Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples
2.2. Hemoglobin Solubility Buffers
2.3. Design and Operation of the Paper-Based Test
2.4. Blood Stain Pattern Interpretation
2.5. Test Performance and Statistical Analysis
3. Results
3.1. Limit of Detection
3.2. Test Readout Time
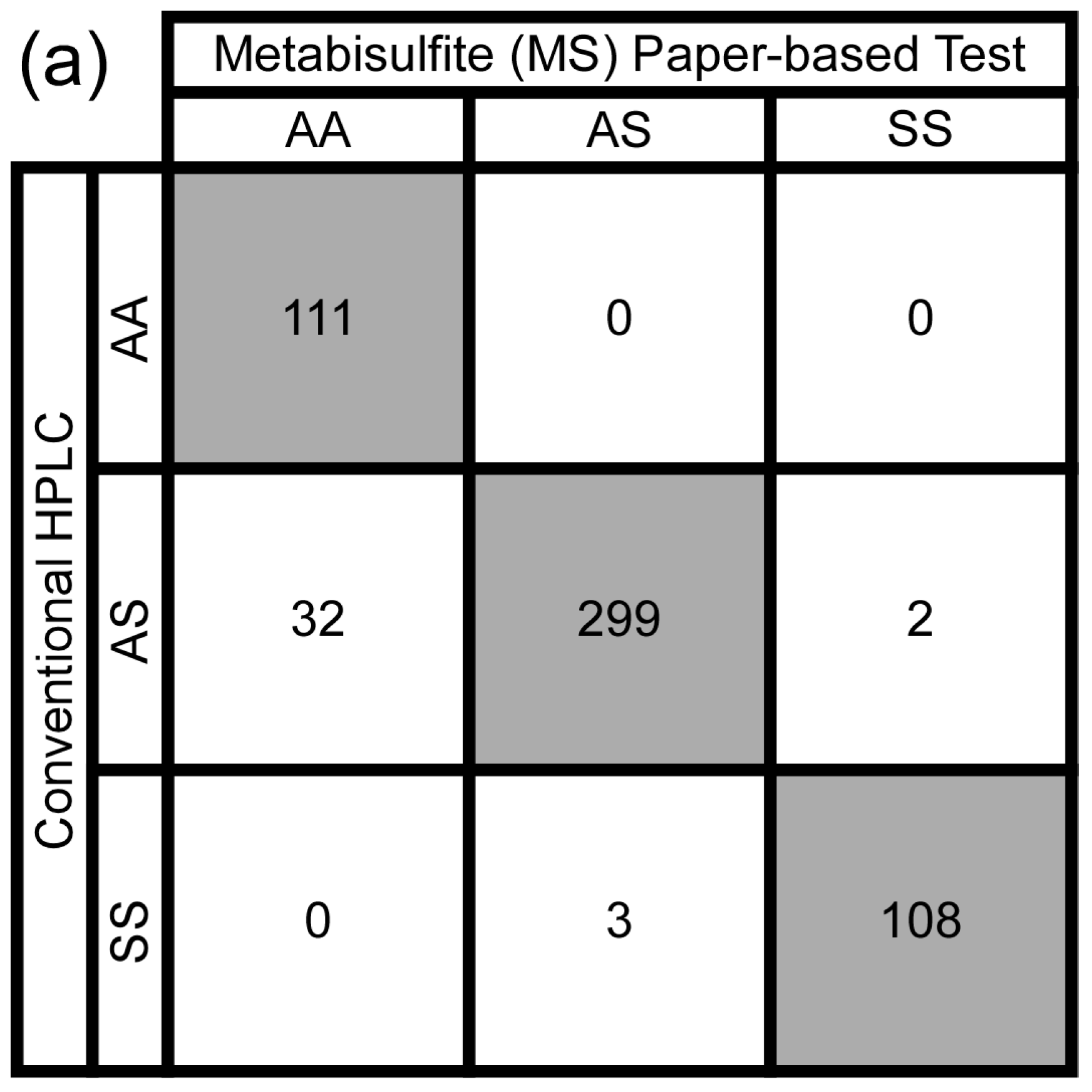
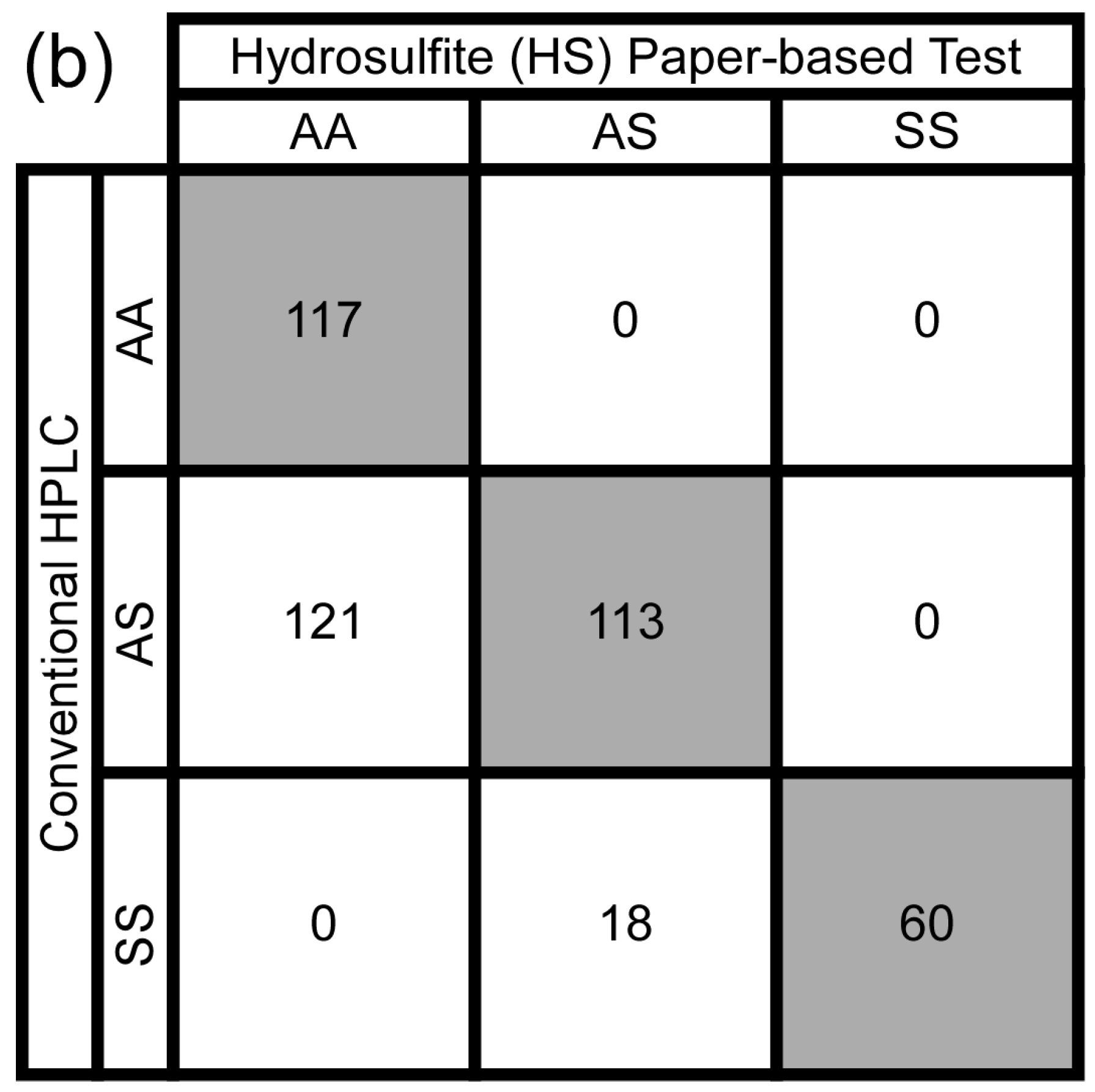
3.3. Test Performance Metrics
3.4. Intra- and Inter-Observer Agreement
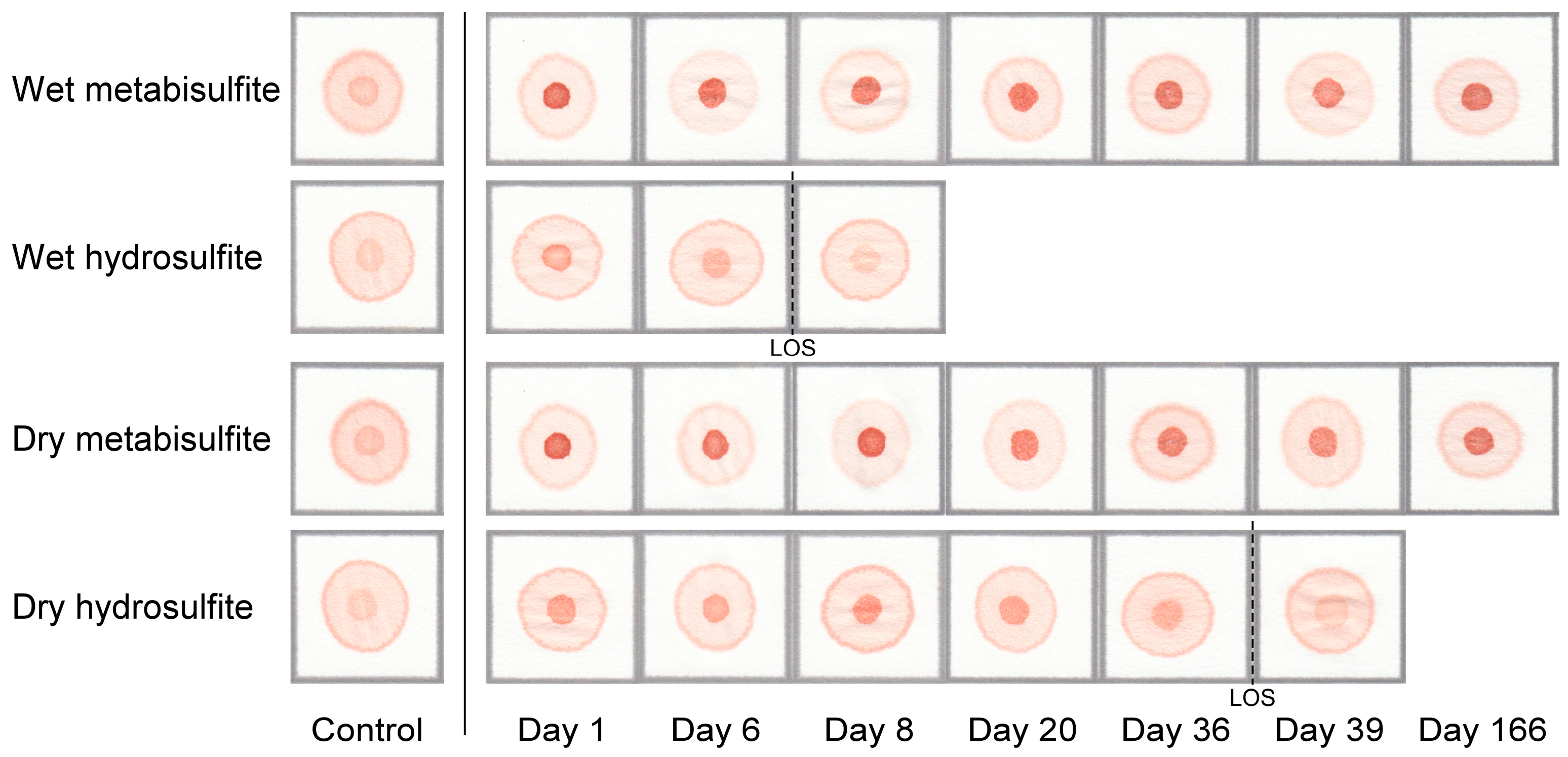
3.5. Test Kit Stability
3.6. Distributable Test Kit Cost
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Powars, D.R.; Chan, L.S.; Hiti, A.; Ramicone, E.; Johnson, C. Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine 2005, 84, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- McGann, P.T.; Ferris, M.G.; Ramamurthy, U.; Santos, B.; de Oliveira, V.; Bernardino, L.; Ware, R.E. A prospective newborn screening and treatment Program for Sickle Cell Anemia in Luanda, Angola. Am. J. Hematol. 2013, 88, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E. Is sickle cell anemia a neglected tropical disease? PLoS Negl. Trop. Dis. 2013, 7, e2120. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Hay, S.I.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global burden of sickle cell anaemia in children under five, 2010–2050: Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.; Hurst, D.; Earles, A.; Kleman, K.; Lubin, B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics 1988, 81, 749–755. [Google Scholar] [PubMed]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Makani, J.; Cox, S.E.; Soka, D.; Komba, A.N.; Oruo, J.; Mwamtemi, H.; Magesa, P.; Rwezaula, S.; Meda, E.; Mgaya, J.; et al. Mortality in sickle cell anemia in africa: A prospective cohort study in tanzania. PLoS ONE 2011, 6, e14699. [Google Scholar] [CrossRef] [PubMed]
- Head, C.E.; Conroy, M.; Jarvis, M.; Phelan, L.; Bain, B.J. Some observations on the measurement of haemoglobin a2 and s percentages by high performance liquid chromatography in the presence and absence of alpha thalassaemia. J. Clin. Pathol. 2004, 57, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.A.; Ratnaike, S. Capillary isoelectric focusing of haemoglobin variants in the clinical laboratory. Clin. Chim. Acta 1999, 289, 121–132. [Google Scholar] [CrossRef]
- Cronin, E.K.; Normand, C.; Henthorn, J.S.; Hickman, M.; Davies, S.C. Costing model for neonatal screening and diagnosis of haemoglobinopathies. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F161–167. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Tchernia, G. Sickle cell disease in africa. Curr. Opin. Hematol. 2002, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Montagu, D.; Yamey, G.; Visconti, A.; Harding, A.; Yoong, J. Where do poor women in developing countries give birth? A multi-country analysis of demographic and health survey data. PLoS ONE 2011, 6, e17155. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kanter, J.; Piety, N.Z.; Benton, M.S.; Vignes, S.M.; Shevkoplyas, S.S. A simple, rapid, low-cost diagnostic test for sickle cell disease. Lab Chip 2013, 13, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Piety, N.Z.; Yang, X.; Lezzar, D.; George, A.; Shevkoplyas, S.S. A rapid paper-based test for quantifying sickle hemoglobin in blood samples from patients with sickle cell disease. Am. J. Hematol. 2015, 90, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Piety, N.Z.; Yang, X.; Kanter, J.; Vignes, S.M.; George, A.; Shevkoplyas, S.S. Validation of a low-cost paper-based screening test for sickle cell anemia. PLoS ONE 2016, 11, e0144901. [Google Scholar] [CrossRef] [PubMed]
- Piety, N.Z.; George, A.; Serrano, S.; Lanzi, M.R.; Patel, P.R.; Noli, M.P.; Kahan, S.; Nirenberg, D.; Camanda, J.F.; Airewele, G.; et al. A paper-based test for screening newborns for sickle cell disease. Sci. Rep. 2017, 7, 45488. [Google Scholar] [CrossRef] [PubMed]
- Safety Data Sheet: Saponin, from Quillaja Bark (cas No.: 8047-15-2). Available online: http://www.sigmaaldrich.com/catalog/product/sigma/s2149?lang=en®ion=US (accessed on 19 September 2017).
- Fleiss, J.L. Measuring nominal scale agreement among Many Raters. Psychol. Bull. 1971, 76, 378. [Google Scholar] [CrossRef]
- Roila, F.; Lupattelli, M.; Sassi, M.; Basurto, C.; Bracarda, S.; Picciafuoco, M.; Boschetti, E.; Milella, G.; Ballatori, E.; Tonato, M.; et al. Intra and interobserver variability in cancer patients’ performance status assessed according to karnofsky and ecog scales. Ann. Oncol. 1991, 2, 437–439. [Google Scholar] [CrossRef] [PubMed]
- El Fadli, K.I.; Cerveny, R.S.; Burt, C.C.; Eden, P.; Parker, D.; Brunet, M.; Peterson, T.C.; Mordacchini, G.; Pelino, V.; Bessemoulin, P.; et al. World meteorological organization assessment of the purported world record 58 °C temperature extreme at el azizia, libya (13 September 1922). Bull. Am. Meteorol. Soc. 2013, 94, 199–204. [Google Scholar] [CrossRef]
- Kanter, J.; Telen, M.J.; Hoppe, C.; Roberts, C.L.; Kim, J.S.; Yang, X. Validation of a novel point of care testing device for sickle cell disease. BMC Med. 2015, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.T.; Schaefer, B.A.; Paniagua, M.; Howard, T.A.; Ware, R.E. Characteristics of a rapid, point-of-care lateral flow immunoassay for the diagnosis of sickle cell disease. Am. J. Hematol. 2016, 91, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Patton, M.R.; Hennek, J.W.; Lee, S.Y.; D’Alesio-Spina, G.; Yang, X.; Kanter, J.; Shevkoplyas, S.S.; Brugnara, C.; Whitesides, G.M. Density-based separation in multiphase systems provides a simple method to identify sickle cell disease. Proc. Natl. Acad. Sci. USA 2014, 111, 14864–14869. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.M.; Sencan, I.; Aytar, Y.; Khoory, J.; Heeney, M.M.; Ghiran, I.C.; Tasoglu, S. Sickle cell detection using a smartphone. Sci. Rep. 2015, 5, 15022. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Chunda-Liyoka, C.; Hennek, J.W.; Mantina, H.; Lee, S.Y.; Patton, M.R.; Sambo, P.; Sinyangwe, S.; Kankasa, C.; Chintu, C.; et al. Evaluation of a density-based rapid diagnostic test for sickle cell disease in a clinical setting in zambia. PLoS ONE 2014, 9, e114540. [Google Scholar] [CrossRef] [PubMed]
- Screening Information Dataset: Initial Assessment Report for Sodium Dithionite (cas No.: 7775-14-6). Available online: http://www.inchem.org/documents/sids/sids/7775146.pdf (accessed on 19 September 2017).
- Asakura, T.; Mayberry, J. Relationship between morphologic characteristics of sickle cells and method of deoxygenation. J. Lab. Clin. Med. 1984, 104, 987–994. [Google Scholar] [PubMed]
- Noguchi, C.T.; Schechter, A.N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood 1981, 58, 1057–1068. [Google Scholar] [PubMed]
- Horiuchi, K.; Ballas, S.K.; Asakura, T. The effect of deoxygenation rate on the formation of irreversibly sickled cells. Blood 1988, 71, 46–51. [Google Scholar] [PubMed]
- Nagel, R.L.; Fabry, M.E.; Steinberg, M.H. The paradox of hemoglobin sc disease. Blood Rev. 2003, 17, 167–178. [Google Scholar] [CrossRef]
- Ryan, K.; Bain, B.J.; Worthington, D.; James, J.; Plews, D.; Mason, A.; Roper, D.; Rees, D.C.; de la Salle, B.; Streetly, A.; et al. Significant haemoglobinopathies: Guidelines for screening and diagnosis. Br. J. Haematol. 2010, 149, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Edoh, D.; Antwi-Bosaiko, C.; Amuzu, D. Fetal hemoglobin during infancy and in sickle cell adults. Afr. Health Sci. 2006, 6, 51–54. [Google Scholar] [PubMed]
- Shevkoplyas, S.S.; Yang, X.; Washko, J.K.; Piety, N.Z. Paper Based Diagnostic Test. United States Patent US 15/215,107. Available online: https://www.google.com/patents/US20170023556 (accessed on 19 September 2017).

| Test Component | Cost Per Test (USD) |
|---|---|
| Foil pouch | $0.02 |
| Reagent dropper | $0.05 |
| Blood dropper | $0.08 |
| Chromatography paper | $0.01 |
| Reagent tube | $0.03 |
| Food grade reagents | $0.02 |
| Total | $0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabian, K.; Lezzar, D.; Piety, N.Z.; George, A.; Shevkoplyas, S.S. Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia. Biosensors 2017, 7, 39. https://doi.org/10.3390/bios7030039
Torabian K, Lezzar D, Piety NZ, George A, Shevkoplyas SS. Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia. Biosensors. 2017; 7(3):39. https://doi.org/10.3390/bios7030039
Chicago/Turabian StyleTorabian, Kian, Dalia Lezzar, Nathaniel Z. Piety, Alex George, and Sergey S. Shevkoplyas. 2017. "Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia" Biosensors 7, no. 3: 39. https://doi.org/10.3390/bios7030039
APA StyleTorabian, K., Lezzar, D., Piety, N. Z., George, A., & Shevkoplyas, S. S. (2017). Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia. Biosensors, 7(3), 39. https://doi.org/10.3390/bios7030039







