Abstract
Background: N-chlorotaurine (NCT), an antiseptic that originates from the human defense system, has broad-spectrum microbicidal activity and is well tolerated by human tissue and applicable to sensitive body regions. Bacteria in short-term biofilms, too, have been shown to be killed by NCT. It was the aim of the present study to demonstrate the activity of NCT against bacteria and yeasts in longer-lasting biofilms, including their co-culture. Materials and methods: Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella variicola biofilms were grown for 14 weeks in MBECTM inoculator with 96 well base. Some pegs were pinched off weekly and incubated in 1% NCT in PBS (PBS only for controls) at pH 7.1 and 37 °C, for 30 and 60 min. Subsequently, bacteria were resuspended by ultrasonication and subjected to quantitative cultures. Similar tests were conducted with C. albicans biofilms grown on metal (A2-steel) discs for 4 weeks. Mixed co-cultures of C. albicans plus each of the three bacterial strains on metal discs were grown for 5–7 weeks and weekly evaluated, as mentioned above. Results: Single biofilms of S. aureus, P. aeruginosa, and K. variicola grew to approximately 1 × 106 colony forming units (CFU)/mL and C. albicans to 1 × 105 CFU/mL. In combined biofilms, the CFU count was about 1 log10 lower. Viable counts of biofilms of single bacteria were reduced by 2.8 to 5.6 log10 in 1% NCT after 60 min (0.9 to 4.7 log10 after 30 min) with Gram-negative bacteria being more susceptible than S. aureus. Significant reduction of C. albicans by 2.0 to 2.9 log10 occurred after 4 h incubation. In combined biofilms, viable counts of C. albicans were reduced by 1.1 to 2.4 log10 after 4 h, while they reached the detection limit after 1 to 2 h with bacteria (2.0 to > 3.5 log10 reduction). Remarkably, older biofilms demonstrated no increase in resistance but constant susceptibility to NCT. This was valid for all tested pathogens. In electron microscopy, morphological differences between NCT-treated and non-treated biofilms could be found. Conclusions: NCT is active against long-term biofilms of up to several months irrespective of their age. Combined biofilm cultures of yeasts and bacteria show a similar susceptibility pattern to NCT as single ones. These results contribute to the explanation of the clinical efficacy of NCT, for instance, in infected chronic wounds and purulently coated crural ulcerations.
1. Introduction
Biofilms are a long-lived, organized community of microorganisms within an extracellular matrix. Biofilm-related infections count as nosocomial infections and are a major cause of death and increased morbidity among hospitalized patients. These infections increase the pressure on medical systems already overwhelmed, in both developed and undeveloped countries [1]. Because of the rising number of patients with this serious complication, the orthopedic community, for example, has become more concerned about the management of implant infections. Those infections are difficult to treat often requiring surgical implant replacement [2]. The most commonly isolated microorganisms in implant infections are Coagulase-Negative Staphylococci (CoNS; primarily S. epidermidis), followed by S. aureus and mixed flora [3,4,5]. In previous decades, resistance of CoNS and several other microorganisms against antibiotics increased. Strong and sometimes dramatic increases in the percentage of resistant isolates were noted particularly for penicillin, oxacillin, ciprofloxacin, clindamycin, erythromycin, and gentamicin [6]. The ability of these bacteria to produce biofilms is an additional mechanism contributing to this phenomenon and negatively affecting the antimicrobial susceptibility of CoNS [7]. Biofilm formation also explains why some normal flora organisms traditionally considered “harmless” become pathogenic when they grow on the surface of foreign bodies [8].
N-chlorotaurine (NCT) is a long-lived oxidant produced by activated human granulocytes and monocytes during the oxidative burst [9,10]. As a sodium salt, it can be used as a mild, but effective, and well tolerated antiseptic and anti-infective for topical treatment of infections of different body sites, for instance, the skin and mucous membranes, the eye, the ear, and the urinary bladder [11,12]. In the last years, also the lower airways became a topic of interest, since inhalation of NCT turned out to be well tolerated [13]. Therefore, acute bronchopulmonary infections including the recent COVID-19 pandemic and chronic diseases such as chronic obstructive pulmonary disease and cystic fibrosis may become indications for NCT treatment [13,14,15].
NCT has demonstrated microbicidal activity against bacteria, viruses, fungi, protozoa, and worm larvae [11]. Viability of biofilms of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa is largely reduced by the preferred pharmacological concentrations of 1% (55 mM) NCT, too, after incubation times of 15 min to 1 h [16,17]. Lower millimolar NCT concentrations kill the biofilm at longer incubation times [16,17], and 300 µM inhibited the formation of biofilms [18]. In clinical studies, NCT has shown significant curative effects in infections or disorders where biofilms are thought to play a role, namely in purulently coated leg ulcers [19], external otitis [20], and dental plaque [21]. In such chronic diseases, persisting biofilms appear to be causative for antibiotic resistance and therapeutic problems. A previous study demonstrated increasing resistance of biofilms grown for 2–3 weeks to antiseptics (hypochlorite, iodine, and chlorhexidine) with no further change of susceptibility up to 8 weeks [22]. The activity of NCT against long-term biofilms is unknown.
Because of the encouraging clinical results, we started to focus on the activity of NCT against biofilms grown over longer time-periods. Using the model of MBEC plates, it was possible to monitor the susceptibility of biofilms to NCT for 3 to 5 months. Another unknown aspect was addressed in this study, which is the activity of NCT against combined biofilms of bacteria and fungi.
2. Materials and Methods
2.1. Bacterial and Fungal Strains
S. aureus ATCC 25923 and ATCC 6538, P. aeruginosa ATCC 27853, Klebsiella variicola (clinical isolate), and Candida albicans strains ATCC 15053, ATCC 5314, and CBS 5982 were used. S. aureus, P. aeruginosa, and C. albicans were chosen as pathogens frequently isolated from human samples, while K. variicola is an emerging opportunistic pathogen widespread in humans and animals with the potential of hypervirulence and biofilm formation, too [23,24]. Bacteria and yeast, deep frozen at minus 80 °C for storage, were thawed and grown on Mueller–Hinton agar plates (Oxoid Ltd., Oxoid, UK) for use. Overnight cultures from the plates were grown at 37 °C in tryptic soy broth (Merck, Darmstadt, Germany) to 3–5 × 109 colony forming units (CFU)/mL for bacteria and grown in tryptic soy broth plus 1% glucose to 1 × 107 CFU/mL for yeasts, respectively.
2.2. N-Chlorotaurine
Pure NCT was prepared as crystalline sodium salt (Cl–HN–CH2–CH2–SO3Na; molecular weight, 181.57 [25]) in pharmaceutical quality and dissolved in 0.1 M phosphate buffer (pH 7.1) to a concentration of 1% (55 mM). This concentration turned out as a standard clinically tolerated in different body sites and demonstrating good microbicidal activity against all kinds of pathogens [11,12,26].
2.3. Devices for Growth of Biofilm
Two devices were applied. The first was MBEC™ biofilm inoculator with 96 well base (No. 19112 MBEC P&G Panel, Innovotech Inc., Edmonton, AB, Canada). The biofilm grows on pegs fixed to the lid and soaked in the wells. For evaluation, the pegs were snapped off and subjected to ultrasonication and quantitative cultures or to electron microscopy (for details, please see below).
The second device was metal discs, A2-steel, DIN 9021, size M2, external diameter 6 mm, internal diameter 2.2 mm, thickness 0.8 mm (Schrauben-Niro.de, SyneriaX, Bramstedt, Germany). The metal discs were put into 96-well flat bottom microtitre plates (Corning, NY, USA) for growth of biofilm (for detailed procedure, please see below).
2.4. Growth of Biofilm of Bacteria in MBEC Inoculator and Incubation in NCT
Aliquots of overnight cultures of the pathogens were 100-fold diluted in tryptic soy broth, and 150 µL of the dilutions each were filled into the wells of the 96-well base of the inoculator. All bacteria were tested separately. The initial concentration of bacteria was 3–5 × 107 colony forming units (CFU)/mL. The lid with the pegs was put on the base, and the device was laid in a plastic chamber with a wet towel to warrant a moist condition. The whole chamber was incubated in a rotatory shaker at 150 rpm and 37 °C for 11–21 weeks for P. aeruginosa, for 11–19 weeks for S. aureus ATCC 25923, and for 14–19 weeks for K. variicola. The medium (tryptic soy broth) was changed twice a week each in all wells.
Tests for susceptibility to NCT were performed weekly. For this, six pegs each were pinched off with forceps and washed in 2 mL PBS in wells of a 24-well plate (Corning, NY, USA). Subsequently, four pegs were transferred into wells containing 2 mL of 1% NCT in PBS (pH 7.1) and two pegs in wells with 2 mL of PBS only for controls, one peg per well. Two of the test pegs were incubated for 30 min, and two for 60 min in NCT at 37 °C. Control pegs were incubated for 60 min. After the incubation time, the pegs were washed twice in PBS. One peg was subjected to quantitative colony counts, the other one to scanning electron microscopy.
2.5. Growth of Biofilm of Yeasts and Mixed Cultures of Yeast and Single Bacteria on Metal Discs and Incubation in NCT
Aliquots of overnight cultures of Candida spp. were 100-fold diluted in tryptic soy broth plus 1% glucose, and 150 µL of the dilutions each were filled into the wells of 96-well flat bottom microtiter plates. The wells of the plates were filled with one metal disc each. All yeast strains were tested separately. The initial concentration of yeast was approximately 1 × 105 colony forming units (CFU)/mL. The plates were put in a moist chamber and agitated at 200 rpm at 37 °C, as mentioned, above for 4 weeks. Medium changes were performed twice a week. Tests for susceptibility to NCT were performed weekly. The procedure was similar to the one with the pegs described in the previous paragraph with the only difference that metal discs were used instead of pegs and that 48-well plates were filled with 1 mL of NCT or PBS for the test and control incubation and washing steps.
For mixed cultures, C. albicans ATCC 5314 and the bacteria were grown separately overnight in tryptic soy broth plus 1% glucose. Subsequently, C. albicans was 100-fold diluted in tryptic soy broth plus 1% glucose, while S. aureus, P. aeruginosa, and K. variicola were 10,000-fold diluted in 0.9% saline. For the mixed biofilms, S. aureus strain ATCC 6538 was used because of its yellow colony color, which renders it easily distinguishable from C. albicans on agar plates. C. albicans was tested with each strain of the bacteria separately in a mixed culture. Aliquots of 0.2 mL of C. albicans and 0.2 mL of each bacterial strain were diluted in 19.6 mL of tryptic soy broth plus 1% glucose. Therefore, the final concentration of the yeast was approximately 1 × 103 CFU/mL, and that of the bacteria approximately 3 × 103 CFU/mL. Again, 150 µL of the dilutions each were filled into the wells of 96-well flat bottom microtiter plates, which contained one metal disc per well. The plates were put in a moist chamber and agitated at 200 rpm at 37 °C, as mentioned above, for 5 to 7 weeks. The pattern of medium changes and susceptibility tests was similar to the tests with C. albicans single biofilm cultures.
2.6. Quantitative Cultures
After incubation in NCT or control PBS, the washed pegs and metal disks, respectively, were transferred into 15 mL Falcon tubes containing 1 mL of PBS. They were vortexed three times for 5 s, sonicated for 1 min in an ultrasound water bath (40 kHz; BactoSonic; Bandelin Electronic, Berlin, Germany), and vortexed again three times to detach the remaining live bacteria from the pegs or disks. Test samples were processed undiluted and 10-fold diluted, and controls were 100-fold diluted. Aliquots of 50 μL of these solutions were spread on Mueller–Hinton agar plates in duplicate, using an automatic spiral plater (model WASP 2; Don Whitley, Shipley, West Yorkshire, UK). The detection limit was 10 CFU/mL, taking into account both plates. Plates were incubated for 48 h (bacteria) to 72 h (fungi) at 37 °C, and the number of CFU was counted.
2.7. Scanning Electron Microscopy
A procedure was used similar to a previous study on the impact of NCT on short-term biofilms [17]. After incubation in NCT or control PBS, the washed pegs and metal disks, respectively, were fixed with 2.5% glutaraldehyde (BioChemika Fluka, Buchs, Switzerland) in 0.1 M phosphate buffer (pH 7.4). After a brief wash in phosphate buffer, the samples were gradually dehydrated with ethanol. After the last step, the discs were placed in an incubator for drying out. The dried discs were placed on aluminum pins and fixed with Leit-C (Göcke, Plano GmbH, Wetzlar, Hessen, Germany). The pins were sputtered with Au 10 nm (Agar Sputter Coater, Agar Scientific Ltd., Stansted, GB, UK) for 1 min and analyzed by scanning electron microscopy (SEM, JSM-6010LV, JEOL GmbH, Freising, Germany).
2.8. Statistics
Several independent repetition experiments were performed for each condition. The results are presented as mean values and standard deviation or standard error of the mean, as indicated in the results. One-way analysis of variance (ANOVA) and Dunnett’s and Tukey’s multiple comparison tests were carried out for comparison of test samples with controls and for different incubation times in NCT, respectively. p values < 0.05 were considered significant.
3. Results
Biofilms developed well and could be kept for many weeks both in the MBEC™ biofilm inoculator and on A2-steel discs. The limiting factor is contamination with other pathogens despite strict aseptic workflow, but several independent repetition experiments over 3.5 months were possible. Single tests reached 21 weeks for P. aeruginosa and 19 weeks for S. aureus and K. variicola, with similar results and no additional information. Overall, the aspect of the biofilm in electron microscopy did not change significantly over the months, and the susceptibility to NCT remained constant.
3.1. Bactericidal Effect of NCT in Long-Term Biofilms of Single Strains
These biofilms were grown in MBEC inoculators at 37 °C for 14 weeks. Bacterial counts reached about 1 × 106 CFU/mL. Overall, the standard concentration of 1% NCT showed bactericidal activity after 30 min incubation time at 37 °C and pH 7.1, which increased after 60 min. The course of susceptibility over 14 weeks is shown in Figure 1, Figure 2 and Figure 3 for S. aureus ATCC 25923, P. aeruginosa ATCC 27853, and K. variicola (clinical isolate). S. aureus was less susceptible than the Gram-negative bacteria. Single time points of evaluation did not reach significance for killing after 30 min incubation in NCT because of high standard deviations. Note the presentation with standard error of the mean in the figures. The 30 min incubation of S. aureus biofilm in NCT may be suggestive of decreasing activity with increasing age of the biofilm, but this was insignificant as well. The Gram-negative bacteria had a higher susceptibility with some (P. aeruginosa) or all (K. variicola) single values significant after 30 min incubation and with a reduction of viable counts nearly or completely to the detection limit of 1 log10 after 60 min incubation.
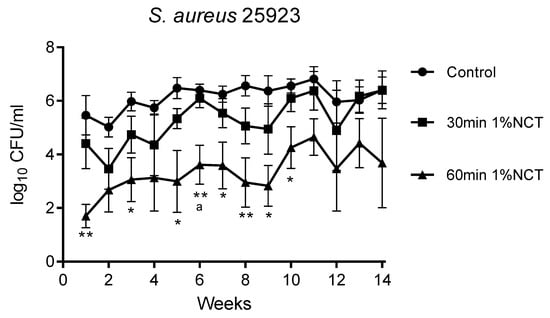
Figure 1.
Bactericidal activity of 1% NCT at pH 7 and 37 °C against biofilms of S. aureus ATCC 25923 grown for 14 weeks. Weekly evaluation by quantitative killing assays. Mean values ± SEM, n = 5 (n = 3 for weeks 12–14); * p < 0.05 versus control; ** p < 0.01 versus control; a p < 0.01 between 30 and 60 min NCT.
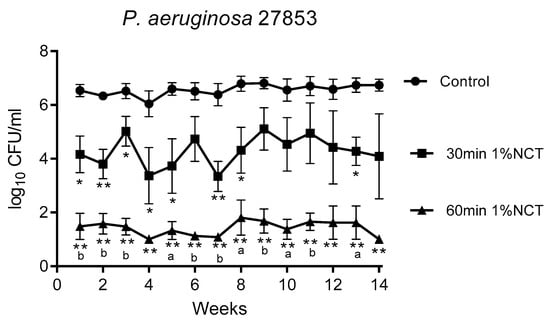
Figure 2.
Bactericidal activity of 1% NCT at pH 7 and 37 °C against biofilms of P. aeruginosa ATCC 27853 grown for 14 weeks. Weekly evaluation by quantitative killing assays. Mean values ± SEM, n = 6 (n = 4 for weeks 12 and 13, n = 3 for week 14); * p < 0.05 versus control; ** p < 0.01 versus control; a p < 0.05 versus 30 min NCT; b p < 0.01 versus 30 min NCT.
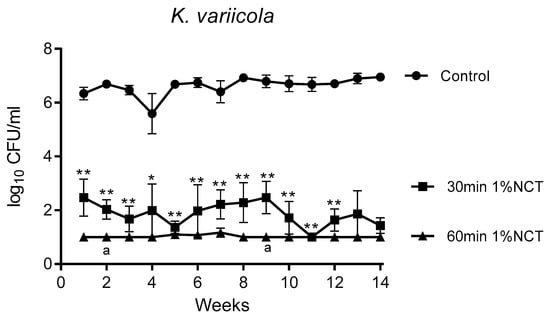
Figure 3.
Bactericidal activity of 1% NCT at pH 7 and 37 °C against biofilms of K. variicola clinical isolate grown for 14 weeks. Weekly evaluation by quantitative killing assays. Mean values ± SEM, n = 5 (n = 4 for weeks 12–14); p < 0.01 for all values of 30 min and 60 min NCT versus control except for p < 0.05 for 30 min NCT after 4 weeks; * p < 0.05 versus control; ** p < 0.01 versus control; a p < 0.05 of 30 min versus 60 min NCT.
The absence of a change of susceptibility over time allowed a calculation over all single values, which is shown in Figure 4. The overall reduction in CFU was highly significant for 30 and 60 min NCT for all strains, coming to 0.91 and 2.83 for S. aureus, 2.27 and 5.13 for P. aeruginosa, and 4.74 and 5.58 for K. variicola, respectively (Figure 4).
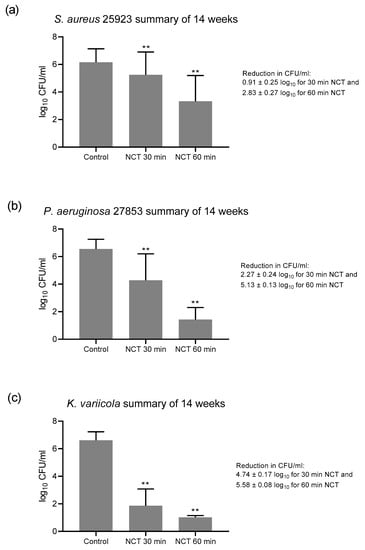
Figure 4.
Summary of all values of NCT and controls over the 14 weeks of Figure 1, Figure 2 and Figure 3. (a) S. aureus, n = 62; (b) P. aeruginosa, n = 73–74; (c) K. variicola, n = 64–65. Mean values ± SD. ** p < 0.01 for controls versus NCT and for 30 min versus 60 min incubation time in all panels.
3.2. Fungicidal Effect of NCT in Long-Term Biofilms of Single C. albicans Strains
These biofilms were grown on A2-steel discs at 37 °C for 4 weeks. Biofilms of all yeast test strains grew to approximately 1 × 105 CFU/mL and were similarly susceptible to NCT. As with bacteria, there was no change in the CFU counts and in the susceptibility to NCT over the whole test period. As an example, the detailed course is shown in Figure 5a for C. albicans ATCC 5314. The summary of the values similar to Figure 4 allowed a good overview and disclosed the longer incubation times of 3 to 4 h in 1% NCT at 37 °C and pH 7.1 needed for significant killing of yeasts compared to bacteria (Figure 5b). The extent of killing was similar using the C. albicans strains ATCC 15053 and CBS 5982 (Figure 6). A reduction in CFU by >3 log10 nearly to the detection limit could be achieved after 5 h incubation in 1% NCT. For detailed values, see Figure 5 and Figure 6.
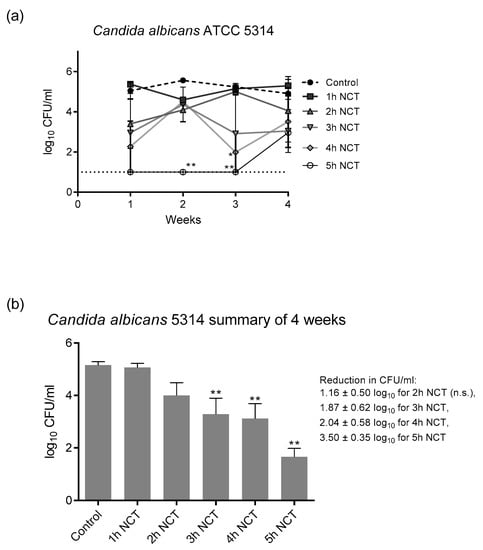
Figure 5.
(a) Fungicidal activity of 1% NCT at pH 7 and 37 °C against biofilms of C. albicans ATCC 5314 grown for 4 weeks. Weekly evaluation by quantitative killing assays. Mean values ± SEM, n = 3 (n = 2 for week 2); * p < 0.05 versus control; ** p < 0.01 versus control. (b) Summary of all values of NCT and controls over the 4 weeks of Figure 5a. Mean values ± SEM, n = 9–12; ** p < 0.01 versus control; n.s.: not significant.
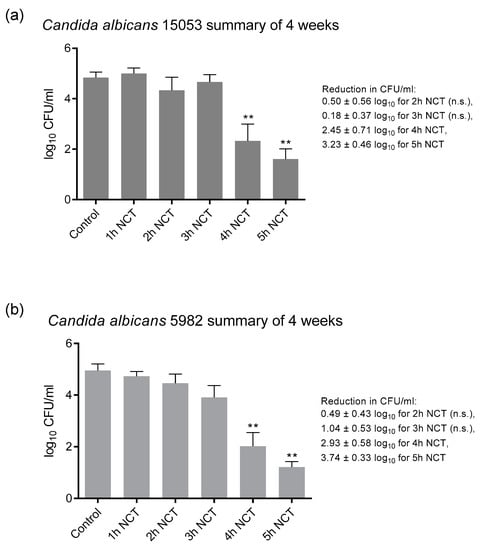
Figure 6.
Fungicidal activity of 1% NCT at pH 7 and 37 °C against biofilms of C. albicans ATCC 15053 (a) and CBS 5982 (b) grown for 4 weeks. Weekly evaluation by quantitative killing assays. Summary of all values of NCT and controls. Mean values ± SEM, n = 9–12 values each from 2–3 independent experiments; ** p < 0.01 versus control; n.s.: not significant.
3.3. Bactericidal and Fungicidal Effect of NCT in Mixed Long-Term Biofilms
These biofilms were grown on A2-steel discs at 37 °C for 5 (S. aureus ATCC 6538) to 7 weeks (P. aeruginosa and K. variicola). C. albicans ATCC 5314 was chosen as the partner for each bacterial strain. The yeast grew to lower colony counts in mixed cultures than in single cultures, coming to roughly 1 × 104 CFU/mL together with P. aeruginosa and 1x 103 CFU/mL with S. aureus and K. variicola. Moreover, S. aureus and K. variicola achieved lower counts by approximately 1 log10 than in single cultures, although this is not directly comparable because of the different biofilm culture systems.
Again, all microorganisms in mixed biofilm cultures were killed by 1% NCT at 37 °C and pH 7.1. The reduction rate of all was similar to that of single strain cultures. The age of the biofilm had no influence, too. The killing with increasing incubation time in NCT as a summary of the values of the weekly evaluation for each microorganism is depicted in Figure 7. The detection limit for the yeast could be nearly achieved after 4 h in NCT, while it was 1–2 h for bacteria.
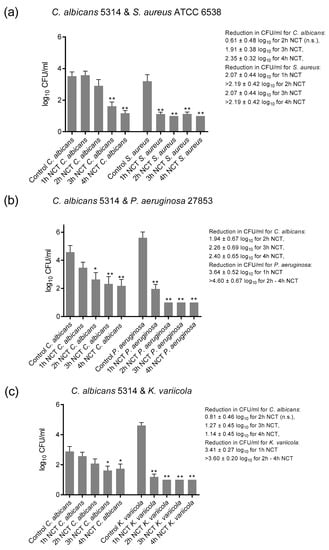
Figure 7.
Fungicidal and bactericidal activity of 1% NCT at pH 7 and 37 °C against mixed biofilms of C. albicans ATCC 5314 plus S. aureus ATCC 6538 (a), P. aeruginosa ATCC 27853 (b) or K. variicola (c) clinical isolate grown for 5 (S. aureus) to 7 weeks. Weekly evaluation by quantitative killing assays. Summary of all values of NCT and controls. Mean values ± SEM, n = 15–17 (S. aureus), 10–15 (P. aeruginosa), and 18–20 (K. variicola) values each from 3–5 independent experiments; * p < 0.05 versus control; ** p < 0.01 versus control; n.s.: not significant.
3.4. Scanning Electron Microscopy
Analyzing the isolated biofilm cultures, we observed that all three strains showed typical biofilm morphology after 1 week of incubation if not treated with NCT. K. variicola biofilms were compact, covered with slime-like and protein-like substance binding between the bacteria. The P. aeruginosa and S. aureus biofilms, likewise, showed a three-dimensional structure although presenting less slime-like substance on their surface. Protein-like structures could be observed connecting the P. aeruginosa rods. The S. aureus biofilms showed a protein-like substance between and underneath the bacteria. After 30 min of treatment with NCT, the typical biofilm morphology of a 3-dimentional structure and the presence of slime-like substance was absent for K. variicola. The protein-like binding structures looked damaged in the P. aeruginosa and S. aureus biofilms, after 30 min of NCT. K. variicola and P. arguginosa, after 30 min of treatment, showed a damaged shape with impressions. S. aureus biofilms showed structural destruction in part of the bacteria. Similar morphological alterations could be observed for biofilms of all three strains after exposition to NCT for 60 min (Figure 8).
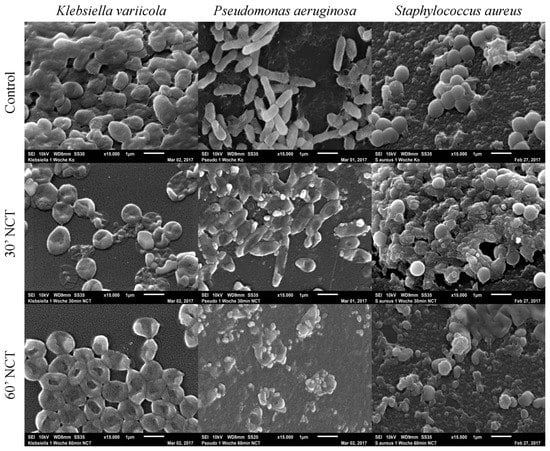
Figure 8.
Scanning electron microscopy of 1-week-old Klebsiella variicola, Pseudomonas aeruginosa, and Staphylococcus au-reus biofilms. Upper row: mock-treated control biofilms (Magnification 15,000×); middle row: biofilms treated with NCT, for 30 min (Magnification 15,000×); lower row: biofilms treated with NCT, for 60 min (Magnification 15,000×). Specimens were analyzed by scanning electron microscopy (SEM, JSM-6010LV, JEOL GmbH, Freising, Germany).
The 14-week-old control biofilms of K. variicola, P. aeruginosa, and S. aureus demonstrated typical mature biofilm morphology. All three strains showed clumps with the presence of water channels and slime-like structures covering and connecting the bacteria. These control biofilms presented morphological cell integrity in all strains. After 30 min of treatment with NCT, the biofilms showed less slime-like structures in all strains, although structural damage could be observed only in the K. variicola biofilms. After 60 min of treatment with NCT, all the strains presented morphological damage and absence of slime-like structures (Figure 9).

Figure 9.
Scanning electron microscopy of 14-week-old Klebsiella variicola, Pseudomonas aeruginosa, and Staphylococcus aureus biofilms. Upper row: mock-treated control biofilms (Magnification: K. variicola 10,000×; P. aeruginosa and S. aureus 15,000×); middle row: biofilms treated with NCT, for 30 min (Magnification: K. variicola 10,000×; P. aeruginosa and S. aureus 15,000×); lower row: biofilms treated with NCT for 60 min (Magnification: K. variicola and P. aeruginosa 10,000×; S. aureus 15,000×). Specimens were analyzed by scanning electron microscopy (SEM, JSM-6010LV, JEOL GmbH, Freising, Germany).
The 3-week-old control biofilms of C. albicans showed agglomerates of cells. The typical C. albicans biofilm morphology could not be observed, but cell morphology was intact. After 60 min treatment with NCT, cell clumps were not visible, and structural cell damage could be observed for all three strains tested. The same could be observed after 120 min of treatment with NCT (Figure 10).
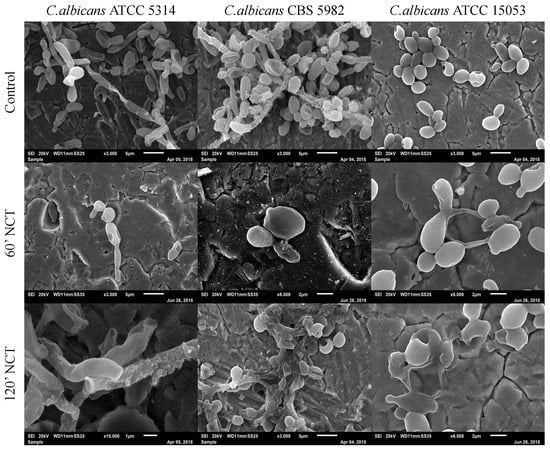
Figure 10.
Scanning electron microscopy of 3-week-old C. albicans ATCC 5314, C. albicans CBS 5982 and C. albicans ATCC 15053 biofilms. Upper row: mock-treated control biofilms (Magnification: 3000×); middle row: biofilms treated with NCT, for 60 min (Magnification: C. albicans ATCC 5314 3000×; C. albicans CBS 5982 and C. albicans ATCC 15053 6000×); lower row: biofilms treated with NCT for 120 min (Magnification: C. albicans ATCC 5314 10,000×; C. albicans CBS 5982 3000× and C. albicans ATCC 15053 6000×). Specimens were analyzed by scanning electron microscopy (SEM, JSM-6010LV, JEOL GmbH, Freising, Germany).
The mixed long-term culture of C. albicans with K. variicola, P. aeruginosa, and S. aureus, after 3 weeks of incubation, showed typical biofilm structure for all strains in the control groups. C. albicans cultivated with K. variicola showed a homogeneous and three-dimensional biofilm formed by both microorganisms. In mixed culture, C. albicans seemed to predominate over P. aeruginosa. The culture of C. albicans together with S. aureus showed the bacteria growing attached to the surface of C. albicans hyphae. After 60 min of treatment with NCT, the C. albicans and K. variicola biofilms showed less volume and morphological alterations. The C. albicans plus P. aeruginosa and C. albicans plus S. aureus biofilms were completely eliminated from the surface of the metal plates after 60 min of NCT activity. The same finding was obtained for the mixed cultures treated for 120 min with NCT (Figure 11).
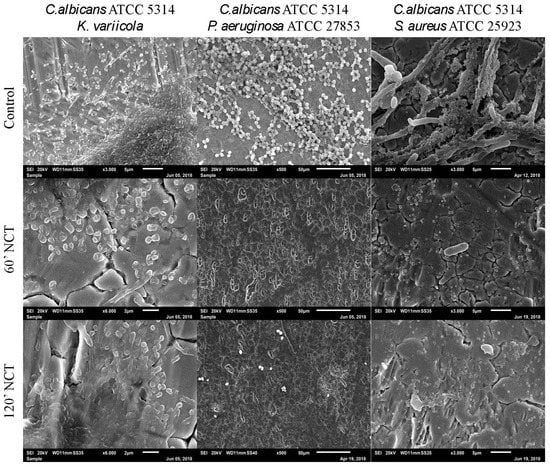
Figure 11.
Scanning electron microscopy of 3-week-old mixed biofilms using C. albicans co-cultured with K. variicola, P. aeruginosa or S. aureus. Upper row: mock-treated control biofilms (Magnification: C. albicans plus K. variicola 3000×; C. albicans plus P. aeruginosa 500×; C. albicans plus S. aureus 3000×); middle row: biofilms treated with NCT, for 60 min (Magnification: C. albicans plus K. variicola 6000×; C. albicans plus P. aeruginosa 500×; C. albicans plus S. aureus 3000×); lower row: biofilms treated with NCT, for 120 min (Magnification: C. albicans plus K. variicola 6000×; C. albicans plus P. aeruginosa 500×; C. albicans plus S. aureus 3000×). Specimens were analyzed by scanning electron microscopy (SEM, JSM-6010LV, JEOL GmbH, Freising, Germany).
4. Discussion
In chronic infections and on foreign bodies, biofilms develop and may persist and be difficult to eradicate if they cannot be removed surgically or by replacement of the foreign body. Particularly in such cases, effective antimicrobial agents are desirable for treatment. Because of their good tolerability and efficacy in infected chronic skin ulcerations, according to a phase II clinical study and additional cases [19,27,28], and NCT may become a valuable therapeutic tool for such patients. It has shown microbicidal activity against bacterial biofilms, which were up to one week old [16,17,18]. Surprisingly, against Staphylococcus epidermidis, this activity appeared to be rather higher in biofilms grown for 7 days, compared with 1 day, without a clear explanation [16]. This might have been specific to the system or to NCT since, in general, mature biofilms seem to be more resistant against antiseptics. According to Stojicic and colleagues, after growing multispecies biofilms on collagen-coated hydroxyapatite disks, bacteria in mature biofilms were more resistant to 1% NaOCl, 0.2/0.4% iodine-potassium iodide, and 2% chlorhexidine than bacteria in young biofilms [22]. In this study, the biofilms were grown for 8 weeks, and a change in the susceptibility was found after 2–3 weeks [22], although biofilms are considered to have reached their last stage of maturation around 9–12 days after the beginning of their formation [22,29].
Considering all these previous knowledges, we wanted to clarify the effect of NCT against long-lasting biofilms over several weeks to months. Both of the devices we used for rapid development and persistence of biofilms, the MBECTM inoculator and metal discs, turned out to be suitable for this purpose. Metal discs are cheap, but not always readily available, while MBECTM inoculators are easier to handle, but more expensive, and their plastic surface was different from the materials used for implants in orthopedics. In the meantime, however, more surface types are available.
As a clear result, the susceptibility of bacterial biofilms to NCT did not change with their age up to 3.5 months and more. The same was true for yeast biofilms and mixed biofilms (bacteria and yeasts) grown for at least one month. As in scanning electron microscopy, the biofilm developed after one week and persisted with some possible increase in thickness but without marked morphological changes on both devices. This is in accordance with the previous findings of maturation after 1–2 weeks [22,29] and may be the reason why the susceptibility of the microorganisms to NCT did not change with the age of the biofilms. In a previous study, a biofilm of Staphylococcus epidermidis grown for 7 days was rather more susceptible to NCT than a biofilm grown for 2 days [16]. Longer periods were not tested. In the same study, the biofilm of S. epidermidis producing poly-N-acetylglucosamine, a main compound for the extracellular matrix, was more resistant to NCT compared with a mutant strain not producing this substance [16]. This indicates that metabolic changes of a biofilm actually can influence its susceptibility to NCT to a limited degree, but does not cause resistance. About the metabolism of the biofilms in the present study, however, no statements are possible since we did not perform functional tests. It can be said that at least no metabolic changes with a significant influence on the susceptibility to NCT occurred.
As it is generally the case with biofilms, their resistance to antiseptics and antimicrobial agents usually is higher than that of planktonic cultures due to hindered penetration and to the heterogeneity of the population of single microorganisms. The same is true for NCT, whereby the present study confirms two previous ones on short-term biofilms [16,17]. The sequence of lower to higher susceptibility of the tested strains turned out to be similar for planktonic and biofilm cultures, with C. albicans > S. aureus > P. aeruginosa > K. variicola (for planktonic cultures for bacteria and yeasts, see for instance [11,30,31]). S. aureus and S. epidermidis needed longer incubation times than P. aeruginosa in a previous biofilm study, too [17]. Incubation times required for killing are determined by penetration of NCT into the microorganisms and therefore by their different coatings [32]. Obviously, penetration is the decisive step in biofilms. Destruction and loss of viability in the presence of NCT at a pH of around 7 and at 37 °C needs more time in biofilms, 0.5–1 h in bacteria and 3–4 h in C. albicans, than against planktonic suspensions (approximately a few—20 min in bacteria, 1–2 h in C. albicans). Killing tests and electron microscopy seem to correlate in the present study.
These times required for inactivation of biofilms by NCT in vitro may appear as relatively long for successful application in vivo. In this regard, however, it is important that the microbicidal activity of NCT is not decreased but increased in the presence of human body fluids and exudates [11,15,33,34,35]. The reason is the transfer of the active chlorine of NCT to other amino compounds and the formation of corresponding chloramines in equilibrium (transchlorination) [36]. Some of them are more lipophilic than NCT, particularly monochloramine (NH2Cl), and penetrate and kill microorganisms faster [11,15,33,34,35]. The efficacy of NCT in chronic wound infections may also indicate that transchlorination plays a significant role in the attack of biofilms by NCT [19,27,28].
Against mixed biofilms, NCT can be an interesting agent as well. The interaction of microbial species in multispecies biofilms leads to the development of antibiotic resistance as well as protective mechanisms against host immune response [37]. Frequently, the interaction of C. albicans and bacteria, either physically or chemically, appears to be causative [38,39]. Physical interaction is achieved through the assistance of cell surface receptors/proteins [40]. By contrast, chemical interactions occur due to the involvement of several secretory molecules or metabolic intermediates released either by fungi or bacteria into the environment. These metabolic products/or secretory molecules facilitate communication between fungal and bacterial cells, either synergistically or antagonistically [41]. As an active chlorine compound, NCT has been shown to oxidize and chlorinate secreted virulence factors of bacteria and fungi and to inactivate these factors, as seen in shiga toxin of Escherichia coli [42], staphylococcal toxins [43], gliotoxin of Aspergillus fumigatus [44], and aspartyl proteinases of Candida albicans [31]. A recent comprehensive investigation disclosed a decrease in a panel of virulence factors in NCT-treated A. fumigatus [45]. Therefore, it is well conceivable that NCT blocks communication and interaction factors of mixed species in biofilms as well. Actually, NCT was equally effective against mixed biofilms compared to the single-species biofilms in the present study. The yeast was killed later than the bacteria, which is explainable by slower penetration into the fungus, as mentioned above. Because of the unspecific oxidizing mechanism of action with multiple targets [46], resistance against NCT and other active chlorine compounds in therapeutic concentration is hardly imaginable and has never been observed [47]. This underlines the general advantage of antiseptics versus antibiotics for topical treatment of infections and can be confirmed by the successful application of NCT in therapy-refractory wound infections [27,28].
5. Conclusions
NCT exerts constant bactericidal and fungicidal activity in biofilms grown for weeks to months. This is also true for biofilms containing a combination of bacteria and yeasts. Therefore, NCT is a promising medication for therapy of infections where long-term biofilms play a role, for instance, infected chronic wounds, which is supported by previous clinical results.
Author Contributions
Conceptualization, M.N. and D.C.C.-H.; methodology, all authors; software, M.N., S.J.M.S. and D.C.C.-H.; validation, M.N. and D.C.C.-H.; formal analysis, V.G. and M.N.; investigation, V.G.; resources, M.N. and D.C.C.-H.; data curation, M.N. and D.C.C.-H.; writing—original draft preparation, V.G. and M.N.; writing—review and editing, M.N. and D.C.C.-H.; visualization, all authors; supervision, M.N. and D.C.C.-H.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Austrian Science Fund (FWF), grant No. KLI 459-B30.
Data Availability Statement
All data are presented in the publication.
Acknowledgments
We are grateful to Andrea Windisch for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwarz, E.M.; McLaren, A.C.; Sculco, T.P.; Brause, B.; Bostrom, M.; Kates, S.L.; Parvizi, J.; Alt, V.; Arnold, W.V.; Carli, A.; et al. Adjuvant antibiotic-loaded bone cement: Concerns with current use and research to make it work. J. Orthop. Res. 2021, 39, 227–239. [Google Scholar] [CrossRef]
- Saeed, K.; McLaren, A.C.; Schwarz, E.M.; Antoci, V.; Arnold, W.V.; Chen, A.F.; Clauss, M.; Esteban, J.; Gant, V.; Hendershot, E.; et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J. Orthop. Res. 2019, 37, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.; Campoccia, D.; An, Y.; Baldassarri, L.; Pirini, V.; Donati, M.B.; Pegreffi, F.; Montanaro, L. Prevalence and Antibiotic Resistance of 15 Minor Staphylococcal Species Colonizing Orthopedic Implants. Int. J. Artif. Organs 2006, 29, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Von Eiff, C.; Pirini, V.; Ravaioli, S.; Becker, K.; Arciola, C.R. Cluster analysis of ribotyping profiles of Staphylococcus epidermidis isolates recovered from foreign body-associated orthopedic infections. J. Biomed. Mater. Res. Part A 2008, 88, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Kresken, M.; Hafner, D.; The Study Group Bacterial Resistance of the Paul-Ehrlich-Society for Chemotherapy. Drug resistance among clinical isolates of frequently encountered bacterial species in central Europe during 1975–1995. Infection 1999, 27, S2–S8. [Google Scholar] [CrossRef]
- Giormezis, N.; Kolonitsiou, F.; Foka, A.; Drougka, E.; Liakopoulos, A.; Makri, A.; Papanastasiou, A.D.; Vogiatzi, A.; Dimitriou, G.; Marangos, M.; et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: The role of biofilm formation and distribution of adhesin and toxin genes. J. Med. Microbiol. 2014, 63, 1500–1508. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Weiss, S.J.; Klein, R.; Slivka, A.; Wei, M. Chlorination of Taurine by Human Neutrophils. J. Clin. Investig. 1982, 70, 598–607. [Google Scholar] [CrossRef]
- Zgliczyński, J.; Stelmaszyńska, T.; Domański, J.; Ostrowski, W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim. Biophys. Acta (BBA) Enzym. 1971, 235, 419–424. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. N-chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob. Chemother. 2010, 65, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Nagl, M.; Arnitz, R.; Lackner, M. N-Chlorotaurine, a Promising Future Candidate for Topical Therapy of Fungal Infections. Mycopathologia 2018, 183, 161–170. [Google Scholar] [CrossRef]
- Arnitz, R.; Stein, M.; Bauer, P.; Lanthaler, B.; Jamnig, H.; Scholl-Bürgi, S.; Stempfl-Al-Jazrawi, K.; Ulmer, H.; Baumgartner, B.; Embacher, S.; et al. Tolerability of inhaled N-chlorotaurine in humans: A double-blind randomized phase I clinical study. Ther. Adv. Respir. Dis. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Lackner, M.; Rössler, A.; Volland, A.; Stadtmüller, M.; Müllauer, B.; Banki, Z.; Ströhle, J.; Luttick, A.; Fenner, J.; Stoiber, H.; et al. N-chlorotaurine, a novel inhaled virucidal antiseptic is highly active against respiratory viruses including SARS-CoV-2 (COVID-19). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Leiter, H.; Toepfer, S.; Messner, P.; Rabensteiner, M.; Gostner, J.M.; Lackner, M.; Hermann, M.; Nagl, M. Microbicidal activity of N-chlorotaurine can be enhanced in the presence of lung epithelial cells. J. Cyst. Fibros. 2020, 19, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Ammann, C.G.; Fille, M.; Hausdorfer, J.; Nogler, M.; Nagl, M.; Coraça-Huber, D.C. Influence of poly-N-acetylglucosamine in the extracellular matrix on N-chlorotaurine mediated killing of Staphylococcus epidermidis. New Microbiol. 2014, 37, 383–386. [Google Scholar]
- Coraca-Huber, D.C.; Ammann, C.; Fille, M.; Hausdorfer, J.; Nogler, M.; Nagl, M. Bactericidal activity of N-chlorotaurine against biofilm forming bacteria grown on metal discs. Antimicrob. Agents Chemother. 2014, 58, 2235–2239. [Google Scholar] [CrossRef][Green Version]
- Marcinkiewicz, J.; Strus, M.; Walczewska, M.; Machul, A.; Mikołajczyk, D. Influence of Taurine Haloamines (TauCl and TauBr) on the Development of Pseudomonas aeruginosa Biofilm: A Preliminary Study. Chem. Biol. Pteridines Folates 2013, 775, 269–283. [Google Scholar] [CrossRef]
- Nagl, M.; Nguyen, V.A.; Gottardi, W.; Ulmer, H.; Höpfl, R. Tolerability and efficacy of N-chlorotaurine compared to chloramine T for treatment of chronic leg ulcers with purulent coating. Br. J. Dermatol. 2003, 149, 590–597. [Google Scholar] [CrossRef]
- Neher, A.; Nagl, M.; Appenroth, E.; Gstöttner, M.; Wischatta, M.; Reisigl, F.; Schindler, M.; Ulmer, H.; Stephan, K. Acute Otitis Externa: Efficacy and Tolerability of N-Chlorotaurine, a Novel Endogenous Antiseptic Agent. Laryngoscope 2004, 114, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Mayer, D.; Bruhn, G.; Noack, B.; Brecx, M.; Heumann, C.; Toutenburg, H.; Netuschil, L.; Nagl, M.; Gottardi, W.; et al. Effect of N-chlorotaurine mouth rinses on plaque regrowth and plaque vitality. Clin. Oral Investig. 2008, 13, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Stojicic, S.; Shen, Y.; Haapasalo, M. Effect of the Source of Biofilm Bacteria, Level of Biofilm Maturation, and Type of Disinfecting Agent on the Susceptibility of Biofilm Bacteria to Antibacterial Agents. J. Endod. 2013, 39, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Devivilla, S.; Lekshmi, M.; Kumar, S.H.; Valappil, R.K.; Roy, S.D.; Nayak, B.B. Effect of Sodium Hypochlorite on Biofilm-Forming Ability of Histamine-Producing Bacteria Isolated from Fish. J. Food Prot. 2019, 82, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Chemical properties of N-chlorotaurine sodium, a key compound in the human defence system. Arch. Pharm. Pharm. Med. Chem. 2002, 335, 411–421. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Less is more, transferring a principle from art to science. Curr. Trends Microbiol. 2019, 13, 47–54. [Google Scholar]
- Kyriakopoulos, A.M.; Grapsa, E.; Marcinkiewicz, J.; Nagl, M. Swift Cure of a Chronic Wound Infected with Multiresistant Staphylococcus aureus in an Elderly Patient with Stage 5 Renal Disease. Int. J. Low. Extrem. Wounds 2019, 18, 192–196. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Nagl, M.; Orth-Höller, D.; Marcinkiewicz, J.; Baliou, S.; Zoumbourlis, V. Successful treatment of a unique chronic multi-bacterial scalp infection with N-chlorotaurine, N-bromotaurine and bromamine T. Access Microbiol. 2020, 2, e000126. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Traore, H.; El Manssouri, N.; Vanderbist, F.; Vaneechoutte, M.; Devleeschouwer, M.J. Evaluation of long-term co-administration of tobramycin and clarithromycin in a mature biofilm model of cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2009, 34, 370–374. [Google Scholar] [CrossRef][Green Version]
- Martini, C.; Hammerer-Lercher, A.; Zuck, M.; Jekle, A.; Debabov, D.; Anderson, M.; Nagl, M. Antimicrobial and anticoagulant activity of N-chlorotaurine (NCT), N,N-dichloro-2,2-dimethyltaurine (NVC-422) and N-monochloro-2,2-dimethyltaurine (NVC-612) in human blood. Antimicrob. Agents Chemother. 2012, 56, 1979–1984. [Google Scholar] [CrossRef][Green Version]
- Nagl, M.; Gruber, A.; Fuchs, A.; Lell, C.P.; Lemberger, E.-M.; Zepelin, M.B.-V.; Würzner, R. Impact of N-Chlorotaurine on Viability and Production of Secreted Aspartyl Proteinases of Candida spp. Antimicrob. Agents Chemother. 2002, 46, 1996–1999. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W.; Nagl, M. Chlorine covers on living bacteria: The initial step in antimicrobial action of active chlorine compounds. J. Antimicrob. Chemother. 2005, 55, 475–482. [Google Scholar] [CrossRef]
- Gruber, M.; Moser, I.; Nagl, M.; Lackner, M. Bactericidal and Fungicidal Activity of N-Chlorotaurine Is Enhanced in Cystic Fibrosis Sputum Medium. Antimicrob. Agents Chemother. 2017, 61, e02527-16. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M.; Binder, U.; Reindl, M.; Gönül, B.; Fankhauser, H.; Mair, C.; Nagl, M. N-Chlorotaurine Exhibits Fungicidal Activity against Therapy-Refractory Scedosporium Species and Lomentospora prolificans. Antimicrob. Agents Chemother. 2015, 59, 6454–6462. [Google Scholar] [CrossRef]
- Nagl, M.; Gottardi, W. Enhancement of the bactericidal efficacy of N-chlorotaurine by inflammation samples and selected N-H compounds. Hyg. Med. 1996, 21, 597–605. [Google Scholar]
- Grisham, M.B.; Jefferson, M.M.; Melton, D.F.; Thomas, E.L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem. 1984, 259, 10404–10413. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It Takes a Village”: Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef]
- Fourie, R.; Ells, R.; Swart, C.W.; Sebolai, O.M.; Albertyn, J.; Pohl, C.H. Candida albicans and Pseudomonas aeruginosa Interaction, with Focus on the Role of Eicosanoids. Front. Physiol. 2016, 7, 64. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.; Krysan, D.J. Candida–streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- Xu, H.; Jenkinson, H.F.; Dongaribagtzoglou, A. Innocent until proven guilty: Mechanisms and roles of Streptococcus–Candida interactions in oral health and disease. Mol. Oral Microbiol. 2014, 29, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Pham, D.T.N.; Tabassum, N.; Khan, M.S.A.; Kim, Y.-M. Mixed biofilms of pathogenic Candida-bacteria: Regulation mechanisms and treatment strategies. Crit. Rev. Microbiol. 2021, 1–29. [Google Scholar] [CrossRef]
- Eitzinger, C.; Ehrlenbach, S.; Lindner, H.; Kremser, L.; Gottardi, W.; Debabov, D.; Anderson, M.; Nagl, M.; Orth-Höller, D. N-Chlorotaurine, a Long-Lived Oxidant Produced by Human Leukocytes, Inactivates Shiga Toxin of Enterohemorrhagic Escherichia coli. PLoS ONE 2012, 7, e47105. [Google Scholar] [CrossRef]
- Jekle, A.; Yoon, J.; Zuck, M.; Najafi, R.; Wang, L.; Shiau, T.; Francavilla, C.; Rani, S.A.; Eitzinger, C.; Nagl, M.; et al. NVC-422 Inactivates Staphylococcus aureus Toxins. Antimicrob. Agents Chemother. 2012, 57, 924–929. [Google Scholar] [CrossRef]
- Reeves, E.P.; Nagl, M.; O’Keeffe, J.; Kelly, J.; Kavanagh, K. Effect of N-chlorotaurine on Aspergillus, with particular reference to destruction of secreted gliotoxin. J. Med Microbiol. 2006, 55, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Nagl, M.; Kavanagh, K. Exposure to N-chlorotaurine induces oxidative stress responses in Aspergillus fumigatus. J. Med. Microbiol. 2019, 68, 279–288. [Google Scholar] [CrossRef]
- Arnitz, R.; Sarg, B.; Ott, H.W.; Neher, A.; Lindner, H.; Nagl, M. Protein sites of attack of N-chlorotaurine in Escherichia coli. Proteomics 2006, 6, 865–869. [Google Scholar] [CrossRef] [PubMed]
- D’Lima, L.; Friedman, L.; Wang, L.; Xu, P.; Anderson, M.; Debabov, D. No Decrease in Susceptibility to NVC-422 in Multiple-Passage Studies with Methicillin-Resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli. Antimicrob. Agents Chemother. 2012, 56, 2753–2755. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).