Abstract
The bifunctional enzyme Dihydrofolate reductase-thymidylate synthase (DHFR-TS) plays a crucial role in the survival of the Leishmania parasite, as folates are essential cofactors for purine and pyrimidine nucleotide biosynthesis. However, DHFR inhibitors are largely ineffective in controlling trypanosomatid infections, largely due to the presence of Pteridine reductase 1 (PTR1). Therefore, the search for structures with dual inhibitory activity against PTR1/DHFR-TS is crucial in the development of new anti-Leishmania chemotherapies. In this research, using the Leishmania major DHFR-TS recombinant protein, enzymatic inhibitory assays were performed on four kauranes and two derivatives that had been previously tested against LmPTR1. The structure 302 (6.3 µM) and its derivative 302a (4.5 µM) showed the lowest IC50 values among the evaluated molecules. To evaluate the mechanism of action of these structures, molecular docking calculations and molecular dynamics simulations were performed using a DHFR-TS hybrid model. Results showed that hydrogen bond interactions are critical for the inhibitory activity against LmDHFR-TS, as well as the presence of the p-hydroxyl group of the phenylpropanoid moiety of 302a. Finally, additional computational studies were performed on DHFR-TS structures from Leishmania species that cause cutaneous and mucocutaneous leishmaniasis in the New World (L. braziliensis, L. panamensis, and L. amazonensis) to explore the targeting potential of these kauranes in these species. It was demonstrated that structures 302 and 302a are multi-Leishmania species compounds with dual DHFR-TS/PTR1 inhibitory activity.
1. Introduction
Leishmaniasis is a neglected tropical disease (NTD) caused by Leishmania parasites, a type of trypanosomatid protozoa [1]. The disease affects 15 million people globally, presenting in three forms: cutaneous (CL), mucocutaneous (ML), and visceral (VL) [2,3]. Despite public health concerns and the need for control, current treatments, including pentavalent antimony salts as first-line drugs or amphotericin B, pentamidine, miltefosine, or paromomycin as second-line drugs, are frequently toxic, as well as expensive with increasing resistance outbreak [3,4,5]. Liposomal amphotericin B is one of the best treatment options for leishmaniasis, but its effectiveness depends on the patient’s immune status, clinical presentation, and location. In addition, its use is limited in developing countries due to its high cost, side effects, and need for injection [6]. Despite attempts to discover more effective and safe alternatives through drug discovery [1,2,7], limited progress has been made, making the search for new antileishmanial chemotherapies necessary [8].
A metabolic pathway that is traditionally considered a crucial target against trypanosomatid parasites involves the inhibition of dihydrofolate reductase (DHFR) in the biosynthesis of folate-like cofactors [9]. DHFR (EC 1.5.1.3) catalyzes the NADPH-dependent reduction of 7,8-dihydrofolates (H2Fs) to 5,6,7,8-tetrahydrofolates (H4Fs) [10], which are necessary for maintaining adequate intracellular folate concentrations [9,10]. In trypanosomatids, a single, fused gene encodes a bifunctional enzyme that has both the DHFR domain and the thymidylate synthase (TS) domain [11]. This bifunctional enzyme is crucial for the parasite’s survival because folates are essential cofactors for the biosynthesis of purine and pyrimidine nucleotides. As a result, inhibition of this single polypeptide can affect two steps of this essential pathway [12]. In contrast, humans have separate monofunctional polypeptides for DHFR and TS, leading to structural differences and unique roles in human folate production [9]. This makes the DHFR-TS combination an attractive molecular target for the development of antimicrobial agents. In fact, antifolate-based antimicrobial drugs such as methotrexate (MTX), trimethoprim, and pyrimethamine are already in use [9,12].
However, Leishmania parasites are auxotrophic for folate, meaning they have a sophisticated metabolic pathway for acquiring folate from the host and incorporating it into intermediate or alternative metabolisms through the action of pteridine reductase (PTR1) [13]. PTR1 (EC 1.5.1.33) transforms conjugated and nonconjugated pterins, including the reduction of biopterin to dihydrobiopterin, and then to tetrahydrobiopterin. This catalytic role is crucial for maintaining vital intracellular levels of tetrahydropterin and has been shown to be an essential component of growth in vivo through gene expression studies [14]. Since PTR1 is less sensitive to the effect of MTX and catalyzes folate reduction, this explains the therapeutic failures of antifolate drugs against trypanosomatid parasites [13,15,16]. Thus, an appropriate strategy would involve searching for dual inhibitors of PTR1 and DHFR as antileishmanial agents [17], and natural compounds are still considered a vast source of bioactive agents [2].
Schmidt et al. conducted a virtual screening of 118 sesquiterpene lactones to evaluate natural products as dual PTR1/DHFR-TS inhibitors against Trypanosoma brucei. Among the 29 virtual hits identified, in vitro assays were performed on 10 selected molecules using recombinant PTR1 and DHFR proteins. Five compounds showed an inhibition of over 50% against TbPTR1, while three compounds exhibited inhibition against TbDHFR, with cynaropicrin being the most interesting hit, inhibiting both TbPTR1 and TbDHFR. [18] Specifically, against Leishmania, Teixeira et al. evaluated 2,4 diaminopyrimidine derivatives as dual and selective inhibitors of pteridine reductase DHFR/PTR1 from Leishmania chagasi. Quinazoline was identified as a selective inhibitor of LcPTR1, and 2,4 diaminopyrimidine derivatives substituted at position 6 were found to be competitive inhibitors of DHFR/PTR1 [19]. Recently, quinoline-linked isatin derivatives showed promising in vitro activity against the promastigote and amastigote form, with 5-bromoindoline, 5-flouroindoline, and 5-trifluoromethoxy indoline derivatives exhibiting significant activity. Folic and folinic acids were able to reverse the antileishmanial effect of these three compounds, confirming their antifolate mechanism via inhibition of DHFR-TS and PTR1 [20].
A class of bioactive naturally-occurring compounds known as kaurane-type diterpenes has been shown to exhibit antileishmanial activity at various levels [21,22,23]. Based on this evidence, a previous in silico and in vitro study was performed on a custom-made library of 360 compounds to select kaurane-type diterpenes against Leishmania major PTR1 (LmPTR1). Two kauranes, structures 135 (2β-hydroxy-menth-6-en-5β-yl ent-kaurenoate) and 302 (3α-cinnamoyloxy-ent-kaur-16-en-19-oic acid), were identified with antileishmanicidal activity against L. major through an in silico approach combining machine learning and molecular docking methodologies. The in vitro results verified the accuracy of the classification model. The top-ranked compounds and two semisynthetic derivatives were found to have half-maximal inhibitory concentrations (IC50) less than 10 µg/mL, which showed that the inhibitory activity of structure 302 was improved by approximately 60% when a 3-p-coumaroyloxy group was used instead of the 3-cinnamoyloxy substituent [24]. These selected compounds can be considered important leads that can be used to obtain more active PTR1 inhibitors. In addition, molecular docking calculations and MD simulations were performed for the entire set of kauranes, and the compounds 302, and 302a (3α-p-coumaroyloxy-ent-kaur-16-en-19-oic acid) were identified as potential multispecies agents against other Leishmania species responsible for the clinical diversity of CL and MCL [24]. Based on the previously obtained results, the present study aims to investigate the selection of kauranes that exhibit activity against L. major DHFR-TS, along with their potential inhibitory activity in Leishmania species of the New World. Furthermore, the study aims to identify potential dual inhibitors of DHFR/PTR1, leveraging the prior findings against PTR1.
2. Results and Discussion
2.1. Kauranes 302 and Its Derivative 302a Have Dual In Vitro Enzymatic Activity against L. major PTR1/DHFR-TS
The potential dual enzymatic activity of L. major PTR1/DHFR-TS for the diterpene esters 135, 301 (3α-cinnamoyloxy-9β-hydroxy-ent-kaur-16-en-19-oic Acid), 302, 301a (3α-p-coumaroyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid), and 302a (which have already been evaluated against L. major PTR1 [24], as shown in Figure 1a), along with structure 4 (6ß,17-isopropylidenedioxy-ent-kauran-3-one), was evaluated using spectrophotometric monitoring of enzymatic activity under a standard DHFR assay. This was performed with a range of test compound concentrations (0.1–128 µM), and methotrexate was used as a positive control. Structure 4 was also selected for the assays, since in previously performed structure-based virtual screening of 360 kaurane-type diterpenes using DHFR-TS of Leishmania species of the New World as targets, it was one of the best-ranked molecules, demonstrating selectivity against this target.
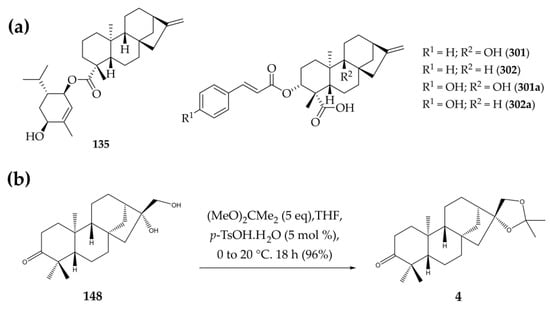
Figure 1.
(a) Structures of selected kaurane-type diterpenes (135, 301, 302) and their derivatives (301a and 302a). (b) Synthesis of compound 4 from structure 148.
The IC50 values were calculated based on the concentration–response behavior within the range of 0.1–128 μM, resulting in values ranging from 4.5 to 11.2 μM (pIC50 values ranging from 4.95 to 5.35). Then, using the Cheng–Prusoff equation and assuming reversible competitive inhibition and a 1:1 stoichiometry [25], the apparent inhibitory constant (Kiapp) was calculated for the selected kauranes using the IC50 results, as shown in Table 1.

Table 1.
Results of enzymatic activity against L. major dihydrofolate reductase (LmDHFR) for selected kaurane-type diterpenes.
The evaluated structures showed similar IC50 values. Among the six tested diterpenes, structure 135 was the least active, which was contrary to what was observed with PTR1. Structure 301, which was classified as inactive against PTR1, showed a different behavior against DHFR-TS with a pIC50 value above 5.0, and was classified as active against this enzyme, according to the cutoff value used to build the machine learning model of L. major (pIC50 = −log IC50) [24].
Using MolpredictX, a recent web tool developed in the Laboratory of Cheminformatics at the Federal University of Paraíba, which provides predictions for 27 different biological activities, including L. major, structures 301 and 301a were classified as active. This tool provides qualitative predictions of molecule activity (active or inactive) and a quantitative probability of activity based on molecular descriptors [26].
For DHFR-TS, the kaurane-type diterpenes 301, 302, 301a, and 302a showed similar pIC50 values above 5.0, indicating that the 9-hydroxyl group at the diterpene moiety is not relevant to the inhibitory activity as observed with PTR1, and suggesting different mechanisms of action for these two enzymes in Leishmania. Additionally, the p-hydroxyl group has a favorable influence on the inhibitory activity of the evaluated kauranes, reducing the inhibitory constant (Kiapp) values by 10–30% for 301a and 302a, respectively, compared to kauranes 301 and 302, which do not have this hydroxyl group present in their structures.
Structures 302 and 302a showed the lowest Kiapp values among the six tested structures against DHFR-TS (despite both having Kiapp values that are higher than MTX). These two structures also displayed a similar behavior to lower Kiapp values in previous enzymatic assays against L. major PTR1 [24], which indicates that these two structures have dual in vitro enzymatic activity against L. major PTR1/DHFR-TS, with the 9-hydroxyl group at the diterpene moiety being the critical structural feature for the observed dual action against these targets.
2.2. Hybrid Model of L. major DHFR-TS and Molecular Docking Calculations
To examine the mechanism of action of the tested kauranes and determine whether the kauranes that previously showed inhibitory activity against pteridine reductase 1 (PTR1) also act against dihydrofolate reductase-thymidylate synthase (DHFR-TS), a molecular docking study was conducted using a LmDHFR-TS hybrid model built in the YASARA software (YASARA Biosciences GmbH, Vienna, Austria; 2018). The model’s reliability and stereochemical qualities were evaluated through Ramachandran, WHAT IF, and VERIFY 3D plots, as well as Z-scores of dihedrals, which describe the deviation of the model’s quality from the average high-resolution X-ray structure. The Ramachandran plot showed that 96.9% of residues were in the most favored regions, with 99.5% in allowed regions and only 0.5% (corresponding to five amino acids) in the outlier region, indicating that the LmDHFR-TS model was satisfactory (Supplementary Material) [27].
The VERIFY 3D (https://services.mbi.ucla.edu/SAVES/, accessed on 3 January 2023) results showed that 92.6% of residues had an averaged 3D-1D score of ≥0.2, indicating a reliable model. The coarse packing quality control of the LmDHFR-TS model, evaluated using WHAT IF, showed a mean score of −0.594, with only 1.7% of residues (8 of 520 amino acids) scoring −5.0 or lower. The dihedral quality was classified as optimal for the LmDHFR-TS hybrid model, with values above 1.085 [28].
Molecular docking calculations were performed for the selected kaurane dataset and derivatives 301a and 302a using Molegro 6.0 software, which employs the MolDock scoring function. The previously validated L. major DHFR-TS hybrid model was used, and the results were consistent with those obtained from enzymatic assays. The MolDock scores ranged from −62.85 to −81.43 kJ/mol, with all structures showing higher scores than the positive control MTX (−107.60 kJ/mol). Kaurane 302 (−76.53 kJ/mol) and its derivative 302a (−81.43 kJ/mol), which exhibited the highest inhibitory activity against L. major DHFR-TS in the enzymatic assay, also had the lowest MolDock scores among the seven evaluated kaurane-type diterpenes (Table 2). In addition, molecular docking calculations were performed for 5-benzyl-6-(cyclohexylmethyl) pyrimidine-2,4-diamine, a 2,4-diamine derivative that exhibited similar affinity to L. chagasi PTR1 and DHFR-TS (with a KiLcPTR1/KiLcDHFR-TS ratio of 0.68). The compound was found to have a similar MolDock score (−72.38 kJ/mol) compared to structure 302 and its derivative 302a, which showed enzymatic inhibition of both L. major PTR1 and DHFR-TS [19].

Table 2.
MolDock scores of six kaurane-type diterpenes, MTX, and a 2,4-diamine derivative (a dual inhibitor of PTR1/DHFR-TS [19]) against L. major DHFR-TS. SD = standard deviation; RMSD values = root-mean-square deviation.
Using a two-dimensional analysis, critical interactions with active site amino acid residues of the enzyme were identified. It was observed that hydrogen bond interactions are directly related to the IC50 values obtained in the enzymatic assay. Structures 302 and 302a, which had the lowest IC50 values, showed two hydrogen bond interactions involving residues I45 and S86 for 302 and W47 for 302a, and the carbon-19 of these two kauranes (Figure 2d–f). The interaction with residue S86 was also observed for structure 301, which had a moderate IC50 value among the tested structures, with the only hydrogen bond interaction being observed in this kaurane (Figure 2c). This behavior was also observed in structure 4 (Figure 2a), which only interacted with residue A32 through hydrogen bonds.
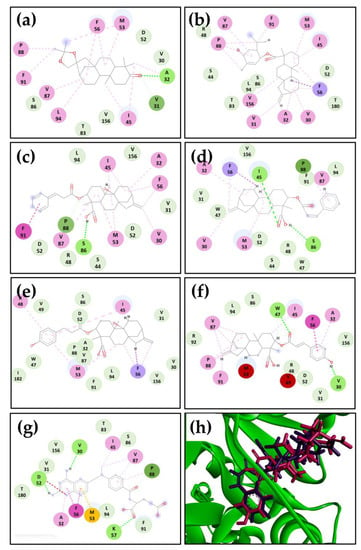
Figure 2.
Two-dimensional residual interaction diagrams in the active site of L. major DHFR-TS for: (a) structure 4, (b) structure 135, (c) structure 301, (d) structure 302, (e) structure 301a, (f) structure 302a and (g) methotrexate (MTX). Interacting residues are shown as colored circles depending on the interactions (as colored dashed lines): H-bond (lime), van der Waals (green), π–σ (purple), π– alkyl (pink), π–π (fuchsia), unfavorable (red), and carbon H-bond (teal) interactions. (h) Docking conformations of structure 302 (purple) and its derivative 302a (pink) in the active site of L. major DHFR-TS (green).
The positive control, MTX, showed three hydrogen bond interactions with residues D52, K57, and V30. The interaction with V30 potentially has a crucial role in the inhibition of DHFR-TS. This interaction was only observed in the derivative 302a, which had the highest inhibitory activity among the tested molecules. Interestingly, this hydrogen bond interaction was formed with the p-hydroxyl group of the phenylpropanoid moiety of 302a, reinforcing the importance of this structural feature in the dual L. major PTR1/DHFR-TS inhibitory activity.
In addition, the analysis of the docking conformations revealed the phenylpropanoid moiety of structures 302 and 302a adopted a similar conformation in the active site of L. major DHFR-TS, with the p-hydroxyl group identified as a crucial feature for the observed inhibitory activity (Figure 2h).
Residue F56 also plays a key role in the inhibition of L. major DHFR-TS when it interacts with aromatic regions in the kaurane series, as the most active molecules exhibited a π–π interaction between the phenyl group of the amino acid and the pteridine ring and phenylpropanoid moiety of MTX and structure 302a, respectively. A different behavior was observed for structure 302 and the derivative 301a, which had intermediate inhibitory activity against DHFR-TS. These two molecules, along with structure 135, showed a π–σ interaction with the hydrogens of the kaurane region. Structure 302a was the only structure that showed an unfavorable interaction with residue M53, which is important for MTX, with a π–sulfur interaction being established (Figure 2f,g).
2.3. Kaurane 302 and Its Derivative 302a May Have the Potential to Inhibit DHFR-TS in Different Species of Leishmania from the New World
Leishmaniasis contracted in North and South America is referred to as “new world leishmaniasis” [29]. Studying this type of species is crucial for the control and elimination of the disease, as there is a high diversity of Leishmania species in the Americas, with high concentrations of different species found in countries such as Brazil and Colombia, leading to a significant disease burden [30]. Some of the main New World Leishmania species include Leishmania panamensis, which is the primary cause of cutaneous leishmaniasis (CL) in Panama and has been found to infect both anthropophilic vectors and mammalian reservoirs [31]; Leishmania braziliensis, a pathogenic agent of CL and mucocutaneous leishmaniasis (MCL), primarily distributed in South and Central America [29,30,31,32]; and Leishmania amazonensis, an etiological agent of diffuse CL and tegumentary leishmaniasis (TL) [33]. In previous research, molecular docking calculations and MD simulations using PTR1 hybrid models of L. braziliensis, L. amazonensis, and L. panamensis identified kauranes 135, 302, and its derivative 302a as potential multispecies agents [24].
To evaluate their potential dual inhibitory activity against PTR1 and DHFR-TS, hybrid models of DHFR-TS for these three Leishmania species were built, and molecular docking calculations and MD simulations were performed using the four kauranes and two derivatives, which were previously tested against the DHFR-TS recombinant. The Ramachandran plot of these three hybrid models showed that the main possible chain conformations included more than 97.2% of residues in the most favored regions for the three hybrid models, with 99.7% of residues in allowed regions. All models showed three residues (0.3%) in disallowed regions (outliers; Supplementary Material).
Additionally, a multiple sequence alignment of DHFR sequences for L. major, L. braziliensis, L. panamensis, and L. amazonensis species, along with the Homo sapiens DHFR sequence, was carried out. The results showed that the percentage of similarity between Homo sapiens and Leishmania species is below 25%, with only five conserved residues out of the main 15 residues associated with the interaction between DHFR with kaurane-type diterpenes. However, among the four Leishmania species used in this study, similarity values close to 80% were obtained.
The analysis of the docking results showed that for L. braziliensis, the tested structures had similar VINA score values, except for derivative 302a, which presented the lowest affinity value (−11.17 kcal/mol). All structures had lower docking values compared to MTX (−9.64 kcal/mol), as seen in Table 3. By analyzing the interactions between the tested kauranes and the flexible residues of the active site of L. braziliensis DHFR-TS, it was found that the unsaturation of carbon-17 is crucial for the inhibition of 301, 302, and their derivatives 301a and 302a with the enzyme, via π-alkyl interactions with Y91 and M53. This interaction was also observed in MTX through a π-sulfur interaction with the thiol group of methionine. In addition, the potential inhibitory activity observed for structure 4 was related to a hydrogen bond interaction between Q48 and the carbonyl group of carbon-3, as well as the presence of a 1,3-dioxolane group. Neither structure 301 nor 302 interacted with the phenylpropanoid moiety of their structures, which is different from what was previously observed with L. major DHFR-TS.

Table 3.
The VINA score values for six tested structures and MTX (methotrexate) for dihydrofolate reductase-thymidylate synthase (DHFR-TS) of Leishmania braziliensis, Leishmania panamensis, and Leishmania amazonensis. SD = standard deviation; RMSD values = root-mean-square deviation.
For L. panamensis, both derivative structures 301a and 302a presented the lowest VINA score values, −12.55 kcal/mol and −12.54 kcal/mol, respectively, showing a higher inhibitory activity compared to the four kauranes and the control, MTX (Table 3). Structures 301 and 302 did not show any π-alkyl interaction with Y91 (Figure 3b), with mainly van der Waals forces observed with flexible residues such as V31, V49, and V156. Kaurane 301 established a hydrogen bond between the carboxylic group of Carbon 4 and residue Q48. This interaction was also observed for MTX, however, a negative–negative unfavorable interaction with D52 affected the affinity value for this compound. A common alkyl interaction between V31 and the unsaturation of carbon-17 of structure 135, and between V31 and the 1,3-dioxolane group of structure 4, was also observed.
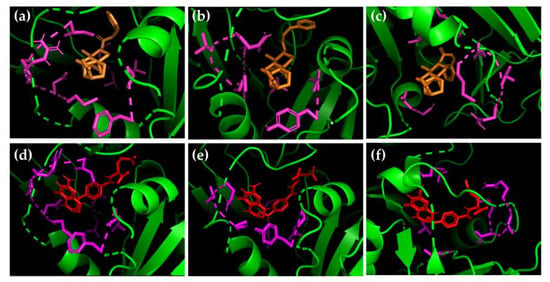
Figure 3.
Best pose of structure 302 (orange) and MTX (red) in the active site of (a–d) L. amazonensis (b–e) L. braziliensis and (c–f) L. panamensis DHFR-TS (green). Flexible amino acids are marked in pink.
In the same manner, L. amazonensis exhibited a behavior similar to that of L. braziliensis, with VINA score values ranging from −10.52 to −11.14 kcal/mol, all of which showed lower affinity values compared to MTX (−9.54 kcal/mol). The latter only showed three interactions with the flexible residues in the active site of the enzyme, including two van der Waals interactions with V49 and Q48 and a π-sulfur interaction between the sulfhydryl group of M53 and the pteridine ring. Structures 301 and 302 displayed the same interactions, which were classified into three groups: π-alkyl with M53 and Y91; alkyl with V87; and van der Waals with V49, V31, and V156. On the other hand, structure 4 was the only kaurane that exhibited a hydrogen bond interaction with Q48, which might explain the slight difference in its affinity value.
Figure 3 displays the complex between the best-docked pose of structure 302, the potential multispecies dual DHFR-TS/PTR1 inhibitor, and each of the three DHFR-TS hybrid models built in this study. For the L. braziliensis and L. amazonensis species (Figure 3a,b), similar poses and intermolecular interactions were observed, highlighting the π-alkyl interactions of Y91 and M53 with the double bond of carbon-17.
In contrast, L. panamensis showed a different three-dimensional conformation in the active site of DHFR-TS, with a different spatial position for the phenylpropanoid moiety compared to the other two species of Leishmania. Additionally, residue Y91, which was a key residue in the interaction of the evaluated structures with the enzyme in L. amazonensis and L. braziliensis species (Figure 3a,b), did not interact with the unsaturation of carbon-17. This same pattern was also observed for MTX, where Y91 did not appear to be a relevant amino acid for the inhibitory activity.
2.4. Molecular Dynamics Simulations for L. major and L. braziliensis DHFR-TS Interacting with 302 and MTX
To validate the hybrid models built for the different Leishmania species used in this study and evaluate the protein–ligand stability of structure 302 and its derivative 302a, molecular dynamics (MD) studies were performed on L. major and L. braziliensis DHFR-TS using MTX as a reference ligand. Among the three species of Leishmania used in this study, Leishmania braziliensis was selected to perform MD simulations, because this species is responsible for the most cases in the New World. L. braziliensis causes CL and MCL, which are prevalent in Brazil, Peru, Colombia, and other countries in Central and South America. L. amazonensis and L. panamensis are also causative agents of CL and MCL, but they are less common than L. braziliensis [34].
Initially, root-mean-square deviation (RMSD) analyses were conducted to assess the structural stability of the receptor frame. These analyses measured the distance between different positions of a set of atoms over time (in nm) [35]. For L. major DHFR-TS, during the first 30 ns, similar levels of perturbation were observed, with RMSD values ranging from 0.10 to 0.35 nm for structures 302, 302a, MTX, and the apoenzyme (apoLmDHFR-TS, the protein without the ligand). After 30 ns, the protein in complex with structure 302 and its derivative 302a showed increased stability, with lower RMSD values compared to apoLmDHFR-TS (Figure 4a). Structure 302a showed a similar pattern to the complex LmDHFR-TS:MTX. In the case of L. braziliensis, the apoLbDHFR-TS complex showed a gradual increase in RMSD values from 0.20 to 0.60 nm over the course of the 100 ns simulation. In contrast, the LbDHFR-TS:302a and LbDHFR-TS: MTX complexes exhibited more stable structures, with RMSD values ranging from 0.20 to 0.25 nm (Figure 4b). This suggests that structure 302a enhances the stability of the complex with DHFR-TS in both Leishmania species, similar to the stability conferred by MTX. In addition, the LbDHFR-TS:302 complex maintained a relatively constant RMSD value after 30 ns until the end of the simulation, which was consistently higher than that observed for MTX and 302a.
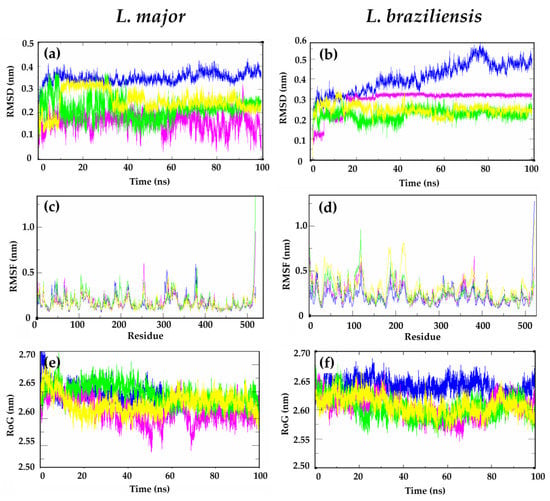
Figure 4.
(a,b) Root-mean-square deviation (RMSD), (c,d) root-mean-square fluctuation (RMSF), and (e,f) radius of gyration (RoG) values within the L. major DHFR-TS and L. braziliensis DHFR-TS binding site, obtained after molecular dynamics simulations. Apoenzyme (blue); DHFR-TS:MTX complex (yellow); DHFR-TS: 302 complex (pink); DHFR-TS: 302a complex (light green).
Afterward, we analyzed the flexibility of residues with different ligands using root-mean-square fluctuation (RMSF) values. Similar patterns were found in both L. major and L. braziliensis during the entire dynamic simulations (Figure 4c,d). Regions with defined tertiary structures (α-helices or β-sheets) showed similar RMSF values (0.1 to 0.3 nm) for structure 302 and its derivative 302a in complex with L. major DHFR-TS, as well as for the apoenzyme. However, the control compound MTX presented higher RMSF values, particularly in loop regions of the protein. On analyzing the RMSF values in L. braziliensis DHFR-TS, the LbDHFR-TS:302 and the apoenzyme showed similar behaviors over the simulation time, while structure 302 had higher fluctuations in loop regions than MTX and the uncomplexed protein, especially in the region from A113 to T121, where values ranging from 0.30 nm to 0.63 nm were observed. Despite this, in regions with defined tertiary structure, both MTX and the kaurane 302a showed RMSF values lower than 0.30 nm, which indicates low flexibility in L. major DHFR-TS when complexed (Figure 4d).
In addition, we observed the evolution of the packing level of L. major and L. braziliensis DHFR-TS through the radius of gyration (RoG) values. For L. major, the complexes with structure 302 and its derivative 302a showed no difference in RoG values compared with the control MTX and apoLmDHFR-TS (ranging from 2.55 nm to 2.70 nm), indicating high stability and low fluctuations in the tertiary structure (Figure 4e).
For L. braziliensis, the RoG values for DHFR-TS were different for the evaluated complexes compared to the apoLbDHFR-TS. During the first 30 ns of the simulation, no differences in RoG values were observed (RoG of approximately 2.68 nm). However, after this time, the complexes LbDHFR-TS:302, LbDHFR-TS:302a and LbDHFR-TS:MTX demonstrated different behaviors, with a reduction in the RoG value (approximately 2.61 nm). This indicates that structure 302 and its derivative 302a stably folded after the simulation, compared to the apoenzyme, which remained at a constant value during the 100 ns test period (Figure 4f).
2.5. Free Energy Calculations by the Molecular Mechanics Poisson–Boltzmann Surface Area Approach (MM/PBSA) Method
After the molecular dynamic simulations were completed, the binding free energies for the complexes of structures 302 and 302a, as well as MTX with L. major DHFR-TS and L. braziliensis DHFR-TS, were calculated using the MM/PBSA method. Kaurane 302 and its derivative 302a in complex with L. major DHFR-TS reached similar binding free energy values of −138.2 kJ/mol and −134.2 kJ/mol, respectively, which were both higher than the value measured for the complex LmDHFR-TS: MTX, which was −140.1 kJ/mol. Conversely, the complexes LbDHFR-TS:302 (−134.8 kJ/mol) and LbDHFR-TS:302a (−144.5 kJ/mol) reached a lower binding free energy value compared to the complex LbDHFR-TS: MTX (−95.9 kJ/mol). Nevertheless, for both Leishmania species, similar energetic contributions were observed, which were linked to the structural features of the evaluated molecules (Table 4).

Table 4.
Binding free energies (kJ/mol) from the MM/PBSA calculations for structure 302 and its derivative 302a for L. major DHFR-TS and L. braziliensis DHFR-TS; In both proteins MTX was used as reference ligand.
For the complexes with 302 and 302a in both Leishmania species, the van der Waals, electrostatic, and solvent-accessible surface area (SASA) parameters showed negative contributions to the binding free energy. The van der Waals parameter had the highest negative contribution in L. braziliensis, and these results are directly related to the molecular docking calculations, where—mainly in New World Leishmania species—this type of interaction is fundamental for the stability of the DHFR-TS-diterpenoid complexes. For L. major, the electrostatic parameter contributed negatively to the binding free energies for 302 and MTX; however, its contribution in 302a was close to 50%. In this same way, for structure 302 and its derivative 302a complexed with L. braziliensis, electrostatic interactions were significatively minor compared to the contribution observed for MTX, which had a higher contribution to the total binding energy. Finally, for all molecules, polar solvation had a positive contribution to the total energy value, with larger contributions to the complexes with MTX in both evaluated Leishmania species.
3. Materials and Methods
3.1. LmDHFR-TS Enzyme Inhibition Assay
Purification and kinetic characterization of the recombinant LmDHFR-TS protein was performed according to the previously reported procedures [36,37]. The in vitro evaluation of selected diterpenoids (i.e., 4, 135, 301, 302, 301a, and 302a) for inhibitory activity against LmDHFR-TS was conducted using a spectrophotometric assay under standard DHFR conditions. The assay consisted of LmDHFR (2.7 nM), bovine serum albumin (BSA, 1 mg/mL), N-[tris(hydroxymethyl)-methyl]-2-aminoethanesulfonic acid (TES) buffer (100 mM, pH 7.0, 150 mM β-mercaptoethanol, 2 mM ethylenediaminetetraacetic acid (EDTA)), and nicotinamide adenine dinucleotide phosphate (NADPH, 100 μM) with varying concentrations of the test compounds (0.1–128 μM). The reaction was initiated by adding the substrate (7,8-dihydrofolate (H2F), 20 μM) and was monitored for 360 s at 340 nm (i.e., oxidation of NADPH to NADP+) to determine the initial reaction rate (Vo) through linear regression analysis of the resulting absorbance profile. All measurements were performed in triplicate and MTX was used as a positive control. The resulting Vo values were used to calculate the % inhibition, as 100 − (Ri/Rc × 100), where Ri is the Vo in the presence of the inhibitor and Rc is the Vo in the absence of inhibitors (1% DMSO v/v final concentration). The % inhibition was measured for at least five concentrations (0.1–128 μM) for each test compound (diterpenoids and MTX), and concentration–response curves (% inhibition vs. Log [inhibitor]) were constructed using nonlinear regression to determine the IC50 using GraphPad Prism 7.0 (GraphPad, San Diego, CA, USA). The Kiapp values were finally calculated using the Cheng–Prusoff equation for competitive inhibition with a 1:1 stoichiometry and reversible inhibitor-binding reactions: Kiapp = IC50/(1 + [S]/Km), where [S] is the substrate (H2F) concentration and Km is the Michaelis constant. The substrate Km was calculated during the kinetic characterization of the purified, recombinant LmDHFR-TS and was determined to be 2.4 ± 0.7 μM.
3.2. Isolation of Compound 148
Kaurane-type diterpene 148 was isolated from Euphorbia graminea Jacq. (Euphorbiaceae), which was propagated under greenhouse conditions from commercially available seeds (Swallowtail Garden Seeds, Santa Rosa, CA, USA). The aerial part (128 g) of two-month-old plants of E. graminea was extracted with 96% ethanol, and the raw extract (11.2 g) was purified by column chromatography (CC) using a gradient elution of n-hexane to methanol, yielding fifteen fractions. The purification of fraction 7 was then performed independently by flash column chromatography on SiO2 using a mobile phase of a 7:3 mixture of n-hexane and ethyl acetate, which resulted in the isolation of diterpene 148 (35.6 mg). Its spectroscopic data, including NMR and HRMS, were found to match those of the previously isolated compound ent-kaurane-3-oxo-16α,17-diol [38].
3.3. Synthesis of 16ß,17-Isopropylidenedioxy-ent-kauran-3-one (4)
Compound 4 was synthesized from 148 using a previously reported procedure [39]. Briefly, compound 148 (24 mg, 0.075 mmol) and tetrahydrofuran (THF) (4 mL) were mixed in a 10 mL round-bottom flask by stirring at 0 °C. Then, 2,2-dimethoxypropane (46 µL, 0.375 mmol) and p-toluenesulfonic acid monohydrate (0.75 mg, 0.375 µmol) were added. The reaction mixture was stirred at 0 °C for 2 h, allowed to warm to 20 °C, and then stirred at this temperature for 16 h. The reaction was then quenched with saturated NaHCO3 (3 mL) and extracted with CH2Cl2 (3 × 3 mL). The CH2Cl2 extract was separated, washed with 10% NaCl (2 × 3 mL), dried over MgSO4, filtered, and concentrated under reduced pressure to obtain the structure 4 (26 mg, 96%); [α]D20 –41.8 (c 0.04, CHCI3); 1H NMR (400 MHz, CDCl3) δH 4.22 (d, J = 8.3 Hz, 1H), 3.61 (d, J = 10.5 Hz, 1H), 2.41 (dd, J = 8.1, 6.3 Hz, 2H), 2.33–2.28 (m, 1H), 1.85 (dd, J = 10.4, 3.6 Hz, 1H), 1.76–1.72 (m, 1H), 1.68–1.65 (m, 2H), 1.64–1.61 (m, 1H), 1.52–1.47 (m, 1H), 1.47–1.44 (m, 1H), 1.37 (s, 3H), 1.33 (s, 3H), 1.27–1.25 (m, 3H), 1.24–1.23 (m, 1H), 1.23–1.19 (m, 3H), 1.14–1.11 (m, 1H), 0.99 (s, 3H), 0.91 (s, 3H), 0.88 (s, 3H), 0.82 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CHCI3) δC 217.6, 193.0, 109.3, 79.6, 69.5, 55.2, 54.7, 52.3, 47.4, 44.1, 40.6, 40.4, 37.8, 37.3, 37.2, 34.5, 27.6, 27.4, 27.2, 26.7, 20.3, 19.4, 17.8; HREIMS [M+H]+ m/z 361.2724 (calcd. for C23H37O3, 361.2743).
3.4. Hybrid Models of Leishmania DHFR-TS
Hybrid models of the dihydrofolate reductase-thymidylate synthase (DHFR-TS) of different Leishmania species were constructed using YASARA software (YASARA 18.4.24, Vienna, Austria: YASARA Biosciences GmbH, 2018) [40]. The FASTA sequences of L. major DHFR-TS (P07382), L. braziliensis DHFR-TS (A4H4P8), L. panamensis DHFR-TS (S5M3K7), and L. amazonensis DHFR-TS (P16126) were obtained from the UNIPROT database (https://www.uniprot.org/, accessed on 28 December 2022). The constructed hybrid models were validated through stereochemical quality assessment using PROCHECK [41]. PROCHECK evaluated molecular diversity through several stereochemical parameters, including the torsional angles of the main chain, side chain torsional angles, bad contacts or steric impediments, and planarity. PROCHECK generated a Ramachandran graph [27], which verified the allowed and prohibited regions of the main amino acid chain. The structural quality was evaluated using VERIFY 3D software (https://services.mbi.ucla.edu/SAVES/, accessed on 3 January 2023) and WHAT IF (https://swift.cmbi.ru.nl/servers/html/index.html, accessed on 5 January 2023). VERIFY 3D software analyzes the compatibility of the protein sequence with its 3D structure based on the chemical environment, while WHAT IF analyzes various structural parameters, such as atomic contacts between residues. The Discovery Studio Visualizer (BIOVIA, Dassault Systèmes, Discovery Studio Visualizer, v21.1.0.20298, San Diego: Dassault Systèmes, 2020) was used to visualize the modeled protein [24].
3.5. Molecular Docking Calculations
The hybrid model of L. major DHFR-TS in complex with methotrexate (PDB ID: MTX) was used for the molecular docking calculations of the six kaurane-type diterpenes using Molegro 6.0.1 software. All water molecules were removed from the enzyme structures and both the enzyme and compound structures were prepared with the same default parameters in the same software package. MolDock was used as the score function, and the internal ES, internal H-bond, and Sp2-Sp2 torsions were all checked as the ligand evaluation criteria. The molecular docking procedure was run 10 times, using the MolDock SE algorithm, with a maximum of 1500 interactions, a maximum population size of 50, a maximum of 300 steps, a neighbor distance factor of 1.00, and a maximum of 5 poses returned. A grid with a 15 Å radius and 0.30 Å resolution was used to cover the ligand-binding site for the enzyme structure [42,43].
For L. braziliensis, L. panamensis, and L. amazonensis DHFR-TS, the docking calculations were performed using the Autodock/Vina (1.1.2) plug-in for PyMOL (1.3r2) under a Python 2.5.2 environment for Windows. The minimized structure was located in a cube with dimensions of 22.5 Å × 22.5 Å × 22.5 Å and a grid spacing of 0.375 Å at the geometric center of the binding pocket (coordinates for L. braziliensis: 43.01, 23.70, 1.67; L. panamensis: 43.64, 24.00, 1.50; and L. amazonensis: 43.49, 24.96, 1.95), which was identified through cavities analysis in Molegro 6.0.1. Flexible residues in the binding site were selected for each model: L. braziliensis I30, V31, Q48, V49, M53, V87, and V156; L. amazonensis: Q30, V31, Q48, V49, M53, V87, and V156; L. panamensis: I30, V31, Q48, V49, D52, M53, S86, and V87. The docking poses were classified based on their docking scores, such as the free energy or affinity, and each calculation was performed in three replicates. Methotrexate (MTX) was used as a control. The two-dimensional residual interaction diagrams were visualized on the Discovery Studio Visualizer (BIOVIA, Dassault Systèmes, Discovery Studio Visualizer, v21.1.0.20298, San Diego: Dassault Systèmes, 2020) [24].
3.6. Molecular Dynamics Simulations
Molecular dynamics simulations were carried out using Gromacs 5.0.5 on an Ubuntu 12.04 server [44,45]. Structure 302, its derivative 302a, MTX, and the hybrid models of L. major and L. braziliensis DHFR-TS were used as inputs for the simulations [40]. Leishmania braziliensis was selected to perform MD simulations, because this species is responsible for the most cases in Central and South America. Kaurane-type diterpene 302 and its derivative 302a were selected for MD simulations because they were previously found to have half-maximal inhibitory concentrations (IC50) of less than 10 µg/mL against PTR1. It was shown that the inhibitory activity of structure 302 improved by approximately 60% when a 3-p-coumaroyloxy group was used instead of the 3-cinnamoyloxy substituent. Additionally, these two structures showed the lowest Kiapp values among the six tested structures against DHFR-TS. These two structures also displayed a similar behavior with lower Kiapp values in previous enzymatic assays against L. major PTR1, indicating their potential as dual PTR1/DHFR inhibitors.
For the tested structures, hydrogen atoms and corresponding charges for the ligands were added using the AM1-BCC charge scheme in UCSF Chimera, and the ligand topologies were generated automatically with the ACPYPE script. The protein topologies were obtained in Gromacs using the Amber 99SB force field and the TIP3P water model. Solvation was performed in a triclinic box with a margin distance of 1.0 nm and 0.1 M NaCl was added to the complexes and proteins by randomly replacing water molecules until neutrality was achieved [35,43,44,45]. The systems were energy-minimized for 2000 steps using the steepest descent method. Then, NVT equilibration was performed at 310 K for 50 ps followed by NPT equilibration for 500 ps, using the Parrinello–Rahman method at 1 bar with position restraints. The solute position restraints were then released, and a production run was performed for 5 ns while maintaining constant temperature and pressure at 310 K and 1 bar, respectively. The coordinates were recorded in a 1 fs time step, and electrostatic forces were calculated using the particle-mesh Ewald method. All simulations used periodic boundary conditions, and covalent bond lengths were constrained by the LINCS algorithm.
3.7. Binding Free Energies Using the Molecular Mechanics Poisson–Boltzmann Surface Area (MM/PBSA) Method
The binding free energies were calculated using the molecular mechanics Poisson–Boltzmann surface area (MM/PBSA) method based on the trajectories obtained from the molecular dynamics simulations [43,44,45]. The calculation of free binding energy of the protein-binding complex in the study of the molecular behavior of enzymes and their respective ligands was evaluated using the molecular mechanics Poisson–Boltzmann surface area approach (MM/PBSA) method [46]. The GROMACS g_mmpbsa module [47,48] was applied to estimate the bond-free energy of the selected complex using the trajectory files obtained in the molecular dynamics simulation. The GROMACS MM-PBSA calculation consisted of three steps. First, the potential energy in the vacuum was calculated, and then, the energies of polar and, finally, nonpolar solvation were estimated. The nonpolar solvation energy was calculated using the solvent-accessible surface area model (SASA). The required input files and solvation energy values were then selected to evaluate the following energetic components: van der Waals energy, electrostatic energy, polar energy of solvation, nonpolar solvation energy, and free energy of bonding.
4. Conclusions
This study identified compounds 302 (3α-cinnamoyloxy-ent-kaur-16-en-19-oic acid) and 302a as potential inhibitors of both PTR1 and DHFR-TS in L. major, building upon previous findings of PTR1 inhibition [20]. Both 302 and 302a displayed in vitro inhibitory activity against L. major DHFR-TS, with IC50 values of 6.3 and 4.5 µM, respectively. Additionally, other kaurane-type diterpenes, such as synthesized structure 4, also inhibited DHFR-TS in vitro with an IC50 value of 7.6 µM. Structures 301 and 301a, which were previously classified as inactive against PTR1, also showed inhibitory activity against DHFR-TS, verifying the results obtained from MolpredictX. Furthermore, molecular docking calculations using a hybrid model of L. major DHFR-TS allowed evaluation of the mechanism of action of the tested kauranes. The p-hydroxyl group of the phenylpropanoid moiety of structure 302a was found to play a crucial role in the inhibition of DHFR-TS.
Additionally, hybrid models for three Leishmania species with high incidence in Central and South America were constructed. The best docked results for structure 302 and its derivative 302a in the three hybrid models showed a correlation between the affinity values obtained from the molecular docking and some structural features of the kauranes, such as the presence of an unsaturation at carbon-17 that interacts with the amino acids of DHFR-TS through π-alkyl interactions, making these two structures potential multispecies inhibitors. Furthermore, the molecular dynamics simulation, in addition to validating the hybrid models, confirmed the results previously obtained from the molecular docking calculations. So, this study presents a valuable approach for identifying potential dual PTR1/DHFR-TS inhibitors, contributing to the development of alternative chemotherapy strategies against these diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12040663/s1. Figure S1: Ramachandran plot for hybrid model of, L. braziliensis; Figure S2: Ramachandran plot for hybrid model of L. panamensis; Figure S3: Ramachandran plot for hybrid model of L. amazonensis; Figure S4: Ramachandran plot for hybrid model of L. major; Figure S5: Results of a multiple sequence alignment using ClustalW software, comparing the sequences of Leishmania and Homo sapiens.
Author Contributions
Conceptualization, E.C.-B. and M.T.S.; methodology, E.C.-B., L.S. and M.T.S.; dataset construction, and calculations, C.H.-A., N.F.d.S. and R.P.B.d.M.; writing—original draft preparation, C.H.-A., N.F.d.S., R.P.B.d.M. and E.C.-B.; writing—review and editing, C.H.-A., E.C.-B., L.S. and M.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number 141341/2020-3, Paraiba State Research Foundation (FAPESQ) grant number 2002/2022, Coordination of Improvement of Higher Education Personnel—Brazil (CAPES)—grant number 88887.513720/2020-00 and Universidad Militar Nueva Granada (UMNG), grant number IMP-CIAS-2924 (2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Supplementary Material can be accesses directly from investigators by email.
Acknowledgments
Authors thank UMNG, CNPq and CAPES for the financial support. This study is a cooperative activity within the Research Network Natural Products against Neglected Diseases (ResNet NPND, www.resnetnpnd.org, accessed on 13 February 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all compounds are available from the authors.
References
- Soni, M.; Pratap, J.V. Development of Novel Anti-Leishmanials: The Case for Structure-Based Approaches. Pathogens 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Gouri, V.; Upreti, S.; Samant, M. Evaluation of target-specific natural compounds for drug discovery against leishmaniasis. Parasitol. Int. 2022, 91, 102622. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Bamorovat, M.; Sharifi, I.; Almani, P.G.N. Global distribution of treatment resistance gene markers for leishmaniasis. J. Clin. Lab. Anal. 2022, 36, e24599. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, P.K.; Yadav, P.K.; Narender, T.; Patil, U.K.; Jain, S.K.; Chourasia, M.K. Emerging strategies and challenges of molecular therapeutics in antileishmanial drug development. Int. Immunopharmacol. 2023, 115, 109649. [Google Scholar] [CrossRef]
- Frézard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics 2023, 15, 99. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Brindha, J.; Balamurali, M.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 622286. [Google Scholar]
- Vickers, T.J.; Beverley, S.M. Folate metabolic pathways in Leishmania. Essays Biochem. 2011, 51, 63–80. [Google Scholar]
- Gilbert, I.H. Inhibitors of dihydrofolate reductase in leishmania and trypanosomes. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1587, 249–257. [Google Scholar] [CrossRef]
- Ivanetich, K.M.; Santi, D.V. Thymidylate synthase-dihydrofolate reductase in protozoa. Exp. Parasitol. 1990, 70, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, H.; Bakri, R.; Mirza, A.Z. Dihydrofolate reductase, thymidylate synthase, and serine hydroxy methyltransferase: Successful targets against some infectious diseases. Mol. Biol. Rep. 2022, 49, 6659–6691. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.B.; Sienkiewicz, N.; Wyllie, S.; Fairlamb, A.H. Dissecting the metabolic roles of pteridine reductase 1 in Trypanosoma brucei and Leishmania major. J. Biol. Chem. 2011, 286, 10429–10438. [Google Scholar] [CrossRef]
- Nare, B.; Luba, J.; Hardy, L.W.; Beverley, S. New approaches to Leishmania chemotherapy: Pteridine Reductase 1 (PTR1) as a target and modulator of antifolate sensitivity. Parasitology 1997, 114, S101–S110. [Google Scholar] [CrossRef]
- Panecka-Hofman, J.; Poehner, I.; Wade, R.C. Anti-trypanosomatid structure-based drug design—Lessons learned from targeting the folate pathway. Expert Opin. Drug Discov. 2022, 17, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, F.; Bandehpour, M.; Haghighi, A.; Mahboudi, F.; Mohebali, M.; Kazemi, B. Inhibition of Leishmania major PTR1 Gene Expression by Antisense in Escherichia coli. Iran. J. Public Health 2012, 41, 65–71. [Google Scholar]
- Das Neves, G.M.H.; Kagami, L.P.; Gonçalves, I.L.; Eifler-Lima, V.L. Targeting pteridine reductase 1 and dihydrofolate reductase: The old is a new trend for leishmaniasis drug discovery. Future Med. Chem. 2019, 11, 207–2130. [Google Scholar] [CrossRef]
- Possart, K.; Herrmann, F.C.; Jose, J.; Costi, M.P.; Schmidt, T.J. Sesquiterpene Lactones with Dual Inhibitory Activity against the Trypanosoma brucei Pteridine Reductase 1 and Dihydrofolate Reductase. Molecules 2022, 27, 149. [Google Scholar] [CrossRef]
- Teixeira, B.V.F.; Teles, A.L.B.; da Silva, S.G.; Brito, C.C.B.; de Freitas, H.F.; Pires, A.B.L.; Froes, T.Q.; Castilho, M.S. Dual and selective inhibitors of pteridine reductase 1 (PTR1) and dihydrofolate reductase-thymidylate synthase (DHFR-TS) from Leishmania chagasi. J. Enzym. Inhib. Med. Chem. 2019, 34, 1439–1450. [Google Scholar] [CrossRef]
- Sabt, A.; Eldehna, W.M.; Ibrahim, T.M.; Bekhit, A.A.; Batran, R.Z. New antileishmanial quinoline linked isatin derivatives targeting DHFR-TS and PTR1: Design, synthesis, and molecular modeling studies. Eur. J. Med. Chem. 2023, 246, 114959. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Da Costa, F.B.; Brun, R.; Kaiser, M.; Schmidt, T.J. Ent-pimarane and ent-kaurane diterpenes from Aldama discolor (Asteraceae) and their antiprotozoal activity. Molecules 2016, 21, 1237. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.M.; Panis, C.; Da Silva, S.S.; Macri, J.A.; Kawakami, N.Y.; Hayashida, T.H.; Madeira, T.B.; Acquaro, V.R.; Nixdorf, S.L.; Pizzatti, L.; et al. Kaurenoic acid possesses leishmanicidal activity by triggering a NLRP12/IL-1 β/cNOS/NO Pathway. Mediat. Inflamm. 2015, 2015, 392918. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; da Veiga-Júnior, V.F.; Vataru Nakamura, C. Antileishmanial activity of diterpene acids in copaiba oil. Mem. Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Acevedo, C.; Flores-Gaspar, A.; Scotti, L.; Mendonça-Junior, F.J.B.; Scotti, M.T.; Coy-Barrera, E. Identification of Kaurane-type diterpenes as inhibitors of Leishmania pteridine reductase I. Molecules 2021, 26, 3076. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Prusoff, W.H. the concentration of inhibitor which causes 50 percent inhibition (I) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Scotti, M.T.; Herrera-Acevedo, C.; Barros de Menezes, R.P.; Martin, H.J.; Muratov, E.N.; Ítalo de Souza Silva, Á.; Albuquerque, E.F.; Calado, L.F.; Coy-Barrera, E.; Scotti, L. MolPredictX: Online Biological Activity Predictions by Machine Learning Models. Mol. Inform. 2022, 41, e2200133. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall Iii, W.B.; De Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ, and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Vriend, G.; Sander, C. Quality control of protein models: Directional atomic contact analysis. J. Appl. Crystallogr. 1993, 26, 47–60. [Google Scholar] [CrossRef]
- Kosaka, A.; Sakamoto, N.; Hikone, M.; Imai, K.; Ota, M.; Washino, T.; Iwabuchi, S. Failure of liposomal-amphotericin B treatment for new world cutaneous leishmaniasis due to leishmania braziliensis. Intern. Med. 2020, 59, 1227–1230. [Google Scholar] [CrossRef]
- Herrera, G.; Barragán, N.; Luna, N.; Martínez, D.; De Martino, F.; Medina, J.; Niño, S.; Páez, L.; Ramírez, A.; Vega, L.; et al. An interactive database of Leishmania species distribution in the Americas. Sci. Data 2020, 7, 110. [Google Scholar] [CrossRef]
- Davila, M.; Pineda, V.; Calzada, J.E.; Saldaña, A.; Samudio, F. Evaluation of cytochrome b sequence to identify Leishmania species and variants: The case of Panama. Memórias Do Inst. Oswaldo Cruz 2021, 116, e200572. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Tomaz, D.C.; de Souza, L.A.; Onofre, T.S.; de Menezes, W.A.; Almeida-Silva, J.; Suarez-Fontes, A.M.; de Almeida, M.R.; da Silva, A.M.; Bressan, G.C.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef]
- Brustolin, A.Á.; Ramos-Milaré, Á.C.F.H.; de Mello, T.F.P.; Aristides, S.M.A.; Lonardoni, M.V.C.; Silveira, T.G.V. In vitro activity of cinnamaldehyde on Leishmania (Leishmania) amazonensis. Exp. Parasitol. 2022, 236, 108244. [Google Scholar] [CrossRef]
- Garcia, L.S.; Nielsen-Saines, K. Leishmaniasis. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Feigin, R.D., Cherry, J.D., Demmler-Harrison, G.J., Kaplan, S.L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; p. 2920. ISBN 978-1-4160-4044-6. [Google Scholar]
- Rozo-Lugo, C.; Cuca-Suárez, L.E.; Schmidt, T.J.; Coy-Barrera, E. Tetrahydrobenzofuran-6 (2 H)-one neolignans from Ocotea heterochroma: Their platelet activating factor (PAF) antagonistic activity and in silico insights into the PAF receptor binding mode. J. Nat. Prod. 2018, 81, 1968–1975. [Google Scholar] [CrossRef]
- Grumont, R.; Sirawaraporn, W.; Santi, D. V Heterologous Expression of the Bifunctional Thymidylate Synthase-Dihydrofolate Reductase from Leishmania major. Biochemistry 1988, 27, 3776–3784. [Google Scholar] [CrossRef]
- Nare, B.; Hardy, L.W.; Beverley, S.M. The roles of pteridine reductase 1 and dihydrofolate reductase- thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J. Biol. Chem. 1997, 272, 13883–13891. [Google Scholar] [CrossRef]
- Yi-Li, D.; Zhong-Jian, J. Tetracyclic diterpenols from Euphorbia sieboldiana. Phytochemistry 1991, 30, 2413–2415. [Google Scholar] [CrossRef]
- Bon, D.J.-Y.D.; Banwell, M.G.; Willis, A.C. A chemoenzymatic total synthesis of the hirsutene-type sesquiterpene (+)-connatusin B from toluene. Tetrahedron 2010, 66, 7807–7814. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Acevedo, C.H.; Scotti, L.; Scotti, M.T. In silico studies designed to select sesquiterpene lactones with potential antichagasic activity from an in-house asteraceae database. ChemMedChem 2018, 13, 634–645. [Google Scholar] [CrossRef]
- Herrera-Acevedo, C.; Maia, M.D.S.; Cavalcanti, É.B.V.S.; Coy-Barrera, E.; Scotti, L.; Scotti, M.T. Selection of antileishmanial sesquiterpene lactones from SistematX database using a combined ligand-/structure-based virtual screening approach. Mol. Divers. 2020, 25, 2411–2427. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson− Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Consortium, O.S.D.D.; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of Nanosystems: Application to Microtubules and the Ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).