Abstract
Introduction: Combination therapy with daptomycin plus ceftaroline to treat methicillin-resistant Staphylococcus aureus bacteremia has been reported to reduce methicillin-resistant Staphylococcus aureus bacteremia-related mortality. The purpose of the current meta-analysis was to compare the clinical outcome of methicillin-resistant Staphylococcus aureus bacteremia in patients treated with daptomycin or vancomycin plus ceftaroline combination therapy versus daptomycin or vancomycin monotherapy. Methods: Studies were included if they directly compared the efficacy of daptomycin or vancomycin plus ceftaroline combination therapy with that of daptomycin or vancomycin monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia in adult patients. Results: One randomized controlled trial and five retrospective studies were included in the meta-analysis. The combination therapy group had an in-hospital mortality, duration of bacteremia, and adverse event rate similar to those patients who had monotherapy. There was less bacteremia recurrence in the combination group. Initial combination therapy with ceftaroline for the treatment of methicillin-resistant Staphylococcus aureus bacteremia showed a trend of reducing the risk of in-hospital mortality in the current meta-analysis. Conclusions: Randomized controlled trials are needed to further study the role of initial combination therapy with daptomycin or vancomycin plus ceftaroline in the treatment of methicillin-resistant Staphylococcus aureus bacteremia.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is a serious infectious disease associated with a high risk of mortality [1,2,3]. The primary parenteral therapy for MRSA infection is vancomycin. Treatment with vancomycin will clinically or microbiologically fail in invasive MRSA infections [4,5,6,7,8]. Daptomycin is an alternative first-line option that is reserved for MRSA bacteremia that has relapsed or persisted despite vancomycin treatment. However, the failure of daptomycin in the treatment of MRSA bacteremia is common, and nonsusceptibility has emerged [9,10,11]. The clinical practice guidelines by the infectious diseases society of America for the treatment of MRSA infections in adults and children recommends the following management strategies for persistent MRSA bacteremia and vancomycin treatment failure in adult patients: 1. If the isolate is susceptible, high-dose daptomycin in combination with another agent (such as a beta-lactam antibiotic) should be considered (B-III strength of recommendation). 2. If a reduced susceptibility to vancomycin and daptomycin are present, options may include quinupristindalfopristin, trimethoprim-sulfamethoxazole, linezolid, or telavancin (C-III strength of recommendation) [12]. Ceftaroline is a fifth-generation cephalosporin that is active against gram-positive pathogens such as Staphylococcus aureus (SA) and Streptococcus pneumoniae as well as their resistant strains (e.g., MRSA, vancomycin-resistant SA, and multidrug-resistant Streptococcus pneumoniae) [13]. The in vitro activity of ceftaroline is also active against common gram-negative pathogens such as Escherichia coli, Klebsiella pneumoniae, and Haemophilus influenzae; it is not active against extended spectrum beta-lactamase-producing gram-negative organisms [14]. Ceftaroline is the only commercially available beta-lactam with bactericidal activity against MRSA; it also increases the bactericidal effect of daptomycin by enhancing daptomycin binding to bacterial cell membranes [15,16,17]. Ceftaroline is currently approved in the United States for bacterial pneumonia and skin/soft tissue infections [18,19]. Observational studies have shown that ceftaroline may be effective in the treatment of MRSA bacteremia. However, studies comparing ceftaroline to standard-of-care therapy for MRSA bacteremia are limited, and ceftaroline is not United States Food and Drug Administration-approved for this indication. Thus, combination therapy with ceftaroline plus daptomycin is an option because both agents have an individual efficacy against MRSA [20,21,22]. In the study by Zasowski et al., ceftaroline was noninferior to daptomycin in the treatment of MRSA bacteremia [23]. Studies have shown that combination therapy, such as the combination of daptomycin and ceftaroline, can reduce the duration of MRSA bacteremia and mortality due to MRSA bacteremia [24,25,26,27,28]. Therefore, we performed a comprehensive and updated meta-analysis of the clinical outcomes associated with daptomycin or vancomycin plus ceftaroline combination therapy in MRSA bacteremia patients. The purpose of the current meta-analysis was to compare the clinical outcomes of the in-hospital mortality, recurrence of MRSA bacteremia, duration of bacteremia, and adverse events between patients treated with daptomycin or vancomycin plus ceftaroline combination therapy and daptomycin or vancomycin monotherapy.
2. Results
2.1. Characteristics of the Included Trials
The details of the study selection process are shown in Figure 1. The numbers of studies from the initial search results from PubMed, Web of Science, and the Cochrane Library were 110, 114, and 27, respectively. There were 105 duplicate articles. A total of 146 irrelevant studies were identified by reading the title and/or abstract. After excluding the duplicates and irrelevant studies, 23 potentially relevant articles remained. After a full-text article review, 17 articles were excluded because they lacked results comparing the outcomes of ceftaroline plus vancomycin or daptomycin combination therapy versus vancomycin or daptomycin monotherapy in adults with MRSA bacteremia. Finally, six studies were included in the meta-analysis [29,30,31,32,33,34]. The main characteristics of the six included studies are shown in Table 1. One was a randomized controlled trial (RCT), and five were retrospective observational studies. The infection sites were all multiple primary infection sites. All of the studies had a high risk of bias.
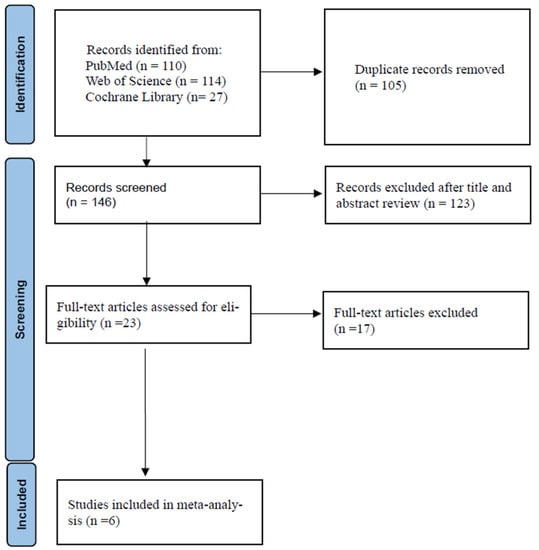
Figure 1.
Flow diagram of the study selection process.

Table 1.
Characteristics of the included studies.
2.2. Efficacy and Safety Outcomes
There were 200 patients in the monotherapy group and 147 patients in the combination therapy group. Five studies involving 299 patients (177 receiving monotherapy, 122 receiving ceftaroline combination therapy) reported bacteremia recurrence. There was a statistically significant difference in the bacteremia recurrence between the patients treated with monotherapy and those treated with ceftaroline combination therapy (OR = 2.95, 95% CI= 1.22–7.15, p = 0.02, I2 = 6%) (Figure 2). All five studies reported that the combination therapy group had a lower bacteremia recurrence than the monotherapy group. Six studies involving 347 patients (200 receiving monotherapy, 147 receiving ceftaroline combination therapy) reported in-hospital mortality. There was no statistically significant difference in the in-hospital mortality between the patients treated with monotherapy and those treated with ceftaroline combination therapy (OR = 1.24, 95% CI= 0.66–2.33, p = 0.50, I2 = 53%) (Figure 3). Three studies favored monotherapy, and two studies favored combination therapy. Merrisette et al. found no deaths in either group [32]. We analyzed the two studies in which the combination therapy group had a better in-hospital mortality than the monotherapy group [30,31]. Geriak et al. showed that combination therapy had mortality benefits as an initial therapy within 72 h of the index culture in the treatment of MRSA bacteremia [30]. McCreary et al. found a lower all-cause mortality at day 30 in the combination therapy group than in those who received standard-of-care monotherapy, and there was a lower mortality in the patients who received daptomycin plus ceftaroline within 72 h of the index culture [31]. Four studies involving 159 patients (82 receiving monotherapy, 77 receiving ceftaroline combination therapy) reported adverse events. There was no statistically significant difference in the adverse events between the patients treated with monotherapy and those treated with ceftaroline combination therapy (OR= 0.59, 95% CI= 0.27–1.27, p = 0.18, I2 = 16%) (Figure 4). There were two cases of eosinophilic pneumonitis and three cases of elevated creatine phosphokinase in the combination therapy group. There was no case of eosinophilic pneumonitis but two cases of elevated creatine phosphokinase in the monotherapy group. Regarding the duration of bacteremia, four studies reported no statistically significant difference in the duration of bacteremia between the patients treated with monotherapy and those treated with ceftaroline combination therapy [29,30,32,33]. In the study of McCreary et al., the mean duration of bacteremia was 4.8 days in the standard-of-care group and 9.3 days in the daptomycin plus ceftaroline group (p < 0.001); following the switch to daptomycin plus ceftaroline, the mean duration of continued bacteremia was 3.3 days for the combination therapy. The longer duration of bacteremia was related to daptomycin plus ceftaroline as a salvage therapy, and there were 6.0 mean bacteremia days before switching to combination therapy [31]. A study by Johnson et al. showed that the mean duration of bacteremia was 5.0 days in the standard-of-care group and 9.0 days in the daptomycin plus ceftaroline group (p = 0.01). The total duration of bacteremia was significantly longer in the daptomycin plus ceftaroline group because the patients failed first-line therapy (after a median of six days) before daptomycin plus ceftaroline combination therapy. The longer duration of bacteremia was related to daptomycin plus ceftaroline as a salvage therapy [34]. We found that daptomycin plus ceftaroline as a salvage therapy was associated with a longer duration of bacteremia in both studies.
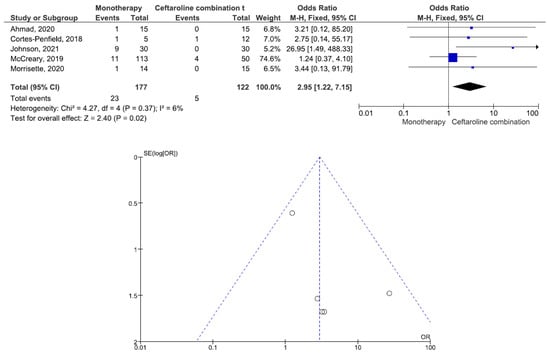
Figure 2.
Forest plots and funnel plots for bcteremia recurrence between monotherapy and combination therapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia.
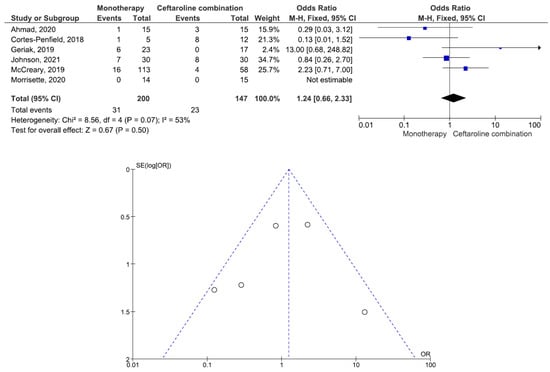
Figure 3.
Forest plots and funnel plots for in-hospital mortality between monotherapy and combination therapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia.
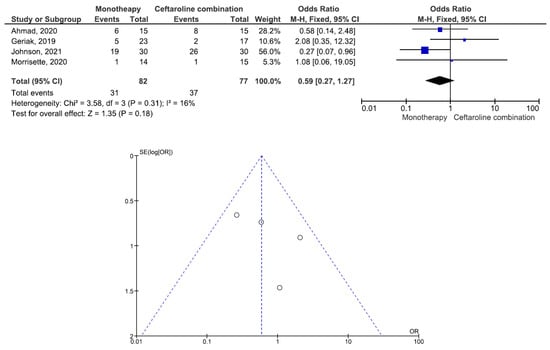
Figure 4.
Forest plots and funnel plots for adverse events between monotherapy and combination therapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia.
3. Methods
3.1. Data Search Strategy
The literature search was performed using the PubMed, Web of Science, and Cochrane Library databases in order to identify all included clinical studies and meta-analyses or systematic reviews on the topic from 1 January 2009. In the databases, we used the following search string: (ceftaroline OR vancomycin OR daptomycin) (bacteremia OR MRSA bacteremia OR methicillin-resistant Staphylococcus aureus bacteremia). We examined treatment studies that directly compared the outcomes of ceftaroline plus vancomycin or daptomycin combination therapy versus vancomycin or daptomycin monotherapy in adults with MRSA bacteremia and searched the relevant articles published from inception to 30 May 2022. Previously published systematic reviews and meta-analyses were reviewed to identify any additional studies that may have been missed in the primary literature search. Articles published in all languages were included.
3.2. Study Selection and Data Extraction
To determine the eligibility of the identified trial reports, each study was independently screened and reviewed for eligibility by two authors. After excluding duplicates, two investigators screened the titles and abstracts of all the studies retrieved to identify eligible records. After excluding irrelevant studies, all of the relevant articles were reviewed by reading the full texts to determine eligibility. Data regarding author, year of publication, country, study design, primary infection sites, total number of patients receiving monotherapy, total number of patients receiving combination therapy, antibiotic dosage, initial therapy or salvage therapy, in-hospital mortality, bacteremia recurrence, duration of bacteremia, and adverse events were extracted from the eligible full text articles. When disagreement occurred, a third author resolved the issue.
3.3. Inclusion and Exclusion Criteria
Due to inadequate levels of evidence, observational studies are not as meaningful as RCTs. There was a very small number of RCTs available. We included retrospective observational studies, prospective observational studies, and RCTs in the current meta-analysis. The studies were considered eligible for inclusion only if they directly compared the outcomes of ceftaroline plus vancomycin or daptomycin combination therapy versus vancomycin or daptomycin monotherapy in adults with MRSA bacteremia. Ceftaroline was administered at dosages ranging from 600 mg every 12 h to 600 mg every 8 h. Daptomycin was administered at a dosage of 5.7–10 mg/kg/day. Vancomycin was administered at a dosage of 15–20 mg/kg every 12 h to every 8 h. All studies were included if they reported one or more of the following outcomes: bacteremia recurrence, in-hospital mortality, duration of bacteremia, and adverse events. Studies with a population of participants who were younger than 18 years were excluded.
3.4. Definitions and Outcomes
The primary outcome was in-hospital mortality. In-hospital mortality was the death rate from all causes of death before patient discharge. The secondary outcomes were bacteremia recurrence, duration of bacteremia, and adverse events. Bacteremia recurrence was defined as at least one positive blood culture for MRSA seven or more days after the initial microbiological cure. The duration of bacteremia cure was defined as the number of days between the first positive blood culture and the first negative blood culture without a subsequent positive blood culture within 72 h of the negative blood culture. The adverse event data recorded were the risk of discontinuing due to adverse events, the incidence of serious adverse events, and some common events, such as diarrhea, nausea, headache, constipation, and seizure.
3.5. Quality Assessment and Statistical Analysis
The methods of quality assessment of the included studies and the statistical analysis of the data were the same as those used in a previous study [35].
4. Discussion
The current meta-analysis of six studies provides evidence that the in-hospital mortality rates, duration of bacteremia, and incidence of adverse drug events were not significantly different between the combination therapy and standard care of MRSA bacteremia. Combination therapy has a lower rate of bacteremia recurrence. In addition, daptomycin or vancomycin plus ceftaroline combination therapy, compared with monotherapy, did not reduce the risk of in-hospital mortality in the treatment of MRSA bacteremia patients.
The standard-of-care therapy for MRSA bacteremia is associated with high morbidity and mortality. Medical experts should explore the use of two antibiotics in combination. Four meta-analyses of combination therapy in the treatment of MRSA bacteremia were published in the literature [36,37,38,39]. Ye et al. (2020) included six studies of vancomycin combined with beta-lactam antibiotics and showed that there was significantly reduced persistent bacteremia and a shortened duration of bacteremia in the combination therapy group. There was no statistically significant difference in the incidence of nephrotoxicity, 30-day mortality, MRSA-related mortality, or bacteremia relapse between the two groups [36]. The study of Wang et al. (2020) included 15 studies of patients treated with daptomycin or vancomycin in combination with beta-lactam antibiotics and showed that the combination therapy significantly reduced the bacteremia recurrence and persistent bacteremia and shortened the duration of bacteremia. There was no statistically significant difference in the risk of crude mortality between the two groups. However, a subgroup analysis of three studies showed that the combination of daptomycin plus beta-lactam antibiotics could reduce the risk of crude mortality [37]. The study by Kale-Pradhan et al. (2020) included nine studies of patients treated with daptomycin or vancomycin in combination with beta-lactam antibiotics and demonstrated that the combination therapy was associated with significantly lower rates of bacteremia relapse and persistent bacteremia. Mortality was not significantly different between the two groups [38]. Yi et al. (2021) included 13 studies of patients treated with daptomycin or vancomycin in combination with beta-lactam antibiotics and found no statistically significant difference in 30-day mortality, in-hospital mortality, or mortality within 60–90 days between the two groups. Combination therapy is associated with a shorter duration of bacteremia, a lower risk of persistent bacteremia, and a lower risk of bacteremia recurrence within 60–90 days [39]. The previous four meta-analyses showed that adding a beta-lactam antibiotic to vancomycin or daptomycin decreased the recurrence of bacteremia and shortened the bacteremia duration in the treatment of patients with MRSA bacteremia. There was no evidence that combination therapy could reduce the risk of MRSA bacteremia mortality. We only included ceftaroline in combination with vancomycin or daptomycin versus vancomycin or daptomycin in the current meta-analysis. Our results were the same as those of the previous four meta-analyses. Combination therapy in the treatment of MRSA bacteremia did not reduce the risk of mortality, implying no significant benefit for patients with MRSA bacteremia.
Lodise et al. suggested that the administration of MRSA bacteremia therapy within the first 24–48 h was strongly related to clinical outcomes [40]. Studies have shown that high-risk MRSA bacteremia patients benefit the most from combination therapy when it is administered early in the treatment course (within 72 h) [41]. It is important to initiate combination therapy early in the treatment of MRSA bacteremia, and it should be initiated within the first 72 h of onset, ideally within the first 24 h to prevent complications from persistent bacteremia [42,43,44]. Many studies have stressed that administering initial therapy within 72 h of the index culture is strongly related to MRSA bacteremia mortality. We analyzed two studies in the current meta-analysis, namely, the study of McCreary et al. and the study of Geriak et al. [30,31]. In the study of McCreary et al., the patients receiving daptomycin with ceftaroline combination therapy for MRSA bacteremia had a lower all-cause mortality at day 30 than those who received standard-of-care monotherapy. A subgroup analysis showed that there was a numerically lower mortality in the patients who received daptomycin plus ceftaroline within 72 h of the index culture. The study suggested that daptomycin and ceftaroline may have mortality benefits when initiated early for MRSA bacteremia [31]. In the study of Geriak et al., vancomycin or daptomycin was used as a monotherapy, and a regimen of daptomycin plus ceftaroline was used as a comparator for the initial treatment of MRSA bacteremia. That study observed an unanticipated in-hospital mortality difference of 0% (0/17) for combination therapy and 26% (6/23) for monotherapy, causing the early termination of the study [30]. That study also showed that daptomycin and ceftaroline combination therapy have mortality benefits as initial therapy within 72 h of the index culture in the treatment of MRSA bacteremia. The current meta-analysis recommended initial combination therapy with ceftaroline for MRSA bacteremia rather than ceftaroline as salvage therapy because initial combination therapy with ceftaroline may reduce the risk of MRSA bacteremia mortality. The current challenges with vancomycin or daptomycin plus ceftaroline combination in the treatment of MRSA bacteremia include a lack of ceftaroline data for the treatment of MRSA bacteremia, and a lack of data on the efficacy and safety of initial combination therapy for MRSA bacteremia. We call into question whether combination therapy works. Which combinations are best for MRSA bacteremia patients? What is the appropriate duration of combination therapy? Is combination therapy necessary for the entire course of treatment? Whether a de-escalation treatment regimen is considered a reasonable alternative to long-term combination therapy in patients with an early clinical response remains to be determined. In the future, four issues need to be explored by medical experts, which are as follows: 1. Initial and early combination therapy with daptomycin or vancomycin plus ceftaroline may be beneficial for mortality in MRSA bacteremia patients. Blinded, randomized, prospective studies are needed to confirm the efficacy and safety of combination therapy in MRSA bacteremia patients. 2. Appropriate dosing strategies for daptomycin or vancomycin plus ceftaroline combination therapy have not been determined. 3. Combination therapy is not necessary for the entire course of treatment. If de-escalation therapy is considered a reasonable alternative to long-term combination therapy in patients with an early clinical response, further investigation is warranted to determine the optimal timing of de-escalation. These drugs, including their dosage regimen and duration of therapy, are optimal for de-escalation therapy. 4. In the study of Geriak et al., a higher mortality was seen in patients with serum interleukin-10 concentrations >5 pg/mL [30]. The authors recommend the use of biomarkers as potential risk indicators for the administration of combination therapy in high-risk patients. Biomarkers related to MRSA bacteremia are a new, attractive area that is worth exploring. The medical community urgently needs advanced knowledge of biomarkers related to MRSA bacteremia to guide clinical decision-making and the management of MRSA bacteremia patients.
Limitations: Few RCTs have explored this issue. We included the findings of observational studies in the current meta-analysis. All of the included studies had a high risk of bias in the current meta-analysis. In addition, the number of included studies and the number of populations were very small, which was a limitation of this meta-analysis. However, vancomycin or daptomycin plus ceftaroline combination in the treatment of MRSA bacteremia constitutes a promising possibility for reducing its mortality. We expect that there will be further studies that will explore this issue so as to provide a new way to treat MRSA bacteremia patients.
5. Conclusions
In the current meta-analysis, there was a trend that showed initial combination therapy with ceftaroline for MRSA bacteremia reducing the risk of MRSA bacteremia mortality. RCTs are needed to further study the role of initial combination therapy with daptomycin or vancomycin plus ceftaroline in the treatment of MRSA bacteremia.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The meta-analysis was registered at the Prospero international prospective register of systematic reviews (registration No. CRD42022341000).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Naber, C.K. Staphylococcus aureus bacteremia: Epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. 2009, 48, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Huang, J.; Jin, N.; Noskin, G.A.; Zembower, T.R.; Bolon, M. Persistent Staphylococcus aureus bacteremia: An analysis of risk factors and outcomes. Arch. Intern. Med. 2007, 167, 1861–1867. [Google Scholar] [CrossRef]
- Forstner, C.; Dungl, C.; Tobudic, S.; Mitteregger, D.; Lagler, H.; Burgmann, H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: A retrospective cohort study in a region with low MRSA prevalence. Clin. Microbiol. Infect. 2013, 19, E291–E297. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Liao, W.H.; Lai, C.C.; Liao, C.H.; Tan, C.K.; Wang, C.Y.; Huang, Y.T.; Hsueh, P.R. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J. Antimicrob. Chemother. 2010, 65, 1792–1798. [Google Scholar] [CrossRef]
- Lodise, T.P.; Graves, J.; Evans, A.; Graffunder, E.; Helmecke, M.; Lomaestro, B.M.; Stellrecht, K. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 2008, 52, 3315–3320. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, J.Y.; Park, D.W.; Sohn, J.W.; Kim, M.J. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 2010, 65, 1015–1018. [Google Scholar] [CrossRef]
- Gould, I.M. Treatment of bacteraemia: Meticillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA). Int. J. Antimicrob. Agents 2013, 42 (Suppl. S1), S17–S21. [Google Scholar] [CrossRef]
- Sharma, M.; Riederer, K.; Chase, P.; Khatib, R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacte¬remia. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 433–437. [Google Scholar] [CrossRef]
- Kelley, P.G.; Gao, W.; Ward, P.B.; Howden, B.P. Daptomycin non-susceptibility in vancomycin- intermediate Staphylococcus aureus (VISA) and heterogeneous- VISA (hVISA): Implications for therapy after vancomycin treatment failure. J. Antimicrob. Chemother. 2011, 66, 1057–1060. [Google Scholar] [CrossRef]
- Gasch, O.; Camoez, M.; Domínguez, M.A.; Padilla, B.; Pintado, V.; Almirante, B.; Martín, C.; López-Medrano, F.; de Gopegui, E.R.; Blanco, J.R.; et al. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J. Antimicrob. Chemother. 2014, 69, 568–571. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.J.; Castanheira, M.; Mendes, R.E.; Sader, H.S.; Jones, R.N. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: A review of published studies and the AWARE Surveillance Program (2008–2010). Clin. Infect. Dis. 2012, 55 (Suppl. S3), S206–S214. [Google Scholar] [CrossRef] [PubMed]
- Laudano, J.B. Ceftaroline fosamil: A new broad-spectrum cephalosporin. J. Antimicrob Chemother. 2011, 66 (Suppl. S3), iii11–iii18. [Google Scholar] [CrossRef] [PubMed]
- Moisan, H.; Pruneau, M.; Malouin, F. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob Chemother. 2010, 65, 713–716. [Google Scholar] [CrossRef]
- De Lencastre, H.; Oliveira, D.; Tomasz, A. Antibiotic resistant Staphylococcus aureus: A paradigm of adaptive power. Curr. Opin. Microbiol. 2007, 10, 428–435. [Google Scholar] [CrossRef]
- Kosowska-Shick, K.; McGhee, P.L.; Appelbaum, P.C. Affinity of ceftaroline and other β-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2010, 54, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- File, T.M.J.r.; Wilcox, M.H.; Stein, G.E. Summary of ceftaroline fosamil clinical trial studies and clinical safety. Clin. Infect. Dis. 2012, 55 (Suppl. S3), S173–S180. [Google Scholar] [CrossRef]
- Cosimi, R.A.; Beik, N.; Kubiak, D.W.; Johnson, J.A. Ceftaroline for severe methicillin-resistant Staphylococcus aureus infections: A systematic review. Open Forum Infect. Dis. 2017, 4, ofx084. [Google Scholar] [CrossRef]
- Casapao, A.M.; Davis, S.L.; Barr, V.O.; Klinker, K.P.; Goff, D.A.; Barber, K.E.; Kaye, K.S.; Mynatt, R.P.; Molloy, L.M.; Pogue, J.M.; et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob. Agents Chemother. 2014, 58, 2541–2546. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Trinh, T.D.; Claeys, K.C.; Casapao, A.M.; Sabagha, N.; Lagnf, A.M.; Klinker, K.P.; Davis, S.L.; Rybak, M.J. Multicenter observational study of ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 2017, 61, e02015–e02016. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Sakoulas, G.; Rose, W.E.; Pogliano, J.; Tewhey, R.; Rybak, M.J. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2013, 57, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Trinh, T.D.; Claeys, K.C.; Lagnf, A.M.; Bhatia, S.; Klinker, K.P.; Veve, M.P.; Estrada, S.J.; Johns, S.T.; Sawyer, A.J.; et al. Multicenter cohort study of ceftaroline versus daptomycin for treatment of methicillin-resistant Staphylococcus aureus bloodstream infection. Open Forum. Infect. Dis. 2021, 9, ofab606. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Sud, A.; O’Sullivan, M.V.N.; Robinson, J.O.; Ferguson, P.E.; Foo, H.; van Hal, S.J.; Ralph, A.P.; Howden, B.P.; Binks, P.M.; et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: A pilot multicenter randomized controlled trial. Clin. Infect. Dis. 2016, 62, 173–180. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- Dhand, A.; Sakoulas, G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin. Ther. 2014, 36, 1303–1316. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Zasowski, E.J.; Trinh, T.D.; Lagnf, A.M.; Bhatia, S.; Sabagha, N.; Abdul-Mutakabbir, J.C.; Alosaimy, S.; Mynatt, R.P.; Davis, S.L.; et al. Daptomycin plus beta-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: A retrospective, comparative cohort study. Clin. Infect. Dis. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.J.; Rybak, M.J. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J. Antimicrob. Chemother. 2015, 70, 505–509. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.; Oliver, N.T.; Hunter, A.; Rodriguez-Barradas, M. Daptomycin and combination daptomycin-ceftaroline as salvage therapy for persistent methicillin-resistant Staphylococcus aureus bacteremia. Infect. Dis. 2018, 50, 643–647. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef]
- McCreary, E.K.; Kullar, R.; Geriak, M.; Zasowski, E.J.; Rizvi, K.; Schulz, L.T.; Ouellette, K.; Vasina, L.; Haddad, F.; Rybak, M.J.; et al. Multicenter cohort of patients with methicillin-resistant Staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum. Infect. Dis. 2019, 7, ofz538. [Google Scholar] [CrossRef] [PubMed]
- Morrisette, T.; Lagnf, A.M.; Alosaimy, S.; Rybak, M.J. A comparison of daptomycin alone and in combination with ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bacteremia complicated by septic pulmonary emboli. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.; Crawford, T.N.; Myint, T. Comparing the outcomes of ceftaroline plus vancomycin or daptomycin combination therapy versus monotherapy in adults with complicated and prolonged methicillin-resistant Staphylococcus aureus bacteremia initially treated with supplemental ceftaroline. Infect. Dis. Ther. 2020, 9, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Molina, K.C.; Miller, M.A.; Kiser, T.H.; Huang, M.; Mueller, S.W. Combination ceftaroline and daptomycin salvage therapy for complicated methicillin-resistant Staphylococcus aureus bacteraemia compared with standard of care. Int. J. Antimicrob. Agents 2021, 57, 106310. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, I.; Yang, Y. Doripenem in the Treatment of Patients with Nosocomial Pneumonia: A Meta-Analysis. J. Clin. Med. 2022, 11, 4014. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Z.; Hu, Y.; Deng, C.; Liao, L.; Sun, L.; Wang, C. Systematic review and meta-analysis of the efficacy and safety of vancomycin combined with β-lactam antibiotics in the treatment of methicillin-resistant Staphylococcus aureus bloodstream infections. J. Glob. Antimicrob. Resist. 2020, 23, 303–310. [Google Scholar] [CrossRef]
- Wang, C.; Ye, C.; Liao, L.; Wang, Z.; Hu, Y.; Deng, C.; Liu, L. Adjuvant β-lactam therapy combined with vancomycin or daptomycin for methicillin-resistant Staphylococcus aureus bacteremia: A systematic review and meta-analysis. Antimicrob. Agents Chemother. 2020, 64, e01377-20. [Google Scholar] [CrossRef]
- Kale-Pradhan, P.B.; Giuliano, C.; Jongekrijg, A.; Rybak, M.J. Combination of vancomycin or daptomycin and beta-lactam antibiotics: A meta-analysis. Pharmacotherapy 2020, 40, 648–658. [Google Scholar] [CrossRef]
- Yi, Y.H.; Wang, J.L.; Yin, W.J.; Xu, W.H. Vancomycin or daptomycin Plus a β-lactam versus vancomycin or daptomycin alone for methicillin-resistant Staphylococcus aureus bloodstream infections: A systematic review and meta-analysis. Microb. Drug Resist. 2021, 27, 1044–1056. [Google Scholar] [CrossRef]
- Lodise, T.P.; Drusano, G.L.; Zasowski, E.; Dihmess, A.; Lazariu, V.; Cosler, L.; McNutt, L.A. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: How much is enough? Clin. Infect. Dis. 2014, 59, 666–675. [Google Scholar] [CrossRef]
- Casapao, A.M.; Jacobs, D.M.; Bowers, D.R.; Beyda, N.D.; Dilworth, T.J. Early administration of adjuvant blactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: A retrospective, multicenter analysis. Pharmacotherapy 2017, 37, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Seifert, H.; Rieg, S.; Kern, W.V.; Kim, H.B.; Kim, E.S.; Liao, C.H.; et al. Defining persistent Staphylococcus aureus bacteraemia: Secondary analysis of a prospective cohort study. Lancet Infect. Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Minejima, E.; Mai, N.; Bui, N.; Mert, M.; Mack, W.J.; She, R.C.; Nieberg, P.; Spellberg, B.; Wong-Beringer, A. Defining the Breakpoint Duration of Staphylococcus aureus Bacteremia Predictive of Poor Outcomes. Clin. Infect. Dis. 2020, 70, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Souli, M.; Ruffin, F.; Choi, S.H.; Park, L.P.; Gao, S.; Lent, N.C.; Sharma-Kuinkel, B.K.; Thaden, J.T.; Maskarinec, S.A.; Wanda, L.; et al. Changing characteristics of Staphylococcus aureus bacteremia: Results from a 21-Year, prospective, longitudinal study. Clin. Infect. Dis. 2019, 69, 1868–1877. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).