Fumonisin B Determination in Maize Products from Belize Using an Immunosensor Based on Screen-Printed Carbon Electrodes
Abstract
1. Introduction
| Fumonisin | Approach | Electrode | Linear Range (µg/L) | LOD (µg/L) | Ref. |
|---|---|---|---|---|---|
| FB1 | Aptasensor | AuNPs/GS-TH/GCE | 0.001–1000 | 0.001 | [37] |
| FB1 | Aptasensor | AuNPs/SPCE | 0.5–500 | 0.14 | [39] |
| FB1 | Aptasensor | AuNPs/PDMS/SPCE | 0.01–50 | 0.0034 | [57] |
| FB1 | Aptasensor | rMoS2-Au/GCE | 0.001–100 | 0.0005 | [58] |
| FB1 | Aptasensor | PGE | 10–40 | 3.69 | [59] |
| FB1 | Aptasensor | AuE | 0.001–100 | 0.00026 | [60] |
| FB1 | Immunosensor | AuNPs/PPy-ErGO/SPCE | 200–4500 | 4.2 | [38] |
| FB1 | Immunosensor | SWCNT/CS/GCE | 0.01–1000 | 0.002 | [61] |
| FB1, FB2 | Immunosensor | SPGE | 10–1000 | 5 | [62] |
| FB1, FB2 | Immunosensor | MBs/SPCE | 0.73–11.2 | 0.33 | [63] |
| FB1 | Immunosensor | AuNPs/ITO | 0.3–140 | 0.097 | [64] |
| FB1 | Immunosensor | SPCE | 0.25–50 | 0.14 | This work |
| FB1 + FB2 | 0.25–50 | 0.15 | |||
| FB1 + FB2 + FB3 | 0.25–10 | 0.12 |
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Blank Maize and Tortilla Samples
2.2.2. Preparation of Fortified Maize Samples
2.2.3. Extraction of Fumonisins
2.2.4. Competitive Immunoassay for Fumonisin (FB) Detection
2.2.5. Electrochemical Detection
3. Results and Discussion
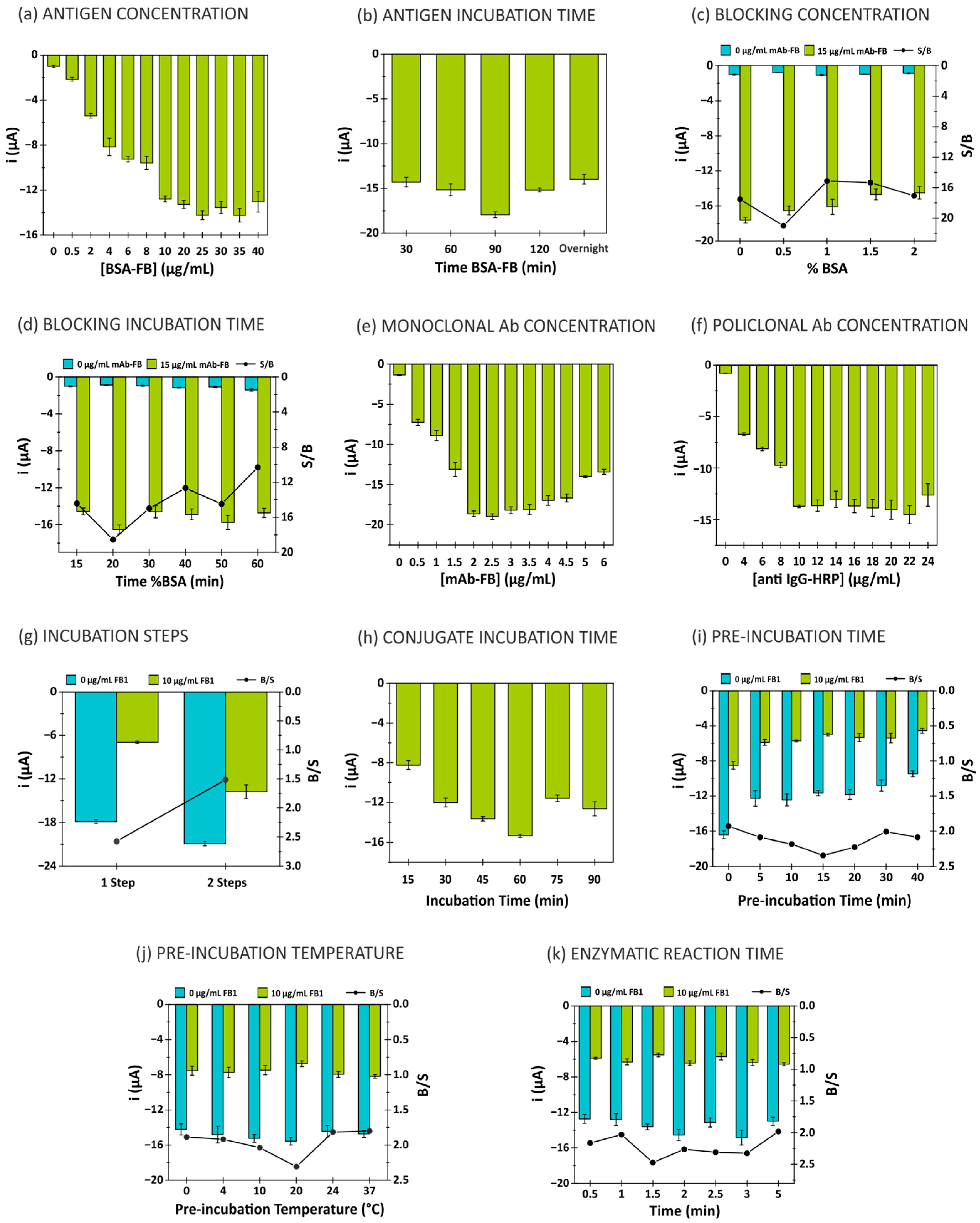
3.1. Evaluation and Adjustment of Key Parameters in the Development of a Fumonisin Immunosensor
3.1.1. Evaluation of Antigen Conditions (BSA-FB)
3.1.2. Evaluation of Blocking Agent Conditions (BSA)
3.1.3. Evaluation of Monoclonal Antibody Concentration (mAb-FB)
3.1.4. Evaluation of Polyclonal Antibody Concentration (Anti-IgG-HRP)
3.1.5. Evaluation of Immunoreaction
3.1.6. Evaluation of Enzymatic Reaction
3.2. Fumonisin Determination: Analytical Performance Evaluation of the Immunosensor
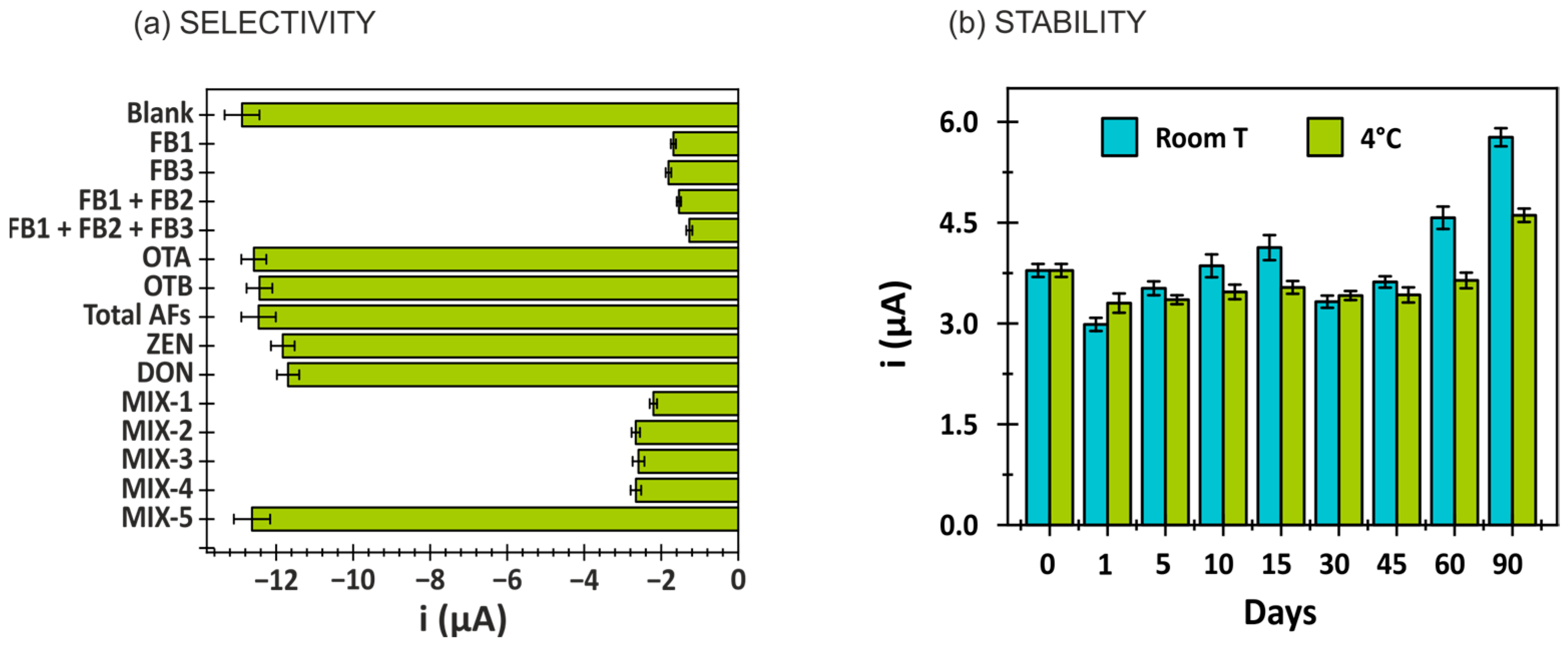
3.3. Selectivity and Stability of the Immunosensor
3.4. Method Validation for Analysis of Fumonisins in Maize Products by LC-MS/MS
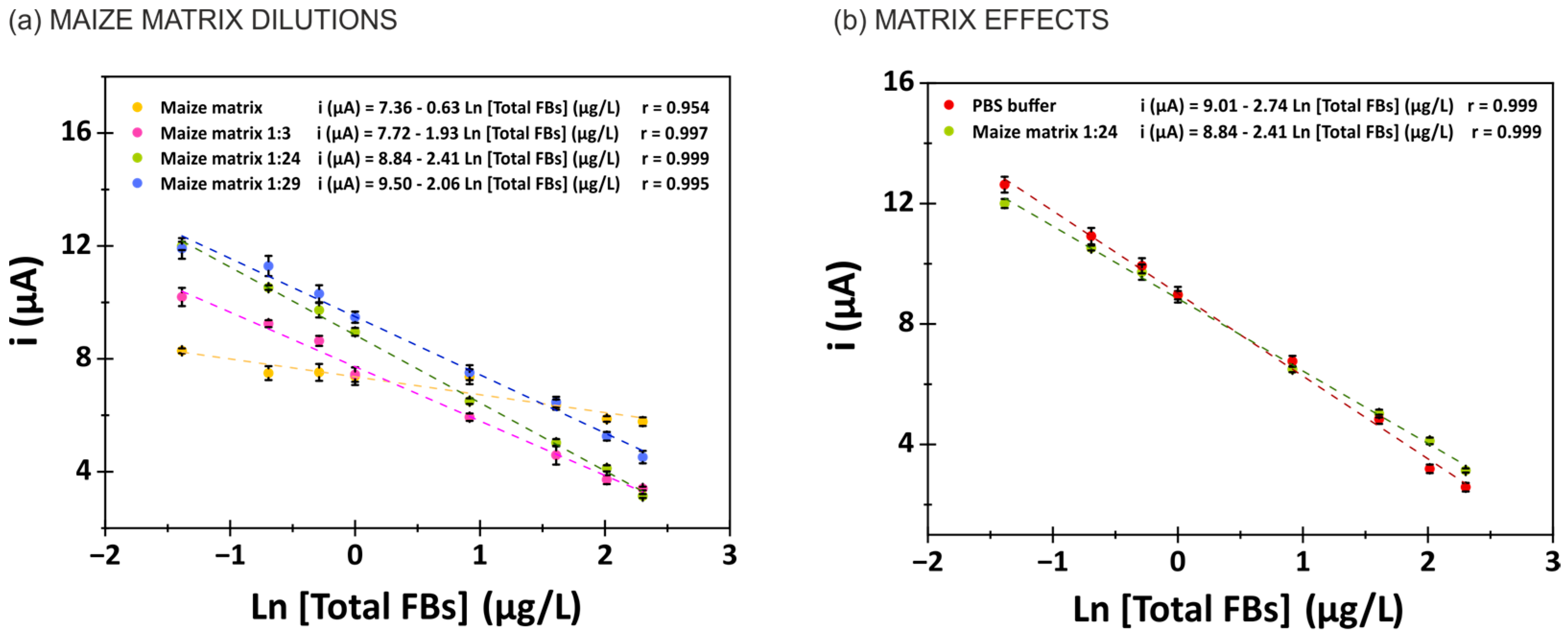
3.5. Evaluation of Matrix Effects in Maize Samples and Naturally Contaminated Tortilla Samples
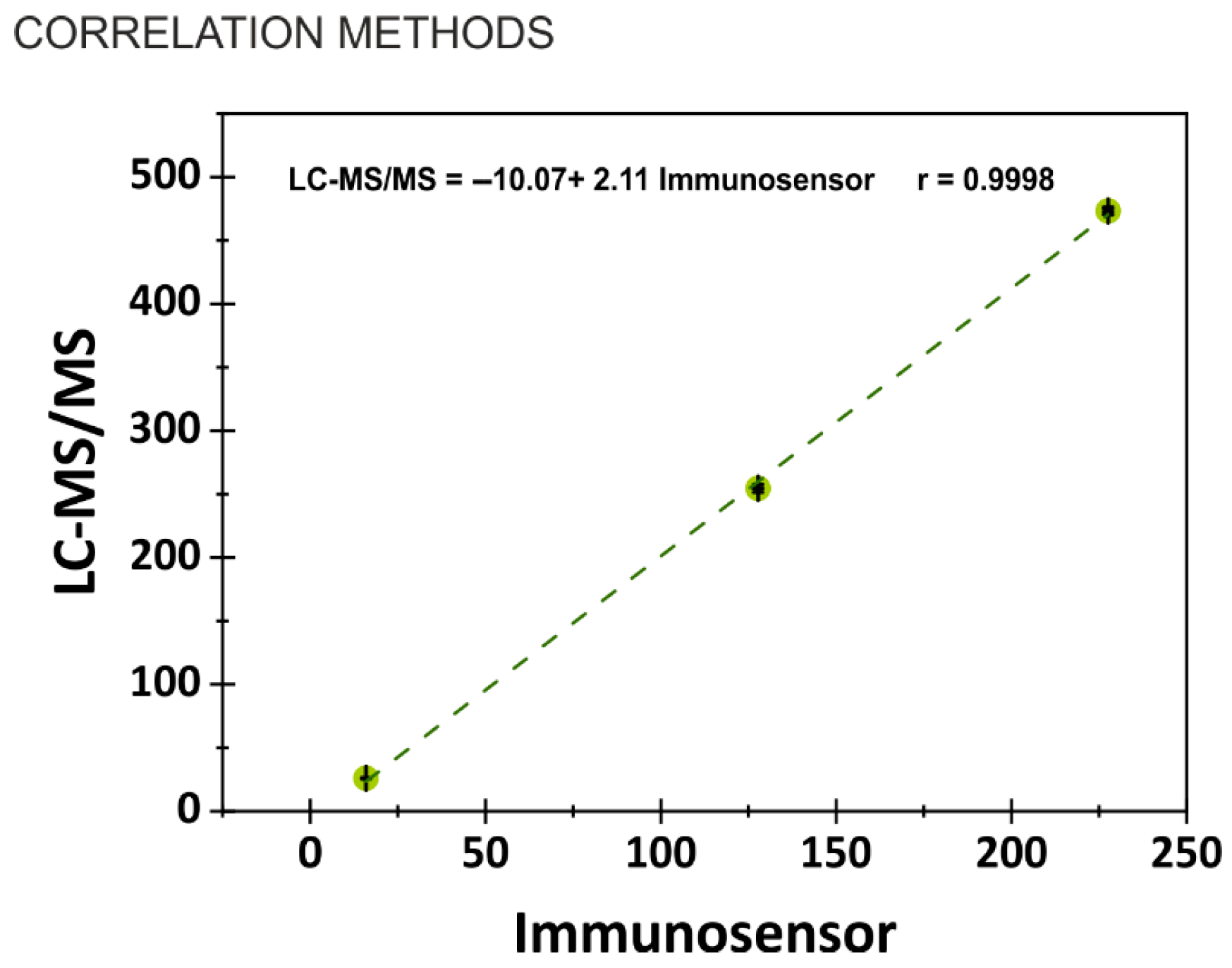
3.6. Cross-Validation of Immunosensor Performance with LC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPCE | Screen-Printed Carbon Electrode |
| FB | Fumonisin |
| IARC | International Agency for Research on Cancer |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectrometry |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| TLC | Thin-Layer Chromatography |
| ELISA | Enzyme-Linked ImmunoSorbent Assays |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| ZEN | Zearalenone |
| OTA | Ochratoxin A |
| OTB | Ochratoxin B |
| DON | Deoxynivalenol |
| AF | Aflatoxins |
| BSA | Bovine Serum Albumin |
| TMB | 3,3′-5,5′-Tetramethylbezidine |
| PBS | Phosphate-Buffered Saline solution |
| BSA-FB | Fumonisin antigen conjugate |
| mAb-FB | Mouse monoclonal antibody specific to Fumonisin |
| Anti-IgG-HRP | Polyclonal rabbit anti-mouse IgG-HRP |
| HRP | Horse Radish Peroxidase |
| ESI | Electrospray ionization |
| MRM | Multiple Reaction Monitoring |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| SB | Standard Deviation |
| RSD | Relative Standard Deviation |
| ME | Matrix Effect |
References
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Jaskiewicz, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P.J. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, e05172. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Improving Public Health Through Mycotoxin Control. IARC Scientific Publication No. 158. Food Chem. Toxicol. 2012, 51, 188–193. [Google Scholar]
- Jackson, L.; Jablonski, J. 16-Fumonisins, in: Mycotoxins in Food Detection and Control. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 367–405. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Mallmann, C.A.; Santurio, J.M.; Dilkin, P. Equine leukoencephalomalacia associated with ingestion of corn contaminated with fumonisin B1. Rev. Microbiol. 1999, 30, 249–252. [Google Scholar] [CrossRef]
- Martins, H.M.L.; Almeida, I.F.M.; Camacho, C.R.L.; Santos, S.M.O.; Costa, J.M.G.; Bernardo, F.M.A. Occurrence of fumonisins in feed for swine and horses. Rev. Iberoam. Micol. 2012, 29, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.F.; Nelson, P.E.; Richard, J.L.; Osweiler, G.D.; Rice, L.G.; Plattner, R.D.; Wilson, T.M. Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl. Environ. Microbiol. 1990, 56, 3225–3226. [Google Scholar] [CrossRef]
- Dutton, M.F. Fumonisins, mycotoxins of increasing importance: Their nature and their effects. Pharmacol. Ther. 1996, 70, 137–161. [Google Scholar] [CrossRef]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EU). 2023/915 of 25 April 2023, on maximum levenls for certain contaminants in food and repealing Regulation (EC) No 1881/2006 (Text with EEA relevance). Off. J. Eur. Union 2023, L119, 103–157. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Faridah, A.; Khatib, A. A UPLC-MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control 2012, 25, 647–653. [Google Scholar] [CrossRef]
- Ocampo-Acuña, Y.D.; Salazar-Rios, E.; Ramírez-Cisneros, M.Á.; Rios, M.Y. Comprehensive review of liquid chromatography methods for fumonisin determination, a 2006–2022 update. Arab. J. Chem. 2023, 16, 104716. [Google Scholar] [CrossRef]
- Ferreira, I.; Fernandes, J.O.; Cunha, S.C. Optimization and validation of a method based in a QuEChERS procedure and gas chromatography-mass spectrometry for the determination of multi-mycotoxins in popcorn. Food Control 2012, 27, 188–193. [Google Scholar] [CrossRef]
- Jiménez, M.; Mateo, R. Determination of mycotoxins produced by Fusarium isolates from banana fruits by capillary gas chromatography and high-performance liquid chromatography. J. Chromatogr. A 1997, 778, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Vrabcheva, T.; Stroka, J.; Anklam, E. Occurrence of fumonisin B1 in Bulgarian maize samples determined by ELISA and TLC methods using different clean up steps. Mycotoxin Res. 2002, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, W.; De Saeger, S.; Shen, J.; Zhang, S.; Wang, Z. Development of a sensitive enzyme-linked immunosorbent assay for the detection of fumonisin B1 in maize. Toxicon 2012, 60, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Xu, Y.; Li, Y.; He, Q.; Chen, B.; Wang, D. Development of a single-chain variable fragment antibody-based enzyme-linked immunosorbent assay for determination of fumonisin B1 in corn samples. J. Sci. Food Agric. 2014, 94, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kalisa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.E.; Gobi, K.V.; Kim, K.H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; de la Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef]
- Reverté, L.; Prieto-Simón, B.; Campàs, M. New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal. Chim. Acta 2016, 908, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Gong, Y.Y.; Goycoolea, F.M. Aptamer-based detection of fumonisin B1: A critical review. Anal. Chim. Acta 2021, 1160, 338395. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; An, K.; Huang, X.; Zhao, L.; Liu, Q.; Hao, N.; Wang, K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens. Bioelectron. 2017, 89, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Electrochemical immuno-and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef]
- Oswald, S.; Karsunke, X.Y.Z.; Dietrich, R.; Märtlbauer, E.; Niessner, R.; Knopp, D. Automated regenerable microarray-based immunoassay for rapid parallel quantification of mycotoxins in cereals. Anal. Bioanal. Chem. 2013, 405, 6405–6415. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
- Ren, W.; Xu, Y.; Huang, Z.; Li, Y.; Tu, Z.; Zou, L.; He, Q.; Fu, J.; Liu, S.; Hammock, B.D. Single-chain variable fragment antibody-based immunochromatographic strip for rapid detection of fumonisin B1 in maize samples. Food Chem. 2020, 319, 126546. [Google Scholar] [CrossRef]
- Zha, C.; An, X.; Zhang, J.; Wei, L.; Zhang, Q.; Yang, Q.; Li, F.; Sun, X.; Guo, Y. Indirect signal amplification strategy with a universal probe-based lateral flow immunoassay for the rapid quantitative detection of fumonisin B1. Anal. Methods 2022, 14, 708–716. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Mao, L.; Ji, K.; Yao, L.; Xue, X.; Wen, W.; Zhang, X.; Wang, S. Molecularly imprinted photoelectrochemical sensor for fumonisin B 1 based on GO-CdS heterojunction. Biosens. Bioelectron. 2019, 127, 57–63. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zheng, Y.T.; Zhang, H.B.; He, C.H.; Wu, W.D.; Zhang, H. Bin DNA Electrochemical Aptasensor for Detecting Fumonisins B1 Based on Graphene and Thionine Nanocomposite. Electroanalysis 2015, 27, 1097–1103. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.C.; Yu, J.H.; Gunasekaran, S. An Electrochemical Immunosensor for Rapid and Sensitive Detection of Mycotoxins Fumonisin B1 and Deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Naghshbandi, B.; Adabi, M.; Pooshang Bagheri, K.; Tavakolipour, H. Design of a new electrochemical aptasensor based on screen printed carbon electrode modified with gold nanoparticles for the detection of fumonisin B1 in maize flour. J. Nanobiotechnology 2022, 20, 534. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- García-González, R.; Fernández-Abedul, M.T.; Pernía, A.; Costa-García, A. Electrochemical characterization of different screen-printed gold electrodes. Electrochim. Acta 2008, 53, 3242–3249. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Mercader, J.V.; Checa-Orrego, B.I.; de la Escosura-Muñiz, A.; Costa-García, A. A monoclonal antibody-based immunosensor for the electrochemical detection of imidacloprid pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Mercader, J.V.; Abad-fuentes, A.; Checa-Orrego, B.I.; Costa-García, A.; de la Escosura-Muñiz, A. Direct competitive immunosensor for Imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 2020, 209, 120465. [Google Scholar] [CrossRef] [PubMed]
- Omrani, N.M.; Hayat, A.; Korri-youssou, H.; Marty, J.L. Electrochemical Biosensors for Food Security: Mycotoxins Detection in Biosensors for Security and Bioterrorism Applications. In Biosensors for Security and Bioterrorism Applications; Springer International Publishing: Cham, Switzerland, 2016; pp. 469–490. ISBN 978-3-319-28924-3. [Google Scholar]
- Pérez-Fernández, B.; Maestroni, B.M.; Nakaya, S.; Bussalino, S.; Vlachou, C.; de la Escosura-Muñiz, A. Development, optimization and validation of an electrochemical immunosensor for determination of total aflatoxins in pistachio. Food Control 2023, 152, 109859. [Google Scholar] [CrossRef]
- Pemberton, R.M.; Pittson, R.; Biddle, N.; Drago, G.A.; Hart, J.P. Studies towards the development of a screen-printed carbon electrochemical immunosensor array for mycotoxins: A sensor for aflatoxin B1. Anal. Lett. 2006, 39, 1573–1586. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Duato, P.; Castillo, J.R. Improved electrochemical competitive immunosensor for ochratoxin A with a biotinylated monoclonal antibody capture probe and colloidal gold nanostructuring. Anal. Methods 2011, 3, 977–984. [Google Scholar] [CrossRef]
- Hsieh, M.-K.; Chen, H.; Chang, J.-L.; She, W.-S.; Chou, C.-C. Electrochemical Detection of Zeranol and Zearalenone Metabolic Analogs in Meats and Grains by Screen-Plated Carbon-Plated Disposable Electrodes. Food Nutr. Sci. 2013, 04, 31–38. [Google Scholar] [CrossRef]
- Ammida, N.H.S.; Micheli, L.; Piermarini, S.; Moscone, D.; Palleschi, G. Detection of aflatoxin B1 in barley: Comparative study of immunosensor and HPLC. Anal. Lett. 2006, 39, 1559–1572. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. Electroanalytical sensing of chromium(iii) and (vi) utilising gold screen printed macro electrodes. Analyst 2012, 137, 896–902. [Google Scholar] [CrossRef]
- Rubino, A.; Queirós, R. Electrochemical determination of heavy metal ions applying screen-printed electrodes based sensors. A review on water and environmental samples analysis. Talanta Open 2023, 7, 100203. [Google Scholar] [CrossRef]
- Chen, C.; Niu, X.; Chai, Y.; Zhao, H.; Lan, M. Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry. Sens. Actuators B Chem. 2013, 178, 339–342. [Google Scholar] [CrossRef]
- Fernández, E.; Vidal, L.; Martín-Yerga, D.; Blanco, M.D.C.; Canals, A.; Costa-García, A. Screen-printed electrode based electrochemical detector coupled with ionic liquid dispersive liquid-liquid microextraction and microvolume back-extraction for determination of mercury in water samples. Talanta 2015, 135, 34–40. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Crew, A.; Northall, H.; Radbourne, S.; Davies, O.; Newman, S.; Hart, J.P. The redox behaviour of diazepam (Valium®) using a disposable screen-printed sensor and its determination in drinks using a novel adsorptive stripping voltammetric assay. Talanta 2013, 116, 300–307. [Google Scholar] [CrossRef]
- Masawat, P.; Slater, J.M. The determination of tetracycline residues in food using a disposable screen-printed gold electrode (SPGE). Sens. Actuators B Chem. 2007, 124, 127–132. [Google Scholar] [CrossRef]
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent electrochemical sensors and biosensors for toxic agents based on screen-printed electrodes equipped with nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addit. Contam. Part A 2009, 26, 507–511. [Google Scholar] [CrossRef]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural mycotoxin contamination of maize (Zea mays L.) in the South region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: London, UK, 2010. [Google Scholar]
- Ren, C.; Li, H.; Lu, X.; Qian, J.; Zhu, M.; Chen, W.; Liu, Q.; Hao, N.; Li, H.; Wang, K. A disposable aptasensing device for label-free detection of fumonisin B1 by integrating PDMS film-based micro-cell and screen-printed carbon electrode. Sens. Actuators B Chem. 2017, 251, 192–199. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Jiang, K.; Huang, Q.; Meng, J.; Nie, D.; Zhao, Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and Au NPs (rMoS2-Au) for multiplex detection of mycotoxins. Biosens. Bioelectron. 2020, 150, 111894. [Google Scholar] [CrossRef]
- Kesici, E.; Erdem, A. Impedimetric detection of Fumonisin B1 and its biointeraction with fsDNA. Int. J. Biol. Macromol. 2019, 139, 1117–1122. [Google Scholar] [CrossRef]
- Wei, M.; Xin, L.; Feng, S.; Liu, Y. Simultaneous electrochemical determination of ochratoxin A and fumonisin B1 with an aptasensor based on the use of a Y-shaped DNA structure on gold nanorods. Microchim. Acta 2020, 187, 102. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A Highly Sensitive Electrochemical Immunosensor for Fumonisin B1 Detection in Corn Using Single-Walled Carbon Nanotubes/Chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Kadir, M.K.A.; Tothill, I.E. Development of an electrochemical immunosensor for fumonisins detection in foods. Toxins 2010, 2, 382–398. [Google Scholar] [CrossRef]
- Jodra, A.; López, M.Á.; Escarpa, A. Disposable and reliable electrochemical magnetoimmunosensor for Fumonisins simplified determination in maize-based foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638. [Google Scholar] [CrossRef]
- Lu, L.; Gunasekaran, S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 2019, 194, 709–716. [Google Scholar] [CrossRef]
- Maestroni, B.; Abu Alnaser, A.; Ghanem, I.; Islam, M.; Cesio, V.; Heinzen, H.; Kelly, S.; Cannavan, A. Validation of an Analytical Method for the Determination of Pesticide Residues in Vine Leaves by GC-MS/MS. J. Agric. Food Chem. 2018, 66, 6421–6430. [Google Scholar] [CrossRef]
- FAO. Guidelines on Performance Criteria for Methods of Analysis for the Determination of Pesticide Residues in Food and Feed; Codex Alimentarius; FAO: Rome, Italy, 2017; pp. 1–13. [Google Scholar]
- Del Mar Gómez-Ramos, M.; Rajski, Ł.; Lozano, A.; Fernández-Alba, A.R. The evaluation of matrix effects in pesticide multi-residue methods: Via matrix fingerprinting using liquid chromatography electrospray high-resolution mass spectrometry. Anal. Methods 2016, 8, 4664–4673. [Google Scholar] [CrossRef]
- Maestroni, B.; Besil, N.; Rezende, S.; Liang, Y.; Gerez, N.; Karunarathna, N.; Islam, M.; Heinzen, H.; Cannavan, A.; Cesio, M.V. Method optimization and validation for multi-class residue analysis in turmeric. Food Control 2021, 121, 107579. [Google Scholar] [CrossRef]
- European Commission Commission implementing regulation (EU) 2023/2782 of 14 December 2023 laying down the methods of sampling and analysis for the control of the levels of mycotoxins in food and repealing Regulation (EC) No 401/2006. Off. J. Eur. Union 2023, 2782, 1–44.
- SANTE/12682/2019 Guidance Document on Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed; Safe Food Chain: Pesticides and Biocides–European Commission: Brussels, Belgium, 2019; pp. 1–48.


| Sample | Fortification Level of Total FBs (µg/kg) | Nominal Concentration a (µg/L) | Current Matrix b (µA) | Current in the Sample Extract (µA) | Measured Concentration (µg/L) ± CV % | Recovery |
|---|---|---|---|---|---|---|
| Maize | 30 | 15 | −10.20 ± 0.10 | −10.26 ± 0.08 | 14.26 ± 4% | 95% |
| 300 | 150 | −4.47 ± 0.10 | −4.54 ± 0.07 | 154.33 ± 4% | 103% | |
| 600 | 300 | −2.84 ± 0.07 | −2.95 ± 0.04 | 302.95 ± 3% | 101% |
| Tortilla Sample | LC-MS/MS (Validated Method) (µg/kg) | Electrochemical Immunosensor (µg/kg) | Relative Error a |
|---|---|---|---|
| S-4 | 84.0 | 79.5 | −5.4% |
| S-295 | 180.4 | 174.2 | −3.4% |
| S-296 | 210.3 | 196.3 | −6.7% |
| S-306 | 119.9 | 123.1 | +2.7% |
| S-311 | 114.6 | 118.0 | +3.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Fernández, B.; Maestroni, B.M.; Cozzani, C.; Eusey, C.; Gibson, N.; de la Escosura-Muñiz, A.; Vlachou, C. Fumonisin B Determination in Maize Products from Belize Using an Immunosensor Based on Screen-Printed Carbon Electrodes. Biosensors 2025, 15, 526. https://doi.org/10.3390/bios15080526
Pérez-Fernández B, Maestroni BM, Cozzani C, Eusey C, Gibson N, de la Escosura-Muñiz A, Vlachou C. Fumonisin B Determination in Maize Products from Belize Using an Immunosensor Based on Screen-Printed Carbon Electrodes. Biosensors. 2025; 15(8):526. https://doi.org/10.3390/bios15080526
Chicago/Turabian StylePérez-Fernández, Beatriz, Britt Marianna Maestroni, Carlotta Cozzani, Colette Eusey, Natalie Gibson, Alfredo de la Escosura-Muñiz, and Christina Vlachou. 2025. "Fumonisin B Determination in Maize Products from Belize Using an Immunosensor Based on Screen-Printed Carbon Electrodes" Biosensors 15, no. 8: 526. https://doi.org/10.3390/bios15080526
APA StylePérez-Fernández, B., Maestroni, B. M., Cozzani, C., Eusey, C., Gibson, N., de la Escosura-Muñiz, A., & Vlachou, C. (2025). Fumonisin B Determination in Maize Products from Belize Using an Immunosensor Based on Screen-Printed Carbon Electrodes. Biosensors, 15(8), 526. https://doi.org/10.3390/bios15080526






