Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection
Abstract
1. Introduction
2. ZnO Nanomaterial Biosensors Based on Different Optical Detection Modes
2.1. Absorption and Colorimetry
2.1.1. Overview of Absorption and Colorimetry in Biodetection
2.1.2. Contributions of ZnO Nanomaterials in Absorption- and Colorimetry-Based Biosensors
2.1.3. Applications of 0D ZnO Nanomaterials in Absorption and Colorimetry Biosensors
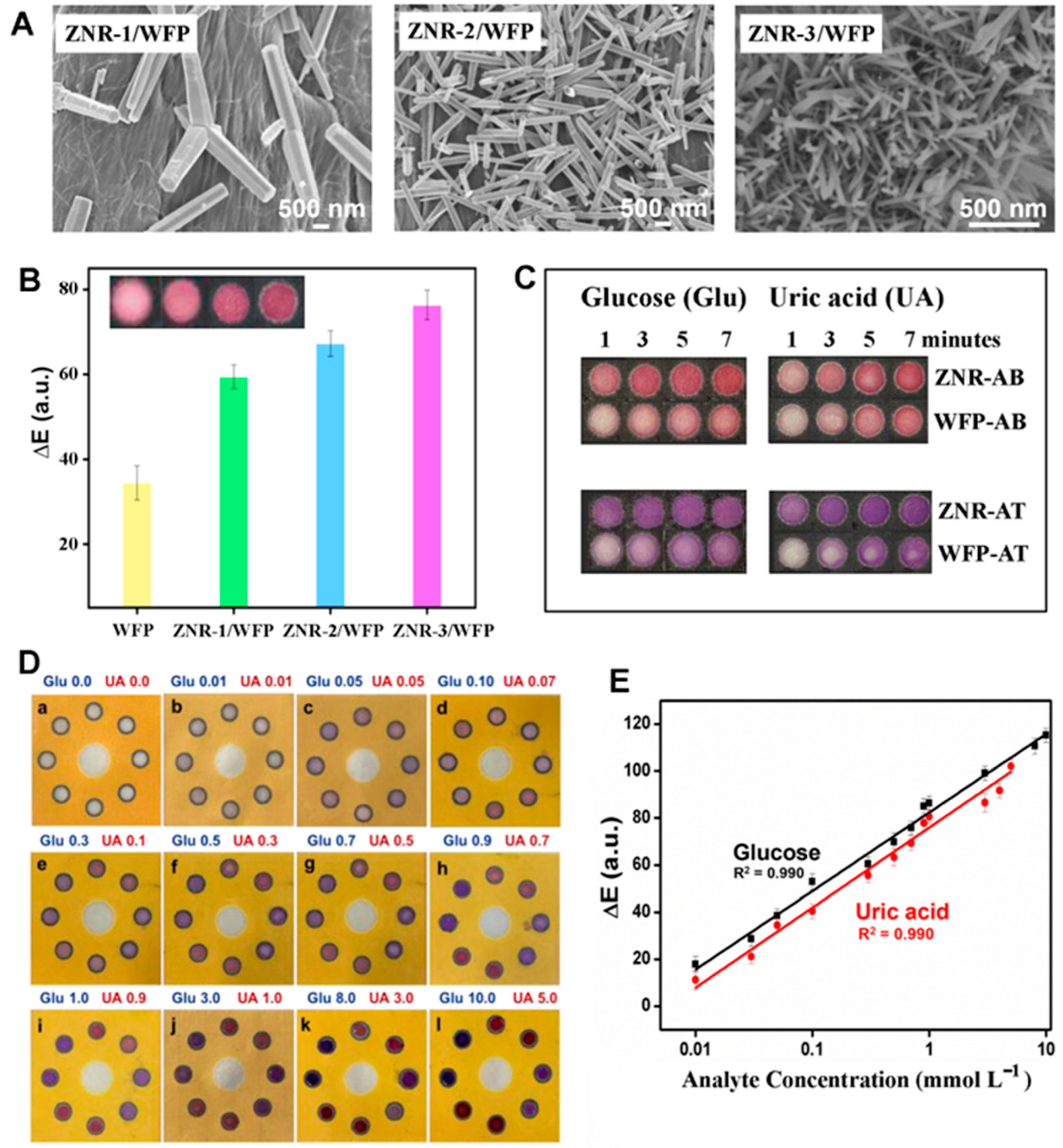
2.1.4. Applications of 1D ZnO Nanomaterials in Absorption and Colorimetry Biosensors
2.2. Fluorescence
2.2.1. Overview of Fluorescence in Biodetection
2.2.2. Contributions of ZnO Nanomaterials in Fluorescence-Based Biodetection
2.2.2.1. Quantum Dot Fluorescence Probes
2.2.2.2. Fluorescence Signal-Enhancing Platforms
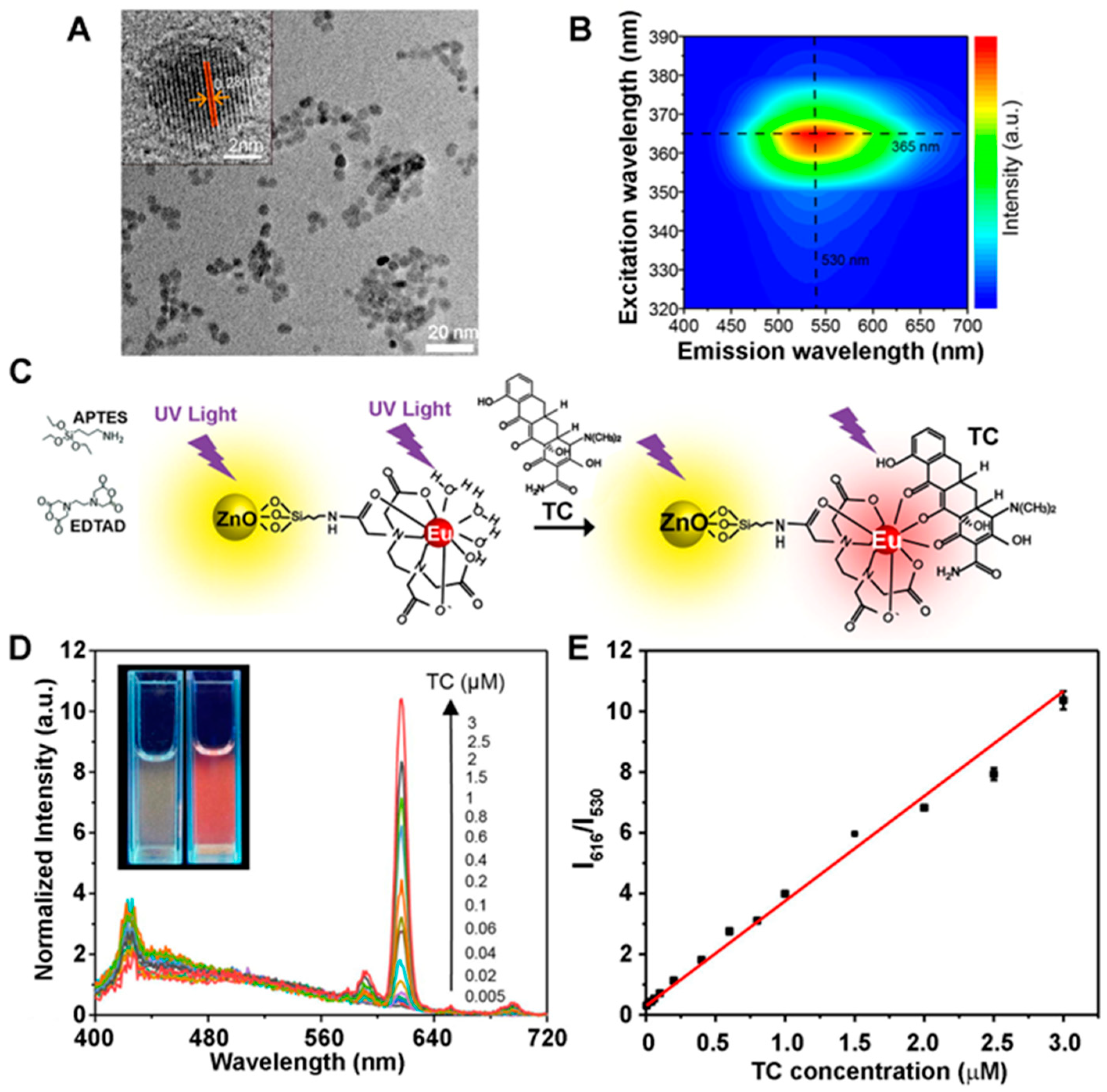
2.2.3. Applications of 0D ZnO Nanomaterials in Fluorescence-Based Biodetection
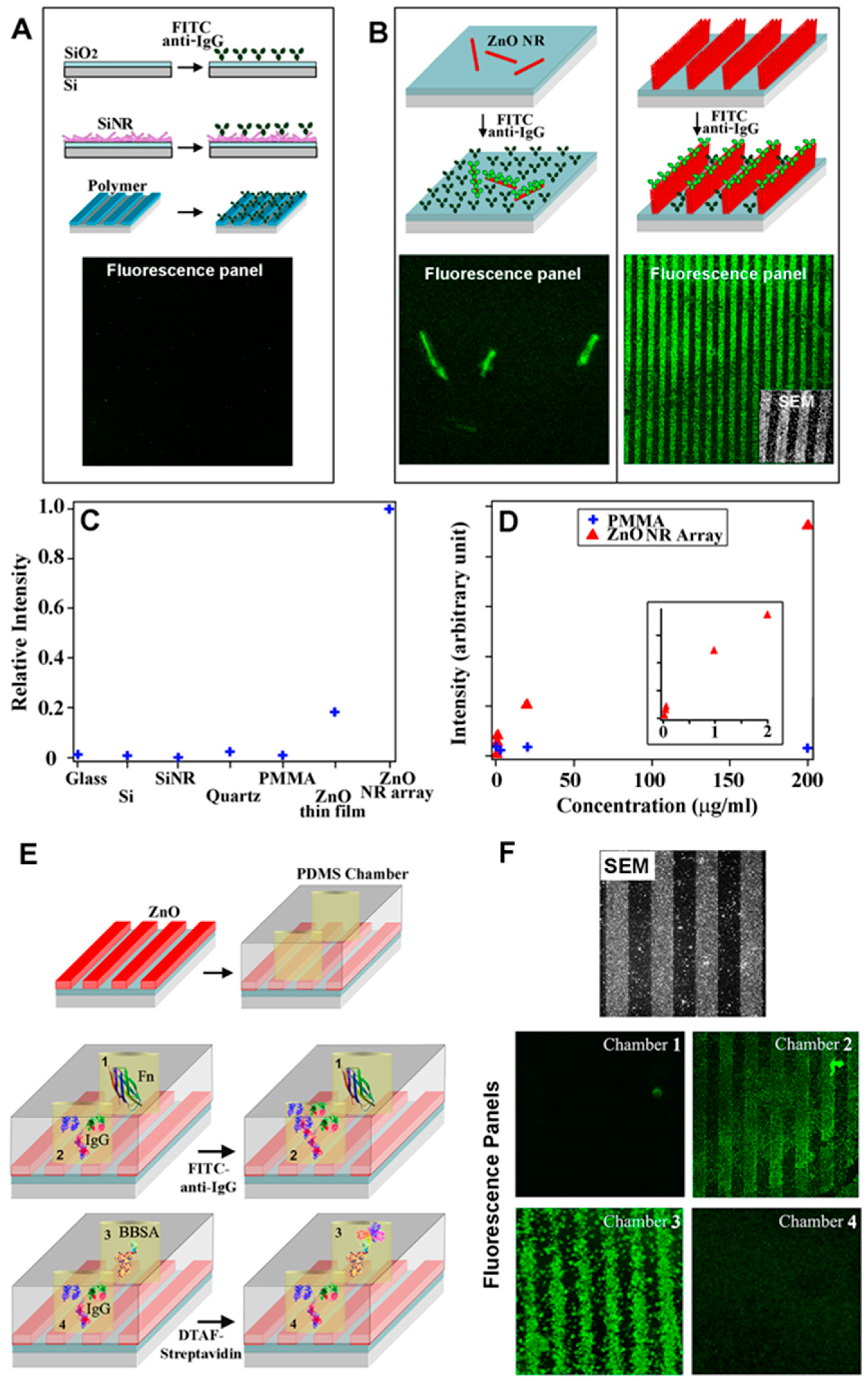
2.2.4. Applications of 1D ZnO Nanomaterials in Fluorescence Biosensors
2.2.4.1. ZnO Nanorods in Fluorescence-Based Biodetection
2.2.4.2. Single ZnO and ZnO-Related Nanorods in Fluorescence-Based Biodetection
2.2.5. Applications of 2D ZnO Nanomaterials in Fluorescence Biosensors
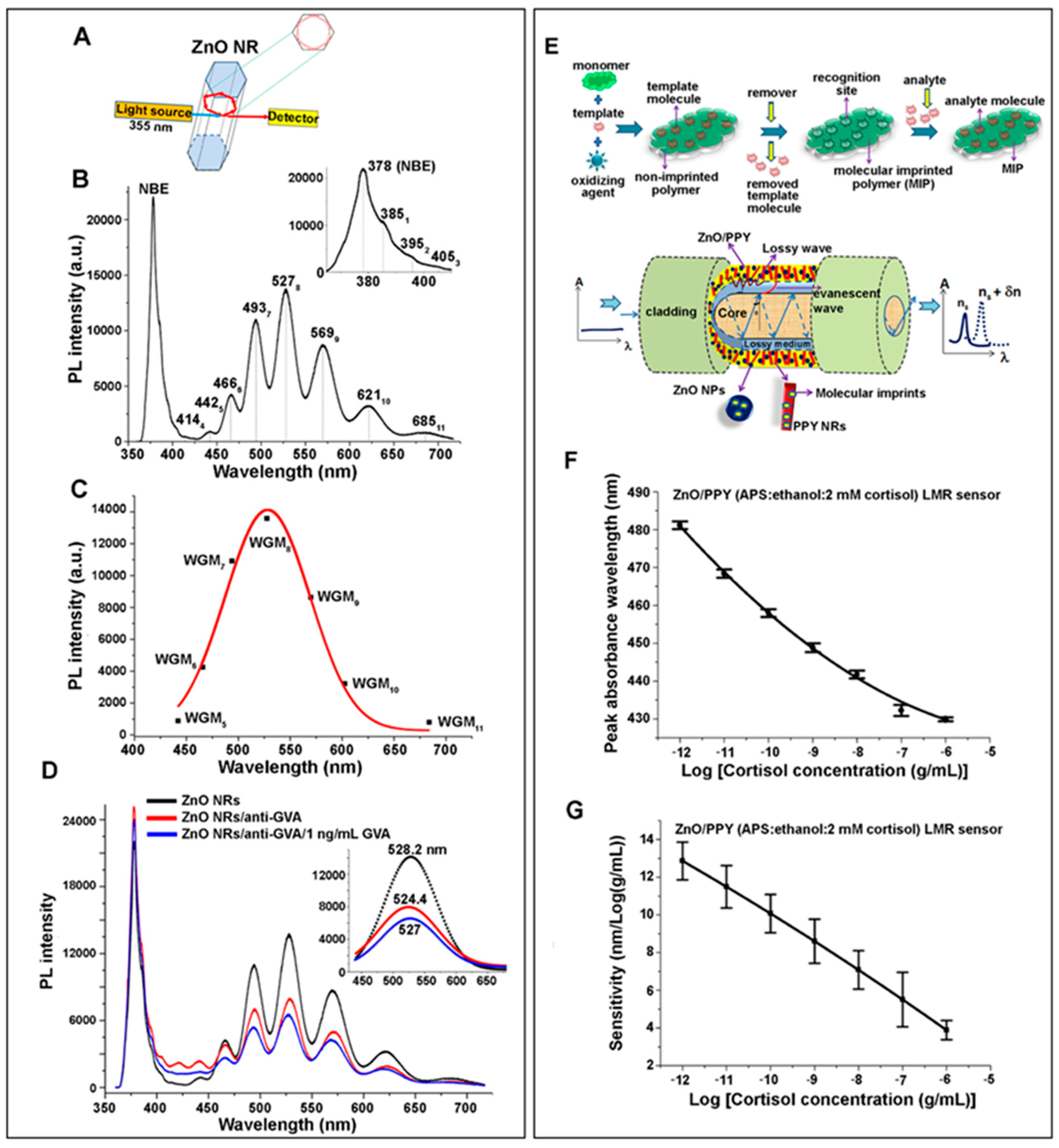
2.3. Photoluminescence: Near-Band-Edge Emission and Deep-Level Emission
2.3.1. Contributions of ZnO Nanomaterials in Photoluminescence-Based Biodetection
2.3.2. Applications of 0D ZnO Nanomaterials in Photoluminescence Biosensors
2.3.3. Applications of 1D ZnO Nanomaterials in Photoluminescence Biosensors
2.3.4. Applications of 2D ZnO Nanomaterials in Photoluminescence Biosensors
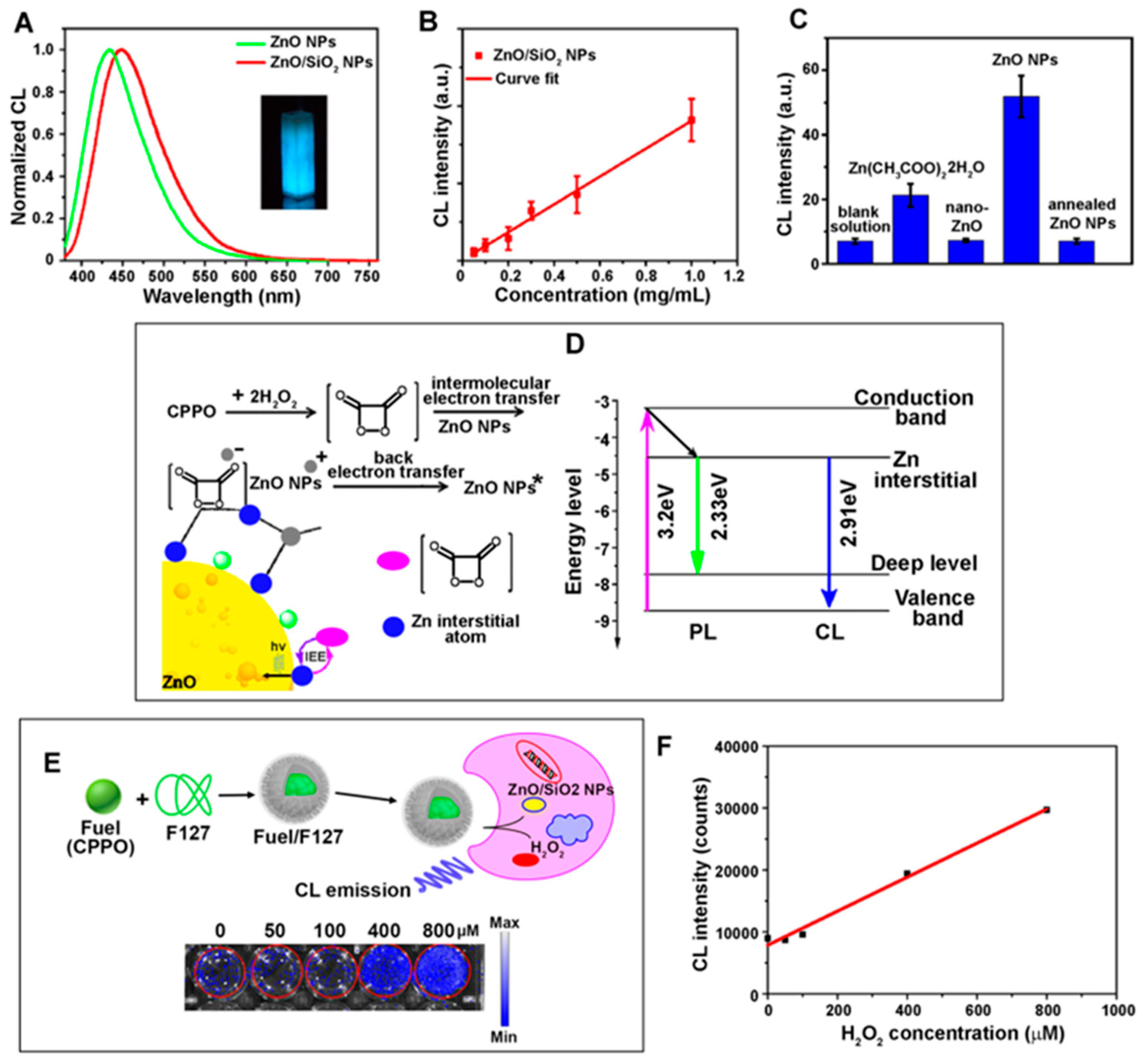
2.4. Chemiluminescence
2.4.1. Overview of Chemiluminescence in Biodetection
2.4.2. Contributions of ZnO Nanomaterials in Chemiluminescence-Based Biodetection
2.4.3. Applications of 0D/1D ZnO Nanomaterials in Chemiluminescence Biosensors
2.5. Surface Evanescence, Whispering Gallery Mode, and Lossy-Mode Resonance
2.5.1. Contributions of ZnO Nanomaterials in Various Guided- and Lossy-Mode Biodetection
2.5.2. Applications of 0D/1D ZnO Nanomaterials in Surface Evanescent Wave Biosensors
2.5.3. Applications of 1D ZnO Nanomaterials in Whispering Gallery Mode Biosensors
2.5.4. Applications of 2D ZnO Nanomaterials in Lossy-Mode Resonance Biosensors
2.6. Surface Plasmon Resonance
2.6.1. Overview of Surface Plasmon Resonance in Biodetection
2.6.2. Contributions of ZnO Nanomaterials in Surface Plasmon Resonance-Based Biodetection
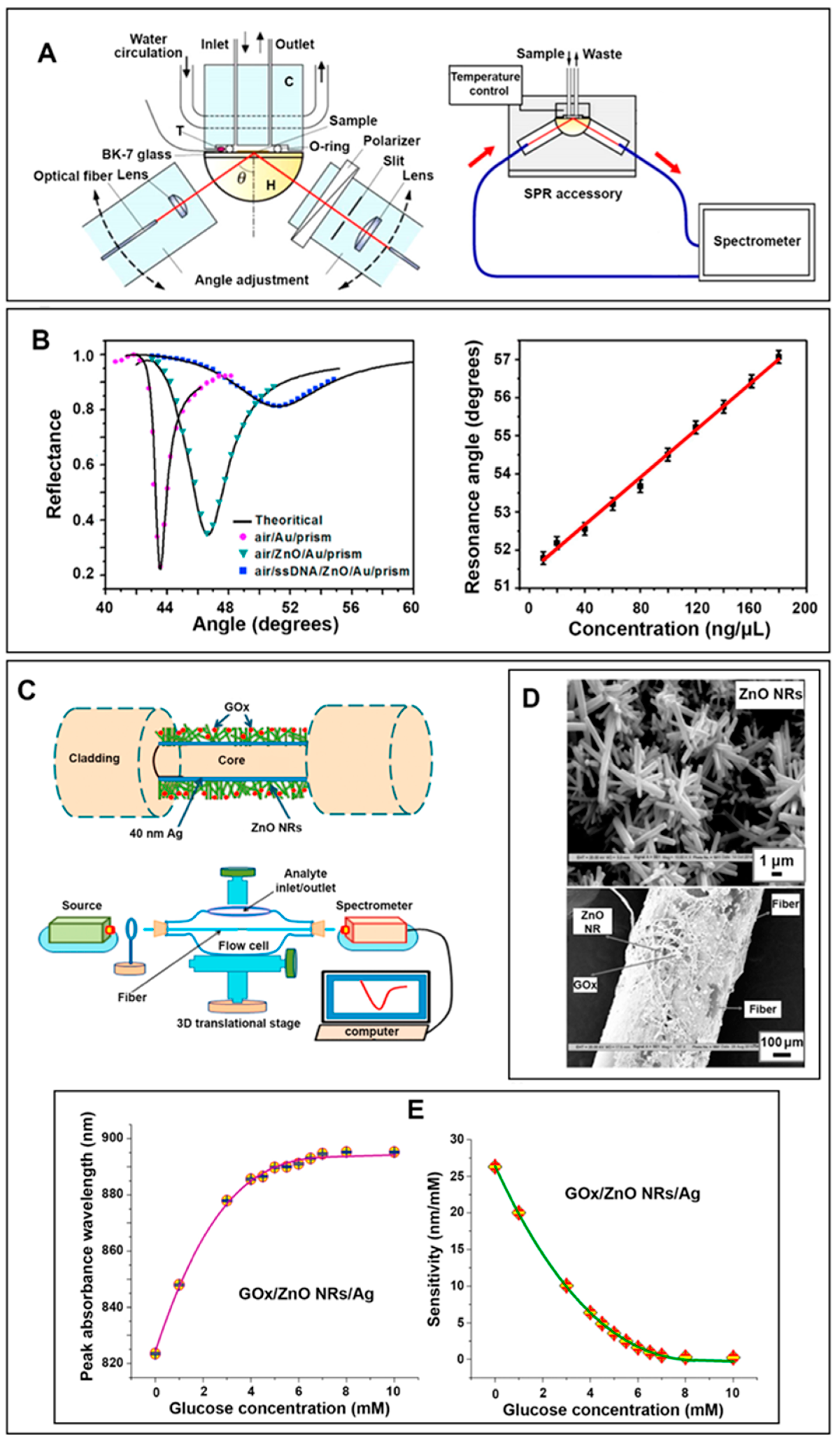
2.6.3. Applications of 0D/2D ZnO Nanomaterials in Surface Plasmon Resonance Biosensors
2.6.4. Applications of 1D ZnO Nanomaterials in Surface Plasmon Resonance Biosensors
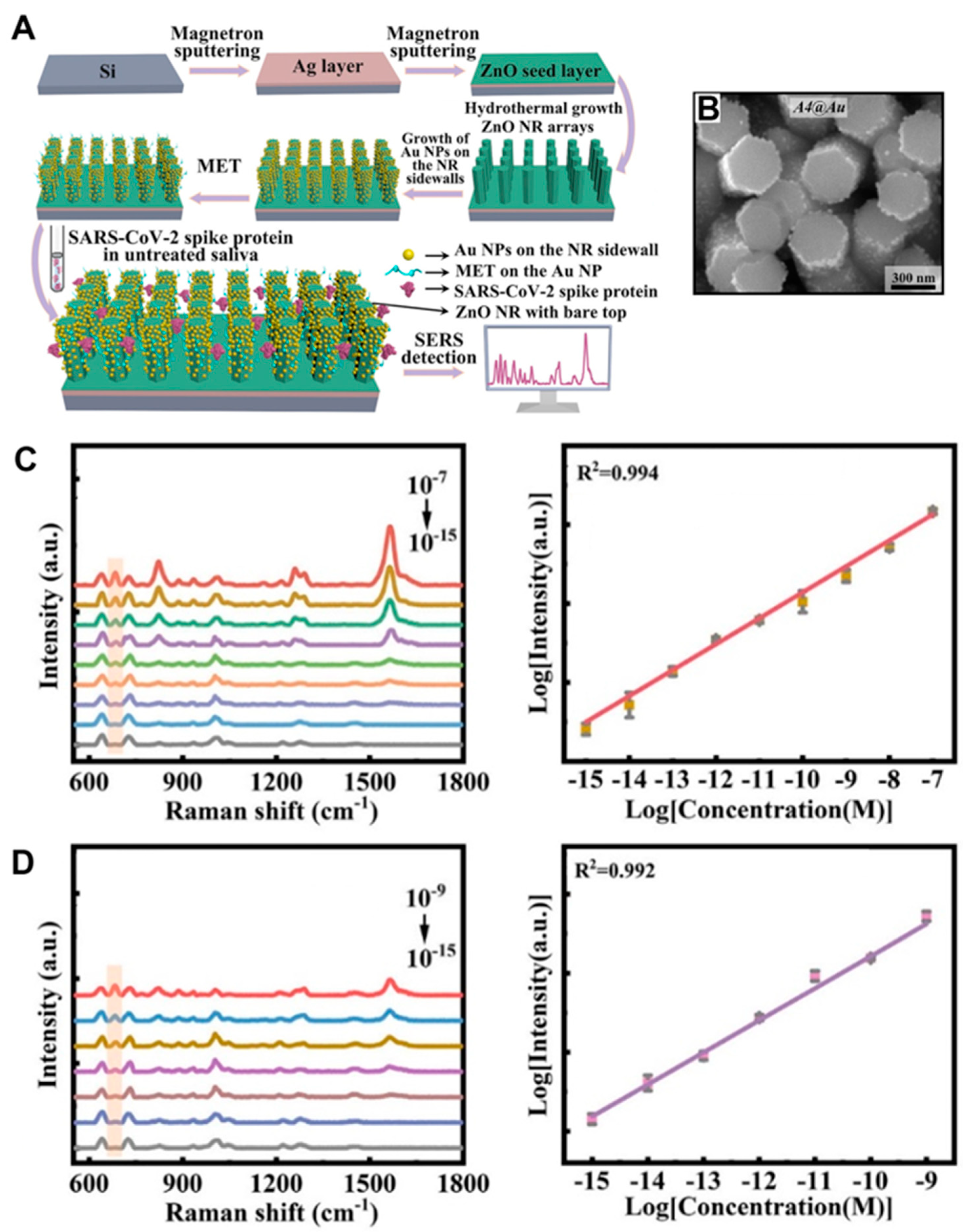
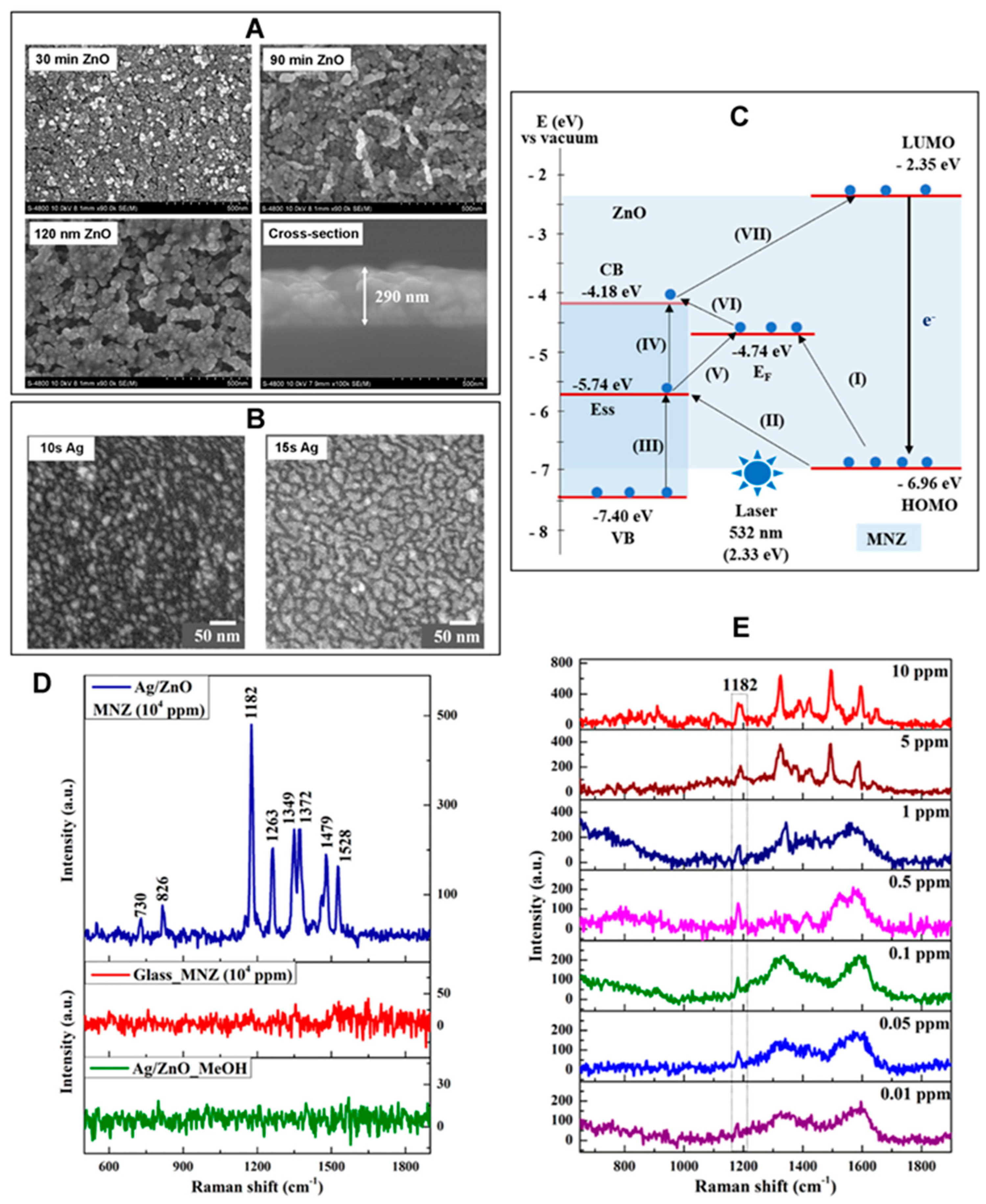
2.7. Surface-Enhanced Raman Scattering
2.7.1. Overview of Surface-Enhanced Raman Scattering in Biodetection
2.7.2. Contributions of ZnO Nanomaterials in Raman Scattering-Based Biodetection
2.7.3. Applications of 0D ZnO Nanomaterials in Surface-Enhanced Raman Sensors
2.7.4. Applications of 1D ZnO Nanomaterials in Surface-Enhanced Raman Sensors
2.7.5. Applications of 2D ZnO Nanomaterials in Surface-Enhanced Raman Sensors
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hahm, J.-I. Fundamental Properties of One-Dimensional Zinc Oxide Nanomaterials and Implementations in Various Detection Modes of Enhanced Biosensing. Annu. Rev. Phys. Chem. 2016, 67, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.S.; Singh, S.; Batool, M.; Fahmy, H.M.; Seku, K.; Shalan, A.E.; Lanceros-Mendez, S.; Zafar, M.N. A Review on 2D-ZnO Nanostructure Based Biosensors: From Materials to Devices. Mater. Adv. 2023, 4, 320–354. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Garkani Nejad, F.; Safaei, M. Recent Advances in ZnO Nanostructure-Based Electrochemical Sensors and Biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Viveros-Palma, K.; Espinosa-Lagunes, F.I.; Rodríguez-Morales, J.A.; Arriaga, L.G.; Macazo, F.C.; Minteer, S.D.; Ledesma-García, J. Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, H.; Jian, X.; Qu, K.; Xu, J.; Li, C.; Gao, Z.; Song, Y.-Y. Wireless Battery-Free Generation of Electric Fields on One-Dimensional Asymmetric Au/ZnO Nanorods for Enhanced Raman Sensing. Anal. Chem. 2021, 93, 9286–9295. [Google Scholar] [CrossRef]
- Vafabakhsh, M.; Dadmehr, M.; Kazemi Noureini, S.; Es’haghi, Z.; Malekkiani, M.; Hosseini, M. Paper-Based Colorimetric Detection of COVID-19 Using Aptasenor Based on Biomimetic Peroxidase Like Activity of ChF/ZnO/CNT Nano-Hybrid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 301, 122980. [Google Scholar] [CrossRef]
- Hayat, A.; Haider, W.; Raza, Y.; Marty, J.L. Colorimetric Cholesterol Sensor Based on Peroxidase Like Activity of Zinc Oxide Nanoparticles Incorporated Carbon Nanotubes. Talanta 2015, 143, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Sodzel, D.; Khranovskyy, V.; Beni, V.; Turner, A.P.F.; Viter, R.; Eriksson, M.O.; Holtz, P.-O.; Janot, J.-M.; Bechelany, M.; Balme, S.; et al. Continuous Sensing of Hydrogen Peroxide and Glucose via Quenching of the UV and Visible Luminescence of ZnO Nanoparticles. Microchim. Acta 2015, 182, 1819–1826. [Google Scholar] [CrossRef]
- Feng, L.-X.; Tang, C.; Han, X.-X.; Zhang, H.-C.; Guo, F.-N.; Yang, T.; Wang, J.-H. Simultaneous and Sensitive Detection of Multiple Small Biological Molecules by Microfluidic Paper-Based Analytical Device Integrated with Zinc Oxide Nanorods. Talanta 2021, 232, 122499. [Google Scholar] [CrossRef]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured Biosensor for Detecting Glucose in Tear by Applying Fluorescence Resonance Energy Transfer Quenching Mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.H.; Janssens, E. Au Nanoparticle–Decorated ZnO Nanorods as Fluorescent Non-Enzymatic Glucose Probe. Microchim. Acta 2020, 187, 577. [Google Scholar] [CrossRef]
- Sung, Y.-M.; Noh, K.; Kwak, W.-C.; Kim, T.G. Enhanced Glucose Detection using Enzyme-Immobilized ZnO/ZnS Core/Sheath Nanowires. Sens. Actuator B Chem. 2012, 161, 453–459. [Google Scholar] [CrossRef]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. FO-SPR Based Dextrose Sensor Using Ag/ZnO nanorods/GOx for Insulinoma Detection. Biosens. Bioelectron. 2016, 85, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Batumalay, M.; Harith, Z.; Rafaie, H.A.; Ahmad, F.; Khasanah, M.; Harun, S.W.; Nor, R.M.; Ahmad, H. Tapered Plastic Optical Fiber Coated with ZnO Nanostructures for the Measurement of Uric Acid Concentrations and Changes in Relative Humidity. Sens. Actuator A Phys. 2014, 210, 190–196. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Kamaci, M. Selective and Sensitive ZnO Quantum Dots Based Fluorescent Biosensor for Detection of Cysteine. J. Fluoresc. 2021, 31, 401–414. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, Q.; Liu, K.-K.; Lu, Y.-J.; Dong, L.; Shan, C.-X. Europium-Decorated ZnO Quantum Dots as a Fluorescent Sensor for the Detection of an Anthrax Biomarker. J. Mater. Chem. C 2017, 5, 1685–1691. [Google Scholar] [CrossRef]
- Wu, W.-J.; Zhao, Q.; Zhou, R.; Liang, Y.-C.; Zhao, W.-B.; Shan, C.-X. Ratiometric Fluorescence Sensor Based on Europium-Grafted ZnO Quantum Dots for Visual and Colorimetric Detection of Tetracycline. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119901. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Liu, B.; Sun, Y.-F.; Yu, Y. DNA-Length-Dependent Fluorescent Sensing Based on Energy Transfer in Self-Assembled Multilayers. Biosens. Bioelectron. 2014, 61, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, W.; Ma, L.; Lei, Y. Glutathione-Functionalized Mn:ZnS/ZnO Core/Shell Quantum Dots as Potential Time-Resolved FRET Bioprobes. RSC Adv. 2014, 4, 9372–9378. [Google Scholar] [CrossRef]
- Dorfman, A.; Kumar, N.; Hahm, J.-I. Highly Sensitive Biomolecular Fluorescence Detection Using Nanoscale ZnO Platforms. Langmuir 2006, 22, 4890–4895. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Song, S.; Hahm, J.-I. Unique Temporal and Spatial Biomolecular Emission Profile on Individual Zinc Oxide Nanorods. Nanoscale 2014, 6, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Jiang, R.; Coia, H.; Choi, D.S.; Alabanza, A.; Chang, J.Y.; Wang, J.; Hahm, J.-I. Insight into Factors Affecting the Presence, Degree, and Temporal Stability of Fluorescence Intensification on ZnO Nanorod Ends. Nanoscale 2015, 7, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dorfman, A.; Hahm, J.-I. Ultrasensitive DNA Sequence Detection Using Nanoscale ZnO Sensor Arrays. Nanotechnology 2006, 17, 2875. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Zhang, Z.; Ma, P.; Wang, X.; Song, D.; Sun, Y. Biotin-Streptavidin Sandwich Integrated PDA-ZnO@Au Nanocomposite Based SPR Sensor for hIgG Detection. Talanta 2022, 246, 123496. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, A.; Kumar, N.; Hahm, J. Nanoscale ZnO-Enhanced Fluorescence Detection of Protein Interactions. Adv. Mater. 2006, 18, 2685–2690. [Google Scholar] [CrossRef]
- Jenrette, E.A.; Farrell, M.J.; Flowers, J.A.; Pradhan, A.K. CdSe-ZnO Core–Shell Quantum Dots for Protein Detection: A Potential Sensing Platform. Nanomanufacturing 2021, 1, 3–13. [Google Scholar] [CrossRef]
- Dorfman, A.; Parajuli, O.; Kumar, N.; Hahm, J.-I. Novel Telomeric Repeat Elongation Assay Performed on Zinc Oxide Nanorod Array Supports. J. Nanosci. Nanotechnol. 2008, 8, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Alabanza, A.; Gonzalez, L.E.; Wang, W.; Reeves, W.B.; Hahm, J.-I. Ultratrace Level Determination and Quantitative Analysis of Kidney Injury Biomarkers in Patient Samples Attained by Zinc Oxide Nanorods. Nanoscale 2016, 8, 4613–4622. [Google Scholar] [CrossRef]
- Sytu, M.R.C.; Stoner, A.; Hahm, J.-I. Strain-Modulated and Nanorod-Waveguided Fluorescence in Single Zinc Oxide Nanorod-Based Immunodetection. Biosensors 2024, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.-Y.; Kwon, K.; Huh, J.; Kim, G.T.; Lee, E.B.; Park, D.; Lee, J. A Sensitive Diagnostic Assay of Rheumatoid Arthritis Using Three-Dimensional ZnO Nanorod Structure. Biosens. Bioelectron. 2011, 28, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Triet, N.M.; Son, Y.-M.; Lee, W.-I.; Lee, N.-E. Seesawed Fluorescence Nano-Aptasensor Based on Highly Vertical ZnO Nanorods and Three-Dimensional Quantitative Fluorescence Imaging for Enhanced Detection Accuracy of ATP. Biosens. Bioelectron. 2017, 90, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Meeseepong, M.; Ghosh, G.; Shrivastava, S.; Lee, N.-E. Fluorescence-Enhanced Microfluidic Biosensor Platform Based on Magnetic Beads with Highly Stable ZnO Nanorods for Biomarker Detection. ACS Appl. Mater. Interfaces 2023, 15, 21754–21765. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Bhand, S. Zinc Oxide Nanoparticle-Enhanced Ultrasensitive Chemiluminescence Immunoassay for the Carcinoma Embryonic Antigen. Microchim. Acta 2015, 182, 1643–1651. [Google Scholar] [CrossRef]
- Du, B.; Tang, C.; Zhao, D.; Zhang, H.; Yu, D.; Yu, M.; Balram, K.C.; Gersen, H.; Yang, B.; Cao, W.; et al. Diameter-Optimized High-Order Waveguide Nanorods for Fluorescence Enhancement Applied in Ultrasensitive Bioassays. Nanoscale 2019, 11, 14322–14329. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, S.; Park, J.-K.; Park, I. Quantum Dot-Based Immunoassay Enhanced by High-Density Vertical ZnO Nanowire Array. Biosens. Bioelectron. 2014, 55, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tawa, K.; Umetsu, M.; Hattori, T.; Kumagai, I. Zinc Oxide-Coated Plasmonic Chip Modified with a Bispecific Antibody for Sensitive Detection of a Fluorescent Labeled-Antigen. Anal. Chem. 2011, 83, 5944–5948. [Google Scholar] [CrossRef] [PubMed]
- Tawa, K.; Umetsu, M.; Nakazawa, H.; Hattori, T.; Kumagai, I. Application of 300× Enhanced Fluorescence on a Plasmonic Chip Modified with a Bispecific Antibody to a Sensitive Immunosensor. ACS Appl. Mater. Interfaces 2013, 5, 8628–8632. [Google Scholar] [CrossRef]
- Vasudevan, S.; Srinivasan, P.; Rayappan, J.B.B.; Solomon, A.P. A Photoluminescence Biosensor for the Detection of N-acyl Homoserine Lactone Using Cysteamine Functionalized ZnO Nanoparticles for the Early Diagnosis of Urinary Tract Infections. J. Mater. Chem. B 2020, 8, 4228–4236. [Google Scholar] [CrossRef]
- Swaminathan, N.; Sharma, N.; Nerthigan, Y.; Wu, H.-F. Self-Asembled Diphenylalanine-Zinc Oxide Hybrid Nanostructures as a Highly Selective Luminescent Biosensor for Trypsin Detection. App. Surf. Sci. 2021, 554, 149600. [Google Scholar] [CrossRef]
- Das, S.; Mukhopadhyay, S.; Chatterjee, S.; Devi, P.S.; Suresh Kumar, G. Fluorescent ZnO–Au Nanocomposite as a Probe for Elucidating Specificity in DNA Interaction. ACS Omega 2018, 3, 7494–7507. [Google Scholar] [CrossRef]
- Das, S.; Pramanik, S.; Chatterjee, S.; Das, P.P.; Devi, P.S.; Suresh Kumar, G. Selective Binding of Genomic Escherichia coli DNA with ZnO Leads to White Light Emission: A New Aspect of Nano–Bio Interaction and Interface. ACS Appl. Mater. Interfaces 2017, 9, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Viter, R.; Savchuk, M.; Iatsunskyi, I.; Pietralik, Z.; Starodub, N.; Shpyrka, N.; Ramanaviciene, A.; Ramanavicius, A. Analytical, Thermodynamical and Kinetic Characteristics of Photoluminescence Immunosensor for the Determination of Ochratoxin A. Biosens. Bioelectron. 2018, 99, 237–243. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Yazdi, G.R.; Konup, I.; Smyntyna, V.; Khranovskyy, V.; Yakimova, R.; Ramanavicius, A. Application of ZnO Nanorods Based Whispering Gallery Mode Resonator in Optical Immunosensors. Colloids Surf. B Biointerfaces 2020, 191, 110999. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO Films Formed by Atomic Layer Deposition as an Optical Biosensor Platform for the Detection of Grapevine Virus A-type Proteins. Biosens. Bioelectron. 2017, 92, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sharma, M.K.; Danielsson, B.; Willander, M.; Chatterjee, R.; Bhand, S. A Miniaturized Nanobiosensor for Choline Analysis. Biosens. Bioelectron. 2014, 54, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Botewad, S.N.; Pahurkar, V.G.; Muley, G.G.; Gaikwad, D.K.; Bodkhe, G.A.; Shirsat, M.D.; Pawar, P.P. PANI-ZnO Cladding-Modified Optical Fiber Biosensor for Urea Sensing Based on Evanescent Wave Absorption. Front. Mater. 2020, 7, 184. [Google Scholar] [CrossRef]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. A Contemporary Approach for Design and Characterization of Fiber-Optic-Cortisol Densor Tailoring LMR and ZnO/PPY Molecularly Imprinted Film. Biosens. Bioelectron. 2017, 87, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Usha, S.P.; Gupta, B.D. Urinary P-cresol Diagnosis Using Nanocomposite of ZnO/MoS2 and Molecular Imprinted Polymer on Optical Fiber Based Lossy Mode Resonance Sensor. Biosens. Bioelectron. 2018, 101, 135–145. [Google Scholar] [CrossRef]
- Kaur, G.; Paliwal, A.; Tomar, M.; Gupta, V. Detection of Neisseria meningitidis Using Surface Plasmon Resonance Based DNA Biosensor. Biosens. Bioelectron. 2016, 78, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Haldavnekar, R.; Venkatakrishnan, K.; Tan, D.B. Boosting the Sub-Cellular Biomolecular Cancer Signals by Self-Functionalized Tag-Free Nano Sensor. Biosens. Bioelectron. 2021, 190, 113407. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Wang, Z.; Cao, W.; Yu, M.; Sun, Y. Antibody- and Aptamer-free SERS Substrate for Ultrasensitive and Anti-Interference Detection of SARS-CoV-2 Spike Protein in Untreated Saliva. Biosens. Bioelectron. 2023, 237, 115457. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Nan, H.; Zhu, Q.; Qin, F.; Manohari, A.G.; Wei, M.; Zhu, Z.; Shi, Z.; Ni, Z. SERS-Active ZnO/Ag hybrid WGM Microcavity for Ultrasensitive Dopamine Detection. Appl. Phys. Lett. 2016, 109, 073701. [Google Scholar] [CrossRef]
- Kamińska, A.; Kowalska, A.A.; Snigurenko, D.; Guziewicz, E.; Lewiński, J.; Waluk, J. ZnO Oxide Films for Ultrasensitive, Rapid, and Label-Free Detection of Neopterin by Surface-Enhanced Raman Spectroscopy. Analyst 2015, 140, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Le Thi Minh, H.; Nguyen, A.T.; Thuy Doan, K.H.; Van Ho Phan, N.; Chi, C.M.; Le Vu Tuan, H. Optimizing the Structure of Zinc Oxide Thin Films and Silver Nanoparticles for Preparing High-Performance and Stability SERS Substrate to Detect Banned Antibiotics in Aquaculture. ACS Appl. Opt. Mater. 2023, 1, 1460–1473. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Ahn, D.; Kim, Y.M.; Chung, S.J. Enzyme Mimetic Activity of ZnO-Pd Nanosheets Synthesized via a Green Route. Molecules 2020, 25, 2585. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, K.V.; Ahmed, S.R.; Weng, X.; Neethirajan, S. Chitosan as a Peroxidase Mimic: Paper Based Sensor for the Detection of Hydrogen Peroxide. Sens. Actuator B Chem. 2018, 272, 8–13. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, X.; Wan, G.; Shi, S.; Wang, G. NiFe2O4/CNTs Fabricated by Atomic Layer Deposition as Highly Stable Peroxidase Mimics for Sensitive Colorimetric Detection of Hydrogen Peroxide and Glucose. Mater. Res. Bull. 2022, 147, 111637. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots Versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Han, M.; Dong, Z.; White, T.J.; Knoll, W. Composition-Tunable ZnxCd1-xSe Nanocrystals with High Luminescence and Stability. J. Am. Chem. Soc. 2003, 125, 8589–8594. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Kumar, R. Journey of ZnO Quantum Dots from Undoped to Rare-Earth and Transition Metal-Doped and Their Applications. RSC Adv. 2021, 11, 2512–2545. [Google Scholar] [CrossRef]
- Moussodia, R.-O.; Balan, L.; Merlin, C.; Mustin, C.; Schneider, R. Biocompatible and Stable ZnO Quantum Dots Generated by Functionalization with Siloxane-Core PAMAM Dendrons. J. Mater. Chem. 2010, 20, 1147–1155. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Gu, H.; Xia, F.; Zuo, X. Recent Development of Sandwich Assay Based on the Nanobiotechnologies for Proteins, Nucleic Acids, Small Molecules, and Ions. Chem. Rev. 2014, 114, 7631–7677. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guo, X.; Mou, F.; Guan, J. Lighting up Micro-/Nanorobots with Fluorescence. Chem. Rev. 2023, 123, 3944–3975. [Google Scholar] [CrossRef]
- Saavedra Rodriguez, G.; Sanchez-Zeferino, R.; Chapa, C.; Alvarez Ramos, M.E. Silica-Coated ZnS Quantum Dots for Multicolor Emission Tuning from Blue to White Light. ACS Appl. Nano Mater. 2021, 4, 12180–12187. [Google Scholar] [CrossRef]
- Bang, J.; Park, J.; Lee, J.H.; Won, N.; Nam, J.; Lim, J.; Chang, B.Y.; Lee, H.J.; Chon, B.; Shin, J.; et al. ZnTe/ZnSe (Core/Shell) Type-II Quantum Dots: Their Optical and Photovoltaic Properties. Chem. Mater. 2010, 22, 233–240. [Google Scholar] [CrossRef]
- Tyrakowski, C.M.; Shamirian, A.; Rowland, C.E.; Shen, H.; Das, A.; Schaller, R.D.; Snee, P.T. Bright Type II Quantum Dots. Chem. Mater. 2015, 27, 7276–7281. [Google Scholar] [CrossRef]
- Liu, X.; Xing, X.; Li, Y.; Chen, N.; Djerdj, I.; Wang, Y. Controllable Synthesis and Change of Emission Color from Green to Orange of ZnO Quantum Dots Using Different Solvents. New J. Chem. 2015, 39, 2881–2888. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y.; Xiang, D. Origin of Visible Photoluminescence of ZnO Quantum Dots: Defect-Dependent and Size-Dependent. J. Phys. Chem. C 2010, 114, 9651–9658. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Luminescent ZnO Quantum Dots for Sensitive and Selective Detection of Dopamine. Talanta 2013, 107, 133–139. [Google Scholar] [CrossRef]
- Algar, W.R.; Krause, K.D. Developing FRET Networks for Sensing. Annu. Rev. Anal. Chem. 2022, 15, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Dos Santos, M.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum Dots for Förster Resonance Energy Transfer (FRET). TrAC Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle Based Fluorescence Resonance Energy Transfer (FRET) for Biosensing Applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum Dots-Fluorescence Resonance Energy Transfer-Based Nanosensors and Their Application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. FÖrster Resonance Energy Transfer (FRET)-Based Biosensors for Biological Applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Tamura, T.; Hamachi, I. Recent Progress in Design of Protein-Based Fluorescent Biosensors and Their Cellular Applications. ACS Chem. Biol. 2014, 9, 2708–2717. [Google Scholar] [CrossRef]
- Foerster, T. Delocalized Excitation and Excitation Transfer. Bulletin No. 18; Florida State University: Tallahassee, FL, USA, 1964; 68p. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sekar, R.B.; Periasamy, A. Fluorescence Resonance Energy Transfer (FRET) Microscopy Imaging of Live Cell Protein Localizations. J. Cell Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Unksov, I.N.; Anttu, N.; Verardo, D.; Höök, F.; Prinz, C.N.; Linke, H. Fluorescence Excitation Enhancement by Waveguiding Nanowires. Nanoscale Adv. 2023, 5, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Y.; Yang, H.; Zhou, X.; Li, C.M. ZnO Nanorods-Enhanced Fluorescence for Sensitive Microarray Detection of Cancers in Serum Without Additional Reporter-Amplification. Biosens. Bioelectron. 2011, 26, 3683–3687. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, D.; Hou, C.; Liu, L.; Chen, J.; Huang, H.; Zhang, Q.; Duan, Y.; Li, Y.; Wang, H. Enhanced Immunofluorescence Detection of a Protein Marker Using a PAA Modified ZnO Nanorod Array-Based Microfluidic Device. Nanoscale 2018, 10, 17663–17670. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced Fluorescence Detection of Proteins using ZnO Nanowires Integrated Inside Microfluidic Chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef]
- Hauschild, R.; Kalt, H. Guided Modes in ZnO Nanorods. Appl. Phys. Lett. 2006, 89, 123107. [Google Scholar] [CrossRef]
- Voss, T.; Svacha, G.T.; Mazur, E.; Müller, S.; Ronning, C.; Konjhodzic, D.; Marlow, F. High-Order Waveguide Modes in ZnO Nanowires. Nano Let. 2007, 7, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.; Kudyk, I.; Wischmeier, L.; Gutowski, J. Nonlinear Optics with ZnO Nanowires. Phys. Status Solidi B 2009, 246, 311–314. [Google Scholar] [CrossRef]
- Sirbuly, D.J.; Law, M.; Pauzauskie, P.; Yan, H.; Maslov, A.V.; Knutsen, K.; Ning, C.-Z.; Saykally, R.J.; Yang, P. Optical Routing and Sensing with Nanowire Assemblies. Proc. Natl. Acad. Sci. USA 2005, 102, 7800–7805. [Google Scholar] [CrossRef]
- Singh, M.; Zhuo, X.; Choi, D.S.; Gonzalez, L.E.; Wang, J.; Hahm, J.-I. Effects of Crystallographic Facet-Specific Peptide Adsorption Along Single ZnO Nanorods on the Characteristic Fluorescence Intensification on Nanorod Ends (FINE) Phenomenon. Nanoscale 2015, 7, 18813–18826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Truong, J.; Singh, M.; Hansen, M.; Hahm, J.-I. Polarization-Resolved Mechanistic Investigation of Fluorescence Signal Intensification on Zinc Oxide Nanorod Ends. Nanoscale 2017, 9, 8164–8175. [Google Scholar] [CrossRef]
- Verardo, D.; Lindberg, F.W.; Anttu, N.; Niman, C.S.; Lard, M.; Dabkowska, A.P.; Nylander, T.; Månsson, A.; Prinz, C.N.; Linke, H. Nanowires for Biosensing: Lightguiding of Fluorescence as a Function of Diameter and Wavelength. Nano Lett. 2018, 18, 4796–4802. [Google Scholar] [CrossRef] [PubMed]
- Truong, J.; Stoner, A.; Sytu, M.R.C.; Tatlock, T.R.; Cho, D.H.; Hahm, J.-I. Elucidation of Strain-Dependent, Zinc Oxide Nanorod Response for Nanorod-Guided Fluorescence Intensity. Nanomaterials 2022, 12, 3558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, Z.; Yu, J.; Wang, H.; Li, Y.; Duan, Y. Highly Sensitive Microfluidic Detection of Carcinoembryonic Antigen via a Synergetic Fluorescence Enhancement Strategy Based on the Micro/Nanostructure Optimization of ZnO Nanorod Arrays and in situ ZIF-8 Coating. Chem. Eng. J. 2020, 383, 123230. [Google Scholar] [CrossRef]
- Park, H.-Y.; Gedi, V.; Kim, J.; Park, H.-C.; Han, S.-H.; Yoon, M.-Y. Ultrasensitive Diagnosis for an Anthrax-Protective Antigen Based on a Polyvalent Directed Peptide Polymer Coupled to Zinc Oxide Nanorods. Adv. Mater. 2011, 23, 5425–5429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, F.; Zheng, W.; Xue, T.; Jin, Z.; Wang, Z.; Cui, X. Plasmonic ZnO Nanorods/Au Substrates for Protein Microarrays with High Sensitivity and Broad Dynamic Range. Sens. Actuator B Chem. 2016, 228, 231–236. [Google Scholar] [CrossRef]
- Wu, K.; Chu, C.; Ma, C.; Yang, H.; Yan, M.; Ge, S.; Yu, J.; Song, X. Immunoassay for Carcinoembryonic Antigen Based on the Zn2+-Enhanced Fluorescence of Magnetic-Fluorescent Nanocomposites. Sens. Actuator B Chem. 2015, 206, 43–49. [Google Scholar] [CrossRef]
- Rafique, S.; Kiyani, F.; Jawaid, S.; Nasir, R.; Ahmad, M.; Bashir, S.; Idress, M.; Khan, J.S.; Akram, R. Reusable, Noninvasive, and Sensitive Fluorescence Enhanced ZnO-Nanorod-Based Microarrays for Quantitative Detection of AFP in Human Serum. Biomed. Res. Int. 2021, 2021, 9916909. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, Z.; Liu, Y.; Chen, T.; Zhou, X.; Li, C.M. A Portable Flow-Through Fluorescent Immunoassay Lab-on-a-Chip Device Using ZnO Nanorod-Decorated Glass Capillaries. Lab Chip 2013, 13, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Y.; Chen, T.; Liu, Y.; Li, C.M. Hybrid ZnO Nanorod-Polymer Brush Hierarchically Nanostructured Substrate for Sensitive Antibody Microarrays. Adv. Mater. 2015, 27, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Go, H.-Y.; Kalme, S.; Mane, R.S.; Han, S.-H.; Yoon, M.-Y. Protective Antigen Detection Using Horizontally Stacked Hexagonal ZnO Platelets. Anal. Chem. 2009, 81, 4280–4284. [Google Scholar] [CrossRef]
- Sang, C.-H.; Chou, S.-J.; Pan, F.M.; Sheu, J.-T. Fluorescence Enhancement and Multiple Protein Detection in ZnO Nanostructure Microfluidic Devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zong, L.; Jiao, Y.; Han, Y.; Zhang, X.; Xu, J.; Li, L.; Zhang, C.-W.; Liu, Z.; Ju, Q.; et al. Signal-Enhanced Detection of Multiplexed Cardiac Biomarkers by a Paper-Based Fluorogenic Immunodevice Integrated with Zinc Oxide Nanowires. Anal. Chem. 2019, 91, 9300–9307. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, S.; Fu, J.; Tang, S.; Wu, X.; Zhao, P.; Zhang, Z. A Near Infrared Fluorescence Imprinted Sensor Based on Zinc Oxide Nanorods for Rapid Determination of Ketoprofen. Anal. Methods 2021, 13, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Yan, H.; Choi, H.-J.; Knutsen, K.; Petersen, P.; Law, M.; Yang, P.; Saykally, R. Single Nanowire Waveguides and Lasers; Proc. SPIE: San Diego, CA, USA, 2003; Volume 5223. [Google Scholar]
- Johnson, J.C.; Yan, H.; Yang, P.; Saykally, R.J. Optical Cavity Effects in ZnO Nanowire Lasers and Waveguides. J. Phys. Chem. B 2003, 107, 8816–8828. [Google Scholar] [CrossRef]
- Sirbuly, D.J.; Tao, A.; Law, M.; Fan, R.; Yang, P. Multifunctional Nanowire Evanescent Wave Optical Sensors. Adv. Mater. 2007, 19, 61–66. [Google Scholar] [CrossRef]
- Börner, S.; Rüter, C.E.; Voss, T.; Kip, D.; Schade, W. Modeling of ZnO Nanorods for Evanescent Field Optical Sensors. Phys. Stat. Sol. 2007, 204, 3487–3495. [Google Scholar] [CrossRef]
- Pauzauskie, P.J.; Yang, P. Nanowire Photonics. Mater. Today 2006, 9, 36–45. [Google Scholar] [CrossRef]
- Chon, B.; Truong, J.; Hansen, M.; Hahm, J.-I.; Lee, Y.J. Position- and Polarization-Specific Waveguiding of Multi-Emissions in Single ZnO Nanorods. ACS Photonics 2019, 6, 1416–1424. [Google Scholar] [CrossRef]
- Yan, R.; Gargas, D.; Yang, P. Nanowire Photonics. Nat. Photonics 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Yan, R.; Park, J.-H.; Choi, Y.; Heo, C.-J.; Yang, S.-M.; Lee, L.P.; Yang, P. Nanowire-Based Single-Cell Endoscopy. Nat. Nanotechnol. 2012, 7, 191–196. [Google Scholar] [CrossRef]
- Lee, S.-C.; Park, H.-H.; Kim, S.-H.; Koh, S.-H.; Han, S.-H.; Yoon, M.-Y. Ultrasensitive Fluorescence Detection of Alzheimer’s Disease Based on Polyvalent Directed Peptide Polymer Coupled to a Nanoporous ZnO Nanoplatform. Anal. Chem. 2019, 91, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, P. Direct Observation of Vapor−Liquid−Solid Nanowire Growth. J. Am. Chem. Soc. 2001, 123, 3165–3166. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.-W.; Yi, G.-C. Metalorganic Vapor-Phase Epitaxial Growth of Vertically Well-Aligned ZnO Nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, M. Synthesis of Visible Light-Sensitive ZnO Nanostructures: Subwavelength Waveguides. J. Phys. Chem. C 2009, 113, 11952–11958. [Google Scholar] [CrossRef]
- Thomas, D.G. The Exciton Spectrum of Zinc Oxide. J. Phys. Chem. Solids 1960, 15, 86–96. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From Basics Towards Applications. Phys. Stat. Sol. 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms Behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Brochen, S.; Granier, C.; Feuillet, G.; Pernot, J. Role of Deep and Shallow Donor Levels on n-Type Conductivity of Hydrothermal ZnO. Appl. Phys. Lett. 2012, 100, 052115. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation Between Photoluminescence and Oxygen Vacancies in ZnO Phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, C.; Liu, X.; Li, G.; Luo, Y.; Quan, Z.; Xiang, H.; Lin, J. Tunable Photoluminescent and Cathodoluminescent Properties of ZnO and ZnO:Zn Phosphors. J. Phys. Chem. B 2006, 110, 9469–9476. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical Biosensors Based on ZnO Nanostructures: Advantages and Perspectives. A Review. Sens. Actuator B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pereira, S.O.; Zanoni, J.; Rodrigues, C.; Brás, M.; Costa, F.M.; Monteiro, T. ZnO Transducers for Photoluminescence-Based Biosensors: A Review. Chemosensors 2022, 10, 39. [Google Scholar] [CrossRef]
- Soundharraj, P.; Dhinasekaran, D.; Rajendran, A.R.; Prakasarao, A.; Ganesan, S. N-Doped Zinc Oxide as an Effective Fluorescence Sensor for Urea Detection. New J. Chem. 2021, 45, 6080–6090. [Google Scholar] [CrossRef]
- Thakur, D.; Sharma, A.; Awasthi, A.; Rana, D.S.; Singh, D.; Pandey, S.; Thakur, S. Manganese-Doped Zinc Oxide Nanostructures as Potential Scaffold for Photocatalytic and Fluorescence Sensing Applications. Chemosensors 2020, 8, 120. [Google Scholar] [CrossRef]
- Mai, H.H.; Pham, V.T.; Nguyen, V.T.; Sai, C.D.; Hoang, C.H.; Nguyen, T.B. Non-Enzymatic Fluorescent Biosensor for Glucose Sensing Based on ZnO Nanorods. J. Electron. Mater. 2017, 46, 3714–3719. [Google Scholar] [CrossRef]
- Mai, H.H.; Tran, D.H.; Janssens, E. Non-Enzymatic Fluorescent Glucose Sensor Using Vertically Aligned ZnO Nanotubes Grown by a One-Step, Seedless Hydrothermal Method. Microchim. Acta 2019, 186, 245. [Google Scholar] [CrossRef] [PubMed]
- Briones, M.; Busó-Rogero, C.; Catalán-Gómez, S.; García-Mendiola, T.; Pariente, F.; Redondo-Cubero, A.; Lorenzo, M.E. ZnO Nanowire-Based Fluorometric Enzymatic Assays for Lactate and Cholesterol. Microchim. Acta 2020, 187, 180. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghosh, R.; Giri, P.K. Tuning the Visible Photoluminescence in Al Doped ZnO Thin Film and its Application in Label-Free Glucose Detection. Sens. Actuator B Chem. 2018, 254, 681–689. [Google Scholar] [CrossRef]
- Tiwari, A.; Dhoble, S.J. Recent Advances and Developments on Integrating Nanotechnology with Chemiluminescence Assays. Talanta 2018, 180, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, G.H.G.; Kricka, L.J. Enhanced Chemiluminescent Reactions Catalyzed by Horseradish Peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 133, pp. 331–353. [Google Scholar]
- Stott, R.A.W. Enhanced Chemiluminescence Immunoassay. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 1089–1096. [Google Scholar]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and Nitrogen Co-Doped Carbon Quantum Dots as the Chemiluminescence Probe for Detection of Cu2+ Ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Biparva, P.; Abedirad, S.M.; Kazemi, S.Y.; Shanehsaz, M. Chemiluminescence Recognition of Berberine Triggered by Biomimetically Synthesized Silver Nanoparticles. Sens. Actuator B Chem. 2016, 234, 278–285. [Google Scholar] [CrossRef]
- Dong, Y.P.; Gao, T.T.; Chu, X.F.; Chen, J.; Wang, C.M. Flow Injection-Chemiluminescence Determination of Ascorbic Acid Based on Luminol–Ferricyanide–Gold Nanoparticles System. J. Lumin. 2014, 154, 350–355. [Google Scholar] [CrossRef]
- Iranifam, M.; Hendekhale, N.R. CuO Nanoparticles-Catalyzed Hydrogen Peroxide–Sodium Hydrogen Carbonate Chemiluminescence System Used for Quenchometric Determination of Atorvastatin, Rivastigmine and Topiramate. Sens. Actuator B Chem. 2017, 243, 532–541. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Shen, C.-L.; Lou, Q.; Zhao, W.-B.; Wei, J.-Y.; Liu, K.-K.; Zang, J.-H.; Dong, L.; Shan, C.-X. Efficient Chemiluminescent ZnO Nanoparticles for Cellular Imaging. J. Lumin. 2020, 221, 117111. [Google Scholar] [CrossRef]
- Eaton, S.W.; Fu, A.; Wong, A.B.; Ning, C.-Z.; Yang, P. Semiconductor Nanowire Lasers. Nat. Rev. Mater. 2016, 1, 16028. [Google Scholar] [CrossRef]
- Nobis, T.; Grundmann, M. Low-Order Optical Whispering-Gallery Modes in Hexagonal Nanocavities. Phys. Rev. A 2005, 72, 063806. [Google Scholar] [CrossRef]
- Baratto, C.; Faglia, G.; Carletti, L.; Angelis, C.D. New Trends in Optical Resonant Bio-Chemical Sensing. IEEE Sens. J. 2021, 21, 12856–12867. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L. Light Management with Patterned Micro- and Nanostructure Arrays for Photocatalysis, Photovoltaics, and Optoelectronic and Optical Devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Wang, N.W.; Yang, Y.H.; Yang, G.W. Fabry–Pérot and Whispering Gallery Modes Enhanced Luminescence from an Individual Hexagonal ZnO Nanocolumn. Appl. Phys. Lett. 2010, 97, 041917. [Google Scholar] [CrossRef]
- Chen, R.; Ling, B.; Sun, X.W.; Sun, H.D. Room Temperature Excitonic Whispering Gallery Mode Lasing from High-Quality Hexagonal ZnO Microdisks. Adv. Mater. 2011, 23, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Czekalla, C.; Nobis, T.; Rahm, A.; Cao, B.; Zúñiga-Pérez, J.; Sturm, C.; Schmidt-Grund, R.; Lorenz, M.; Grundmann, M. Whispering Gallery Modes in Zinc Oxide Micro- and Nanowires. Phys. Status Solidi B 2010, 247, 1282–1293. [Google Scholar] [CrossRef]
- Schmidt-Grund, R.; Michalsky, T.; Wille, M.; Grundmann, M. Coherent Polariton Modes and Lasing in ZnO Nano- and Microwires. Phys. Status Solidi B 2019, 256, 1800462. [Google Scholar] [CrossRef]
- Rühle, S.; van Vugt, L.K.; Li, H.Y.; Keizer, N.A.; Kuipers, L.; Vanmaekelbergh, D. Nature of Sub-Band Gap Luminescent Eigenmodes in a ZnO Nanowire. Nano Lett. 2008, 8, 119–123. [Google Scholar] [CrossRef]
- Yusof, H.H.M.; Rahim, H.R.A.; Thokchom, S.; Dimyati, K.; Harun, S.W. Uric Acid Sensing Using Tapered Silica Optical Fiber Coated with Zinc Oxide Nanorods. Microw. Opt. Technol. Lett. 2018, 60, 645–650. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D.; van Vugt, L.K. ZnO Nanowire Lasers. Nanoscale 2011, 3, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Kolhep, M.; Bläsing, J.; Strittmatter, A.; Zacharias, M. Spontaneous and Position-Controlled Epitaxial Growth of ZnO Nanowires on AlN/Si by CVD. Cryst. Growth Des. 2023, 23, 7095–7102. [Google Scholar] [CrossRef]
- Hassan, M.A.; Johar, M.A.; Yu, S.Y.; Ryu, S.-W. Facile Synthesis of Well-Aligned ZnO Nanowires on Various Substrates by MOCVD for Enhanced Photoelectrochemical Water-Splitting Performance. ACS Sustainable Chem. Eng. 2018, 6, 16047–16054. [Google Scholar] [CrossRef]
- Corres, J.M.; Villar, I.D.; Arregui, F.J.; Matias, I.R. Analysis of Lossy Mode Resonances on Thin-Film Coated Cladding Removed Plastic Fiber. Opt. Lett. 2015, 40, 4867–4870. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Einziger, P.D.; Adam, D.R. Broadband Absorption Spectroscopy via Excitation of Lossy Resonance Modes in Thin Films. Phys. Rev. Lett. 2005, 95, 018101. [Google Scholar] [CrossRef]
- Socorro, A.B.; Del Villar, I.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Spectral Width Reduction in Lossy Mode Resonance-Based Sensors by Means of Tapered Optical Fibre Structures. Sens. Actuator B Chem. 2014, 200, 53–60. [Google Scholar] [CrossRef]
- Del Villar, I.; Arregui, F.J.; Zamarreño, C.R.; Corres, J.M.; Bariain, C.; Goicoechea, J.; Elosua, C.; Hernaez, M.; Rivero, P.J.; Socorro, A.B.; et al. Optical Sensors Based on Lossy-Mode Resonances. Sens. Actuator B Chem. 2017, 240, 174–185. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, S.K.; Gupta, B.D. Sensitivity Enhancement of a Surface Plasmon Resonance Based Fibre Optic Refractive Index Sensor Utilizing an Additional Layer of Oxides. Sens. Actuator A Phys. 2013, 193, 136–140. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A Comprehensive Review on Plasmonic-Based Biosensors Used in Viral Diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Z.-H.; Zhao, W.-M.; Wang, L.; Yan, X.; Zhu, A.-S.; Qiu, F.-M.; Zhang, K.-K. Research Advances on Surface Plasmon Resonance Biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Anwar, M.; Pradhan, R.; Ashar, M.S.; Rai, N.; Dey, S. Surface Plasmon Resonance Based-Optical Biosensor: Emerging Diagnostic Tool for Early Detection of Diseases. J. Biophotonics 2023, 16, e202200380. [Google Scholar] [CrossRef]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Murillo Almuzara, C.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef]
- Salamon, Z.; Tollin, G. Optical Anisotropy in Lipid Bilayer Membranes: Coupled Plasmon-Waveguide Resonance Measurements of Molecular Orientation, Polarizability, and Shape. Biophys. J. 2001, 80, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Boruah, R.; Mohanta, D.; Choudhury, A.; Nath, P.; Ahmed, G.A. Surface Plasmon Resonance-Based Protein Bio-Sensing Using a Kretschmann Configured Double Prism Arrangement. IEEE Sens. J. 2015, 15, 6791–6796. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A.V. Phase-Sensitive Surface Plasmon Resonance Biosensors: Methodology, Instrumentation and Applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.-M.; Yaacob, M.H. Highly Sensitive SPR Response of Au/Chitosan/Graphene Oxide Nanostructured Thin Films Toward Pb (II) Ions. Sens. Actuator B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Kong, W.; Zheng, Z.; Wan, Y.; Li, S.; Liu, J. High-Sensitivity Sensing Based on Intensity-Interrogated Bloch Surface Wave Sensors. Sens. Actuator B Chem. 2014, 193, 467–471. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Wu, S.-Y.; He, J.; Qu, J.; Li, X.; Ho, H.-P.; Gu, D.; Gao, B.Z.; Shao, Y. Wavelength-Scanning SPR Imaging Sensors Based on an Acousto-Optic Tunable Filter and a White Light Laser. Sensors 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Güell, F.; Galdámez-Martínez, A.; Martínez-Alanis, P.R.; Catto, A.C.; da Silva, L.F.; Mastelaro, V.R.; Santana, G.; Dutt, A. ZnO-Based Nanomaterials Approach for Photocatalytic and Sensing Applications: Recent Progress and Trends. Mater. Adv. 2023, 4, 3685–3707. [Google Scholar] [CrossRef]
- Kuranaga, Y.; Matsui, H.; Ikehata, A.; Shimoda, Y.; Noiri, M.; Ho, Y.-L.; Delaunay, J.-J.; Teramura, Y.; Tabata, H. Enhancing Detection Sensitivity of ZnO-Based Infrared Plasmonic Sensors Using Capped Dielectric Ga2O3 Layers for Real-Time Monitoring of Biological Interactions. ACS Appl. Bio Mater. 2020, 3, 6331–6342. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhang, S.; Sun, Y.; Zhu, X.; Cao, Y.; Wang, X.; Zhang, H.; Song, D. Surface Plasmon Resonance Biosensor Based on Water-Soluble ZnO–Au nanocomposites. Anal. Chim. Acta 2009, 653, 109–115. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Wang, J.; Wang, J.; Yu, A.; Zhang, H.; Song, D. Water-Soluble ZnO–Au Nanocomposite-Based Probe for Enhanced Protein Detection in a SPR Biosensor System. J. Colloid Interface Sci. 2010, 351, 392–397. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Lee, S.-K. Fiber Optic Sensor Based on ZnO Nanowires Decorated by Au Nanoparticles for Improved Plasmonic Biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Wang, Y.; Marques, C.; Zhang, B.; Kumar, S. Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review. Biosensors 2022, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Esfahani Monfared, Y. Overview of Recent Advances in the Design of Plasmonic Fiber-Optic Biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef]
- Dillen, A.; Scarpellini, C.; Daenen, W.; Driesen, S.; Zijlstra, P.; Lammertyn, J. Integrated Signal Amplification on a Fiber Optic SPR Sensor Using Duplexed Aptamers. ACS Sens. 2023, 8, 811–821. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy for Bioanalysis and Diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Tukova, A.; Wang, Y. Emerging SERS Biosensors for the Analysis of Cells and Extracellular Vesicles. Nanoscale 2022, 14, 15242–15268. [Google Scholar] [CrossRef]
- Käll, M.; Xu, H.; Johansson, P. Field Enhancement and Molecular Response in Surface-Enhanced Raman Scattering and Fluorescence Spectroscopy. J. Raman Spectrosc. 2005, 36, 510–514. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Colniță, A.; Toma, V.-A.; Brezeștean, I.A.; Tahir, M.A.; Dina, N.E. A Review on Integrated ZnO-Based SERS Biosensors and Their Potential in Detecting Biomarkers of Neurodegenerative Diseases. Biosensors 2023, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Saxena, K.; Rawal, R.; Yadav, L.; Jain, U. Advances in Surface-Enhanced Raman Spectroscopy-Based Sensors for Detection of Various Biomarkers. Prog. Biophys. Mol. Biol. 2023, 184, 32–41. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtuluş, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent Progress in SERS Biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and Applications of Surface-Enhanced Raman Spectroscopy–Based Biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, E.; Shi, H.; Tao, Y.; Ren, X. Semiconductor-Based Surface Enhanced Raman Scattering (SERS): From Active Materials to Performance Improvement. Analyst 2022, 147, 1257–1272. [Google Scholar] [CrossRef]
- Willets, K.A. New Tools for Investigating Electromagnetic Hot Spots in Single-Molecule Surface-Enhanced Raman Scattering. ChemPhysChem 2013, 14, 3186–3195. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Daqiqeh Rezaei, S.; Middha, E.; Tan, Y.N. Synthesis and Simulation Study of Right Silver Bipyramids via Seed-Mediated Growth cum Selective Oxidative Etching Approach. Part. Part. Syst. Charact. 2020, 37, 2000027. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Daqiqeh Rezaei, S.; Tan, Y.N. Simulation Guided Design of Silver Nanostructures for Plasmon-Enhanced Fluorescence, Singlet Oxygen Generation and SERS Applications. Phys. Chem. Chem. Phys. 2020, 22, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Yan, S.; Zeng, Z.; Huang, A.; Liu, A.; Yuan, Y.; Huang, Y. Organic Molecule Detection Based on SERS in Microfluidics. Sci. Rep. 2019, 9, 17634. [Google Scholar] [CrossRef]
- Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Liz-Marzán, L.M.; García de Abajo, F.J. Optimization of Nanoparticle-Based SERS Substrates through Large-Scale Realistic Simulations. ACS Photonics 2017, 4, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruan, W.; Zhang, J.; Yang, B.; Xu, W.; Zhao, B.; Lombardi, J.R. Direct Observation of Surface-Enhanced Raman Scattering in ZnO Nanocrystals. J. Raman Spectrosc. 2009, 40, 1072–1077. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, F.; Zhang, S.; Zhang, X.; Wu, H.; Zhang, X.; Xing, Z.; Qin, W.; Liu, Y.; Jiang, C. A General Method for Large-Scale Fabrication of Semiconducting Oxides with High SERS Sensitivity. ACS Appl. Mater. Interfaces 2017, 9, 14534–14544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, W.; Jin, Z.; Huang, W.; Lin, J.; Ma, G.; Li, S.; Guo, L. Remarkable SERS Activity Observed from Amorphous ZnO Nanocages. Angew. Chem. Int. Ed. 2017, 56, 9851–9855. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman Investigation of Nanosized TiO2: Effect of Crystallite Size and Quantum Confinement. J. Phys. Chem. C 2012, 116, 8792–8797. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Time-Dependent Picture of the Charge-Transfer Contributions to Surface Enhanced Raman Spectroscopy. J. Chem. Phys. 2007, 126, 244709. [Google Scholar] [CrossRef] [PubMed]
- Sinha, G.; Depero, L.E.; Alessandri, I. Recyclable SERS Substrates Based on Au-Coated ZnO Nanorods. ACS Appl. Mater. Interfaces 2011, 3, 2557–2563. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Wang, Y.; Schlücker, S. Rational Design and Synthesis of SERS Labels. Analyst 2013, 138, 2224–2238. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef]
- Adesoye, S.; Dellinger, K. ZnO and TiO2 Nanostructures for Surface-Enhanced Raman Scattering-Based Bio-Sensing: A Review. Sens. Bio-Sens. Res. 2022, 37, 100499. [Google Scholar] [CrossRef]
- Lin, C.; Li, Y.; Peng, Y.; Zhao, S.; Xu, M.; Zhang, L.; Huang, Z.; Shi, J.; Yang, Y. Recent Development of Surface-Enhanced Raman Scattering for Biosensing. J. Nanobiotechnol. 2023, 21, 149. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Yang, X.; Yuan, R.; Chai, Y. A SERS Biosensor Constructed by Calcined ZnO Substrate with High-Efficiency Charge Transfer for Sensitive Detection of Pb2+. Sens. Actuator B Chem. 2021, 343, 130142. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Jing, S.; Wang, Y.; Sun, Z.; Zhao, B.; Zhao, C.; Lombardi, J.R. Enhanced Raman Scattering as a Probe for 4-Mercaptopyridine Surface-modified Copper Oxide Nanocrystals. Anal. Sci. 2007, 23, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Wang, C.; Qiu, G.; Ye, W.; Li, Y.; Wang, D. ZnO/Ag Nanorods as a Prominent SERS Substrate Contributed by Synergistic Charge Transfer Effect for Simultaneous Detection of Oral Antidiabetic Drugs Pioglitazone and Phenformin. Sens. Actuator B Chem. 2020, 307, 127634. [Google Scholar] [CrossRef]
- Pal, A.K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ultra-Sensitive, Reusable, and Superhydrophobic Ag/ZnO/Ag 3D Hybrid Surface Enhanced Raman Scattering Substrate for Hemoglobin Detection. J. Appl. Phys. 2020, 127, 164501. [Google Scholar] [CrossRef]
- Xie, L.; Yang, X.; He, Y.; Yuan, R.; Chai, Y. Polyacrylamide Gel-Contained Zinc Finger Peptide as the “Lock” and Zinc Ions as the “Key” for Construction of Ultrasensitive Prostate-Specific Antigen SERS Immunosensor. ACS Appl. Mater. Interfaces 2018, 10, 15200–15206. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Wang, S.; Fei, X.; Liu, Y.; Yang, G. Heterostructured ZnO/Au Nanoparticles-Based Resonant Raman Scattering for Protein Detection. J. Phys. Chem. B 2009, 113, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Pagal, S.; Prashanth, K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ag/ZnO/Au 3D Hybrid Structured Reusable SERS Substrate as Highly Sensitive Platform for DNA Detection. Sens. Actuator B Chem. 2019, 279, 157–169. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, C.; Nie, Y.; Ren, Y.; Hao, N.; Xu, Z.; Dong, L.; Zhang, J.X.J. Silver Nanoparticle on Zinc Oxide Array for Label-Free Detection of Opioids Through Surface-Enhanced Raman Spectroscopy. RSC Adv. 2021, 11, 11329–11337. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Hsu, L.-C.; Chen, C.-H.; Chen, L.-Y.; Lai, C.-S. Investigation of SERS Studies on Periodic Patterned ZnO Nanorod Array Fabricated Using Silica Inverse Opal Nanostructure as a Template. J. Phys. Chem. C 2024, 128, 8288–8295. [Google Scholar] [CrossRef]
| ZnO Nanomaterial Biosensors According to Optical Detection Modes | ||
|---|---|---|
| Absorption (Abs) and Colorimetry (Col) [Section 2.1] | Overview of abs/col in biodetection [Section 2.1.1] | |
| Contributions of ZnO nanomaterials in abs-/col-based biodetection [Section 2.1.2] | ||
| Applications of 0D ZnO NPs in abs/col biosensors [Section 2.1.3] | ||
| Applications of 1D ZnO NRs in abs/col biosensors [Section 2.1.4] | ||
| Fluorescence (Fluo) [Section 2.2] | Overview of fluo in biodetection [Section 2.2.1] | |
| Contributions of ZnO nanomaterials in fluo-based biodetection [Section 2.2.2] | ||
| QD fluorescent probes [Section 2.2.2.1] | Fluo signal-enhancing platforms [Section 2.2.2.2] | |
| Applications of 0D ZnO NPs in fluo biosensors [Section 2.2.3] | ||
| Applications of 1D ZnO NRs in fluo biosensors [Section 2.2.4] | ||
| ZnO NRs in fluo-based biodetection [Section 2.2.4.1] | Single ZnO and ZnO-related NRs in fluo-based biodetection [Section 2.2.4.2] | |
| Applications of 2D ZnO thin films in fluo biosensors [Section 2.2.5] | ||
| Photoluminescence (PL): Near-band-edge emission (NBE), Deep-level emission (DLE) [Section 2.3] | Contributions of ZnO nanomaterials in PL-based biodetection [Section 2.3.1] | |
| Applications of 0D ZnO NPs in PL biosensors [Section 2.3.2] | ||
| Applications of 1D ZnO NRs in PL biosensors [Section 2.3.3] | ||
| Applications of 2D ZnO thin films in PL biosensors [Section 2.3.4] | ||
| Chemiluminescence (CL) [Section 2.4] | Overview of CL in biodetection [Section 2.4.1] | |
| Contributions of ZnO nanomaterials in CL-based biodetection [Section 2.4.2] | ||
| Applications of 0D/1D ZnO nanomaterials in CL biosensors [Section 2.4.3] | ||
| Surface evanescent wave (Surface EW), whispering gallery mode (WGM), Lossy-mode resonance (LMR) [Section 2.5] | Contributions of ZnO nanomaterials in various guided- and lossy-mode biodetection [Section 2.5.1] | |
| Applications of 0D/1D ZnO nanomaterials in surface EW biosensors [Section 2.5.2] | ||
| Applications of 1D ZnO NRs in WGM biosensors [Section 2.5.3] | ||
| Applications of 2D ZnO thin films in LMR biosensors [Section 2.5.4] | ||
| Surface plasmon resonance (SPR) [Section 2.6] | Overview of SPR in biodetection [Section 2.6.1] | |
| Contributions of ZnO nanomaterials in SPR-based biodetection [Section 2.6.2] | ||
| Applications of 0D/2D ZnO nanomaterials in SPR biosensors [Section 2.6.3] | ||
| Applications of 1D ZnO NRs in SPR biosensors [Section 2.6.4] | ||
| Surface-enhanced Raman scattering (SERS) [Section 2.7] | Overview of SERS in biodetection [Section 2.7.1] | |
| Contributions of ZnO nanomaterials in SERS-based biodetection [Section 2.7.2] | ||
| Applications of 0D ZnO NPs in SERS biosensors [Section 2.7.3] | ||
| Applications of 1D ZnO NRs in SERS biosensors [Section 2.7.4] | ||
| Applications of 2D ZnO thin films in SERS biosensors [Section 2.7.5] | ||
| Bioanalytes | Nanomaterials | Detection Mode (Section) | Role of ZnO | Detection Range Limit of Detection | Ref. |
|---|---|---|---|---|---|
| Lactate | Fe2O3-ZnO NPs | Abs (Section 2.1.3) | Peroxidase mimic | 50–1000 μM (l) | [5] |
| 9.4 μM | |||||
| AuNPs/ZnO NRs | SERS (Section 2.7.4) | Promoting CE and LSPR excitation, piezotronic enhancement | 3–35 mM (l) | [6] | |
| 2 mM | |||||
| COVID-19 virus | Chitosan/ZnO NPs/carbon nanotubes (CNTs) | Abs (Section 2.1.3) | Peroxidase mimic | 1–500 pg/mL (l) | [7] |
| 0.05 pg/mL | |||||
| Col (Section 2.1.3) | Peroxidase mimic | 50–500 pg/mL (l) | |||
| 8 pg/mL | |||||
| Cholesterol | ZnO NPs/CNTs | Col (Section 2.1.3) | Peroxidase mimic | 0.5–500 nM (l) | [8] |
| 0.2 nM | |||||
| Glucose | ZnO NPs | PL (Section 2.3.2) | Near-band-edge (NBE) emission source | 30–130 mM (l) | [9] |
| 10 mM | |||||
| ZnO NRs | Col (Section 2.1.4) | Greater enzyme loading | 0.01–10 mM (l) | [10] | |
| 3 µM | |||||
| Fluo (Section 2.2.4.1) | Greater CdSe/ZnS QD loading | 0.03–3 mM (l) | [11] | ||
| N/A | |||||
| ZnO NRs/ Au NPs | PL (Section 2.3.3) | NBE emission source | 0.01–2 mM (l) | [12] | |
| 0.01 mM | |||||
| ZnO/ZnS NRs | PL (Section 2.3.3) | NBE emission source | 3.5–24 mM (l) | [13] | |
| 0.14 mM | |||||
| ZnO NRs/ Ag film | SPR (Section 2.6.4) | Greater enzyme binding, high index material | 0–10 mM | [14] | |
| 0.012 mM | |||||
| Uric Acid | ZnO NRs | Col (Section 2.1.4) | Greater enzyme loading | 0.01–5 mM (l) | [10] |
| 4 µM | |||||
| Surface EW (Section 2.5.2) | Surface evanescence generation | 0–500 ppm (l) | [15] | ||
| 5.6 ppm | |||||
| Cysteine | Melamine/ ZnO QDs | Fluo (Section 2.2.3) | QD fluorophore | 0.1–600 µM (l) | [16] |
| 0.642 µM | |||||
| Calcium dipicolinate | Eu/ZnO QDs | Fluo (Section 2.2.3) | QD fluorophore | 0–4 µM (l) | [17] |
| 3 nM | |||||
| Tetracycline | Eu/ZnO QDs | Fluo (Section 2.2.3) | QD fluorophore | 5 nM–3 µM (l) | [18] |
| 4 nM | |||||
| 40 base-pair-long DNA | ZnO/CdS QDs | Fluo (Section 2.2.3) | FRET donor | 75.82 pM–15.28 nM (l) | [19] |
| 8.289 pM | |||||
| Avidin | Mn-doped ZnS/ZnO QDs | Fluo (Section 2.2.3) | Higher quantum yield | 10–100 nM (l) | [20] |
| 3 nM | |||||
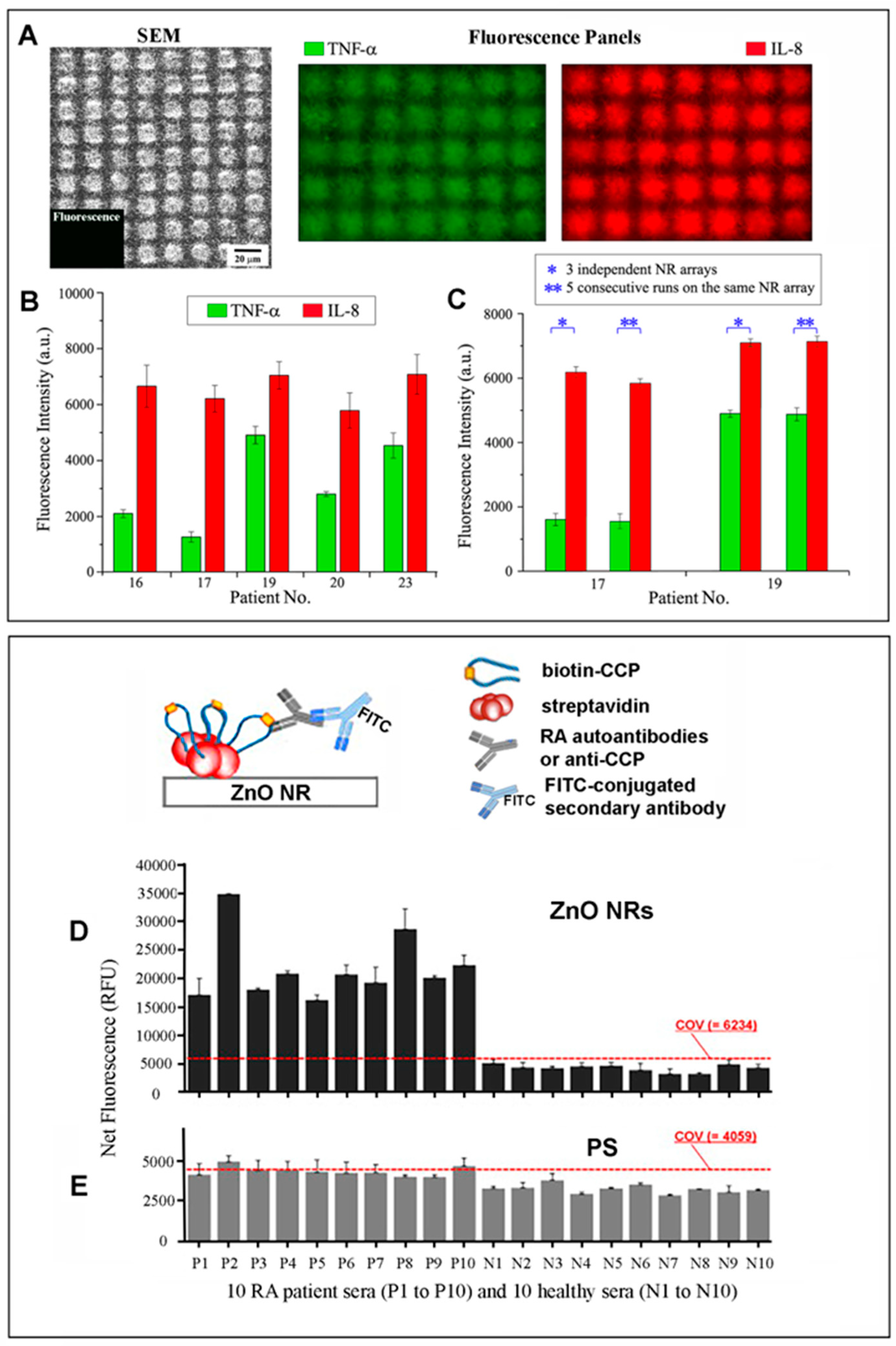
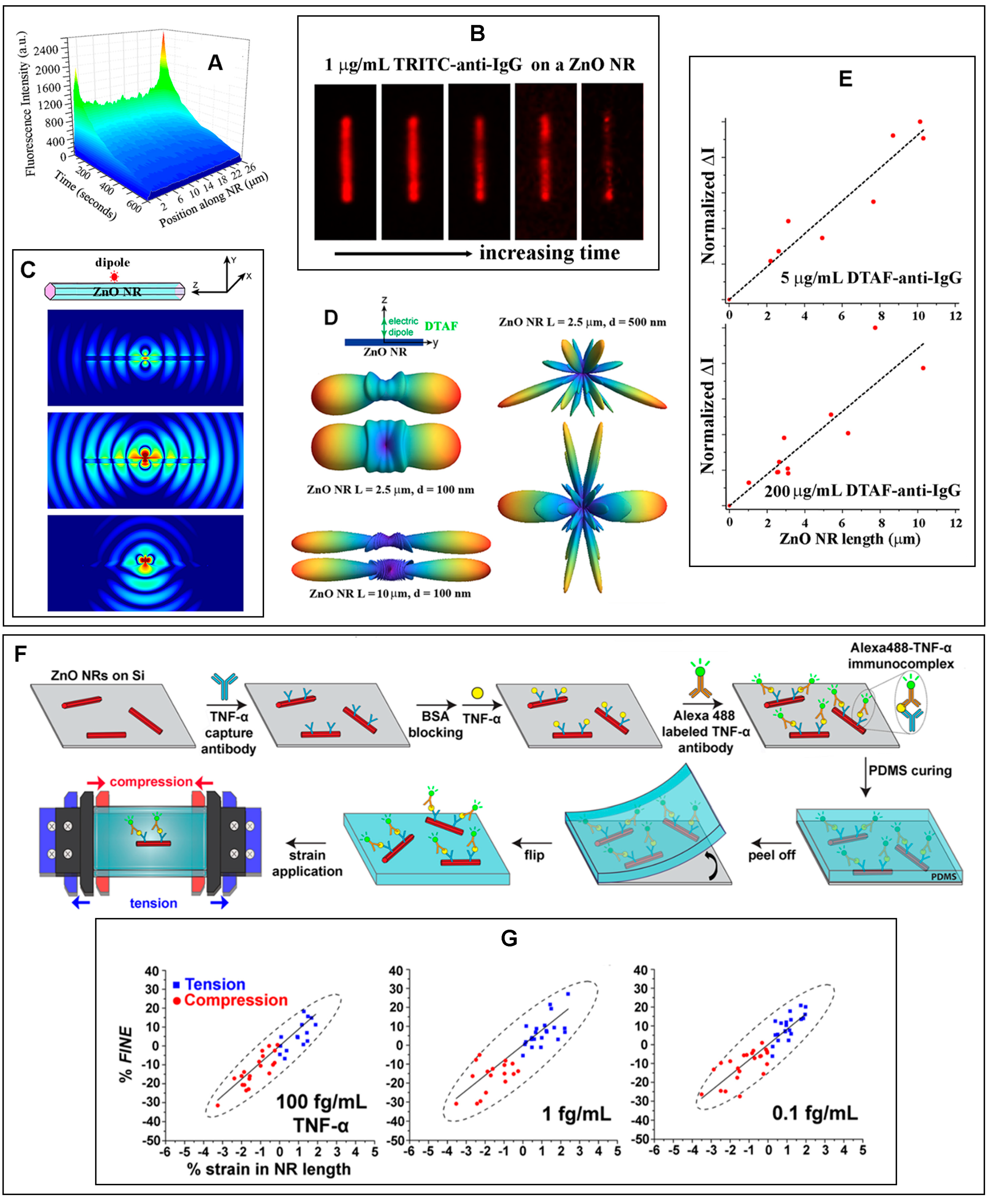
| Immunoglobulin G antibodies | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [21] |
| Fluo (Section 2.2.4.2) | Signal-enhancing platform | N/A | [22,23] | ||
| Bacillus anthracis DNA | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [24] |
| Immunoglobulin G | ZnO NPs coated with Au | SPR (Section 2.6.3) | Greater biomolecule loading | 0.0375–40 µg/mL | [25] |
| 0.0375 µg/mL | |||||
| ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [26] | |
| Bovine serum albumin | CdSe/ZnO QDs | SERS (Section 2.7.3) | Promoting chemical enhancement (CE) | N/A | [27] |
| 2.5 × 10−6 M | |||||
| ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [26] | |
| Telomerase | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [28] |
| Interleukin-8 | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | 10 fg/mL–10 ng/mL (l) | [29] |
| 5.5 fg/mL | |||||
| Tumor necrosis factor-α | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | 10 fg/mL–1 ng/mL (l) | [29] |
| 4.2 fg/mL | |||||
| ZnO NR (i) | Fluo (Section 2.2.4.2) | Subwavelength waveguide | N/A | [30] | |
| Rheumatoid arthritis autoantibodies | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [31] |
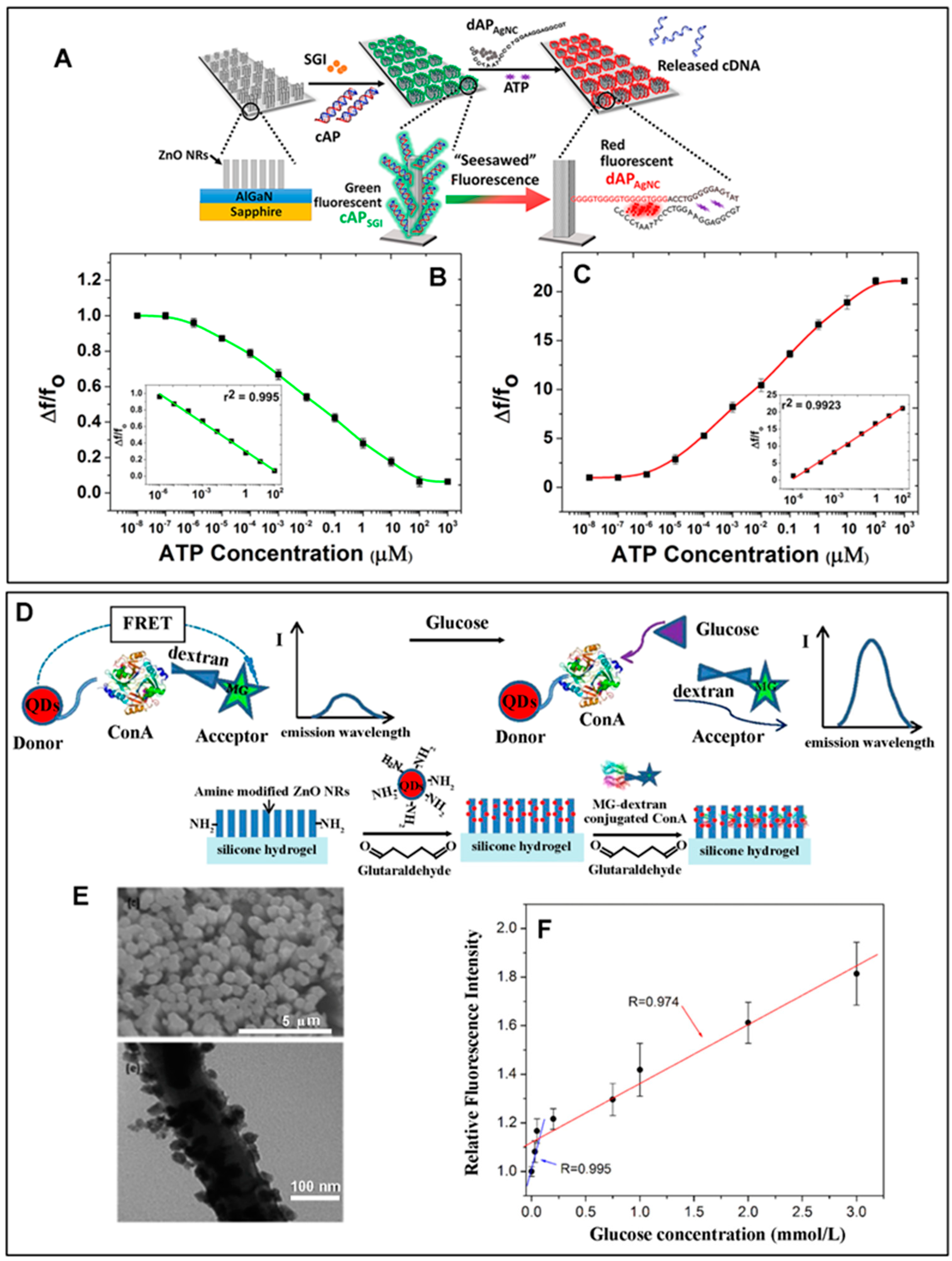
| Adenosine triphosphate | ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | 1 pM–100 µM (l) | [32] |
| 1 pM | |||||
| Cardiac troponin I | Magnetic beads-ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [33] |
| 252.4 pg/mL | |||||
| Carcinoembryonic antigen | ZnO NPs | CL (Section 2.4.3) | Catalyzing radical and electron processes in CL | 0.001–20 ng/mL (l) | [34] |
| 0.001 ng/mL | |||||
| ZnO NRs | Fluo (Section 2.2.4.1) | Signal-enhancing platform | N/A | [35] | |
| 10 fg/mL | |||||
| Fluo (Section 2.2.4.1) | Greater biomolecule loading, FRET acceptor | 0.001–100 ng/mL | [36] | ||
| 0.001 ng/mL | |||||
| Green fluorescent protein | ZnO/Ag/PMMA thin film | Fluo (Section 2.2.5) | Greater antibody loading | 10 pM–100 nM | [37] |
| 7 pM | |||||
| Soluble epidermal growth factor receptor | ZnO/Ag/PMMA thin film | Fluo (Section 2.2.5) | Greater antibody loading | 700 fM–10 nM | [38] |
| 700 fM | |||||
| N-acyl homoserine lactone | Cysteamine/ ZnO NPs | PL (Section 2.3.2) | Deep-level emission (DLE) source | 10–120 nM (l) | [39] |
| N/A | |||||
| Trypsin | Diphenylalanine/ZnO NPs | PL (Section 2.3.2) | DLE source | 0–160 ng/mL (l) | [40] |
| 0.1 ng/mL | |||||
| Calf thymus DNA | ZnO/Au NPs | PL (Section 2.3.2) | DLE source | 0.1–0.7 µM (l) | [41] |
| 36 nM | |||||
| Escherichia coli DNA | ZnO NRs | PL (Section 2.3.3) | DLE source | 0.102–0.894 µM | [42] |
| 28.4 nM | |||||
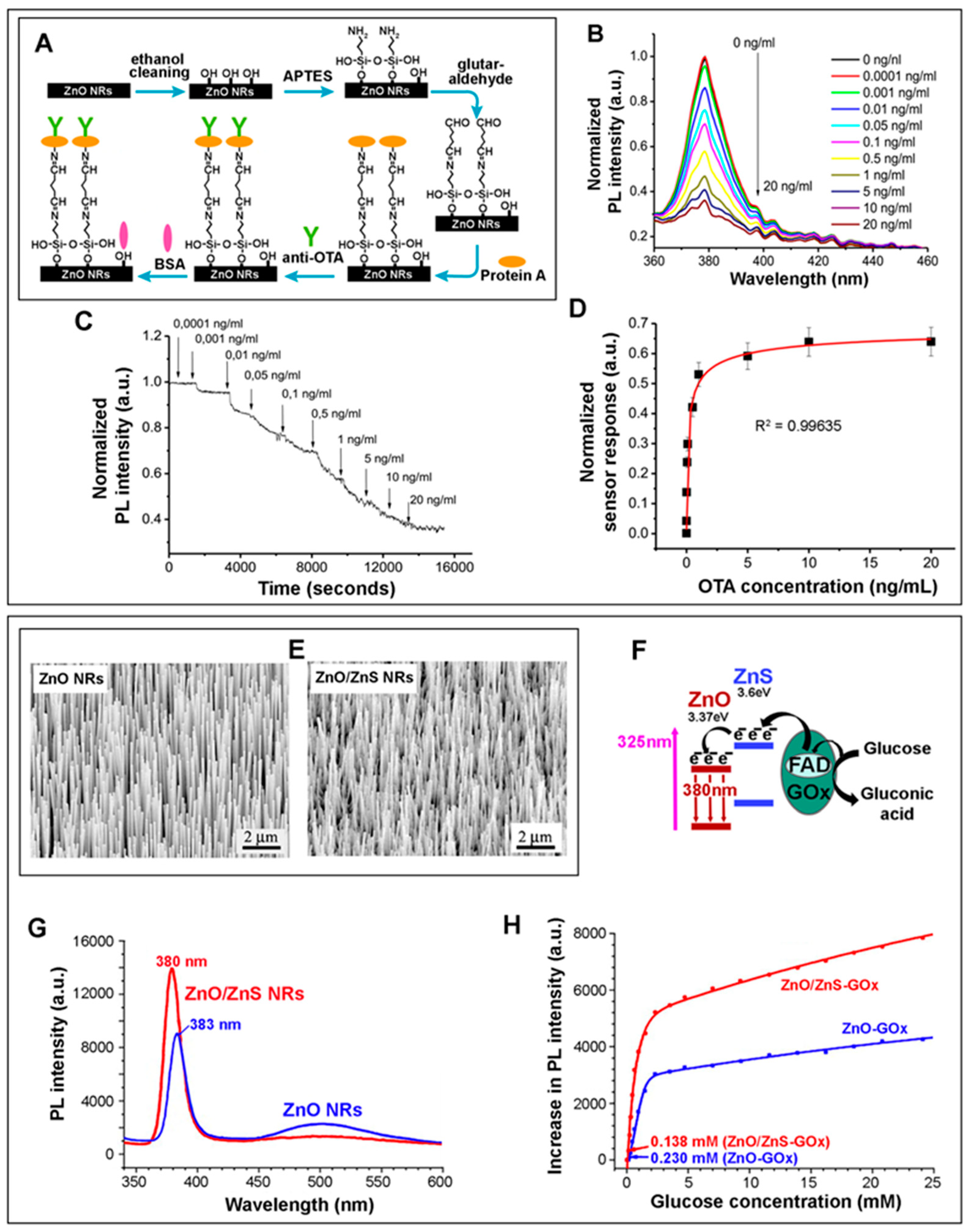
| Ochratoxin A | ZnO NRs | PL (Section 2.3.3) | NBE emission source | 0.1–1 ng/mL | [43] |
| 0.1 ng/mL | |||||
| Grapevine virus A-type proteins | Mn-doped ZnO NRs | WGM (Section 2.5.3) | WGM resonator | 1–200 ng/mL | [44] |
| N/A | |||||
| ZnO thin film | PL (Section 2.3.4) | DLE source | 0.001–10 ng/mL | [45] | |
| N/A | |||||
| Choline | ZnO NRs | CL (Section 2.4.3) | Greater enzyme loading | 0.006–2 mM (l) | [46] |
| 0.0005 mM | |||||
| Urea | Polyaniline/ ZnO NPs | Surface EW (Section 2.5.2) | Surface evanescence generation | 10 nM–1 M (l) | [47] |
| 10 nM | |||||
| Cortisol | Polypyrrole/ ZnO thin film | LMR (Section 2.5.4) | Lossy-mode resonator | 10–105 pg/mL (l) | [48] |
| 25.9 fg/mL | |||||
| p-Cresol | ZnO/MoS2 thin film | LMR (Section 2.5.4) | Lossy-mode resonator | 0.028–1000 µM | [49] |
| 28 nM | |||||
| Neisseria meningitidis DNA | ZnO thin film/Au | SPR (Section 2.6.3) | Greater DNA loading, high-index material | 10–180 ng/µL (l) | [50] |
| 5 ng/µL | |||||
| Cellular DNA, lipids, proteins | ZnO NPs | SERS (Section 2.7.3) | CE, greater analyte binding | N/A | [51] |
| SARS-CoV-2 spike protein | AuNPs/ZnO NRs/Ag film | SERS (Section 2.7.4) | Promoting LSPR excitation | N/A | [52] |
| 0.36 (1.6) × 10−16 M in PBS (saliva) | |||||
| Dopamine | AgNPs/ ZnO microrods (i) | SERS (Section 2.7.4) | WGM resonator | N/A | [53] |
| 1 × 10−12 M | |||||
| Neopterin | Au-coated ZnO thin film | SERS (Section 2.7.5) | Promoting CE and LSPR excitation | N/A | [54] |
| 1.4 nM | |||||
| Metronidazole | Ag-coated ZnO thin film | SERS (Section 2.7.5) | Promoting CE and LSPR excitation | N/A | [55] |
| 0.01 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sytu, M.R.C.; Hahm, J.-I. Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection. Biosensors 2024, 14, 480. https://doi.org/10.3390/bios14100480
Sytu MRC, Hahm J-I. Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection. Biosensors. 2024; 14(10):480. https://doi.org/10.3390/bios14100480
Chicago/Turabian StyleSytu, Marion Ryan C., and Jong-In Hahm. 2024. "Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection" Biosensors 14, no. 10: 480. https://doi.org/10.3390/bios14100480
APA StyleSytu, M. R. C., & Hahm, J.-I. (2024). Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection. Biosensors, 14(10), 480. https://doi.org/10.3390/bios14100480






