime and ceftazidime of E. coli and Klebsiella spp. is possibly due to their possession of acquired plasmids encoding ESBLs. ESBLs hydrolyse the β-lactam ring inactivating β-lactam antibiotics. Apart from spreading resistance to third-generation cephalosporins, these plasmids rapidly spread resistance against other antibiotics as well, rendering the bacteria to be MDR. This is because the ESBL-encoding plasmids generally also contain genes for resistance against aminoglycosides, sulfonamides, and quinolones [1]. Furthermore, E. coli and Klebsiella spp. are responsible for the presence of ESBLs in other Enterobacterales. This is because incautious administration of cephalosporins in hospital settings and transferrable elements bearing ESBL-encoding genes together create a suitable setting for antibiotic resistance selection in bacteria [1]. Therefore, the careless and excessive misuse of antibiotics in the region is likely to cause this rapid emergence of cephalosporin resistance [14].
In addition to cefuroxime and ceftazidime, we found 44.06% to 62.13% of E. coli and Klebsiella spp. to exhibit resistance to trimethoprim–sulfamethoxazole, amoxicillin–clavulanate, netilmicin, and ciprofloxacin. A similar finding has been reported by Kothari and Sagar [46] in India, where uropathogens exhibited high levels of resistance to trimethoprim–sulfamethoxazole, amoxicillin–clavulanate, and ciprofloxacin. Additionally, several studies across Asia have consistently documented a substantial resistance of E. coli and Klebsiella spp. to these antibiotics [47,48,49,50]. The widespread resistance observed may be attributed to the frequent use of these antibiotics as first-line or empirical treatments for uncomplicated UTIs, given their broad spectrum of activity, favourable pharmacokinetics, and general tolerability [38,51].
3.9. Susceptibility of E. coli and Klebsiella spp. to Amikacin
We found that 96.14% of E. coli and 93.01% of Klebsiella spp. showed sensitivity to amikacin, and this was the case throughout most months of the year. Our findings are consistent with studies from South Korea, where 0.7% of E. coli and 3.4% of Klebsiella spp. were amikacin resistant [49]; Iran, where 1–5% E. coli and 13–37% Klebsiella spp. were amikacin resistant [16]; India, where 0% of both the uropathogens were amikacin resistant [52]; and Bangladesh, where 8.8% of E. coli and 13.2% of Klebsiella spp. were amikacin resistant [36]. In addition to E. coli and Klebsiella spp., the majority of uropathogens in this study were observed to be sensitive to this antibiotic, aligning with similar findings by Setu et al. [17] and Mohapatra et al. [37]. The remarkable effectiveness of amikacin against the uropathogens among the tested antimicrobials may be attributed to its uncommon use in empirical UTI treatment, as successful amikacin therapy requires precise patient selection and close monitoring of renal function and potential toxicity [53].
3.10. Contributing Factors to the Soaring Antibiotic Resistance
The higher percentages of antibiotic resistance to potent antibiotics observed in our study can be linked to several key risk factors contributing to the rise of antibiotic resistance. A significant factor is the excessive consumption of antibiotics, facilitated by their widespread availability as over-the-counter drugs that can be purchased without a doctor’s prescription. Since Bangladesh has one of the highest numbers of unlicensed stores dispensing antibiotics, even for mild ailments [54], the easy access, combined with their relatively low cost and the convenience of oral administration, has led to inappropriate self-medication and the misuse of antibiotics. Additionally, uropathogens in this study exhibited high resistance to cephalosporins, which are commonly prescribed antibiotics for various infections in Bangladesh [55]. Consequently, factors such as improper dosing, unnecessary prescriptions of effective antibiotics to patients who do not require them, and frequent use of antibiotics for various infections are contributing to the rising antibiotic resistance in local hospitals and the community. Nevertheless, external factors like gender, patient status, or age group did not affect the resistance profile of the bacterial isolates in our study, as deduced from the lack of obvious clustering trends observed in the PCoA plots. Additionally, the PCoA highlighted that the level of resistance or susceptibility is not bacteria-specific and cannot be used to guide general antibiotic stewardship.
3.11. Limitations of the Study
Our study has several limitations. Firstly, reliance on retrospective data from a single hospital may limit the generalizability of the findings to other regions. Secondly, detailed information on patient comorbidities or whether the inpatients were catheterized was not documented. This lack of data may have resulted in the inclusion of cases of colonization or asymptomatic bacteriuria. Thirdly, the analysis primarily focused on the predominant bacterial isolates, which may have led to the underestimation of the significance of uropathogens isolated in smaller numbers to ensure reliable data interpretation. Finally, as antimicrobial resistance (AMR) patterns evolve over time, the data from 2018 may not fully reflect current AMR trends and treatment strategies. Additionally, the study’s duration of one year was slightly compromised, as data for August 2018 could not be collected.
4. Materials and Methods
4.1. Sample Collection
This retrospective study was conducted at Uttara Adhunik Medical College and Hospital (UAMCH) in Dhaka, Bangladesh. Antibiogram data were conveniently collected from hospital records over a one-year period, from January 2018 to December 2018. All patients suspected to have a UTI, regardless of gender (male or female) and age (0 to 100 years), from both outpatient and inpatient departments, were included in this study, except for those already on antibiotic treatment. A total of 6549 samples were collected during this period. UTI was diagnosed depending on microscopic findings of >5 pus cells per high-power field (1000× for high power) and a colony count of ≥105 CFU/mL of one pathogen. In cases where three or more microorganisms were identified at ≥105 CFU/mL and no single species predominated, the infection was classified as a polymicrobial UTI. A sterile, leak-proof, wide-mouthed container was provided to the patients by the laboratory to collect a clean-catch midstream urine sample. Within 1 h of the sample collection, they were sent to the laboratory and processed within 2–4 h at room temperature.
4.2. Sample Processing
A standard bacteriological loopful (0.01 mL) of each urine sample was spread over the surface of sterile cystine lactose electrolyte-deficient (CLED) agar plates and left on the bench for some time in order to allow the urine to dry onto the agar medium. The plates were then incubated at 37 °C for 18–24 h in an inverted position. A significant bacterial count was taken as any count equal to or in excess of 105 CFU/mL. Positive samples for both significant bacterial colonies and pus cell tests were cultured aseptically on sterile MacConkey agar, blood agar, and chocolate agar plates, along with other conventional biochemical tests that were performed to identify pure isolates [56,57]. The inoculated plates were incubated at 37 °C for 24 h. Following that, all the identified isolates were subjected to antibiotic sensitivity testing, apart from the fungal isolates.
4.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing of the isolated uropathogens were performed by the Kirby–Bauer disc diffusion technique [56]. The inoculum of the uropathogen was streaked onto sterile Mueller Hinton agar plates aseptically using a sterile inoculating wire loop. Prior to that, the turbidity of the inoculum was adjusted to the 0.5 McFarland standard or 108 cells/mL, according to the recommendations of the Clinical Laboratory Standard Institute (CLSI) [58].
The appropriate antibiotic discs containing amikacin (AK; 30 µg), amoxicillin–clavulanate (AMC; 30 µg), aztreonam (ATM; 30 µg), cefixime (CFM; 5 µg), cefpodoxime (CPD; 10 µg), ceftazidime (CAZ; 30 µg), cefuroxime (CXM; 30 µg), ciprofloxacin (CIP; 5 µg), levofloxacin (LEV; 5 µg), netilmicin (NET; 30 µg), and trimethoprim–sulfamethoxazole (SXT; 25 µg) were then aseptically impregnated onto the surface of the dried plates using sterile forceps. The antibiotic discs used in the study were all obtained from Oxoid Ltd. (Oxoid, Ogdensburg, NY, USA). The plates were left at room temperature for 1 h to allow for the diffusion of the different antibiotics from the disc into the medium. All plates were then incubated at 37 °C for 18–24 h. The results were interpreted depending on the diameter of the inhibition zone according to the CLSI guidelines [58].
4.4. Statistical Analysis
These data were collected and analyzed using Microsoft Excel 2007 and MATLAB2019b (MathWorks, Natick, MA, USA). For each sample, a multivariate resistant profile to all the above-mentioned antibiotics was recorded, where ‘1’ indicates resistance to a particular antibiotic and ‘0’ represents susceptibility to this antibiotic. The Hamming distance was used to measure the dissimilarity between each pair of resistant profiles and a principal coordinate analysis (PCoA) was applied to square-rooted Hamming distances to visualize the pattern of these profiles [59].
5. Conclusions
In conclusion, 6549 urine samples were analyzed in this study, identifying an alarming prevalence of MDR uropathogens among patients with UTI in a tertiary care hospital in Bangladesh, with E. coli and Klebsiella spp. being the most prevalent. An elevated resistance of these uropathogens was observed against cephalosporins and other antibiotics commonly used for UTI treatment. However, we found amikacin to be the most effective antibiotic for treating UTIs caused by E. coli and Klebsiella spp. Although demographic factors such as gender, age, and patient status did not significantly influence AMR patterns of the bacterial uropathogens, our findings suggest continuous surveillance and strategic antibiotic stewardship is inevitable to manage resistance trends effectively and guide empirical treatment strategies, thereby addressing the rise of MDR bacteria and improving treatment outcomes for UTIs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13100925/s1. Table S1: Frequency of uropathogens isolated from patients with UTI; Table S2: Antibiotic resistance profile of bacteria isolated from patients positive for UTI. The table shows the number and percentage of antibiotic-resistant bacterial isolates for each antibiotic. Bacterial isolates include Gram-negative E. coli, Klebsiella spp., Enterobacter spp., Pseudomonas spp., Proteus spp., Acinetobacter spp., Acetobacter spp., Citrobacter spp., and Morganella morganii, and Gram-positive Enterococcus spp., Staphylococcus aureus, and Lactobacillus spp.; Table S3: Trends in resistance rates of E. coli, Klebsiella spp., Enterobacter spp., and Enterococcus spp. over time (covering all months of 2018, except August); Figure S1: Graphical representation of trends in resistance rates of (A) E. coli; (B) Klebsiella spp.; (C) Enterobacter spp.; and (D) Enterococcus spp. over time (covering all months of 2018, except August).
Author Contributions
S.S.C.: conceptualization, investigation, data curation, visualization, writing—original draft, and project administration; P.T.: conceptualization, investigation, data curation, and manuscript preparation; Y.X.: software, validation, formal analysis, and data curation; A.S.M.M.: methodology, resources, and data curation; H.M.: conceptualization, manuscript preparation, and supervision; R.G.: conceptualization, formal analysis, and supervision; all authors: review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the Ethical Review Committee of Uttara Adhunik Medical College (affiliated with Uttara Adhunik Medical College Hospital, Dhaka, Bangladesh) on 4 January 2018 (reference number: UAMC/ERC/02/2018).
Informed Consent Statement
Patient consent was waived by the Ethical Review Committee, Uttara Adhunik Medical College, because this was a retrospective study in which the samples were collected for diagnostic purposes independently of the study, and the data were provided to us anonymously. This study complied with the Declaration of Helsinki.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors thank the University of Liverpool for their funding of S.S.C.’s PhD and the Centre for Metabolomics Research for support. We also gratefully acknowledge Most. Fahmida Begum and Shahabuddin Choudhuri for their helpful guidance, as well as Abida Aktar Akhi and Sharmin Ummah Khayar Kulsum for their assistance in data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK436013/ (accessed on 12 November 2023).
- Wilson, M.L.; Gaido, L. Laboratory Diagnosis of Urinary Tract Infections in Adult Patients. Clin. Infect. Dis. 2004, 38, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, D.J.; Rycyk, M.T.; Chen, M.C.; Thumbikat, P.; Sengupta, S.; Schaeffer, A.J. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect. Immun. 2006, 74, 5106–5113. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Tsegaye, W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in jimma university specialized hospital, southwest ethiopia. Ethiop. J. Health Sci. 2011, 21, 141–146. [Google Scholar] [CrossRef]
- Haque, R.; Akter, M.L.; Salam, M.A. Prevalence and susceptibility of uropathogens: A recent report from a teaching hospital in Bangladesh. BMC Res. Notes 2015, 8, 416. [Google Scholar] [CrossRef]
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary tract infections caused by uropathogenic Escherichia coli strains—New strategies for an old pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef]
- Singhal, A.; Sharma, R.; Jain, M.; Vyas, L. Hospital and community isolates of uropathogens and their antibiotic sensitivity pattern from a tertiary care hospital in North West India. Ann. Med. Health Sci. Res. 2014, 4, 51–56. [Google Scholar] [CrossRef]
- Mosharraf, F.B.; Chowdhury, S.S.; Ahmed, A.; Hossain, M.M. A Comparative Study of Static Biofilm Formation and Antibiotic Resistant Pattern between Environmental and Clinical Isolate of Pseudomonas aeruginosa. Adv. Microbiol. 2020, 10, 663–672. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Hopkins, S.; Llewelyn, M.J.; Walker, A.S.; McNulty, C.A.; Robotham, J.V. Duration of antibiotic treatment for common infections in English primary care: Cross sectional analysis and comparison with guidelines. BMJ 2019, 364. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Islam, M.R.; Khan, R.; Amin, M.B.; Rahman, M.; Hossain, M.I.; Ahmed, D.; Asaduzzaman, M.; Riley, L.W. Prevalence, etiology and antibiotic resistance patterns of community-acquired urinary tract infections in Dhaka, Bangladesh. PLoS ONE 2022, 17, e0274423. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Chowdhury, O.A.; Hoque, M.M.; Hoque, S.A.; Chowdhury, S.M.R.; Rahman, M.A. Antimicrobial Resistance Pattern of Uropathogenic Escherichia coli and Klebsiella species Isolated in a Tertiary Care Hospital of Sylhet. Med. Today 2018, 30, 61–66. [Google Scholar] [CrossRef][Green Version]
- Alkhawaldeh, R.; Abu Farha, R.; Abu Hammour, K.; Alefishat, E. Optimizing antimicrobial therapy in urinary tract infections: A focus on urine culture and sensitivity testing. Front. Pharmacol. 2022, 13, 1058669. [Google Scholar] [CrossRef] [PubMed]
- Mihankhah, A.; Khoshbakht, R.; Raeisi, M.; Raeisi, V. Prevalence and antibiotic resistance pattern of bacteria isolated from urinary tract infections in Northern Iran. J. Res. Med. Sci. 2017, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Setu, S.K.; Sattar, A.N.I.; Saleh, A.A.; Roy, C.K.; Ahmed, M.; Muhammadullah, S.; Kabir, M.H. Study of Bacterial pathogens in Urinary Tract Infection and their antibiotic resistance profile in a tertiary care hospital of Bangladesh. Bangladesh J. Med. Microbiol. 2016, 10, 22–26. [Google Scholar] [CrossRef]
- Al-Badr, A.; Al-Shaikh, G. Recurrent urinary tract infections management in women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359. [Google Scholar] [CrossRef]
- Dougherty, J.M.; Leslie, S.W.; Aeddula, N.R. Male Urinary Retention: Acute and Chronic. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538499/ (accessed on 20 April 2024).
- Oli, A.N.; Akabueze, V.B.; Ezeudu, C.E.; Eleje, G.U.; Ejiofor, O.S.; Ezebialu, I.U.; Oguejiofor, C.B.; Ekejindu, I.M.; Emechebe, G.O.; Okeke, K.N. Bacteriology and antibiogram of urinary tract infection among female patients in a tertiary health facility in south eastern Nigeria. Open Microbiol. J. 2017, 11, 292. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Jesudason, M.R.; Prakasah, J.A.J.; Anandan, S.; Sahni, R.D.; Pragasam, A.K.; Bakthavatchalam, Y.D.; Selvakumar, R.J.; Dhole, T.; Rodrigues, C. Antimicrobial susceptibility profiles of gram-negative bacteria causing infections collected across India during 2014–2016: Study for monitoring antimicrobial resistance trend report. Indian J. Med. Microbiol. 2018, 36, 32–36. [Google Scholar] [CrossRef]
- Musa-Aisien, A.; Ibadin, O.; Ukoh, G.; Akpede, G. Prevalence and antimicrobial sensitivity pattern in urinary tract infection in febrile under-5s at a children’s emergency unit in Nigeria. Ann. Trop. Paediatr. 2003, 23, 39–45. [Google Scholar] [CrossRef]
- Nuutinen, M.; Uhari, M. Recurrence and follow-up after urinary tract infection under the age of 1 year. Pediatr. Nephrol. 2001, 16, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.P.; Sarangi, R.; Padhy, R.N. Prevalence of multidrug resistant uropathogenic bacteria in pediatric patients of a tertiary care hospital in eastern India. J. Infect. Public Health 2016, 9, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Finlay, J.C.; Lang, M.E.; Bortolussi, R.; Society, C.P.; Committee, C.P.; Diseases, I.; Committee, I. Urinary tract infection in infants and children: Diagnosis and management. Paediatr. Child Health 2014, 19, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Behzadi, E. The microbial agents of urinary tract infections at central laboratory of Dr. Shariati Hospital, Tehran, Iran. Turk Klin Tip Bilim 2008, 28, 445. [Google Scholar]
- Behzadi, P.; Behzadi, E.; Yazdanbod, H.; Aghapour, R.; Cheshmeh, M.A.; Omran, D.S. Urinary tract infections associated with Candida albicans. Maedica 2010, 5, 277. [Google Scholar]
- Helbig, S.; Achkar, J.; Jain, N.; Wang, X.; Gialanella, P.; Levi, M.; Fries, B. Diagnosis and inflammatory response of patients with candiduria. Mycoses 2013, 56, 61–69. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary tract infections and Candida albicans. Cent. Eur. J. Urol. 2015, 68, 96. [Google Scholar] [CrossRef]
- Maillet, F.; Passeron, A.; Podglajen, I.; Ranque, B.; Pouchot, J. Lactobacillus delbrueckii urinary tract infection in a male patient. Médecine Et Mal. Infect. 2019, 49, 226–228. [Google Scholar] [CrossRef]
- Darbro, B.W.; Petroelje, B.K.; Doern, G.V. Lactobacillus delbrueckii as the cause of urinary tract infection. J. Clin. Microbiol. 2009, 47, 275–277. [Google Scholar] [CrossRef]
- Malmartel, A.; Ghasarossian, C. Epidemiology of urinary tract infections, bacterial species and resistances in primary care in France. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 447–451. [Google Scholar] [CrossRef]
- Codelia-Anjum, A.; Lerner, L.B.; Elterman, D.; Zorn, K.C.; Bhojani, N.; Chughtai, B. Enterococcal urinary tract infections: A review of the pathogenicity, epidemiology, and treatment. Antibiotics 2023, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Chandrashekhar, T.; Joshi, H.; Gurung, M.; Shrestha, N.; Shivananda, P. Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal. Singap. Med. J. 2006, 47, 281. [Google Scholar]
- Shakya, S.; Edwards, J.; Gupte, H.; Shrestha, S.; Shakya, B.; Parajuli, K.; Kattel, H.; Shrestha, P.; Ghimire, R.; Thekkur, P. High multidrug resistance in urinary tract infections in a tertiary hospital, Kathmandu, Nepal. Public Health Action 2021, 11, 24–31. [Google Scholar] [CrossRef]
- Dasgupta, C.; Rafi, M.A.; Salam, M.A. High prevalence of multidrug resistant uropathogens: A recent audit of antimicrobial susceptibility testing from a tertiary care hospital in Bangladesh. Pak. J. Med. Sci. 2020, 36, 1297. [Google Scholar] [CrossRef]
- Mohapatra, S.; Panigrahy, R.; Tak, V.; JV, S.; KC, S.; Chaudhuri, S.; Pundir, S.; Kocher, D.; Gautam, H.; Sood, S. Prevalence and resistance pattern of uropathogens from community settings of different regions: An experience from India. Access Microbiol. 2022, 4, 000321. [Google Scholar] [CrossRef]
- Sugianli, A.K.; Ginting, F.; Parwati, I.; de Jong, M.D.; van Leth, F.; Schultsz, C. Antimicrobial resistance among uropathogens in the Asia-Pacific region: A systematic review. JAC-Antimicrob. Resist. 2021, 3, dlab003. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Saud, B.; Paudel, G.; Bajracharya, D. Emergence of antimicrobial drug resistant bacteria in Nepal: A current scenario. PBCR 2019, 1, 31–33. [Google Scholar]
- Shakya, P.; Shrestha, D.; Maharjan, E.; Sharma, V.K.; Paudyal, R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: A hospital based study. Open Microbiol. J. 2017, 11, 23. [Google Scholar] [CrossRef]
- Baral, P.; Neupane, S.; Marasini, B.P.; Ghimire, K.R.; Lekhak, B.; Shrestha, B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res. Notes 2012, 5, 38. [Google Scholar] [CrossRef]
- Iqbal, Z.; Mumtaz, M.Z.; Malik, A. Extensive drug-resistance in strains of Escherichia coli and Klebsiella pneumoniae isolated from paediatric urinary tract infections. J. Taibah Univ. Med. Sci. 2021, 16, 565–574. [Google Scholar] [CrossRef]
- Jalil, M.B.; Al Atbee, M.Y.N. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J. Clin. Lab. Anal. 2022, 36, e24619. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Hasani, A.; Pormohammad, A.; Rezaee, M.A.; Reza, M.; Nahaie, A.H.; Haghi, M.H.S.B.; Gholizade, P.; Arbatan, A.E. qnrA implicated quinolone resistance in Escherichia coli and Klebsiella pneumoniae clinical isolates from a University Teaching Hospital. Life Sci. J. 2014, 11, 1032–1035. [Google Scholar]
- Kang, K.T.; Ng, K.; Kendrick, J.; Tilley, P.; Ting, J.; Rassekh, S.; Murthy, S.; Roberts, A. Third-generation cephalosporin-resistant urinary tract infections in children presenting to the paediatric emergency department. Paediatr. Child Health 2020, 25, 166–172. [Google Scholar] [CrossRef]
- Kothari, A.; Sagar, V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: A multicenter study. J. Infect. Dev. Ctries. 2008, 2, 354–358. [Google Scholar] [CrossRef]
- Fasugba, O.; Mitchell, B.G.; Mnatzaganian, G.; Das, A.; Collignon, P.; Gardner, A. Five-year antimicrobial resistance patterns of urinary Escherichia coli at an Australian tertiary hospital: Time series analyses of prevalence data. PLoS ONE 2016, 11, e0164306. [Google Scholar] [CrossRef]
- Hossain, M.D.; Ahsan, S.; Kabir, M.S. Antibiotic resistance patterns of uropathogens isolated from catheterized and noncatheterized patients in Dhaka, Bangladesh. Tzu Chi Med. J. 2014, 26, 127–131. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, E.-J.; Kim, D.; Jeong, S.H.; Won, E.J.; Shin, J.H.; Kim, S.H.; Shin, J.H.; Shin, K.S.; Kim, Y.A. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: First one-year report from Kor-GLASS. Eurosurveillance 2018, 23, 1800047. [Google Scholar] [CrossRef]
- Turnidge, J.D.; Gottlieb, T.; Mitchell, D.H.; Coombs, G.W.; Daly, D.A.; Bell, J.M.; Australian Group on Antimicrobial Resistance. Community-onset Gram-negative Surveillance Program annual report, 2012. Commun. Dis. Intell. Q. Rep. 2014, 38, E54–E58. [Google Scholar]
- Jancel, T.; Dudas, V. Management of uncomplicated urinary tract infections. West. J. Med. 2002, 176, 51. [Google Scholar] [CrossRef]
- Devi, A.; Rajkumar, J. A study on antibiotic susceptibility and resistance profiles of bacterial strains isolated from patients with urinary tract infection (UTI) at Kanchipuram District, Tamilnadu, India. Int. J. Pharm. Pharm. Sci. 2013, 5, 817–820. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Choi, S.-M.; Park, S.H.; Lee, D.-G.; Choi, J.-H.; Yoo, J.-H. Amikacin therapy for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Korean J. Intern. Med. 2016, 31, 156. [Google Scholar] [CrossRef] [PubMed]
- Do, N.T.; Vu, H.T.; Nguyen, C.T.; Punpuing, S.; Khan, W.A.; Gyapong, M.; Asante, K.P.; Munguambe, K.; Gómez-Olivé, F.X.; John-Langba, J. Community-based antibiotic access and use in six low-income and middle-income countries: A mixed-method approach. Lancet Glob. Health 2021, 9, e610–e619. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Roy, D.N.; Tajmim, A.; Rajib, S.S.; Hossain, M.; Farzana, F.; Yasmen, N. Prescription antibiotics for outpatients in Bangladesh: A cross-sectional health survey conducted in three cities. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria; Lippinccot, Williams & Williams: Baltimore, MD, USA, 2000. [Google Scholar]
- Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S. Diagnostic Microbiology; Mosby: St Louis, MO, USA, 2007. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Document M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Borg, I.; Groenen, P.J. Modern Multidimensional Scaling: Theory and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).