Abstract
Few studies have addressed drug resistance of Enterobacterales in rural communities in developing countries. This study aimed to determine the coexistence of extended-spectrum β-lactamase (ESBL) and carbapenemase genes in Escherichia coli and Klebsiella pneumoniae strains carrying the mcr-1 gene in rural communities in Ecuador from healthy humans and their backyard animals. Sixty-two strains, thirty E. coli and thirty-two K. pneumoniae strains carrying the mcr-1 gene were selected from a previous study. PCR were performed for the presence of ESBLs and carbapenemase genes. The strains were further characterized, and the genetic relationship was studied with multi-locus sequencing typing (MLST) of seven housekeeping genes. Fifty-nine of the sixty-two mcr-1 isolates (95%) harbored at least on β-lactam resistance gene. The most prevalent ESBL genes were the blaTEM genes (present in in 80% of the E. coli strains) and the blaSHV gene (present in 84% of the K. pneumoniae strains). MSLT analysis revealed 28 different sequence types (ST); 15 for E. coli and 12 for K. pneumoniae, with most ST never described in humans and animals. The coexistence of mcr-1 and β-lactams resistant genes in E. coli and K. pneumoniae strains is alarming and threatens the efficacy of last-resort antibiotics. Our findings highlight backyard animals as a reservoir of mcr-1/β-lactams resistant genes.
1. Introduction
The emergence of multidrug-resistant (MDR) Enterobacterales isolated from humans and animals has become a great concern worldwide, and resistance to colistin in coexistence with β-lactams resistant genes compromises the effectiveness of antimicrobial drugs. Multidrug-resistant (MDR) Enterobacterales such as Escherichia coli and, more recently, Klebsiella pneumoniae have emerged as one of the significant causes of healthcare-associated communitywide infections [1,2]. It has also been shown that these MDR bacteria not only have shown up in hospital settings but can be isolated from healthy humans or from backyard animals, and both hosts can act as a reservoir for resistance genes [3,4].
During the last few decades, the emergence of colistin-resistant isolates has been frequently reported. The antibiotic colistin is a highly effective drug against most Enterobacterales species and non-fermenting Gram-negative bacteria and is considered a last resort antimicrobial for the treatment of difficult-to-treat infections caused by MDR. However, the uncontrolled use of colistin in human and veterinary medicine has resulted in the emergence of colistin resistance. Of serious concern are plasmid-borne or mobile colistin resistance (mcr) genes, whose first variant (mcr-1) was reported in China in 2016 [5]. These mcr genes assist in the dissemination of colistin resistance to other pathogenic bacteria. After this first report, the mcr genes have been found in more than 70 countries and 10 different variants have been described (mcr-1–mcr-10). In Ecuador, several recent studies have shown an increase of the prevalence of mcr genes in different hosts and sources [6,7]. The widespread of these resistance genes can be attributed to selective pressure due to the use of colistin in livestock and, consequently, the increase of the horizontal transfer of plasmids that contains this type of resistance gene [8].
Another worrying phenomena is that the plasmid-mediated resistance to colistin can co-occur with other resistance genes such as extended-spectrum beta-lactamases (ESBL) and carbapenemases [9,10]. The appearance of this type of resistance is also largely due to the irrational use of antibiotics for preventive prophylactic and/or growth-promoting purposes [11,12]; and threatens the One Health approach that seeks the well-being of humans and animals [13]. Beta-lactam antibiotics are used worldwide to treat Gram-negative bacterial infections and represent about 65% of the total antibiotics on the market [14]. The extensive use of those antibiotics for medical, veterinary, and animal-production purposes has triggered a natural selection of ESBL- and carbapenemase-gene–producing microorganisms as pathogens. These phenomena have caused resistances against cephalosporins, penicillin, carbapenems, and monobactams. ESBL genes are ubiquitous, with ESBL-producing Enterobacterales having been found in a wide range of settings—including livestock [15], companion animals [16], hospitals (involving human-to-human transmission) [17], the environment [18], and food—as well as being spread through travel [19]. Although β-lactamases are widely detected and reported in hospital areas, those same enzymes are not so frequently reported in animals, but mainly in those intended for food production [20].
CTX-M-type enzymes have surpassed TEM and SHV as the most prevalent group of extended-spectrum β-lactamases (ESBLs) and have been reported in various members of the Enterobacterales order, as well as in P. aeruginosa and Acinetobacter species [21]. In South-American countries, CTX enzymes are also more common than other ESBL types [22,23]. These β-lactamase CTX-M-producing bacteria have emerged globally as the primary cause of urinary tract infections, leading to what is now known as the “CTX-M pandemic” [24]. Among the CTX-M variants, the CTX-M-15 type within the CTX-M-1 sublineage is particularly concerning due to its heightened activity against ceftazidime and its association with other β-lactamases, such as VIM, OXA, and KPC [25]. Additionally, several dangerous clones have been identified, including the E. coli clones ST131 and ST10 [26,27].
The presence of both mobile colistin-resistant (mcr) genes and β-lactamase-encoding genes in Enterobacterales presents a significant threat to global health, as their dissemination could lead to untreatable infections, increased mortality rates, prolonged hospital stays, and higher healthcare costs. Therefore, monitoring the spread of these resistance genes is critical. Despite the importance of this issue, the co-occurrence of β-lactamases and mcr-1 genes has not been extensively studied in Ecuador. In this study, we investigated the presence of this combination of resistance genes in two rural communities in the amazon and the coast of Ecuador. Therefore, this study aimed to examined for six types of ESBL and four carbapenemase genes coexisting with the mcr-1 gene in E. coli and K. pneumoniae isolates from both humans and their backyard animals in rural low-income communities of Ecuador.
2. Results
The majority of colistin-resistant E. coli and K. pneumoniae isolates (harboring the mcr-1 gene) in our study were found to co-harbor with eight different β-lactam resistance genes. Specifically, 95% [87.8–100] 95% CI of all isolates (n = 59/62) carried at least one β-lactam resistance gene across the three hosts included in the study. For individual E. coli isolates, co-harboring of mcr-1 and β-lactam resistance genes was observed in 100% of human (n = 10/10) and pig (n = 9/9), and 80% [49–100] 95% CI of the chicken (n = 9/11) isolates. For K. pneumoniae, co-harboring of mcr-1 and β-lactam resistance genes was observed in 100% of chicken (n = 3/3) and pig (n = 6/6) isolates, as well as in 91% [75–99] 95% CI of the human isolates (n = 21/23). Notably, we only detected the carbapenemase gene blaKPC in one K. pneumoniae isolate, which was found in co-existence with another β-lactam resistance gene.
2.1. Genomic Coexistence of β-Lactamase and Mcr-1 Resistance Genes
For E. coli, 30 mcr-1 positive colistin-resistant isolates, 7 ß-lactam–resistance genes were detected, with blaTEM being the most frequent in 24 positive isolates (80%); followed by blaCTX-M-9 in 6 isolates (20%), blaCTX-M-1 in 5 (17%), and blaOXA-48 in 2 (7%). Finally, blaNDM or blaCTX-M-8/25 was present in only a single isolate. No isolates were positive for blaCTX-M-2, blaVIM, or blaKPC. Table 1 lists the detailed frequency and percentages for E coli.

Table 1.
Frequencies and percentages of mcr-1-positive E. coli isolates coharboring β-lactamase genes from three different hosts in rural communities of Ecuador (N = 30). * Frequency, (percentage), [95% CI].
Among the 32 colistin-resistant K. pneumoniae isolates that tested positive for mcr-1, we detected the coexistence of seven different β-lactam resistance genes. The most prevalent resistance gene was blaSHV, which was found in 27 isolates with 84% [67.8–100] 95% CI, followed by blaTEM in 19 isolates (59%) [37–81.7] 95% CI and blaCTX-M-9 in 4 isolates (13%) [0–17.4] 95% CI. We also found blaCTX-M-1 in three isolates (9%), which co-existed with blaCTX-M-8/25 in one isolate, and with blaNDM and blaKPC in one isolate each. Notably, none of the isolates were positive for blaCTX-M-2, blaVIM, or blaOXA-48 (Table 2).

Table 2.
Frequencies and percentages of mcr-1-positive K. Pneumoniae isolates coharboring β-lactamase genes from three different hosts in rural communities of Ecuador (N = 32). * Frequency, (percentage), [95% CI].
Figure 1 and Figure 2 provide a comprehensive overview of the phylogenetic relationships, resistance genes, and hosts of the E. coli and K. pneumoniae strains isolated from healthy humans, pigs, and chickens. It is noteworthy that K. pneumoniae demonstrated a higher prevalence of the coexistence of β-lactam resistance genes than E. coli. K. pneumoniae LR63 from a healthy human in Santo Domingo de los Tsachilas showed the highest number of resistance genes with four different β-lactam resistance genes. Additionally, 4 isolates had three, 15 isolates had two, and 10 isolates had one β-lactam resistance gene. Intriguingly, a sample (PSJ22) obtained from a healthy human showed the presence of both an E. coli and K. pneumoniae strain with two β-lactam resistance genes each: blaCTX-M-1, blaTEM in E. coli, and blaSHV, blaTEM in K. pneumoniae, respectively.
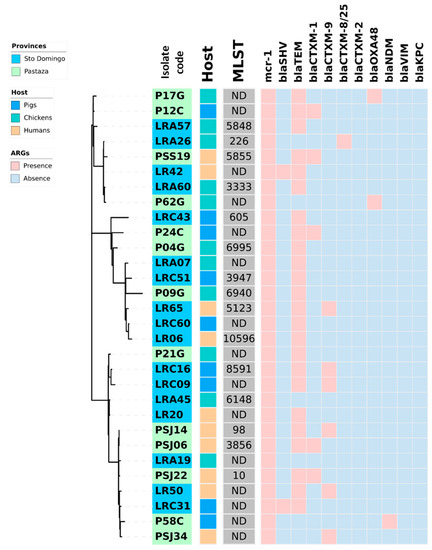
Figure 1.
Heat map showing the coexistence of β-lactamases genes and mcr-1 in 30 E. coli strains isolated from fecal swabs from three hosts (healthy humans, pigs, and chickens) of two rural areas (Pastaza and Santo Domingo de los Tsachilas) of Ecuador. In the center of the figure a phylogram of concatenated sequences of 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) for the 30 analyzed E. coli strains is shown. The evolutionary history was inferred by using the maximum likelihood method and the Tamura-Nei model. The tree with the highest log likelihood (−14986.75) is shown. The lab number of the strains is indicated in the first inner circle with the origin of the strains in blue (Santo Domingo) or green (Pastaza). The host of the strains, pigs, chickens, and humans, is indicated in the second inner circle with, respectively, a blue, green, or orange color. Abbreviations: MLST, multi-locus sequence type in grey; ARG; antibiotic-resistance genes.
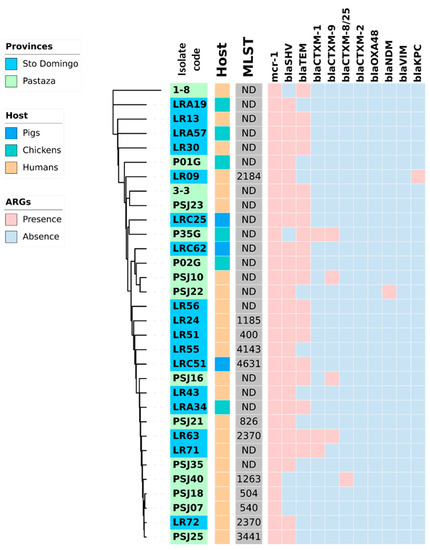
Figure 2.
Heat map showing the coexistence of β-lactamases genes with the mcr-1 gen in 32 K. pneumoniae strains isolated from fecal swabs from three hosts (healthy humans, pigs, and chickens) and two rural areas (Pastaza and Santo Domingo de los Tsachilas) of Ecuador. In the center of the figure a phylogram of concatenated sequences of 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) for the 32 K. pneumoniae strains is shown. The evolutionary history was inferred by using the maximum likelihood method and the Tamura-Nei model. The tree with the highest log likelihood (−14986.75) is shown. The lab number of the strains is indicated in the first inner circle with the origin of the strains in blue (Santo Domingo) or green (Pastaza). The host of the strains, pigs, chickens, and humans, is indicated in the second inner circle with, respectively, a blue, green, or orange color. Abbreviations: MLST, multi-locus sequence type in grey; ARG; antibiotic-resistance genes.
2.2. MLST Analysis
From the 7 conserved genes of the 62 isolates (30 from E. coli and 32 from K. pneumoniae), 434 sequences were obtained (Supplementary Tables S1 and S2). From those sequences and the construction of allelic profiles, 27 different strain types (ST) were identified (15 for E. coli and 12 for K. pneumoniae), whereas 35 isolates (15 E. coli and 20 K. pneumoniae) neither presented matches for any ST in the Pasteur MLST database. The minimum-spanning tree (MST) illustrates the classification and distribution of each strain type detected. For E. coli, we observed that ST10 was the central one for most strains. Accordingly, that strain type is the node that interconnects a large part of the strains (Figure 3). In addition, two strain types (ST98 and ST10) share the same clonal complex (CC), CC10.

Figure 3.
Minimum spanning tree (MST) generated using the PHYLOViz tool, from the allelic profiles of 30 mcr1-positive E. coli isolates. Allelic profiles were constructed according to the 7-genes scheme (adk, fumC, gyrB, icd, mdh, purA, recA) from the PubMLST database. Each ST is represented by a circle. The lengths of the lines between each ST proportionally demonstrates the number of different alleles. Each circle is labeled with the corresponding ST or with the designation ND (not determined). The samples were collected in two provinces of Ecuador, Santo Domingo (dark green circle) and Pastaza (light green circle) between March and December 2019. Based on SNP differences, most strains were derivates of the Escherichia coli ST10 and ST3856 clones.
The MST shown in Figure 4 establishes the relationship between the STs of K. pneumoniae. In this case, the ST founder is ST504. Two isolates shared the sequence type ST2370. For the majority of the K. pneumoniae strains, the ST was not found in the Pasteur MLST database. (See also the limitations of this study.) The phylogenetic analysis showed intermixing between strains of animals and humans. Examples are clones ST400, ST1263, and ST2370.

Figure 4.
Minimum spanning tree (MST) generated using the PHYLOViz tool from the allelic profiles of 30 mcr1-positive K. pneumoniae isolates. Allelic profiles were constructed according to the 7-genes scheme (tonB, phoE, rpoB, pgi, infB, gapA, mdh) from the Institute Pasteur database. Each ST is represented by a circle. The lengths of the lines between each ST proportionally demonstrate the number of different alleles. Each circle is labeled with the corresponding ST or with the designation ND (not determined). The samples were collected in two provinces of Ecuador, Santo Domingo (dark green) and Pastaza (light green) in March and December 2019. Based on SNP differences, most strains were derivates of the K. pneumoniae ST504, ST400, and ST2370 clones.
3. Discussion
Our study represents the first report of the coexistence of the mcr-1 gene with ESBL and carbapenemase loci in two major bacterial pathogens, E. coli and K. pneumoniae, detected in backyard animals and healthy humans in rural Ecuadorian communities. We found a high frequency of ESBL genes in both bacterial species, with the blaSHV, blaTEM, and blaCTX-M as the most prevalent genes, and only a single co-occurrence of carbapenemase genes.
3.1. Co-Harboring of Resistance Genes
Our investigation revealed that the distribution of ESBL and carbapenemase resistance genes was similar across hosts, including humans, chickens, and pigs, as well as between two provinces, Pastaza, and Santo Domingo de los Tsachilas, located in the Amazon and Coastal regions of Ecuador, respectively. These results suggest a general dispersion of these genes within nonhospital settings regardless of region or host. Our findings are consistent with several other studies conducted in South America, which have also identified β-lactam resistance genes in various enterobacteria and hosts [23,28,29].
The ESBL genes have been found historically in clinical isolates in Ecuador of Enterobacterales, such as E. coli [30], K. pneumoniae [31], Acinetobacter baumanii [32], and Raoultella ornithinolytica [33]. Others studies in Ecuador indicated the presence of β-lactam-resistance genes in food animals [27,34] and in the environment [6,18].
The distribution of ESBL genes varies in different bacterial species. In particular, mcr-1 and blaTEM were found to coexist most frequently in E. coli, while blaSHV was the most common in K. pneumoniae. Previous research has also suggested a higher prevalence of TEM enzymes in E. coli compared to other ESBL enzymes. Studies in South America have shown that the blaTEM variant is more prevalent in turkeys, chickens, and humans in Brazil [35,36] and Ecuador [34], respectively. However, in Germany and Iran, 93% and 51,3% of ESBL-positive E. coli strains isolated from adult urinary tract infections were of the blaSHV type, respectively [37]. In Libya, the blaTEM gene was detected in 30% of the isolates, the blaOXA gene in 43% isolates, and the blaCTX-M gene in 26% isolates in humans; but, this study analyzed a relatively low number of 23 ESBL-positive E. coli strains [38].
Our study showed a high incidence of blaTEM in E. coli, which contrasts with other studies that have reported blaCTX-M as the most prevalent ESBL variant [39,40,41,42]. This difference may be attributed to the varying effectiveness of CTX-M enzymes in different epidemiological environments [43,44]. A recent study conducted in broiler farms in Ecuador found that blaCTX-M-9 and blaCTX-M-1 were the most common sublineages [27]. However, this was not observed in our study and, instead, blaTEM was found to be more prevalent in our samples. It is worth noting that a previous study conducted in Venezuela reported blaCTX-M-1 as the prevalent ESBL gene in healthy children [45], which is also inconsistent with our findings. This disparity may be due to differences in the origin and types of samples used, with previous studies focusing on clinical settings and urban areas. Additionally, the low frequency of screening at the interface of backyard animals and humans may contribute to differences in the epidemiology of ESBL genes [23]. Our results suggest that resistant clones and/or resistance elements in E. coli strains may be geographically segregated.
In K. pneumoniae, the most frequently detected ESBL gene combination was mcr-1 and blaSHV (n = 27/32), which is in agreement with previous studies that have found blaSHV to be the most dominant β-lactamase gene worldwide, followed by blaTEM [46]. In our study, we only found six blaCTX-M genes in K pneumoniae, highlighting the dependence of ESBL gene prevalence on location, community, and sample type. The lack of reports on ESBL and carbapenemase genes in K. pneumoniae in non-hospital settings may bias the estimation of their real prevalence [47].
It is possible that the incidence of blaCTX-M in Ecuador has decreased over time due to a lack of selective pressure towards these genes. However, it is important to note that this hypothesis cannot be confirmed without previous studies on the human–backyard–animal interface. Another explanation for the prevalence of blaTEM and blaSHV in E. coli and K. pneumoniae, respectively, compared to blaCTX-M, could be the greater selection and maintenance of these genes. This selection may be due to a plasmid addiction system that contributes to the stability and maintenance of the former genes [48,49]. Plasmid adaptations, such as the presence of a multicopy plasmid linked to blaTEM and blaSHV genes, are associated to fitness increase under conditions of strong selection for β-lactam resistance and provide an evolutionary advantage by the horizontal gene transfer [50,51].
Within this context, it is noteworthy that the selective pressure exerted by colistin use in livestock on these bacteria does not significantly influence by the selection of other antibiotic resistance groups such as blaCTX-M or carbapenemases, including blaOXA-48, blaKPC, blaNDM, or blaVIM, unlike β-lactams like cefotaxime. This independence could partly explain the low incidence of these types of enzymes [52]. Although colistin use has been banned in Ecuador since 2019, our findings suggest that bacteria circulating within the human–backyard–animal interface reflect the state of antimicrobial resistance in the rural environment and act as reservoirs for the spread of not only mcr genes, but also other plasmid-mediated antimicrobial resistance loci such as ESBLs and carbapenemases.
3.2. MLST Analysis
The MLST analysis for E. coli conducted in this study revealed a high heterogenicity, where ST10 was found to be a central node. This clone has been previously reported in various sources, including humans [53,54], pigs [55], chickens [56,57], sewage [58], and rivers [23]. ST10, belonging to CC10 (clonal complex 10), is globally a highly prevalent clone and has been identified as one of the main reservoirs of mcr-1, particularly in fecal and food samples [59]. A 2017 global phylogenetic analysis of E. coli found that ST10 was the most common clone identified in 40 out of 312 isolates from different continents [60]. Furthermore, an emerging clone of foodborne extraintestinal pathogenic E. coli, ST10, was found to comprise 50% of isolated samples in another study [61]. These findings suggest that ST10 is a widely dispersed founding strain across various environments and hosts.
Furthermore, ST98, also a clone previously described in Ecuador, was found to be closely related to ST10 and belongs to CC10 and shown to be highly transmissible across various hosts, including water sources, food, diseased cattle, pigs, and humans [18]. Other E. coli strain types (3856, 5855, 10596, 5123, ST226, and ST5848) identified in this study have been linked to the presence of mcr-1 in other studies and reported in animal such as pigs, poultry, cattle, and ducks, as well as in healthy humans (farm workers) [57,61,62,63,64,65,66].
Likewise, the diversity of K. pneumoniae clones identified in this study reflects their heterogeneity. The founding clone was ST504, reported in a clinical sample of a human [67], and the others are derived from this central strain type. ST2370 was the only strain type with two isolates and has also been reported in clinical fecal samples from outpatients in China [68]. Clones ST4143 and ST1263, identified in this study from animals, have also been reported in hospital areas such as intensive-care units and hematology, and neonatology services in China and France [69,70]. The K. pneumoniae clones ST540 and ST3441, detected in our study in human samples, have been identified in studies from patients with pneumonia in China [71] as well as in patients related to a suspected outbreak of bubonic plague in Madagascar [72]. Another K. pneumoniae clone, ST4631, which was identified in this study from pig fecal samples, has been previously reported in Sweden in patients with sepsis caused by an ESBL-producing K. pneumoniae [73]. Overall, these findings highlight the adaptability and potential dissemination routes of these clones, emphasizing the importance of monitoring antimicrobial resistance in both clinical and non-clinical settings.
4. Conclusions
In conclusion, our study has identified a concerning co-occurrence of β-lactam and mcr-1 resistance genes in Enterobacterales species, posing a significant threat to public health in the communities we investigated. The blaSHV, blaTEM, and blaCTX-M were the most prevalent genes. Of particular concern is the presence of these strain types in animals raised for human consumption, which could contribute to the transmission of MDR (multidrug-resistant) strain types to humans. As such, there is an urgent need for increased surveillance and epidemiological control to prevent the emergence of antibiotic resistance and implement timely intervention strategies. Low-income rural areas in Latin-American countries, where backyard animal husbandry is prevalent [74], are particularly vulnerable to the spread of antibiotic-resistant pathogens. Of special concern is the presence of several ST identified in this study that have been previously associated with epidemiologically relevant clones in the hospital and non-hospital environments, highlighting the need for further surveillance addressed to control the spread of MDR bacteria and underscores the need for a coordinated One-Health approach to combat the global threat of antibiotic resistance.
Despite the ban of colistin use in animal husbandry in Ecuador since 2019, the continuous use of other antibiotics, such as β-lactams in agricultural production, may contribute to the permanent co-harboring of these genes in animal and human carriers [74]. This, in turn, increases the risk of zoonotic transmission of resistant bacteria from animals to humans, given the close coexistence between humans and backyard animals. Hence, the enhanced surveillance of antibiotic usage and resistance patterns in both human and animal populations is necessary to mitigate this risk. It is essential to raise awareness and implement effective measures to mitigate the spread of antibiotic-resistant pathogens in animal and human populations. Failure to act decisively risks a continued rise in antibiotic resistance, resulting in increased morbidity and mortality, and threatens the foundations of modern medicine.
5. Material and Methods
5.1. Bacterial Isolates
The colistin-resistant isolates used in this study were obtained from a previously collected bacterial collection at Universidad de las Americas, originating from a study that aimed to investigate the prevalence of antibiotic-resistant bacteria in various hosts within two rural localities of Ecuador: La Reforma and Santo Domingo de los Tsáchilas (coastal region), and San José and Samasunchi, Pastaza (Amazon region) [74]. In total, 30 isolates of E. coli and 32 of K. pneumoniae that harbored both the mcr-1 gene and a minimum inhibitory concentration of ≥ 4µg/mL were selected for analysis (Supplementary Figure S1).
5.2. DNA Extraction
The DNA extraction of the isolates was performed by following the Chelex-100 protocol with certain modifications [75]. To obtain a high quality and quantity of DNA, 6–10 colonies of each isolate were resuspended on 200 µL of 10% (w/v) Chelex (Sigma-Aldrich, USA). After vortexing for 2 min, 10 µL of proteinase K, 20 mg/mL (Invitrogen, Carlsbad, CA, USA) were added. The samples were next centrifuged at 10,000 rpm for 2 min and incubated in a water bath at 56 °C for 1 h. The samples were then vortexed for 5 min and centrifuged in the same manner for 2 min to continue with a second incubation in a thermoblock (Sigma-Aldrich, St. Louis, MO, USA) at 96 °C for 20 min. After another centrifugation at 10,000 rpm for 3 min, the supernatant that contained the DNA was transferred to a clean 1.5 mL microtube and stored at −20 °C.
5.3. DNA Quantification
The concentration and purity of the DNA were determined by means of a Thermo Scientific™ NanoDrop™ (Thermo Fisher Scientific, Waltham, MA, USA). Replicate samples of bacterial DNA were placed and a reading performed at 260 and 280 nm to obtain the concentration in ng/µL. In addition, the 260/280 ratio is at the same time a measure of the purity. The sample was then diluted to a concentration of 10 ng/µL.
5.4. Molecular-Genetic Identification of β-Lactamase-Encoding Genes
For β-lactamase-encoding genes detection, a single-endpoint polymerase chain reaction (PCR) was performed for six ESBL genes (blaTEM, blaSHV, blaCTX-M1, blaCTX-M2, blaCTX-M-8/25, and blaCTX-M-9) with the primers as described by Le et al. [76]. Furthermore, for detection of the carbapenemase genes blaOXA48, blaKPC, blaNDM, and blaVIM, a single-endpoint PCR was performed according to previously described procedures [77].
We analyzed the PCR products in a 2% (w/w) agarose gel using SYBR™ Safe (Invitrogen, Carlsbad, CA, USA) and 1X TBE buffer. The electrophoresis was programmed at 100 V for 35 min in a Labnet Enduro Gel XL horizontal chamber (Labnet International, Inc., Edison, NJ, USA). The agarose gel was visualized in a gel-documentation system (ChemiDoc™ Imaging Systems BioRad, California, USA) and through the use of Image-Lab™ (BioRad, California, USA) software. The length of each amplicon observed in the gel was determined by comparison with a DNA ladder of 100 bp (Invitrogen, Carlsbad, CA, USA). The DNA-positive controls for the various genes were kindly donated by the Osaka Institute of Public Health, Japan. A non–ESBL-producing E. coli strain was used as a negative control (E. coli ATCC 25922). The PCR products were sequenced, and sequence identities were confirmed by blast analysis.
5.5. Multilocus Sequence Typing (MLST)
In order to determine the strain types circulating between the three hosts of the study, the MLST technique was performed according to the indications of the Pasteur MLST database (http://pubmlst.org). The PCR conditions of seven housekeeping genes—adk, fumC, gyrB, icd, mdh, purA, recA for E. coli, and gapA, tonB, rpoB, phoE, mdh, infB, pgi for K. pneumoniae—were amplified and sequenced as described by Wirth et al. (2006). The PCR products were sequenced by the Sanger sequencing technique in an ABI 3500xL genetic analyzer (Applied Biosystems, USA) and a BigDye 3.1® capillary electrophoresis matrix. Moreover, the allelic profile and strain-type determination were also confirmed through the use of the above PubMLST website. Finally, the MLST analysis was performed by constructing minimum-spanning trees (MSTs). The construction stated in brief: the PHYLOViz online software (https://online.phyloviz.net/index, accessed on 2 February 2023) was used to analyze sequence-typing methods from allelic profiles and epidemiologic data by means of the goeBURST algorithm, which is based on the determination of descent patterns for bacteria based on the number of differences between allelic profiles [78].
5.6. Phylogenetic Analysis
For visualization of the co-harboring resistance genes, an evolutionary history was inferred by using the maximum likelihood method and the Tamura-Nei model for both bacteria species. Evolutionary analyses were conducted in MEGA X (Philadelphia, PA, USA), and the tree was analyzed and annotated using iTOL platform [79]. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura-Nei model, and then selecting the topology with superior log likelihood value. Evolutionary analyses were conducted in MEGA X, and the tree was modified using iTOL.
6. Limitations of This Study
The present study is subject to certain limitations. Firstly, the ESBL and carbapenemase resistance genes were not characterized phenotypically and, therefore, a relationship between their presence and antimicrobial susceptibility patterns on the phenotypic level was not established. It is worth noting that discordant results have been reported previously for strains harboring genetic resistance elements and their phenotypic susceptibility profiles. Despite detecting the presence of these genes through molecular biology and sequencing, the expression of the enzymes and the levels of resistance in vivo may vary [80].
Furthermore, while the classical seven-genes MLST was used to analyze all strains in the study, no ST could be assigned to most of our strains due to the limitations of this method. Nowadays, whole-genome sequencing (WGS) is the standard technique for bacterial genotyping and has proven high discriminatory power. Therefore, the use of WGS in future studies could provide more accurate and detailed information about strain classification and genetic diversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12050856/s1, Supplementary Figure S1: Map of Ecuador indicating the sampling locations; Table S1: List of mcr-1 positive E. coli isolates, allelic profile, and MLST sequences; Table S2: List of mcr-1 positive K. pneumoniae isolates, allelic profile, and MLST sequences.
Author Contributions
Conceptualization, C.B.-C.; methodology, E.C.-V., A.Z. and A.M.-M.; software, W.C.-C. and C.B.-C.; validation, C.B.-C., J.H.d.W. and M.C.; formal analysis, C.B.-C. and E.C.-V.; investigation, C.B.-C.; resources, M.C.; data curation, C.B.-C., E.C-V. and A.Z.; writing—original draft preparation, C.B.-C.; writing—review and editing, J.R., J.H.d.W., Y.Y., M.Y. and M.C.; visualization, W.C.-C., A.Z. and C.B.-C.; supervision, J.R., J.H.d.W. and M.C.; project administration, J.H.d.W.; funding acquisition, Y.Y. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Universidad de las Américas”, grant number VET.JDW.20.03 and “Junta de Extremadura (Consejería de Economía e Infraestructuras)”, grant number IB18047. The APC was funded by the “Universidad de las Américas”.
Institutional Review Board Statement
For stool sampling and isolation of E. coli and K. pneumoniae in humans, permission of the Ethics Committee for Investigation on Human Beings of the Universidad San Francisco de Quito was obtained (code P2019-117E). A consent was signed by the human subjects in this study. Regarding animal sampling, it is important to note that no ethical commission has been established in Ecuador. Nonetheless, sampling was conducted in accordance with ethical guidelines with the assistance of a professional in veterinary sciences.
Informed Consent Statement
All human participants signed an Informed Consent Statement of the Ethics Committee for Investigation on Human Beings of the Universidad San Francisco de Quito was obtained (code P2019-117E).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our gratitude to the Ministerio de Salud del Ecuador and Agrocalidad for their support in this study, as well as to the human volunteers from the communities in Santo Domingo and Pastaza who participated. We would also like to thank Donald F. Haggerty, a retired academic investigator and native English speaker, for editing the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO; Advisory Group on Integrated Surveillance (AGISAR). Critically Important Antimicrobials for Human Medicine 6th Revision 2018 Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J.; Aarestrup, F.M.; Schwarz, S.; Shen, J.; Cavaco, L. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cáceres, W.; Medina, J.; Ortuño-Gutiérrez, N.; Sunyoto, T.; Bastidas-Caldes, C.; Ramírez, M.S.; Harries, A.D. Genomic Insights of Mcr-1 Harboring Escherichia coli by Geographical Region and a One-Health Perspective. Front. Microbiol. 2023, 13, 1032753. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Calvopina, M.; Izurieta, R.; Villacres, I.; Kawahara, R.; Sasaki, M.; Yamamoto, M. Colistin-Resistant Escherichia coli with Mcr Genes in the Livestock of Rural Small-Scale Farms in Ecuador. BMC Res. Notes 2019, 12, 121. [Google Scholar] [CrossRef]
- Nang, S.C.; Li, J.; Velkov, T. The Rise and Spread of Mcr Plasmid-Mediated Polymyxin Resistance. Crit. Rev. Microbiol. 2019, 45, 131–161. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Xu, Y.; Bai, X.; Wang, J.; Zhang, Z.; Liu, X.; Miao, Y.; Zhang, L.; Li, X.; et al. Multidrug-Resistant Escherichia Albertii: Co-Occurrence of β-Lactamase and MCR-1 Encoding Genes. Front. Microbiol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Kong, L.H.; Lei, C.W.; Ma, S.Z.; Jiang, W.; Liu, B.H.; Wang, Y.X.; Guan, R.; Men, S.; Yuan, Q.W.; Cheng, G.Y.; et al. Various Sequence Types of Escherichia coli Isolates Coharboring BlaNDM-5 and Mcr-1 Genes from a Commercial Swine Farm in China. Antimicrob. Agents Chemother. 2017, 61, e02167-16. [Google Scholar] [CrossRef]
- Gupta, C.L.; Blum, S.E.; Kattusamy, K.; Daniel, T.; Druyan, S.; Shapira, R.; Krifucks, O.; Zhu, Y.G.; Zhou, X.Y.; Su, J.Q.; et al. Longitudinal Study on the Effects of Growth-Promoting and Therapeutic Antibiotics on the Dynamics of Chicken Cloacal and Litter Microbiomes and Resistomes. Microbiome 2021, 9, 178. [Google Scholar] [CrossRef]
- Plata, G.; Baxter, N.T.; Susanti, D.; Volland-Munson, A.; Gangaiah, D.; Nagireddy, A.; Mane, S.P.; Balakuntla, J.; Hawkins, T.B.; Kumar Mahajan, A. Growth Promotion and Antibiotic Induced Metabolic Shifts in the Chicken Gut Microbiome. Commun. Biol. 2022, 5, 293. [Google Scholar] [CrossRef]
- Collignon, P.; McEwen, S. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, B.; Lahon, K. The Beta Lactam Antibiotics as an Empirical Therapy in a Developing Country: An Update on Their Current Status and Recommendations to Counter the Resistance against Them. J. Clin. Diagn. Res. 2013, 7, 1207–1214. [Google Scholar] [CrossRef]
- Alegría, Á.; Arias-Temprano, M.; Fernández-Natal, I.; Rodríguez-Calleja, J.M.; García-López, M.L.; Santos, J.A. Molecular Diversity of ESBL-Producing Escherichia coli from Foods of Animal Origin and Human Patients. Int. J. Environ. Res. Public Health 2020, 17, 1312. [Google Scholar] [CrossRef]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The Ecology of Extended-Spectrum β-Lactamases (ESBLs) in the Developed World. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Woerther, P.L.; Burdet, C.; Chachaty, E.; Andremont, A. Trends in Human Fecal Carriage of Extended-Spectrum β-Lactamases in the Community: Toward the Globalization of CTX-M. Clin. Microbiol. Rev. 2013, 26, 744–758. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Ochoa, J.; Guerrero-Latorre, L.; Moyota-Tello, C.; Tapia, W.; Rey-Pérez, J.M.; Baroja, M.I. Removal of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli, ST98, in Water for Human Consumption by Black Ceramic Water Filters in Low-Income Ecuadorian Highlands. Int. J. Environ. Res. Public Health 2022, 19, 4736. [Google Scholar] [CrossRef]
- Peirano, G.; Richardson, D.; Nigrin, J.; McGeer, A.; Loo, V.; Toye, B.; Alfa, M.; Pienaar, C.; Kibsey, P.; Pitout, J.D.D. High Prevalence of ST131 Isolates Producing CTX-M-15 and CTX-M-14 among Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolates from Canada. Antimicrob. Agents Chemother. 2010, 54, 1327–1330. [Google Scholar] [CrossRef]
- Carattoli, A. Animal Reservoirs for Extended Spectrum Beta-Lactamase Producers. Clin. Microbiol. Infect. 2008, 14 (Suppl. S1), 117–123. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Soria Segarra, C.; Soria Baquero, E.; Cartelle Gestal, M. High Prevalence of CTX-M-1-Like Enzymes in Urinary Isolates of Escherichia coli in Guayaquil, Ecuador. Microb. Drug Resist. 2018, 24, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Caldes, C.; Romero-Alvarez, D.; Valdez-Vélez, V.; Morales, R.D.; Montalvo-Hernández, A.; Gomes-Dias, C.; Calvopiña, M. Extended-Spectrum Beta-Lactamases Producing Escherichia coli in South America: A Systematic Review with a One Health Perspective. Infect. Drug Resist. 2022, 15, 5759–5779. [Google Scholar] [CrossRef]
- Cantón, R.; Coque, T.M. The CTX-M β-Lactamase Pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Pitout, J.D.D. Molecular Epidemiology of Escherichia coli Producing CTX-M β-Lactamases: The Worldwide Emergence of Clone ST131 O25:H4. Int. J. Antimicrob. Agents 2010, 35, 316–321. [Google Scholar] [CrossRef]
- Mitsuda, T.; Muto, T.; Yamada, M.; Kobayashi, N.; Toba, M.; Aihara, Y.; Ito, A.; Yokota, S. Epidemiological Study of a Food-Borne Outbreak of Enterotoxigenic Escherichia coli O25:NM by Pulsed-Field Gel Electrophoresis and Randomly Amplified Polymorphic DNA Analysis. J. Clin. Microbiol. 1998, 36, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Vinueza-Burgos, C.; Ortega-Paredes, D.; Narvaéz, C.; De Zutter, L.; Zurita, J. Characterization of Cefotaxime Resistant Escherichia coli Isolated from Broiler Farms in Ecuador. PLoS ONE 2019, 14, e0207567. [Google Scholar] [CrossRef]
- Villegas, M.V.; Pallares, C.J.; Escandón-Vargas, K.; Hernández-Gómez, C.; Correa, A.; Álvarez, C.; Rosso, F.; Matta, L.; Luna, C.; Zurita, J.; et al. Characterization and Clinical Impact of Bloodstream Infection Caused by Carbapenemase-Producing Enterobacteriaceae in Seven Latin American Countries. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Zurita, J.; Yánez, F.; Sevillano, G.; Ortega-Paredes, D.; Miño, A.P.Y. Ready-to-Eat Street Food: A Potential Source for Dissemination of Multidrug-Resistant. Lett. Appl. Microbiol. 2020, 70, 203–209. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Zurita, J. Escherichia coli Hyperepidemic Clone ST410-A Harboring blaCTX-M-15 Isolated from Fresh Vegetables in a Municipal Market in Quito-Ecuador. Int. J. Food Microbiol. 2018, 280, 41–45. [Google Scholar] [CrossRef]
- Villacís, J.E.; Reyes, J.A.; Castelán-Sánchez, H.G.; Dávila-Ramos, S.; Lazo, M.A.; Wali, A.; Bodero, L.A.; Toapanta, Y.; Naranjo, C.; Montero, L.; et al. OXA-48 Carbapenemase in Klebsiella pneumoniae Sequence Type 307 in Ecuador. Microorganisms 2020, 8, 435. [Google Scholar] [CrossRef]
- Nuñez Quezada, T.; Rodríguez, C.H.; Castro Cañarte, G.; Nastro, M.; Balderrama Yarhui, N.; Dabos, L.; Acosta Mosquera, Y.; Plaza Moreira, N.; Famiglietti, A. Outbreak of BlaOXA-72-Producing Acinetobacter Baumannii in South America. J. Chemother. 2017, 29, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.A.; Villavicencio, F.; Villacís, J.E.; Pavón, E.; Campoverde, N.; Espinel, M.; Núñez, B.; Trueba, G. First Report of a Clinical Isolate of BlaOXA-48- Carbapenemase Producing Raoultella Ornithinolytica in South America. Rev. Argent. Microbiol. 2020, 52, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; de Janon, S.; Villavicencio, F.; Ruales, K.J.; De La Torre, K.; Villacís, J.E.; Wagenaar, J.A.; Matheu, J.; Bravo-Vallejo, C.; Fernández-Moreira, E.; et al. Broiler Farms and Carcasses Are an Important Reservoir of Multi-Drug Resistant Escherichia coli in Ecuador. Front. Vet. Sci. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Hoepers, P.G.; Silva, P.L.; Rossi, D.A.; Valadares Júnior, E.C.; Ferreira, B.C.; Zuffo, J.P.; Koerich, P.K.; Fonseca, B.B. The Association between Extended Spectrum Beta-Lactamase (ESBL) and Ampicillin C (AmpC) Beta-Lactamase Genes with Multidrug Resistance in Escherichia coli Isolates Recovered from Turkeys in Brazil. Br. Poult. Sci. 2018, 59, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Crecencio, R.B.; Brisola, M.C.; Bitner, D.; Frigo, A.; Rampazzo, L.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.S.; Faria, G.A.; et al. Antimicrobial Susceptibility, Biofilm Formation and Genetic Profiles of Escherichia coli Isolated from Retail Chicken Meat. Infect. Genet. Evol. 2020, 84, 104355. [Google Scholar] [CrossRef]
- Jena, J.; Sahoo, R.K.; Debata, N.K.; Subudhi, E. Prevalence of TEM, SHV, and CTX-M Genes of Extended-Spectrum β-Lactamase-Producing Escherichia coli Strains Isolated from Urinary Tract Infections in Adults. 3 Biotech 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Abujnah, A.A.; Zorgani, A.; Sabri, M.A.M.; El-Mohammady, H.; Khalek, R.A.; Ghenghesh, K.S. Multidrug Resistance and Extended-Spectrum β-Lactamases Genes among Escherichia coli from Patients with Urinary Tract Infections in Northwestern Libya. Libyan J. Med. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Shi, X.; Wang, S.; Ren, H.; Shen, Z.; Wang, Y.; Lin, J.; Wang, S. Rapid Rise of the ESBL and Mcr-1 Genes in Escherichia coli of Chicken Origin in China, 2008–2014. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Moghanni, M.; Ghazvini, K.; Farsiani, H.; Namaei, M.H.; Derakhshan, M.; Yousefi, M.; Maragheh, A.; Jamehdar, S.A. High Prevalence of Sequence Type 131 Isolates Producing CTX-M-15 among Extended-Spectrum β-Lactamase-Producing Escherichia coli Strains in Northeast Iran. J. Glob. Antimicrob. Resist. 2018, 15, 74–78. [Google Scholar] [CrossRef]
- Amin, M.B.; Sraboni, A.S.; Hossain, M.I.; Roy, S.; Mozmader, T.A.U.; Unicomb, L.; Rousham, E.K.; Islam, M.A. Occurrence and Genetic Characteristics of Mcr-1-Positive Colistin-Resistant E. coli from Poultry Environments in Bangladesh. J. Glob. Antimicrob. Resist. 2020, 22, 546–552. [Google Scholar] [CrossRef]
- Shafiq, M.; Huang, J.; Ur Rahman, S.; Shah, J.M.; Chen, L.; Gao, Y.; Wang, M.; Wang, L. High Incidence of Multidrug-Resistant Escherichia coli Coharboring Mcr-1 and blaCTX-M-15 Recovered from Pigs. Infect. Drug Resist. 2019, 12, 2135–2149. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Arena, F.; Pallecchi, L.; Rossolini, G.M. CTX-M-Type β-Lactamases: A Successful Story of Antibiotic Resistance. Int. J. Med. Microbiol. 2013, 303, 305–317. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef] [PubMed]
- Araque, M.; Labrador, I. Prevalence of Fecal Carriage of CTX-M-15 Beta-Lactamase-Producing Escherichia coli in Healthy Children from a Rural Andean Village in Venezuela. Osong Public Health Res. Perspect. 2018, 9, 9–15. [Google Scholar] [CrossRef]
- Ryoo, N.H.; Kim, E.C.; Hong, S.G.; Park, Y.J.; Lee, K.; Bae, I.K.; Song, E.H.; Jeong, S.H. Dissemination of SHV-12 and CTX-M-Type Extended-Spectrum β-Lactamases among Clinical Isolates of Escherichia coli and Klebsiella pneumoniae and Emergence of GES-3 in Korea. J. Antimicrob. Chemother. 2005, 56, 698–702. [Google Scholar] [CrossRef]
- Komijani, M.; Bouzari, M.; Rahimi, F. Detection of TEM, SHV and CTX-M Antibiotic Resistance Genes in Escherichia coli Isolates from Infected Wounds. Med. Lab. J. 2017, 11, 30–35. [Google Scholar]
- Hülter, N.; Ilhan, J.; Wein, T.; Kadibalban, A.S.; Hammerschmidt, K.; Dagan, T. An Evolutionary Perspective on Plasmid Lifestyle Modes. Curr. Opin. Microbiol. 2017, 38, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J. Bacterial Plasmid Addiction Systems and Their Implications for Antibiotic Drug Development. In Postdoc Journal: A Journal of Postdoctoral Research and Postdoctoral Affairs; NIH: Washington, DC, USA, 2017. [Google Scholar]
- Touati, A.; Mairi, A. Plasmid-Determined Colistin Resistance in the North African Countries: A Systematic Review. Microb. Drug Resist. 2021, 27, 121–133. [Google Scholar] [CrossRef]
- Millan, A.S.; Escudero, J.A.; Gifford, D.R.; Mazel, D.; MacLean, R.C. Multicopy Plasmids Potentiate the Evolution of Antibiotic Resistance in Bacteria. Nat. Ecol. Evol. 2016, 1, 0010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, J.; Wang, X.; Bai, X.; Ma, J.; Dang, R.; Xiong, Y.; Fanning, S.; Bai, L.; Yang, Z. Characterization of Five Escherichia coli Isolates Co-Expressing ESBL and Mcr-1 Resistance Mechanisms from Different Origins in China. Front. Microbiol. 2019, 10, 1994. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.; García-Meniño, I.; Herrera, A.; García, V.; López-Beceiro, A.M.; Alonso, M.P.; Blanco, J.; Mora, A. Genomic Characterization of Escherichia coli Isolates Belonging to a New Hybrid Aepec/Expec Pathotype O153:H10-a-St10 Eae-Beta1 Occurred in Meat, Poultry, Wildlife and Human Diarrheagenic Samples. Antibiotics 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Hagiya, H.; Akeda, Y.; Takeuchi, D.; Sakamoto, N.; Matsumoto, Y.; Motooka, D.; Nishi, I.; Tomono, K.; Hamada, S. Community Spread and Acquisition of Clinically Relevant Escherichia coli Harbouring BlaNDMamong Healthy Japanese Residents of Yangon, Myanmar. J. Antimicrob. Chemother. 2021, 76, 1448–1454. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y.; Cao, S.; Liu, H.; Li, X.; Sun, J.; Li, F.; Ishfaq, M.; Zhang, X. Prevalence and Characteristic of Swine-Origin Mcr-1-Positive Escherichia coli in Northeastern China. Front. Microbiol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ćwiek, K.; Woźniak-Biel, A.; Karwańska, M.; Siedlecka, M.; Lammens, C.; Rebelo, A.R.; Hendriksen, R.S.; Kuczkowski, M.; Chmielewska-Władyka, M.; Wieliczko, A. Phenotypic and Genotypic Characterization of Mcr-1-Positive Multidrug-Resistant Escherichia coli ST93, ST117, ST156, ST10, and ST744 Isolated from Poultry in Poland. Brazilian J. Microbiol. 2021, 52, 1597–1609. [Google Scholar] [CrossRef]
- He, W.Y.; Zhang, X.X.; Gao, G.L.; Gao, M.Y.; Zhong, F.G.; Lv, L.C.; Cai, Z.P.; Si, X.F.; Yang, J.; Liu, J.H. Clonal Spread of Escherichia coli O101:H9-St10 and O101:H9-St167 Strains Carrying fosA3 and blaCTX-M-14 among Diarrheal Calves in a Chinese Farm, with Australian Chroicocephalus as the Possible Origin of E. coli O101:H9-St10. Zool. Res. 2021, 42, 461–468. [Google Scholar] [CrossRef]
- Blaak, H.; De Kruijf, P.; Hamidjaja, R.A.; Van Hoek, A.H.A.M.; De Roda Husman, A.M.; Schets, F.M. Prevalence and Characteristics of ESBL-Producing E. coli in Dutch Recreational Waters Influenced by Wastewater Treatment Plants. Vet. Microbiol. 2014, 171, 448–459. [Google Scholar] [CrossRef] [PubMed]
- MK, A.; JKP, K.; RS, H.; EC, O.; Thakur, S. Genetic Relatedness of Multidrug Resistant Escherichia coli Isolated from Humans, Chickens and Poultry Environments. Antimicrob. Resist. Infect. Control. 2021, 10, 58. [Google Scholar]
- Matamoros, S.; Van Hattem, J.M.; Arcilla, M.S.; Willemse, N.; Melles, D.C.; Penders, J.; Vinh, T.N.; Thi Hoa, N.; Bootsma, M.C.J.; Van Genderen, P.J.; et al. Global Phylogenetic Analysis of Escherichia coli and Plasmids Carrying the Mcr-1 Gene Indicates Bacterial Diversity but Plasmid Restriction. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Li, H.; Yan, S.; Jiang, F.; Cai, W.; Li, G. Whole Genome Sequencing Analysis of Avian Pathogenic Escherichia coli from China. Vet. Microbiol. 2021, 259, 109158. [Google Scholar] [CrossRef]
- Geyer, C.N.; Fowler, R.C.; Johnson, J.R.; Johnston, B.; Weissman, S.J.; Hawkey, P.; Hanson, N.D. Evaluation of CTX-M Steady-State MRNA, MRNA Half-Life and Protein Production in Various STs of Escherichia coli. J. Antimicrob. Chemother. 2016, 71, 607–616. [Google Scholar] [CrossRef]
- Chen, B.; Berglund, B.; Wang, S.; Börjesson, S.; Bi, Z.; Nilsson, M.; Yin, H.; Zheng, B.; Xiao, Y.; Bi, Z.; et al. Rapid Increase in Occurrence of Carbapenem-Resistant Enterobacteriaceae in Healthy Rural Residents in Shandong Province, China, from 2015 to 2017. J. Glob. Antimicrob. Resist. 2022, 28, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.Q.; Hounmanou, Y.M.G.; Dang, S.T.T.; Olsen, J.E.; Truong, G.T.H.; Tran, N.T.; Scheutz, F.; Dalsgaard, A. Genetic Comparison of Esbl-Producing Escherichia coli from Workers and Pigs at Vietnamese Pig Farms. Antibiotics 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Xi, W.; Liu, S.; Liu, J.; Mu, H.; Chen, B.; He, H.; Fan, Y.; Ma, W.; et al. Genetic Features of Plasmid- and Chromosome-Mediated Mcr-1 in Escherichia coli Isolates From Animal Organs With Lesions. Front. Microbiol. 2021, 12, 707332. [Google Scholar] [CrossRef]
- Novovic, K.; Vasiljevic, Z.; Kuzmanovic, M.; Lozo, J.; Begovic, J.; Kojic, M.; Jovcic, B.; Novel, E. Coli ST5123 Containing BlaNDM-1 Carried by IncF Plasmid Isolated from a Pediatric Patient in Serbia. Microb. Drug Resist. 2016, 22, 707–711. [Google Scholar] [CrossRef]
- Abril, D.; Vergara, E.; Palacios, D.; Leal, A.L.; Marquez-Ortiz, R.A.; Madroñero, J.; Corredor Rozo, Z.L.; De La Rosa, Z.; Nieto, C.A.; Vanegas, N.; et al. Within Patient Genetic Diversity of Bla KPC Harboring Klebsiella pneumoniae in a Colombian Hospital and Identification of a New NTEKPC Platform. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Lu, B.; Zhou, H.; Zhang, X.; Qu, M.; Huang, Y.; Wang, Q. Molecular Characterization of Klebsiella pneumoniae Isolates from Stool Specimens of Outpatients in Sentinel Hospitals Beijing, China, 2010-2015. Gut Pathog. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Yin, L.; He, L.; Miao, J.; Yang, W.; Wang, X.; Ma, J.; Wu, N.; Cao, Y.; Wang, L.; Lu, G.; et al. Active Surveillance and Appropriate Patient Placement in Contact Isolation Dramatically Decreased Carbapenem-Resistant Enterobacterales Infection and Colonization in Paediatric Patients in China. J. Hosp. Infect. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Compain, F.; Decré, D.; Fulgencio, J.P.; Berraho, S.; Arlet, G.; Verdet, C. Molecular Characterization of DHA-1-Producing Klebsiella pneumoniae Isolates Collected during a 4-Year Period in an Intensive Care Unit. Diagn. Microbiol. Infect. Dis. 2014, 80, 159–161. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, Z.F.; Li, P.D.; Qu, P.H. Characterization of Carbapenem-Resistant Acinetobacter Baumannii ST540 and Klebsiella pneumoniae ST2237 Isolates in a Pneumonia Case from China. J. Appl. Microbiol. 2022, 133, 1434–1445. [Google Scholar] [CrossRef]
- Rakotondrasoa, A.; Andrianonimiadana, L.M.; Rahajandraibe, S.; Razafimahatratra, S.; Andrianaivoarimanana, V.; Rahelinirina, S.; Crucitti, T.; Brisse, S.; Jeannoda, V.; Rajerison, M.; et al. Characterization of Klebsiella pneumoniae Isolated from Patients Suspected of Pulmonary or Bubonic Plague during the Madagascar Epidemic in 2017. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saxenborn, P.; Baxter, J.; Tilevik, A.; Fagerlind, M.; Dyrkell, F.; Pernestig, A.K.; Enroth, H.; Tilevik, D. Genotypic Characterization of Clinical Klebsiella Spp. Isolates Collected From Patients With Suspected Community-Onset Sepsis, Sweden. Front. Microbiol. 2021, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Caldes, C.; Guerrero-Freire, S.; Ortuño-Gutiérrez, N.; Sunyoto, T.; Gomes-Dias, C.A.; Ramírez, M.S.; Calero-Cáceres, W.; Harries, A.D.; Rey, J.; de Waard, J.H.; et al. Colistin resistance in Escherichia coli and Klebsiella pneumoniae in humans and backyard animals in Ecuador. Revista Panamericana Salud Pública 2023, 47, e48. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, E.; Nakamura, H. Evaluation of Three Methods for Effective Extraction of DNA from Human Hair. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 820, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.P.; Ueda, S.; Nguyen, T.N.H.; Dao, T.V.K.; Van Hoang, T.A.; Tran, T.T.N.; Hirai, I.; Nakayama, T.; Kawahara, R.; Do, T.H.; et al. Characteristics of Extended-Spectrum β-Lactamase-Producing Escherichia coli in Retail Meats and Shrimp at a Local Market in Vietnam. Foodborne Pathog. Dis. 2015, 12, 719–725. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Feil, E.J.; Li, B.C.; Aanensen, D.M.; Hanage, W.P.; Spratt, B.G. EBURST: Inferring Patterns of Evolutionary Descent among Clusters of Related Bacterial Genotypes from Multilocus Sequence Typing Data. J. Bacteriol. 2004, 186, 1518–1530. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Leekitcharoenphon, P.; Hansen, D.S.; Nielsen, H.L.; Ellermann-Eriksen, S.; Kemp, M.; Røder, B.L.; Frimodt-Møller, N.; Søndergaard, T.S.; et al. One Day in Denmark: Comparison of Phenotypic and Genotypic Antimicrobial Susceptibility Testing in Bacterial Isolates From Clinical Settings. Front. Microbiol. 2022, 13, 804627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).