Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major causes of a variety of infections in hospitals and the community. Their spread poses a serious public health problem worldwide. Nevertheless, in Tunisia and other African countries, very little molecular typing data on MRSA strains is currently available. In our study, a total of 64 MRSA isolates were isolated from clinical samples collected from burned patients hospitalized in the Traumatology and Burns Center of Ben Arous in Tunisia. The identification of the collection was based on conventional methods (phenotypic and molecular characterization). The characterization of the genetic support for methicillin resistance was performed by amplification of the mecA gene by polymerase chain reaction (PCR), which revealed that 78.12% of S. aureus harbors the gene. The resistance of all the collection to different antibiotic families was studied. Indeed, the analysis of strain antibiotic susceptibility confirmed their multi-resistant phenotype, with high resistance to ciprofloxacin, gentamicin, penicillin, erythromycin, and tetracycline. The resistance to the last three antibiotics was conferred by the blaZ gene (73.43%), the erm(C) gene (1.56%), the msr(A) gene (6.25%), and tet(M) gene (7.81%), respectively. The clonal diversity of these strains was studied by molecular typing of the accessory gene regulator (agr) system, characterization of the SCCmec type, and spa-typing. The results revealed the prevalence of agr types II and III groups, the SCCmec type III and II cassettes, and the dominance of spa type t233. The characterization of the eight enterotoxins genes, the Panton-Valentine leukocidin and the toxic shock syndrome toxin, was determined by PCR. The percentage of virulence genes detected was for enterotoxins (55%), tst (71.88%), leukocidin E/D (79.69%), and pvl (1.56%) factors. Furthermore, our results revealed that the majority of the strains harbor IEC complex genes (94%) with different types. Our findings highlighted the emergence of MRSA strains with a wide variety of toxins, leukocidin associated with resistance genes, and specific genetic determinants, which could constitute a risk of their spread in hospitals and the environment and complicate infection treatment.
1. Introduction
The high frequency of Staphylococcus aureus infections in burn units constitutes a serious problem for infection treatment. Loss of the functional skin barrier and the depression of the immune responses caused by burns have increased the incidence of various infections [1]. The skin is the first barrier of defense against microbial infection, and it becomes more sensitive once it gets burned. Many pathogens are responsible for burn wound infections, including Staphylococcus, Enterococcus, Pseudomonas, Acinetobacter, and fungi [1]. Staphylococcus aureus bacteria can cause a wide variety of infections, from minor skin infections to life-threatening infections such as pneumonia, endocarditis, and sepsis. During the last five decades, the overuse of antimicrobial agents in human medicine to treat bacterial infections has favored the emergence of multidrug-resistant bacteria, including MRSA, that have spread as human hospital-acquired pathogens (HA-MRSA) throughout the world [2].
The emergence and transmission of methicillin-resistant S. aureus (MRSA) in burn centers results in adverse effects such as prolonged hospitalization, bacteremia or sepsis, and even death, which require further prevention and treatment efforts. In Tunisia, the Traumatology and Burn Center (CTGB) is the only burn center that treats different types of burn wounds.
Methicillin resistance in staphylococci is primary mediated by the expression of the mecA gene, or its homologue mecC, that contains a diverse type of staphylococcal cassette chromosome mec (SCCmec), based on a mobile genetic element, and encodes an altered penicillin-binding protein with a very low affinity to β-lactam antibiotics [2]. This emergence of multidrug-resistant strains presents a global health issue. In fact, the World Health Organization predicts that by 2050, bacterial resistance will be responsible for 10 million more deaths than cancer [3].
To date, there is no staphylococcal vaccine, and the alternative antibiotic used as an anti-MRSA is vancomycin. The main objective for successful clinical treatment of bacterial infections depends on the analysis of the antibiotic susceptibility profiles and the antibiotic resistance mechanisms of pathogenic bacteria. In addition to antibiotic resistance, S. aureus isolates can harbor a diversity of virulence factor genes, including the Panton-Valentine leukocidin (PVL), the toxic shock syndrome toxin 1 (TSST), and the staphylococcal enterotoxins (SE) [4], immune evasion factors like staphylokinase (Sak), staphylococcal inhibitor of complement (SCIN), and chemotaxis inhibitory protein (CIP), including CHIPS. These virulence factors may contribute to human or animal skin and soft tissue infections, as well as cases of severe pneumonia and food poisoning [4]. The number and combinations of toxin genes may contribute to the pathogenicity of S. aureus. It is important to highlight that the expression of virulence genes is under the control of a global quorum-sensing regulator system named agr (accessory gene regulator), which is associated with the pathogenesis and molecular typing of antibiotic resistance in S. aureus [5]. Mobile genetic elements (MGEs) carrying resistance genes such as plasmids, transposons, and genomic islands frequently harbor virulence factor genes [2,6].
The molecular characterization of S. aureus is very interesting for the identification and knowledge of the circulation of virulent and resistant clones in hospital settings [6]. In fact, the most useful typing tool for epidemiological studies of S. aureus that gives an excellent discriminatory result is spa-typing based on the sequence variation in a hypervariable region of the staphylococcal protein A spa gene. The spa gene encodes a surface protein that plays a role in the adhesion and colonization of S. aureus [7]. The prevalence of spa types of S. aureus isolates varies among different origins and countries [6,7].
To develop effective control and treatment of human infections, it is important to study the genetic diversity, antimicrobial resistance, and virulence of S. aureus associated with infectious diseases. These types of data are limited in Tunisia. Therefore, the aim of this study was to determine the genetic lineages, antibiotic resistance genes, and virulence determinants of S. aureus isolates from clinical samples of burned patients in Tunisia.
2. Results
2.1. Confirmation of S. aureus Isolates
The biochemical and molecular identification was performed on the sixty-four isolates collected from the Microbiology laboratory of CTGB. All the isolates were identified as S. aureus since they presented the ability to coagulate rabbit plasma and were confirmed by a species-specific nuc gene PCR assay.
2.2. Antibiotic Resistance Rates
The occurrence of antibiotic resistance in the 64 S. aureus isolates is presented in Figure 1. All S. aureus strains were confirmed to be MRSA and presented oxacillin and/or cefoxitin resistance. A high resistance rate for the β-lactam, quinolone, and aminoglycoside families was observed. The percentages were as follows: cefoxitin (100%), ciprofloxacin (86%), gentamicin (83%), and penicillin (75%), while moderate and low resistance rates were detected for: fosfomycin (28%), oxacillin (26%), fusidic acid (25%), amikacin (23%), tobramycin (22%), ampicillin (22%), kanamycin (20%), tetracycline (17%), erythromycin (8%), and teicoplanin (1.5%). Sixty S. aureus isolates were multiresistant to at least three antibiotic families.
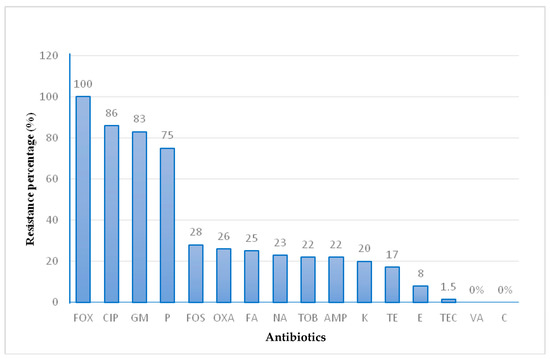
Figure 1.
Phenotypic resistance rate of MRSA isolates. Penicillin (P), Oxacillin (OXA), Cefoxitin (FOX), Gentamicin (GM), Kanamycin (K), Tobramycin (TOB), Tetracycline (TE), Ciprofloxacin (CIP), Erythromycin (E), Fusidic Acid (FA), Ampicillin (AMP), Teicoplanin (TEC), Amikacin (NA), Fosfomycin (FOS), Vancomycin (VA) and Chloramphenicol (C).
2.3. Genetic Support of Antibiotic Resistance
The characteristics of resistance genes in S. aureus isolates are shown in Figure 2. The molecular characterization of methicillin resistance by PCR amplification showed that 50 MRSA strains harbored the mecA gene. Penicillin resistance is coded by the blaZ gene in 47 MRSA isolates. Furthermore, tetracycline resistance was conferred by tet(M) genes in only five isolates; tet(L) and tet(K) genes were not detected. For the five erythromycin-resistant MRSA isolates, erm(C) gene was detected in only one strain and msr(A) gene in four isolates. The erm(A), erm(B), and msr(B) genes were not detected in all the collection.
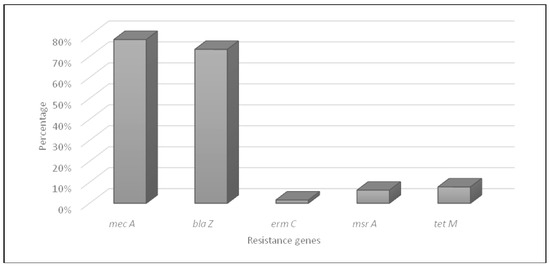
Figure 2.
Distribution of antibiotic resistance genes in MRSA isolates.
2.4. Molecular Typing of MRSA Isolates
Table 1 presents molecular typing by SCCmec cassettes, spa-typing, and agr-typing. The characterization of SCCmec cassettes was performed by multiplex PCR amplification of genes encoding ccr recombination.

Table 1.
Molecular typing of MRSA isolates by ccr genes, spa type, and agr type.
The results revealed that 37 of the tested strains (57%) exhibited different ccr profiles. Indeed, the most dominant recombination was ccrA3-ccrB, assigned to SCCmec type III in 22 strains, of which 4 were mecA gene negative, followed by ccrA2-ccrB recombination assigned to SCCmec type II in 13 strains, of which only 1 was mecA gene negative. In addition, the ccrA1-ccrB recombination allocated to SCCmec type I was detected in two MRSA strains, one of which was mecA-negative (Table 1).
The analysis of the results of spa type for all MRSA strains showed the presence of 10 different spa types (spa type number of isolates): t233 (20), t2524 (6), t067 (3), t1192 (3), t1209 (3), t037 (3), t2453 (1), t2612 (5), t9082 (1), and t808 (1). Nevertheless, the spa type in eighteen strains was not typable.
The amplification of the agr locus by multiplex PCR showed the dominance of the agr II type in 28 strains, followed by the agr III type in 23 strains; the remaining isolates were ascribed to the agr I type.
2.5. Virulence Genes and IEC Profile of MRSA Isolates

Table 2.
Resistance phenotype profiles, resistance genes, agr-typing, virulence factors, and the Immune Evasion Cluster detected in MRSA isolates.
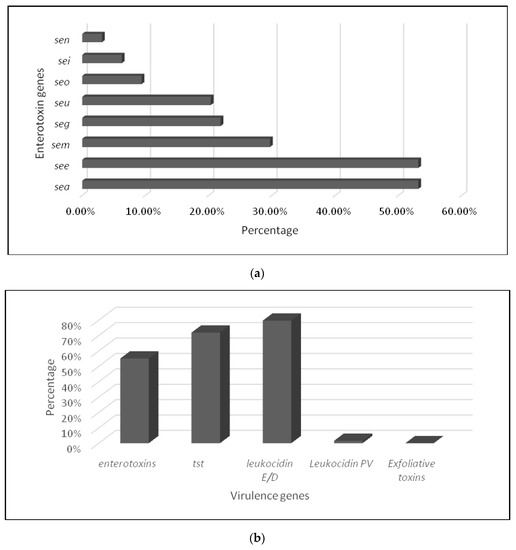
Figure 3.
Prevalence of virulence genes in MRSA isolates: (a) enterotoxins and (b) different virulence genes.
The multiplex PCR of different virulence factors revealed that 63 MRSA isolates (98.44%) harbored virulence genes. Eight enterotoxin genes were detected (number of isolates, percentage) (Figure 3a): seg (14, 21.87%), sea (34, 53.12%), see (34, 53.12%), seo (6, 9.37%), sem (19, 29.68%), sei (4, 6.25%), sen (2, 3.12%), and seu (13, 20.31%). While the genes coding for the toxic shock protein TSST were found in 46 (71.88%) strains, four MRSA harbored the gene lukS-lukF encoding for PVL and were associated with leukocidin E/D factor in three strains. In addition, 51 isolates carried the gene for leukocidin E/D factor; none of them was etA- or etB-positive (Figure 3b). Different gene combinations coding the IEC complex were found in almost all S. aureus strains (n = 60) and assigned to 3 IEC types: IEC type B with the association of scn, sak, and chp; type E with the association of scn and sak; and type D with the association of scn, sak, and sea.
3. Discussion
Staphylococcus aureus is one of the main causes of nosocomial and hospital infections, leading to serious health problems [2]. Our study focused on the characterization of antibiotic resistance, mainly methicillin resistance, in S. aureus strains isolated from different clinical samples of burned patients hosted in the Burn and Traumatology Center in Tunisia. The research on the genetic support of various resistance mechanisms and virulence factors, as well as molecular typing, was intended to provide insight into their clonal diversity and the spread of resistant and virulent clones. Our study included 64 staphylococcus strains recovered from clinical samples taken from burned patients. Biochemical and molecular identification revealed that all isolates are staphylococcus aureus. The presence of S. aureus in burned patients in the Tunisian Center could indicate that this species is involved in cutaneous superinfections of burns due to its commensalism and its diverse virulence factors. Our results are in agreement with those of the study of Thabet et al. [8], performed in two Tunisian hospital structures (CTGB and Aziza Othman Tunis) and reporting that S. aureus is the main bacteria isolated in burns in the new hospital center. Its implication in different types of infections was in agreement with recent reports from China, Iran, and Africa describing S. aureus as the main pathogen bacteria frequently isolated from burned patients and hospital settings [6].
The analysis of antibiotic resistance in the current study revealed that all strains have been confirmed to be methicillin-resistant. This could be due to the widespread and uncontrolled use of β-lactam drugs, which are the first-line treatment for staphylococcal infection [9]. Our feeding is in agreement with different studies in Tunisia [10,11,12], Morocco [13], and Africa [6]. This resistance to beta-lactams has evolved in successive waves as specific resistance mechanisms have been acquired [14]. In the same context, Fallagas et al. [15] showed that in most of the high and medium Human Development Index countries analyzed, the most pronounced increase was observed in Tunisia, with an increase up to 41–46% after 2005, as compared with a prevalence of 12%–18% years before. Thus far, in South Africa, the prevalence of MRSA decreased from 36% in 2006 to 24% during 2007–2011, probably due to the implementation of effective infection control policies. In Algeria and Egypt, according to the same study, the prevalence of MRSA between 2003 and 2005 was 45% and 52%, respectively. Morocco is the only country where a low prevalence of MRSA seems to have stabilized during 2003–2008.
The MRSA strains showed high resistance to all β-lactam antibiotics. The molecular analysis of methicillin resistance showed that 78% of MRSA isolates harbored the mecA gene; the remaining strains (22%) were mecA-negative; this suggests that they may contain further variant cassette genes of the mecA gene, such as the mecC, mecB, or mecD genes recently detected [2]. Indeed, the dissemination of MRSA encoded by the mecA gene in clinical settings has been reported in various African and European countries. This resistance has emerged through the SCCmec cassette genes, which can disseminate by horizontal transfer [16].
In addition to the resistance to methicillin, MRSA isolates showed resistance, especially to penicillin, conferred by the blaZ gene (73.43% of isolates). The resistance to tetracycline and erythromycin is encoded, respectively, by tet(M), erm(C), and msr(A) genes. Our findings conflict with the study of Zmantar et al. [17], which reported a higher frequency of erm(A) and erm(C) genes detected in MRSA isolates from the oral cavity of Tunisian children. Nevertheless, our results were similar to those of Mkhize et al. [17], who described the presence of the erm(C) gene and the absence of the erm(A) and erm(B) genes in S. aureus isolates collected from public hospitals in South Africa [18].
The characterization of SCCmec types in our analyzed collection by multiplex PCR amplification of genes encoding ccr recombination revealed that 37 strains (57%) exhibited different ccr profiles. Indeed, the most dominant recombination was ccrA3-ccrB, assigned to SCCmec type III (3A), followed by the ccrA2-ccrB recombination assigned to SCCmec type II (2A), with only two strains having the ccrA1-ccrB recombination (SCCmec type I (1A)). Nevertheless, SCCmec types I, II, and III were the most common during a study conducted at Charles Nicole Hospital in Tunis [19] and SCCmec type IV is the most often reported cassette in the majority of research performed in clinical settings in Tunisia [6,10,11,20].
Furthermore, SCCmec type I was also reported in the hospital environment in Tunisia [21]. Chen K. et al. [1] demonstrated that the most prevalent clone of MRSA in the burn center in Southeastern China was ST239-SCCmecIII-t030. According to research performed in Brazil in 2013, SCCmec type III had the highest prevalence in burn units [22]. Nevertheless, another study in Iran reported several types of SCCmec (47.5% type III, 25% type IV, 10% type V, 10% type II, and 7.5% type I) in MRSA isolates from burned patients at Motahari Hospital (Iran) [23].
In the present study, the molecular characterization of the polymorphic X region of the spa gene showed the presence of 10 different spa types (t233, t2524, t067, t1192, t1209, t037, t2453, t2612, t9082, and t808) among 46 MRSA isolates. Noteworthy, the spa type t233 was the most prevalent in 20 MRSA isolates. These findings are not consistent with those reported in a recent study that described the distribution of the most prevalent spa types in the world. Indeed, in Africa, t037, t064, and t084 are frequently found. In Europe, it is rather the t032, t008, and t002, and in Asia, the spa type t037 and t002 are the most often reported [24]. This suggests that the spa type t233 could be a new clone circulating in the CTGB hospital environment.
The molecular typing by amplification of the agr locus revealed the dominance of agr types II and III, which is in agreement with the study of Kechrid et al. (2011) at the Children’s Hospital of Tunis [11]. On the other hand, the study reported by Elhani et al. [25] as well as that of Gharsa et al. [21] showed that the agr I group was the most present. The pathogenic and biofilm growth of S. aureus are primarily regulated by the accessory gene regulator (agr) quorum-sensing system. In a cell density-dependent manner, this system inhibits the transcription of numerous cell wall-associated proteins, such as protein A and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), and activates a number of exoproteins, such as hemolysins, exfoliative toxins (ETs), and toxic shock syndrome toxins (TSSTs) [26]. Numerous investigations have documented the association between particular agr classes, particular clonal complexes, disease kinds, and associated virulence factors. For instance, Holtfreter et al. [27] discovered a correlation between particular S. aureus lineages and different agr types. Many studies showed that TSST-1 was predominately carried by agr III-type isolates, while phages and plasmids commonly occur in agr IV isolates that contain etA or etB genes [28]. The agr I type was also the most typical type, particularly among MRSA isolates [28].
Investigation of the virulence determinants and their implication in the infection by MRSA isolates was scarce in Tunisia and Africa. To the best of our knowledge, the current study was the first to characterize the virulence factors in MRSA strains isolated from hospital patients, especially burned ones. Indeed, our findings highlighted the occurrence of MRSA strains with a wider range of virulence genes. The enterotoxin genes were detected in the majority of strains, indicating that despite the fact that enterotoxin genes are encoded by a bacteriophage, the bacteriophage has spread easily among strains with the same genetic background [29]. In addition, we detected that all strains have a high incidence of sea and see genes, which was in accord with other findings [30].
According to the characterization of virulence factors in our collection, the prevalence of the lukF/S-PV gene was low (1.56%). In the same context, Viquez-Molina et al. [31] detected the lukF/S-PV gene in 6.9% of S. aureus isolated from patients with diabetic foot infections; however, another study from Lisbon did not detect the lukF/S-PV gene from the same origin [32]. Many researchers have suggested that the PVL locus is carried on a bacteriophage, and this locus is associated with skin infections and occasionally severe necrotizing pneumonia [33,34]. The presence of PVL gene in MRSA strains was mostly associated with skin and soft tissue infections and community-associated clones [6]. Reported data from Tunisia and Algeria described high PVL prevalence in MRSA isolates, while studies from South Africa revealed low prevalence [6].
The toxic shock syndrome (TSST), which is generated by Staphylococcus aureus, has been associated with a number of acute illnesses [35]. The tst gene was found to have a very high percentage in all strains (71.88%). Our results contradicted other research [36] that described a high prevalence of tst-carrying isolates among methicillin-susceptible isolates as compared to MRSA isolates, indicating a possible link between the drug resistance of the strains and the occurrence of their virulence genes. In addition, our results reported that tsst toxin is produced by MRSA strains affiliated with agr types I, II, and III, whereas other research reported that tsst is produced preferentially by isolates harboring agr III in MRSA strains isolated from hospitals [28].
The genes encoding etA and etB exfoliatins were no longer detected in our collection; this can be suggested by the fact that the plasmids and phages carrying these exfoliatins are linked to isolates expressing agr type IV [28].
There is evidence that some agr types are associated with many clinical characteristics. Most toxic shock syndrome (TSST-1) strains, for instance, are classified as agr group III, while most strains with leukocidin-induced necrotizing pneumonia are classified as agr group II [37,38]. In our study, lukE/D was related to agr type III. Our findings disagree with the report of Ben Nejma et al. [10], who have revealed that PVL negative strains are classified as agr type III. Similarly, Xu et al. [31] demonstrated that all lukS/F-PV-positive isolates belong to agr group I.
4. Material and Methods
4.1. Bacterial Isolates
The study was carried out on a collection of 64 non-duplicated S. aureus isolates collected from 64 different clinical samples from burned patients hospitalized in the Traumatology and Burn Center (CTGB) of Tunisia between January and December 2016. The clinical samples were distributed as follows: blood culture (18), cytobacteriological examination of sputum (3), puncture (4), nasal pus (1), catheters (6), and sebum (32).
The isolates were recovered on petri dishes of Brain Heart Infusion Agar (BHI) from the Microbiology laboratory of CTGB and transferred to the laboratory in a cooler for analysis.
4.2. Strain Identification
The isolates were identified by conventional biochemical tests (Gram staining oxidase, catalase, DNase, and ability to coagulate rabbit plasma (Bio-Rad, France) [4,39]. Molecular identification was performed by species-specific PCR amplification of the nuc gene, as previously described [39], with S. aureus (ATCC 43300) being used as a control strain.
4.3. Antimicrobial Susceptibility Testing
The determination of antibiotic susceptibility was performed using the disk diffusion method in accordance with the Clinical and Laboratory Standards Institute (CLSI) recommendations [40]. The antimicrobial agents tested (charge in µg) were as follows: penicillin (10), oxacillin (1), cefoxitin (30), vancomycin (30), gentamicin (10), kanamycin (30), tobramycin (10), tetracycline (30), ciprofloxacin (5), erythromycin (15), amikacin, fusidic acid (10), teicoplanin (30), fosfomycin (200), chloramphenicol (30), and ampicillin (30).
4.4. Detection of the mecA Gene
Methicillin resistance was detected by oxacillin and cefoxitin susceptibilities on disk diffusion agar, according to CLSI [40]. Confirmation of methicillin resistance was performed by conventional PCR targeting the mecA gene [41]. S. aureus ATCC 43300 was used as a control strain.
4.5. SCCmec-Typing in MRSA Isolates
The presence of SCCmec types I to V was investigated in MRSA isolates by PCR of the ccr recombinases (1–5) and the mec gene complex type (A to C), as recommended by the International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) [42] (Table S1).
4.6. Molecular Typing of S. aureus Isolates
S. aureus protein A spa-typing was performed in all S. aureus isolates (n = 64) as elsewhere described [43]. The polymorphic X region of the spa gene was amplified by PCR, sequenced, and analyzed using Ridom staph-type software version 1.5.21 (Ridom GmbH). It automatically detects spa repeats and assigns a spa type, according to http://spaserver.ridom.de/ (accessed on 10 December 2005). To determine the type of agr in MRSA, two multiplex PCRs were performed; the first one allowed the amplification of agr types I and II, and the second PCR amplified agr types III and IV [44].
4.7. Detection of Antimicrobial Resistance Genes
The detection of antimicrobial resistance genes (blaZ, erm(A), erm(B), erm(C), msr(A), msr(B), tet(K), tet(M), and tet(L)) was investigated in resistant isolates by specific PCRs [45]. Positive and negative controls used in each PCR assay were from the collection of the laboratory of Institute Pasteur of Tunis.
4.8. Detection of Staphylococcal Toxin Genes
All isolates were tested by PCR for the presence of genes coding for the various staphylococcal enterotoxins (sea, see, seg, sei, sem, sen, seo, and seu), toxic shock syndrome toxin 1 (tst), leukocidin of Panton Valentine (PVL, lukF-lukS-PV), and exfoliative ETA and ETB toxins (etA and etB) [46].
4.9. Detection of the Immune Evasion Gene Cluster
All isolates were tested by PCR for the presence of five genes (scn, chp, sak, sea, and sep) of the immune evasion cluster (IEC) system. These genes are enclosed in the φ3 bacteriophage and encode the IEC system, which helps bacteria survive in the human host by evading the innate immune system. Detected alleles allowed the classification of seven IEC types (from A to G) [47], Table S2.
5. Conclusions
In this study, all the isolates from clinical samples of burned patients were confirmed as MRSA with high rates of resistance to ciprofloxacin and gentamicin conferred by different antibiotic resistance genes. In addition, our data reported the detection of resistance genes and a different virulence profile in MRSA isolates. It is important to report the molecular diversity of spa and agr types in the study collection.
This genetic diversity can lead to the emergence and spread of virulent and drug resistant clones within the hospital. Therefore, enhanced antimicrobial surveillance efforts are needed to control and regulate the use of antimicrobials in Tunisian hospital settings in order to reduce the risks associated with the acquisition of multidrug-resistant clones containing virulence determinants and the spread of pathogenic bacteria in humans and their environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12061030/s1. Table S1: Different SCCmec types identified in S. aureus. Table S2: IEC type detected according to the combination of five genes (scn, chp, sak, sea, and sep) of the immune evasion cluster (IEC) system in S. aureus.
Author Contributions
Conceptualization, A.J.; methodology, S.K., A.J., N.Z., S.H. and L.T.; validation, A.J.; formal analysis, A.J. and N.Z.; investigation, A.J., S.K. and N.Z.; resources, S.H. and A.M.; writing–original draft, S.K.; writing–review and editing, S.K., A.J. and A.M.; supervision, A.J. and A.M.; project administration, A.J. and A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Tunisian Ministry of Higher Education, Scientific Research, and Technology (LR16IPT03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Abdeljelil Ghram for his effort in English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, K.; Lin, S.; Li, P.; Song, Q.; Luo, D.; Liu, T.; Zeng, L.; Zhang, W. Characterization of Staphylococcus aureus Isolated from Patients with Burns in a Regional Burn Center, Southeastern China. BMC. Infect. Dis. 2018, 18, 51. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news/item/29-04-2019 (accessed on 29 April 2020).
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of Virulence in Enterotoxin-Mediated Staphylococcal Food Poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Abdulgader, S.M.; Shittu, A.O.; Nicol, M.P.; Kaba, M. Molecular Epidemiology of Methicillin-Resistant Staphylococcus aureus in Africa: A Systematic Review. Front. Microbiol. 2015, 6, 348. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, F.P.; Suaya, J.A.; Ray, G.T.; Baxter, R.; Brown, M.L.; Mera, R.M.; Close, N.M.; Thomas, E.; Amrine-Madsen, H. Spa typing and multilocus sequence typing show comparable performance in a macroepidemiologic study of Staphylococcus aureus in the United States. Microb. Drug Resist. 2016, 22, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Thabet, L.; Zoghlami, A.; Boukadida, J.; Ghanem, A.; Messadi, A.A. Comparative study of antibiotic resistance in bacteria isolated from burned patients during two periods (2005–2008, 2008–2011) and in two hospitals (Hospital Aziza Othmana, Trauma and Burn Center). Tunis. Med. 2013, 91, 134–138. [Google Scholar]
- Bæk, K.T.; Gründling, A.; Mogensen, R.G.; Thøgersen, L.; Petersen, A.; Paulander, W.; Frees, D. β-Lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob. Agents Chemother. 2014, 58, 4593–4603. [Google Scholar] [CrossRef]
- Ben Nejma, M.; Mastouri, M.; Bel Hadj Jrad, B.; Nour, M. Characterization of ST80 Panton-Valentine Leukocidin-positive community-acquired methicillin resistant Staphylococcus aureus clone in Tunisia. Diagn. Microbiol. Infect. Dis. 2013, 77, 20–24. [Google Scholar] [CrossRef]
- Kechrid, A.; Pérez-Vázquez, M.; Smaoui, H.; Hariga, D.; Rodríguez-Baños, M.; Vindel, A.; Baquero, F.; Cantón, R.; del Campo, R. Molecular Analysis of Community-Acquired Methicillin-Susceptible and Resistant Staphylococcus aureus Isolates Recovered from Bacteraemic and Osteomyelitis Infections in Children from Tunisia. Clin. Microbiol. Infect. 2011, 17, 1020–1026. [Google Scholar] [CrossRef]
- Mariem, B.J.J.; Ito, T.; Zhang, M.; Jin, J.; Li, S.; Ilhem, B.-B.B.; Adnan, H.; Han, X.; Hiramatsu, K. Molecular Characterization of Methicillin-Resistant Panton-Valentine Leukocidin positive Staphylococcus aureus clones disseminating in Tunisian Hospitals and in the Community. BMC Microbiol. 2013, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Benouda, A.; Elhamzaui, S. Staphylococcus aureus: Epidemiologie et prevalence des souches resistantes a la methicilline (SARM) au Maroc. Rev. Tunis. D’Infect. 2009, 3, 15–20. [Google Scholar]
- Rice, L.B. Mechanisms of Resistance and Clinical Relevance of Resistance to β-Lactams, Glycopeptides, and Fluoroquinolones. Mayo Clin. Proc. 2012, 87, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Karageorgopoulos, D.E.; Leptidis, J.; Korbila, I.P. MRSA in Africa: Filling the Global Map of Antimicrobial Resistance. PLoS ONE 2013, 8, e68024. [Google Scholar] [CrossRef]
- Baig, S.; Johannesen, T.B.; Overballe-Petersen, S.; Larsen, J.; Larsen, A.R.; Stegger, M. Novel SCCmec Type XIII (9A) identified in an ST152 Methicillin-Resistant Staphylococcus aureus. Infect. Genet. Evol. 2018, 61, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Zmantar, T.; Kouidhi, B.; Hentati, H.; Bakhrouf, A. Detection of disinfectant and antibiotic resistance genes in Staphylococcus aureus isolated from the oral cavity of Tunisian children. Ann. Microbiol. 2012, 62, 123–128. [Google Scholar] [CrossRef]
- Goolam Mahomed, T.; Kock, M.M.; Masekela, R.; Hoosien, E.; Ehlers, M.M. Genetic relatedness of Staphylococcus aureus isolates obtained from cystic fibrosis patients at a tertiary academic hospital in Pretoria, South Africa. Sci. Rep. 2018, 8, 12222. [Google Scholar] [CrossRef]
- Jemili-Ben Jomaa, M.; Boutiba-Ben Boubaker, I.; Ben Redjeb, S. Identification of staphylococcal cassette chromosome mec encoding methicillin resistance in Staphylococcus aureus isolates at Charles Nicolle Hospital of Tunis. Pathol. Biol. 2006, 54, 453–455. [Google Scholar] [CrossRef]
- Bouchami, O.; Achour, A.; Ben Hassan, A. Typing of staphylococcal cassette chromosome mec encoding methicillin resistance in Staphylococcus aureus strains isolated at the bone marrow transplant centre of Tunisia. Curr. Microbiol. 2009, 59, 380–385. [Google Scholar] [CrossRef]
- Gharsa, H.; Dziri, R.; Klibi, N.; Chairat, S.; Lozano, C.; Torres, C.; Bellaaj, R.; Slama, K.B. Environmental Staphylococcus aureus contamination in a Tunisian hospital. J. Chemother. 2016, 28, 506–509. [Google Scholar] [CrossRef]
- Rodrigues, M.V.P.; Fortaleza, C.M.C.B.; Riboli, D.F.M.; Rocha, R.S.; Rocha, C.; de Souza da Cunha, M.d.L.R. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a burn unit from Brazil. Burns 2013, 39, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Namvar, A.E.; Afshar, M.; Asghari, B.; Rastegar Lari, A. Characterisation of SCCmec elements in methicillin-resistant Staphylococcus aureus isolated from burn patients. Burns 2014, 40, 708–712. [Google Scholar] [CrossRef]
- Asadollahi, P.; Farahani, N.N.; Mirzaii, M.; Khoramrooz, S.S.; van Belkum, A.; Asadollahi, K.; Dadashi, M.; Darban-Sarokhalil, D. Distribution of the most prevalent spa-types among clinical isolates of methicillin-resistant and susceptible Staphylococcus aureus around the world: A Review. Front. Microbiol. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Elhani, D.; Gharsa, H.; Kalai, D.; Lozano, C.; Gómez, P.; Boutheina, J.; Aouni, M.; Barguellil, F.; Torres, C.; Ben Slama, K. Clonal lineages detected amongst tetracycline resistant meticillin resistant Staphylococcus aureus isolates of a Tunisian hospital, with detection of lineage ST398. J. Med. Microbiol. 2015, 64, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, A.; Vasdeki, A.; Kristo, I.; Maniatis, A.N.; Tsakris, A.; Malizos, K.N.; Pournaras, S. Association of Biofilm Formation and Methicillin-Resistance with Accessory Gene Regulator (Agr) Loci in Greek Staphylococcus aureus Clones. Microb. Pathog. 2009, 47, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, S.; Grumann, D.; Schmudde, M.; Nguyen, H.T.T.; Eichler, P.; Strommenger, B.; Kopron, K.; Kolata, J.; Giedrys-Kalemba, S.; Steinmetz, I.; et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 2007, 45, 2669–2680. [Google Scholar] [CrossRef]
- Robinson, D.A.; Enright, M.C. Evolutionary models of the emergence of methicillin resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 3926–3934. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S.-Y.; Yao, K.-H.; Dong, F.; Song, W.-Q.; Sun, C.; Yang, X.; Zhen, J.-H.; Liu, X.-Q.; Lv, Z.-Y.; et al. Clinical and molecular characteristics of Staphylococcus aureus isolated from chinese children: Association among the agr groups and genotypes, Virulence Genes and Disease Types. World J. Pediatr. 2021, 17, 180–188. [Google Scholar] [CrossRef]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.-P. Staphylococcus aureus toxins and diabetic foot ulcers: Role in pathogenesis and interest in diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef]
- Víquez-Molina, G.; Aragón-Sánchez, J.; Pérez-Corrales, C.; Murillo-Vargas, C.; López-Valverde, M.E.; Lipsky, B.A. Virulence factor genes in Staphylococcus aureus isolated from diabetic foot soft tissue and bone infections. Int. J. Low. Extrem. Wounds 2018, 17, 36–41. [Google Scholar] [CrossRef]
- Mottola, C.; Semedo-Lemsaddek, T.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Molecular typing, virulence traits and antimicrobial resistance of diabetic foot staphylococci. J. Biomed. Sci. 2016, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Piemont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.-C.; Lina, G.; Bes, M.; Vandenesch, F.; Piémont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine Leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Schlievert, P.M. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome: Superantigen outside-in signaling. FEBS J. 2011, 278, 4649–4667. [Google Scholar] [CrossRef]
- Budzyńska, A.; Skowron, K.; Kaczmarek, A.; Wietlicka-Piszcz, M.; Gospodarek-Komkowska, E. virulence factor genes and antimicrobial susceptibility of Staphylococcus aureus strains isolated from blood and chronic wounds. Toxins 2021, 13, 491. [Google Scholar] [CrossRef]
- Ji, G.; Beavis, R.C.; Novick, R.P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 1995, 92, 12055–12059. [Google Scholar] [CrossRef]
- Sakoulas, G. The cacessory gene regulator (agr) in methicillin resistant Staphylococcus aureus: Role in virulence and reduced susceptibility to glycopeptide Antibiotics. Drug. Discov. Today 2006, 3, 287–294. [Google Scholar] [CrossRef]
- Klibi, A.; Jouini, A.; Gómez, P.; Slimene, K.; Ceballos, S.; Torres, C.; Maaroufi, A. Molecular characterization and clonal diversity of methicillin resistant and susceptible Staphylococcus aureus isolates of milk of cows with clinical mastitis in Tunisia. Microb. Drug. Resist. 2018, 24, 1210–1216. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI document M100-S25: Clinical Laboratory Standard Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.; Witte, W.; Rothganger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Shopsin, B.; Mathema, B.; Alcabes, P.; Said-Salim, B.; Lina, G.; Matsuka, A.; Martinez, J.; Kreiswirth, B.N. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 2003, 41, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Gharsa, H.; Ben Slama, K.; Lozano, C.; Gómez-Sanz, E.; Klibi, N.; Ben Sallem, R.; Gómez, P.; Zarazaga, M.; Boudabous, A.; Torres, C. Prevalence, antibiotic resistance, virulence traits and genetic lineages of Staphylococcus aureus in healthy sheep in Tunisia. Vet. Microbiol. 2012, 156, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Kim, S.H.; Jang, E.J.; Kwon, N.H.; Park, Y.K.; Koo, H.C.; Jung, W.K.; Kim, J.M.; Park, Y.H. Novel multiplex PCR for the detection of the Staphylococcus aureus superantigen and its application to raw meat isolates in Korea. Int. J. Food Microbiol. 2007, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; Van Kessel, K.P.M.; Van Strijp, J.A.G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).