Abstract
In recent years, sodium hypochlorite and chlorhexidine digluconate have been the gold standard of irrigation solutions utilized within the disinfection protocol during root canal treatments. Nowadays, it is known that, during chemical disinfection of the root canal, consecutive application of sodium hypochlorite and chlorhexidine digluconate leads to the formation of an orange-brown precipitate. This precipitate is described as being chemically similar to para-chloroaniline, which is suspected to have cytotoxic and carcinogenic effects. Concerns also exist regarding its influence on the leakage of root canal fillings, coronal restorations, and tooth discoloration. The purpose of this article is to review the literature on the interaction of sodium hypochlorite and chlorhexidine digluconate on the tooth and its surrounding tissues, and to discuss the effect of the precipitate formed during root canal treatment. We further address options to avoid the formation of the precipitate and describe alternative irrigation solutions that should not interact with sodium hypochlorite or chlorhexidine digluconate.
1. Introduction
Complete cleaning and disinfection of the root canal system are considered mandatory for long-term success in root canal treatment [1,2]. However, even after thorough mechanical cleaning, residual pulp tissue, bacteria, and dentin debris can remain in the root canal system [3,4]. Therefore, a variety of irrigating solutions are used in combination with the mechanical processing, such as sodium hypochlorite (NaOCl), chlorhexidine digluconate (CHX) [5], 17% ethylenediaminetetraacetic acid (EDTA), citric acid (CA), BioPure® MTAD® (Dentsply Tulsa Dental Specialties, Tulsa, OK, USA), and 37% phosphoric acid (PA) [6], as well as etidronate, alexidine (ALX), and Octenisept® (Schülke & Mayr, Norderstedt, Germany) [7]. Following internationally accepted quality guidelines, the main goals of irrigation are: eliminating microorganisms, flushing out debris, lubricating root canal instruments, and dissolving organic debris. Therefore, the used irrigation solution should preferably have disinfectant and organic-debris-dissolving properties, whilst not irritating the periradicular tissues [8]. For this purpose, sodium hypochlorite and chlorhexidine digluconate are widely recommended and well accepted in endodontics [9,10].
Unfortunately, endodontic irrigation solutions may interact chemically with each other during an alternating irrigation technique, potentially forming unwanted by-products, which may be toxic or cause allergic reactions [7]. Sodium hypochlorite and chlorhexidine are the best known and, at least in recent years, most frequently recommended irrigating solutions used for eliminating residual bacteria in chemo-mechanical root canal processing [5,6]. The undesirable adverse effects, after sodium hypochlorite and chlorhexidine interaction, of building precipitates are known, published and discussed controversially [11]. However, it is recommended that, until this precipitate is studied further, its formation should be avoided by removing the NaOCl before placing CHX into the canal [11]. Since 2006, the number of articles in PubMed concerning the interaction of NaOCl and CHX have grown significantly, and the topic was greatly debated [12,13,14,15,16]. Therefore, the aim of the present review is to summarize and discuss recently published papers focusing on the different outcomes regarding the interactions between sodium hypochlorite and chlorhexidine. Furthermore, based on the results of the review, the possible impact for the clinical disinfection protocol in endodontic therapy is summarized.
1.1. Sodium Hypochlorite (NaOCl)
Sodium hypochlorite (Figure 1) is the most used irrigating solution in endodontics, because its mechanism of action causes biosynthetic alterations in cellular metabolism and phospholipid destruction, the formation of chloramines that interfere in cellular metabolism, oxidative action with irreversible enzymatic inactivation of bacteria, and lipid and fatty acid degradation [17].
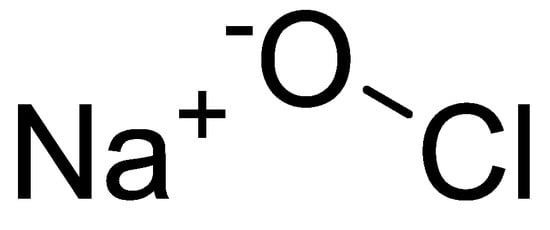
Figure 1.
Structural formula of sodium hypochlorite.
Sodium hypochlorite (NaOCl) is the most common irrigant used in root canal treatments. NaOCl is an effective tissue solvent and antimicrobial agent. It is usually used in a concentration range from 0.5 to 8.25% [18,19,20]. Its germicidal ability is related to the formation of hypochlorous acid when in contact with organic debris. In high concentrations, NaOCl is toxic and can cause inflammation in the periapical tissues [21], whereas in low concentrations, it is ineffective against specific microorganisms. NaOCl is not a substantive antimicrobial agent; it tends to discolor and corrode surgical instruments; and it has a very unpleasant odor [11].
1.2. Chlorhexidine (CHX)
Chlorhexidine digluconate (CHX) is the gluconate salt form of chlorhexidine, a biguanide compound used as an antiseptic agent with topical antibacterial activity (Figure 2). Chlorhexidine digluconate is positively charged and reacts with the negatively charged microbial cell surface, thereby destroying the integrity of the cell membrane. Subsequently, chlorhexidine gluconate penetrates into the cell and causes a leakage of intracellular components, leading to cell death. Since gram-positive bacteria are more negatively charged, they are more sensitive to this agent [22].
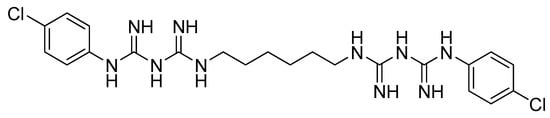
Figure 2.
Structural formula of chlorhexidine gluconate.
Chlorhexidine digluconate (CHX) can be used as a complement to increase the antibacterial action of NaOCl solutions during root canal preparation. CHX shows similar antimicrobial effects to sodium hypochlorite [23,24] in vitro and possesses a lower toxicity [25,26]. A disadvantage compared to NaOCl is its lack of ability to dissolve vital and necrotic tissue [27].
Chlorhexidine digluconate is a broad-spectrum antibacterial agent with substantivity to tooth structures, i.e., it binds to the hydroxyapatite of the enamel and dentin or to anionic groups of glycoproteins, is slowly released and, due to the moderate concentration decrease, its antibacterial effects are prolonged for an extended period of time [28].
1.3. Proteolysis
When NaOCl and CHX are mixed, NaOCl dissociates into different ions (H+, O2−, and Cl−). The chloride group then reacts with the chlorhexidine molecule in the guanine group (NH). This leads to the formation of chlorhexidine chloride (N+ and Cl−). In this reaction, the formation of an orange-brown precipitate is described. This precipitate contaminates the dentin and adheres to the canal walls [29]. Furthermore, CHX is a dicationic acid and has the ability to donate protons, whereas NaOCl is alkaline and can absorb protons from the dicationic acid. This proton exchange leads to the formation of a neutral and insoluble precipitate [11,30,31]. A color change due to the reaction can already be seen from a concentration of 0.023% NaOCl and the formation of the precipitate from 0.19% NaOCl by means of X-ray Photoelectron Spectroscopy (XPS), and the absolute amount by means of Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) [11].
While the undesirable effects of the initial developing substances have been well studied and are classified as acceptable [5], the precipitate with regard to its ingredients and undesirable effects still gives rise to discussions [32,33].
Figure 3 demonstrates microtubes filled with 2% CHX mixed with different concentrations of NaOCl. The first microtube is a control sample with 2% CHX alone. From left to right, a color change, which becomes brighter as the concentration of NaOCl decreases, can be observed.
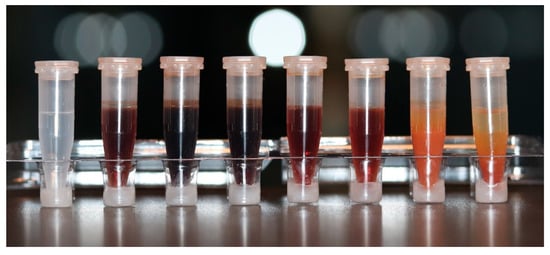
Figure 3.
Microtubes containing 2% CHX mixed with different concentrations of NaOCl, to illustrate the precipitate formation. From left to right: (1) control sample with 0% NaOCl; (2) 0.5%; (3) 1%; (4) 1.5%; (5) 2.5%; (6) 3%; (7) 4%; (8) 5%.
2. Materials and Methods
An unlimited search in all fields of the PubMed database (https://pubmed.ncbi.nlm.nih.gov/, accessed on 4 October 2022) was carried out through the website of the National Center for Biotechnology Information (NCBI), utilizing the combination of the Medical Subject Headings (MeSH terms) “sodium hypochlorite” (NaOCl) AND “chlorhexidine” (CHX) and yielded 955 results from the years 1974–2022. Specifying the search term to “chlorhexidine AND sodium hypochlorite AND interaction”, 64 publications remained from the original result. By individually reviewing the references and abstracts of these 64 publications the keywords “precipitate” and “para-chloroaniline” were regularly found in the keywords of the relevant articles (Figure 4).
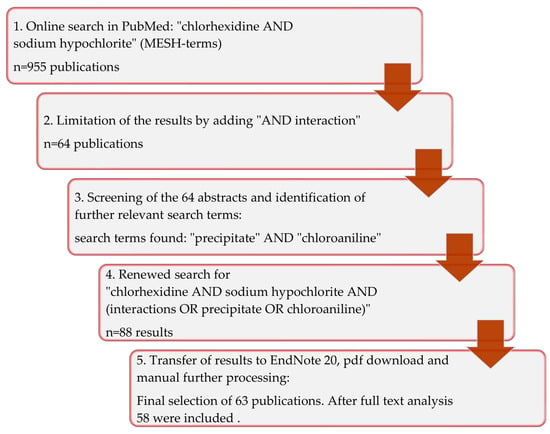
Figure 4.
Graphical representation of the search strategy used in the present review.
Finally, the search term was further expanded to include “chlorhexidine AND sodium hypochlorite AND (interaction OR precipitate OR chloroaniline)”, which resulted in a selection of 88 articles that included the manually determined references. The abstracts of all articles of the final online search result were evaluated and 25 articles that showed no relevance to the question were sorted out (Table 1, Figure 4).

Table 1.
Inclusion and exclusion criteria.
3. Results
The 63 papers included in this review are listed in Table 2, where the title and objective were summarized. These publications were read in full and evaluated.

Table 2.
Included papers of the review.
58 publications (49 studies, 8 reviews, 1 short communication) were relevant to the topic; another 5 were excluded after reading the full texts. Sources to which the research publications referred were included if they were relevant to the topic, even if the date of their publication was before 1994.
4. Discussion
The PubMed search found 63 publications, 58 of which were relevant. After full text analysis, 49 were studies that have been published since 2006 in the medical and especially in the dental-endodontic field, which have dealt with the interaction of NaOCl and CHX. Eight reviews with different focuses giving an overview of the state of knowledge at the date of publication were also selected. Furthermore, one article made recommendations on how to avoid formation of the precipitate, and thus was included.
4.1. Methodology
The 1998 study by Kuruvilla and Kamath [83] indicated that the alternating use of NaOCl and CHX reduces the microbial flora to a greater percentage (84.6%) than the use of NaOCl (69.4%) or CHX (70%) on its own. In order to optimize the tissue-dissolving properties of NaOCl and the antiseptic properties of the CHX against gram-positive germs, it was considered to use a combination of both irrigation solutions. However, by mixing the two solutions, for example through consecutive use in the root canal, a peach-colored to brown precipitate is formed [7], which is difficult to remove [10,13,16,17]. It is undisputed in the literature that the precipitate forms due to the acid–base reaction of NaOCl and CHX. The exact composition and, in particular, the question of whether the precipitate contains para-chloroaniline (PCA), motivated studies in the period from 2007 to 2021. Controversial views on the suitability of test methods for the analysis of the precipitate [13,78,80] and partly contradicting test results from the same test methods [47,59,76] leave doubts as to whether free PCA arises from the reaction of NaOCl and CHX [32]. However, recent studies emphasize the use of multiple non-destructive test methods and always examine 98% PCA as a comparison group, and they could not detect any free PCA in the precipitate [47,55]. In a review carried out by two independent authors on the basis of 13 included articles from different databases, Khatib et al. [33] concluded that the brown precipitate, which forms after mixing NaOCl and CHX, may contain a proportion of para-chloramide rather than free PCA and that PCA may be the by-product of the breakdown of highly concentrated CHX. It is also disputed whether PCA has mutagenic potential. While Gomes et al. [6] were citing publications from 1986 and 1995 according to which PCA was found to be mutagenic in microorganisms, Patil et al. [54] found no significant difference in the mutagenicity of the precipitate and the comparison group.
4.2. Toxicity
Regarding the toxicity of the precipitate, Cintra et al. [63] found a short-term increased toxicity compared to the starting substances, while Vouzara et al. [53] identified a predominantly antagonistic effect in the combination of NaOCl and CHX, indicating that the precipitate was less toxic than the starting substances. Surrender et al. [51] found the precipitate to be less toxic than either NaOCl or CHX alone. Furthermore, Jeong, Sarmast, Terlier, van der Hoeven, Holland and Parikh [35] all concluded that the precipitate has a toxic effect against human gingival fibroblasts, but highly concentrated NaOCl has an even greater cytotoxic effect. Nocca et al. [14] also observed a lower mortality of fibroblast cells to which the precipitate was applied than in those treated with the supernatant.
Marchesan et al. [84] evaluated the metals present in the precipitate of NaOCl and CHX by means of atomic absorption spectrophotometry and identified statistically significant proportions of copper (Cu), tin (Zn), iron (Fe), manganese (Mn), magnesium (Mg) and calcium (Ca). Siddique et al. [16] found selenium (Se) with inductively coupled plasma mass spectrometry. A discoloration of enamel and dentin was found by Souza et al. [65] on bovine anterior teeth that were placed in CHX gel and NaOCl consecutively. Therefore, it could be concluded that the combined use of NaOCl and CHX solution can cause dentin discoloration during endodontic treatment.
4.3. Recommended Irrigation Protocol
In order to prevent the formation of precipitates when using NaOCl and CHX, an irrigation protocol that includes intermediate rinses has frequently been recommended. For example, Zehnder [5] recommended rinsing the root canals exclusively with NaOCl during the mechanical preparation, which he ascribed to “unique tissue-dissolving properties”. Before a final rinse with CHX recommended by him for chronic pulpitis and revisions, Zehnder [5] advised an intermediate rinse with EDTA or citric acid in order to prevent the formation of precipitates. It should be noted here that the root dentin can soften, if it is exposed to strong chelating agents, such as EDTA, for a long time [85]. Bueso et al. [34] used stereomicroscopic analysis to compare the effect of EDTA, distilled water and sodium thiosulfate (STS) as an intermediate rinse to prevent the formation of brown precipitates. In this context, 5% STS significantly reduced the intensity of brown precipitates, compared to no intermediate rinse. Alberto et al. [37] were able to demonstrate this effect ex vivo when CHX was added 10 min after the application of STS. Subsequent studies evaluated the endodontic irrigation regimen. The formation of precipitates was also demonstrated for the mixture of EDTA and NaOCl, but not for citric acid and CHX [72].
In addition, Mortenson et al. [13] found the least amounts of precipitate after intermediate flushing with 50% citric acid, compared to EDTA and saline. Intermediate rinsing with pure alcohol, distilled water, or saline solutions could also prevent or reduce the formation of precipitates [79]. However, a precipitate present in the root canal system represents a layer that occludes the dentinal tubules [12,30], is difficult to remove [29,79], and compromises the tightness of a root filling using AH 26 sealer (Dentsply Sirona, Konstanz, Germany) and gutta-percha proportionally to the amount of precipitate [61]. Whether the precipitate affects sealer adhesion has been controversially discussed: While Gupta et al. [70] came to the conclusion that the precipitate reduced the bonding capacity of an epoxy-based sealer (AH Plus®, Dentsply Sirona, Konstanz, Germany) significantly, it was subsequently shown that the adhesion of Resilon®-Epiphany SE obturation system (Pentron Clinical Technologies, Wallingford, CT, USA) was not affected by the precipitate. In addition, Magro et al. [58,62] found no correlation between the penetration depth of an epoxy sealer into the dentin and bond strength values between groups treated with or without CHX. However, the investigated CHX variants led to more precipitate in all root canal areas previously rinsed with NaOCl, though they did not reduce the bond strength of the sealer in the push-out test, which was traced back to the protocol for canal drying and covalent bonds between the sealer and the dentin surface [58,62].
Even by activating the rinsing solutions, the removal of the precipitate is only possible to a limited extent. However, it was found that activation of the chelating agents EDTA and citric acid, in particular using sonic (Eddy®, VDW, Munich, Germany) or ultrasound devices, is superior to syringe rinsing [32,52,57].
4.4. Alternative Irrigation Solutions
Furthermore, CHX alternatives were also considered and examined. The substitution of CHX by the herbal antimicrobial substances neem, tulsi, aloe vera, and garlic was not successful, as the amount of precipitate resulting from these substances in combination with NaOCl was a factor of 4–7.5 higher than that with CHX [42]. ALX, a substance from the biguanide family, similar to CHX, developed only a slightly yellowish color, but no precipitate formation with NaOCl [46]. In studies by Thomas et al. [41] the effectiveness of the combination of ALX and NaOCl against Enterococcus faecalis was not significantly higher than that of NaOCl alone. Nevertheless, the authors propagated that it can be used in 1% concentration as an alternative to CHX in the endodontic irrigation protocol if used for a sufficiently long time (>5 min). In contrast to the combination of NaOCl and CHX, Kim et al. [86] found no PCA in the mixture of NaOCl and ALX and considered it to be a CHX alternative because it is just as effective against all bacteria and fungi. Czopik [36] described a yellowish precipitate when mixing NaOCl and ALX, which could be identified as aliphatic amines by using the UHPLC-MS (ultra-high-performance liquid chromatography-mass spectrophotometry) method. Surender et al. [51] found a significantly higher effectiveness of NaOCl with ALX against Enterococcus faecalis than with the combination of NaOCl and CHX. Octenisept®, an octenidine-based preparation, which also contains 2% phenoxyethanol, led to a sparse, whitish deposits that partially closed the dentinal tubules and became transparent over time. Thaha et al. [50] saw potential for a combined application with NaOCl, but also a need for further investigations, for example, with regard to the effect on sealer adhesion to dentin. MTAD®, which contains 3% doxycycline, 4.25% CA, and 0.55% polysorbate, forms a green-yellow precipitate with NaOCl, the color of which changes to brown when exposed to light. Intermediate rinsing with ascorbic acid can prevent precipitation [7]. SmearOFFTM (Vista Apex, Raxine, WI, USA) and QMix® (Dentsply Sirona, Bensheim, Germany) are products that combine a biguanide and a chelator. After their application, the penetration depths of the sealer into the dentin were greater than those after sequential rinsing with 17% EDTA, saline solution, and CHX. While QMix® is used after saline or distilled water (2-phase), SmearOFFTM combines the intermediate rinse and the final rinse, which simplifies and shortens the rinsing protocol. According to the manufacturer, the use of SmearOFFTM after NaOCl in the root canal does not lead to the formation of precipitates; this is also indicated by the sealer penetration depths. Since the manufacturers have not disclosed the formulation of the preparations, further studies on effectiveness and interactions are required [39].
The possibility of exchanging NaOCl in the combination of NaOCl and CHX was only considered possible by Buyukozer et al. [38]. Chlorine dioxide (ClO2) can be utilized as an alternative means of root canal irrigation instead of NaOCl, due to its antimicrobial activity, biocompatibility, and ability to dissolve organic tissue [87,88,89].
4.5. Clinical Impact on Endodontic Therapy
In 2023, the best possible cleaning and disinfection of the root canal system by means of chemomechanical preparation is still an indispensable prerequisite for the success of endodontic treatment [2,90,91].
The desirable properties of the various irrigation solutions are:
- -
- Dissolution of necrotic and vital tissue;
- -
- Effectiveness against bacteria;
- -
- Effectiveness against fungi;
- -
- Neutralization of endotoxins;
- -
- Opening of the dentinal tubules;
- -
- Removal of iatrogenic impurities;
- -
- Economic efficiency;
- -
- Practicality.
Unwanted properties are:
- -
- Irritation of neighboring tissues;
- -
- Cytotoxicity;
- -
- Mutagenicity;
- -
- Changes in the color of dentin or tooth enamel;
- -
- Occlusion of the dentinal tubules;
- -
- Undesirable interactions with other endodontic irrigating solutions and materials.
Since no irrigation solution is known that combines all the necessary properties and can be solely applied clinically, different solutions are used consecutively [92]. Certain combinations can interact with each other and result in undesirable effects or by-products. When in contact with each other in the root canal system, NaOCl and CHX interact in an acid–base reaction, forming an orange-brown precipitate, which has undesirable effects. The occlusion of the dentinal tubules [12,30] is indisputable and, depending on the sealer, can have a negative effect on its adhesive force or tightness [29,61,79]. The dyes of the precipitate can discolor the tooth substances, particularly the dentin.
Although it can be considered unlikely that the precipitate of NaOCl and CHX contains free para-chloroaniline, a substance that is suspected of being mutagenic, it is important to avoid precipitation in the root canal. After formation, the complete removal of the precipitate from the root canal system is difficult or impossible, even with advanced methods of activating irrigation solutions with sound, ultrasound, or laser pulses [32,57]. The safest method to avoid a precipitate from forming after use of NaOCl and CHX is to dispense only one of the two substances, which is the preferred option. Because of the sum of its properties, especially due to its ability to dissolve tissue, NaOCl is still the irrigation solution of choice during the mechanical preparation of the root canal. As long as the properties of potential alternatives, such as chlorine dioxide, have not been researched in more detail, it can still be regarded as the “gold standard” to use NaOCl exclusively in this phase [10].
In earlier studies, the combination of CHX and NaOCl was determined to have a better effect against Enterococcus faecalis and gram-positive germs compared to NaOCl alone [27,93,94,95]. Therefore, it was seen as an ideal complement to NaOCl. Some studies have since denied that the effectiveness of this combination against Enterococcus faecalis is better than that of NaOCl alone. However, this point is actually discussed controversially in the international literature [27,93,94,95,96].
A strict avoidance of the possible interaction between NaOCl and CHX in all its variants (including CHX solutions, CHX gels) is desired. According to the literature evaluated, this works best when an intermediate rinse with citric acid is utilized, as this removes the smear layer of the mechanical treatment without causing a precipitate with NaOCl or CHX and thereby triggering other complications.
In rinsing protocols that use NaOCl as the sole antimicrobial rinse, based on current knowledge, the final rinse to remove the smear layer should be carried out with citric acid or EDTA before the final use of NaOCl in an activated manner [10].
5. Conclusions
Since 2006, there has been a sharp increase in publications addressing the interactions between NaOCl and CHX. A total of 88 publications from the PubMed database were identified and evaluated. Of those, 58 publications were relevant to the topic. The results of the studies examined are often controversial, but certain aspects show a tendency over time.
The following findings relate to the endodontic irrigation protocol:
- -
- The chemo-mechanical preparation of the root canal system is currently the gold standard;
- -
- NaOCl should be used as the sole agent during mechanical reprocessing, due to its tissue-dissolving and antimicrobial properties;
- -
- The smear layer can be removed with CA or EDTA after the mechanical preparation. NaOCl should not be mixed with CA or EDTA, since chelators neutralize the tissue-dissolving effect of NaOCl;
- -
- The consecutive use of NaOCl and CHX is obsolete due to the precipitate that forms;
- -
- If NaOCl and CHX (or CHX derivatives) are used in the same tooth, intermediate rinsing is required. Since CHX also forms a precipitate with EDTA, CA is recommended for this.
These recommendations are useful in clinical practice to effectively avoid the formation of the undesirable precipitate.
Author Contributions
Conceptualization, D.-J.D. and C.R.G.; methodology, D.-J.D. and C.R.G.; formal analysis, D.-J.D. and C.R.G.; investigation, D.-J.D.; writing—original draft preparation, D.-J.D., A.D.N., A.D. and C.R.G.; writing—review and editing, A.D.N. and C.R.G.; visualization, D.-J.D. and A.D.; supervision, C.R.G.; project administration, C.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kandaswamy, D.; Venkateshbabu, N. Root canal irrigants. J Conserv. Dent. 2010, 13, 256–264. [Google Scholar] [CrossRef]
- Kojima, K.; Inamoto, K.; Nagamatsu, K.; Hara, A.; Nakata, K.; Morita, I.; Nakagaki, H.; Nakamura, H. Success rate of endodontic treatment of teeth with vital and nonvital pulps: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 95–99. [Google Scholar] [CrossRef]
- Abou-Rass, M.; Piccinino, M.V. The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 323–328. [Google Scholar] [CrossRef]
- Bystrom, A.; Sundqvist, G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand. J. Dent. Res. 1981, 89, 321–328. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Gomes, B.P.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.; Souza-Filho, F.J.; Ferraz, C.C. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102. [Google Scholar] [CrossRef]
- Wright, P.P.; Kahler, B.; Walsh, L.J. Alkaline Sodium Hypochlorite Irrigant and Its Chemical Interactions. Materials 2017, 10, 1147. [Google Scholar] [CrossRef]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Good, M.; El, K.I.; Hussey, D.L. Endodontic ‘solutions’ part 1: A literature review on the use of endodontic lubricants, irrigants and medicaments. Dent. Update 2012, 39, 239–240. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. S3), 588–612. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B.R.; Manek, S.; Sodhi, R.N.; Fillery, E.; Manzur, A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J. Endod. 2007, 33, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Akisue, E.; Tomita, V.S.; Gavini, G.; Poli de Figueiredo, J.A. Effect of the combination of sodium hypochlorite and chlorhexidine on dentinal permeability and scanning electron microscopy precipitate observation. J. Endod. 2010, 36, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, D.; Sadilek, M.; Flake, N.M.; Paranjpe, A.; Heling, I.; Johnson, J.D.; Cohenca, N. The effect of using an alternative irrigant between sodium hypochlorite and chlorhexidine to prevent the formation of para-chloroaniline within the root canal system. Int. Endod. J. 2012, 45, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Nocca, G.; Ahmed, H.M.A.; Martorana, G.E.; Callà, C.; Gambarini, G.; Rengo, S.; Spagnuolo, G. Chromographic Analysis and Cytotoxic Effects of Chlorhexidine and Sodium Hypochlorite Reaction Mixtures. J. Endod. 2017, 43, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Gangaramani, S.; Singbal, K.P.; Desai, K.; Gupta, K. Efficacy of various solutions in preventing orange-brown precipitate formed during alternate use of sodium hypochlorite and chlorhexidine: An in vitro study. J. Conserv. Dent. 2018, 21, 428–432. [Google Scholar] [CrossRef]
- Siddique, R.; Sureshbabu, N.M.; Somasundaram, J.; Jacob, B.; Selvam, D. Qualitative and quantitative analysis of precipitate formation following interaction of chlorhexidine with sodium hypochlorite, neem, and tulsi. J. Conserv. Dent. 2019, 22, 40–47. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spano, J.C.; Marchesan, M.A.; Pecora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Cullen, J.K.; Wealleans, J.A.; Kirkpatrick, T.C.; Yaccino, J.M. The effect of 8.25% sodium hypochlorite on dental pulp dissolution and dentin flexural strength and modulus. J. Endod. 2015, 41, 920–924. [Google Scholar] [CrossRef]
- Hulsmann, M.; Hahn, W. Complications during root canal irrigation—Literature review and case reports. Int. Endod. J. 2000, 33, 186–193. [Google Scholar] [CrossRef]
- Karkare, S.R.; Ahire, N.P.; Khedkar, S.U. Comparative evaluation of antimicrobial activity of hydroalcoholic extract of Aloe vera, garlic, and 5% sodium hypochlorite as root canal irrigants against Enterococcus faecalis: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 274–278. [Google Scholar] [CrossRef]
- Gernhardt, C.R.; Eppendorf, K.; Kozlowski, A.; Brandt, M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int. Endod. J. 2004, 37, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, H.; Sultan, N.; Cirak, M.; Ruhi, M.Z.; Bodur, H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int. Endod. J. 1999, 32, 99–102. [Google Scholar] [CrossRef]
- Ercan, E.; Ozekinci, T.; Atakul, F.; Gul, K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: In vivo study. J. Endod. 2004, 30, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Rocas, I.N.; Siqueira, J.F., Jr. Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorhexidine used as root canal irrigants: A molecular microbiology study. J. Endod. 2011, 37, 143–150. [Google Scholar] [CrossRef]
- Klein, U.; Kleier, D.J. Sodium hypochlorite accident in a pediatric patient. Pediatr. Dent. 2013, 35, 534–538. [Google Scholar]
- Peters, O.A.; Paqué, F. Root canal preparation of maxillary molars with the self-adjusting file: A micro-computed tomography study. J. Endod. 2011, 37, 53–57. [Google Scholar] [CrossRef]
- Gomes, B.P.; Martinho, F.C.; Vianna, M.E. Comparison of 2.5% sodium hypochlorite and 2% chlorhexidine gel on oral bacterial lipopolysaccharide reduction from primarily infected root canals. J. Endod. 2009, 35, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Teixeira, C.S. The properties of chlorhexidine and undesired effects of its use in endodontics. Quintessence Int. 2015, 46, 575–582. [Google Scholar] [CrossRef]
- Vivacqua-Gomes, N.; Ferraz, C.C.; Gomes, B.P.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Influence of irrigants on the coronal microleakage of laterally condensed gutta-percha root fillings. Int. Endod. J. 2002, 35, 791–795. [Google Scholar] [CrossRef]
- Bui, T.B.; Baumgartner, J.C.; Mitchell, J.C. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J. Endod. 2008, 34, 181–185. [Google Scholar] [CrossRef]
- Kim, J.W. Precipitate from a combination of sodium hypochlorite and chlorhexidine. Restor. Dent. Endod. 2012, 37, 185–186. [Google Scholar] [CrossRef]
- Keles, A.; Ors, S.A.; Yilmaz, Z. Effect of various solutions on the removal of orange-brown precipitate formed by interaction of sodium hypochlorite and chlorhexidine with or without ultrasonic activationZ. Niger. J. Clin. Pract. 2020, 23, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.S.; Ameer, B.; Ajit Mannur, N.; Ramalingaiahsetty, A.M.; Peerzade, S.M.; Bambawale, A. Decoding the Perplexing Mystery of Para-Chloroaniline Formation: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 142–147. [Google Scholar] [CrossRef]
- Bueso, V.; Parikh, N.; Terlier, T.; Holland, J.N.; Sarmast, N.D.; Jeong, J.W. Comparative evaluation of intermediate solutions in prevention of brown precipitate formed from sodium hypochlorite and chlorhexidine gluconate. Clin. Exp. Dent. Res. 2022, 8, 1591–1597. [Google Scholar] [CrossRef]
- Jeong, J.W.; Sarmast, N.D.; Terlier, T.; van der Hoeven, R.; Holland, J.N.; Parikh, N. Assessment of the cytotoxic effects and chemical composition of the insoluble precipitate formed from sodium hypochlorite and chlorhexidine gluconate. Int. Endod. J. 2021, 54, 1892–1901. [Google Scholar] [CrossRef]
- Czopik, B.; Ciechomska, M.; Zarzecka, J.; Góra, M.; Woźniakiewicz, M. Insight into the Reaction of Alexidine with Sodium Hypochlorite: A Potential Error in Endodontic Treatment. Molecules 2021, 26, 1623. [Google Scholar] [CrossRef]
- Alberto, A.P.L.; Oliveira, D.D.S.; Oliveira, H.E.; Maciel, A.C.C.; Belladonna, F.G.; Silva, E. Does sodium thiosulphate avoid the formation of the brown-coloured precipitate as an intermediate irrigant between NaOCl and chlorhexidine? Aust. Endod. J. 2022, 48, 72–76. [Google Scholar] [CrossRef]
- Buyukozer Ozkan, H.; Terlemez, A.; Orhan, E.O. Proton Nuclear Magnetic Resonance Spectroscopy Analysis of Mixtures of Chlorhexidine with Different Oxidizing Agents Activated by Photon-Induced Photoacoustic Streaming for Root Canal Irrigation. Photobiomodul. Photomed. Laser Surg. 2020, 38, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Abusteit, O.E. Evaluation of resin sealer penetration of dentin following different final rinses for endodontic irrigation using confocal laser scanning microscopy. Aust. Endod. J. 2020, 47, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, S.T.; Alamoudi, R.A.; Merdad, K. Morphological and chemical analysis of surface interaction of irrigant-endosequence root repair material. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3383–3392. [Google Scholar] [CrossRef]
- Thomas, A.R.; Mani, R.; Reddy, T.V.; Ravichandran, A.; Sivakumar, M.; Krishnakumar, S. Evaluation of the Antibacterial Efficiency of a Combination of 1% Alexidine and Sodium Hypochlorite on Enterococcus faecalis Biofilm Models: An In Vitro Study. J. Contemp. Dent. Pract. 2019, 20, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Nivedhitha, M.S.; Jacob, B. Quantitative analysis for detection of toxic elements in various irrigants, their combination (precipitate), and para-chloroaniline: An inductively coupled plasma mass spectrometry study. J. Conserv. Dent. 2019, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Žižka, R.; Šedý, J.; Gregor, L.; Voborná, I. Discoloration after Regenerative Endodontic Procedures: A Critical Review. Iran. Endod. J. 2018, 13, 278–284. [Google Scholar] [CrossRef]
- Ravinanthanan, M.; Hegde, M.N.; Shetty, V.; Kumari, S. Cytotoxicity Evaluation of Combination Irrigant Regimens with MTAD on Two Different Cell Lines. Contemp. Clin. Dent. 2018, 9, 255–259. [Google Scholar] [CrossRef]
- Piperidou, M.; Sodhi, R.N.S.; Kolosowski, K.P.; Basrani, B.R. Effects of Final Irrigation with SmearOFF on the Surface of Dentin Using Surface Analytical Methods. J. Endod. 2018, 44, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Agarwal, P.; Jain, S.; Seal, M.; Adlakha, T. Alexidine versus chlorhexidine for endodontic irrigation with sodium hypochlorite. Eur. J. Dent. 2018, 12, 398–402. [Google Scholar] [CrossRef]
- Irmak, Ö.; Orhan, E.O.; Görgün, K.; Yaman, B.C. Nuclear magnetic resonance spectroscopy and infrared spectroscopy analysis of precipitate formed after mixing sodium hypochlorite and QMix 2in1. PLoS ONE 2018, 13, e0202081. [Google Scholar] [CrossRef]
- Gonzalez, C.; Forner, L.; Llena, C.; Lozano, A. Temperature changes in 2% chlorhexidine gluconate using two activation methods with different intensity levels. J. Clin. Exp. Dent. 2018, 10, e458–e461. [Google Scholar] [CrossRef]
- Campbell, S.T.; Goodnough, L.H.; Bennett, C.G.; Giori, N.J. Antiseptics Commonly Used in Total Joint Arthroplasty Interact and May Form Toxic Products. J. Arthroplasty 2018, 33, 844–846. [Google Scholar] [CrossRef]
- Thaha, K.A.; Varma, R.L.; Nair, M.G.; Sam Joseph, V.G.; Krishnan, U. Interaction between Octenidine-based Solution and Sodium Hypochlorite: A Mass Spectroscopy, Proton Nuclear Magnetic Resonance, and Scanning Electron Microscopy-based Observational Study. J. Endod. 2017, 43, 135–140. [Google Scholar] [CrossRef]
- Surender, L.R.; Shikha, A.; Swathi, A.; Manaswini, C.; Habeeb, A.; Prabha, S.S. Alexidine: A Safer and an Effective Root Canal Irrigant than Chlorhexidine. J. Clin. Diagn. Res. 2017, 11, ZC18–ZC21. [Google Scholar] [CrossRef]
- Guneser, M.B.; Dincer, A.N.; Arslan, D. Comparison of Conventional Syringe, CanalBrush, EndoActivator, Photon-Induced Photoacoustic Streaming, and Manual Instrumentation in Removing Orange-Brown Precipitate: An In Vitro Study. Photomed. Laser Surg. 2017, 35, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Vouzara, T.; Koulaouzidou, E.; Ziouti, F.; Economides, N. Combined and independent cytotoxicity of sodium hypochlorite, ethylenediaminetetraacetic acid and chlorhexidine. Int. Endod. J. 2016, 49, 764–773. [Google Scholar] [CrossRef]
- Patil, P.; Aminoshariae, A.; Harding, J.; Montagnese, T.A.; Mickel, A. Determination of mutagenicity of the precipitate formed by sodium hypochlorite and chlorhexidine using the Ames test. Aust. Endod. J. 2016, 42, 16–21. [Google Scholar] [CrossRef]
- Orhan, E.O.; Irmak, Ö.; Hür, D.; Yaman, B.C.; Karabucak, B. Does Para-chloroaniline Really Form after Mixing Sodium Hypochlorite and Chlorhexidine? J. Endod. 2016, 42, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Giardino, L.; Palazzi, F.; Asgary, S. Agonistic and Antagonistic Interactions between Chlorhexidine and Other Endodontic Agents: A Critical Review. Iran. Endod. J. 2015, 10, 1–5. [Google Scholar] [PubMed]
- Metri, M.; Hegde, S.; Dinesh, K.; Indiresha, H.N.; Nagaraj, S.; Bhandi, S.H. Comparative Evaluation of Two Final Irrigation Techniques for the Removal of Precipitate Formed by the Interaction between Sodium Hypochlorite and Chlorhexidine. J. Contemp. Dent. Pract. 2015, 16, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.G.; Kuga, M.C.; Aranda-Garcia, A.J.; Victorino, K.R.; Chávez-Andrade, G.M.; Faria, G.; Keine, K.C.; Só, M.V. Effectiveness of several solutions to prevent the formation of precipitate due to the interaction between sodium hypochlorite and chlorhexidine and its effect on bond strength of an epoxy-based sealer. Int. Endod. J. 2015, 48, 478–483. [Google Scholar] [CrossRef]
- Arslan, H.; Uygun, A.D.; Keskin, A.; Karatas, E.; Seçkin, F.; Yıldırım, A. Evaluation of orange-brown precipitate formed in root canals after irrigation with chlorhexidine and QMix and spectroscopic analysis of precipitates produced by a mixture of chlorhexidine/NaOCl and QMix/NaOCl. Int. Endod. J. 2015, 48, 1199–1203. [Google Scholar] [CrossRef]
- Kolosowski, K.P.; Sodhi, R.N.; Kishen, A.; Basrani, B.R. Qualitative analysis of precipitate formation on the surface and in the tubules of dentin irrigated with sodium hypochlorite and a final rinse of chlorhexidine or QMiX. J. Endod. 2014, 40, 2036–2040. [Google Scholar] [CrossRef]
- Homayouni, H.; Majd, N.M.; Zohrehei, H.; Mosavari, B.; Adel, M.; Dajmar, R.; Homayouni, A. The Effect of Root Canal Irrigation with Combination of Sodium Hypo-chlorite and Chlorhexidine Gluconate on the Sealing Ability of Obturation Materials. Open Dent. J. 2014, 8, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.M.; Kuga, M.C.; Regina Victorino, K.; Vazquez-Garcia, F.A.; Aranda-Garcia, A.J.; Faria-Junior, N.B.; Faria, G.; Luis Shinohara, A. Evaluation of the interaction between sodium hypochlorite and several formulations containing chlorhexidine and its effect on the radicular dentin—SEM and push-out bond strength analysis. Microsc. Res. Tech. 2014, 77, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cintra, L.T.; Watanabe, S.; Samuel, R.O.; da Silva Facundo, A.C.; de Azevedo Queiroz, I.O.; Dezan-Júnior, E.; Gomes-Filho, J.E. The use of NaOCl in combination with CHX produces cytotoxic product. Clin. Oral Investig. 2014, 18, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Gok, T.; Saygili, G.; Altintop, H.; Akcay, M.; Capar, I.D. Evaluation of effectiveness of various irrigating solutions on removal of calcium hydroxide mixed with 2% chlorhexidine gel and detection of orange-brown precipitate after removal. J. Endod. 2014, 40, 1820–1823. [Google Scholar] [CrossRef]
- Souza, M.; Cecchin, D.; Barbizam, J.V.; Almeida, J.F.; Zaia, A.A.; Gomes, B.P.; Ferraz, C.C. Evaluation of the colour change in enamel and dentine promoted by the interaction between 2% chlorhexidine and auxiliary chemical solutions. Aust. Endod. J. 2013, 39, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Bolla, N.; Sarath, R.K.; Ram, C.H. Assessment of precipitate formation on interaction of irrigants used in different combinations: An in vitro study. Indian J. Dent. Res. 2013, 24, 451–455. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Doğramacı, E.J.; Steier, L.; de Figueiredo, J.A. Interaction between chlorhexidine-impregnated gutta-percha points and several chlorine-containing endodontic irrigating solutions. Int. Endod. J. 2013, 46, 675–680. [Google Scholar] [CrossRef]
- Prado, M.; Santos Júnior, H.M.; Rezende, C.M.; Pinto, A.C.; Faria, R.B.; Simão, R.A.; Gomes, B.P. Interactions between irrigants commonly used in endodontic practice: A chemical analysis. J. Endod. 2013, 39, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Pasich, E.; Bialecka, A.; Marcinkiewicz, J. Efficacy of taurine haloamines and chlorhexidine against selected oral microbiome species. Med. Dosw. Mikrobiol. 2013, 65, 187–196. [Google Scholar]
- Gupta, H.; Kandaswamy, D.; Manchanda, S.K.; Shourie, S. Evaluation of the sealing ability of two sealers after using chlorhexidine as a final irrigant: An in vitro study. J. Conserv. Dent. 2013, 16, 75–78. [Google Scholar] [CrossRef]
- Vilanova, W.V.; Carvalho-Junior, J.R.; Alfredo, E.; Sousa-Neto, M.D.; Silva-Sousa, Y.T. Effect of intracanal irrigants on the bond strength of epoxy resin-based and methacrylate resin-based sealers to root canal walls. Int. Endod. J. 2012, 45, 42–48. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Doğramaci, E.J.; Guastalli, A.R.; Steier, L.; de Figueiredo, J.A. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J. Endod. 2012, 38, 426–431. [Google Scholar] [CrossRef]
- Kim, H.S.; Zhu, Q.; Baek, S.H.; Jung, I.Y.; Son, W.J.; Chang, S.W.; Lee, W.; Gu, Y.; Lee, Y.; Hong, S.T.; et al. Chemical interaction of alexidine and sodium hypochlorite. J. Endod. 2012, 38, 112–116. [Google Scholar] [CrossRef]
- Gasic, J.; Popovic, J.; Zivkovic, S.; Petrovic, A.; Barac, R.; Nikolic, M. Ultrastructural analysis of the root canal walls after simultaneous irrigation of different sodium hypochlorite concentration and 0.2% chlorhexidine gluconate. Microsc. Res. Tech. 2012, 75, 1099–1103. [Google Scholar] [CrossRef]
- Prado, M.; de Assis, D.F.; Gomes, B.P.; Simão, R.A. Effect of disinfectant solutions on the surface free energy and wettability of filling material. J. Endod. 2011, 37, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, J.B.; Sem, D.S. An in vitro spectroscopic analysis to determine the chemical composition of the precipitate formed by mixing sodium hypochlorite and chlorhexidine. J. Endod. 2011, 37, 983–988. [Google Scholar] [CrossRef] [PubMed]
- De Assis, D.F.; Prado, M.; Simão, R.A. Evaluation of the interaction between endodontic sealers and dentin treated with different irrigant solutions. J. Endod. 2011, 37, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.E.; Sem, D.S. An in vitro spectroscopic analysis to determine whether para-chloroaniline is produced from mixing sodium hypochlorite and chlorhexidine. J. Endod. 2010, 36, 315–317. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Sudhakaran, S. Evaluation and prevention of the precipitate formed on interaction between sodium hypochlorite and chlorhexidine. J. Endod. 2010, 36, 1154–1157. [Google Scholar] [CrossRef]
- Basrani, B.R.; Manek, S.; Mathers, D.; Fillery, E.; Sodhi, R.N. Determination of 4-chloroaniline and its derivatives formed in the interaction of sodium hypochlorite and chlorhexidine by using gas chromatography. J. Endod. 2010, 36, 312–314. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef]
- Basrani, B.R.; Manek, S.; Fillery, E. Using diazotization to characterize the effect of heat or sodium hypochlorite on 2.0% chlorhexidine. J. Endod. 2009, 35, 1296–1299. [Google Scholar] [CrossRef]
- Kuruvilla, J.R.; Kamath, M.P. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J. Endod. 1998, 24, 472–476. [Google Scholar] [CrossRef]
- Marchesan, M.A.; Pasternak Junior, B.; Afonso, M.M.; Sousa-Neto, M.D.; Paschoalato, C. Chemical analysis of the flocculate formed by the association of sodium hypochlorite and chlorhexidine. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, e103–e105. [Google Scholar] [CrossRef] [PubMed]
- Calt, S.; Serper, A. Time-dependent effects of EDTA on dentin structures. J. Endod. 2002, 28, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Woo Chang, S.; Baek, S.H.; Han, S.H.; Lee, Y.; Zhu, Q.; Kum, K.Y. Antimicrobial effect of alexidine and chlorhexidine against Enterococcus faecalis infection. Int. J. Oral Sci. 2013, 5, 26–31. [Google Scholar] [CrossRef]
- Cobankara, F.K.; Ozkan, H.B.; Terlemez, A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J. Endod. 2010, 36, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Herczegh, A.; Ghidan, A.; Friedreich, D.; Gyurkovics, M.; Bendo, Z.; Lohinai, Z. Effectiveness of a high purity chlorine dioxide solution in eliminating intracanal Enterococcus faecalis biofilm. Acta Microbiol. Immunol. Hung. 2013, 60, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, R.; Nomura, Y.; Sawajiri, M.; Masuki, K.; Hirata, I.; Okazaki, M. Influence of chlorine dioxide on cell death and cell cycle of human gingival fibroblasts. J. Dent. 2008, 36, 993–998. [Google Scholar] [CrossRef]
- Fleming, C.H.; Litaker, M.S.; Alley, L.W.; Eleazer, P.D. Comparison of classic endodontic techniques versus contemporary techniques on endodontic treatment success. J. Endod. 2010, 36, 414–418. [Google Scholar] [CrossRef]
- Friedman, S.; Mor, C. The success of endodontic therapy—Healing and functionality. J. Calif. Dent. Assoc. 2004, 32, 493–503. [Google Scholar] [PubMed]
- Regan, J.D.; Fleury, A.A. Irrigants in non-surgical endodontic treatment. J. Ir. Dent. Assoc. 2006, 52, 84–92. [Google Scholar]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.R.; Hirsch, S.; Hiergeist, A.; Kirschneck, C.; Muehler, D.; Hiller, K.A.; Maisch, T.; Al-Ahmad, A.; Gessner, A.; Buchalla, W.; et al. Limited antimicrobial efficacy of oral care antiseptics in microcosm biofilms and phenotypic adaptation of bacteria upon repeated exposure. Clin. Oral Investig. 2021, 25, 2939–2950. [Google Scholar] [CrossRef]
- Yesilsoy, C.; Whitaker, E.; Cleveland, D.; Phillips, E.; Trope, M. Antimicrobial and toxic effects of established and potential root canal irrigants. J. Endod. 1995, 21, 513–515. [Google Scholar] [CrossRef]
- Kandaswamy, D.; Venkateshbabu, N.; Gogulnath, D.; Kindo, A.J. Dentinal tubule disinfection with 2% chlorhexidine gel, propolis, morinda citrifolia juice, 2% povidone iodine, and calcium hydroxide. Int. Endod. J. 2010, 43, 419–423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).