Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanostructured Ag/Si Substrates and Modification with 3-Mercaptopropyl-trimethoxysilane (3-MPTMS)
2.3. Biotinylation of Anti-Survivin Detection Antibody
2.4. Preparation of Raman Label
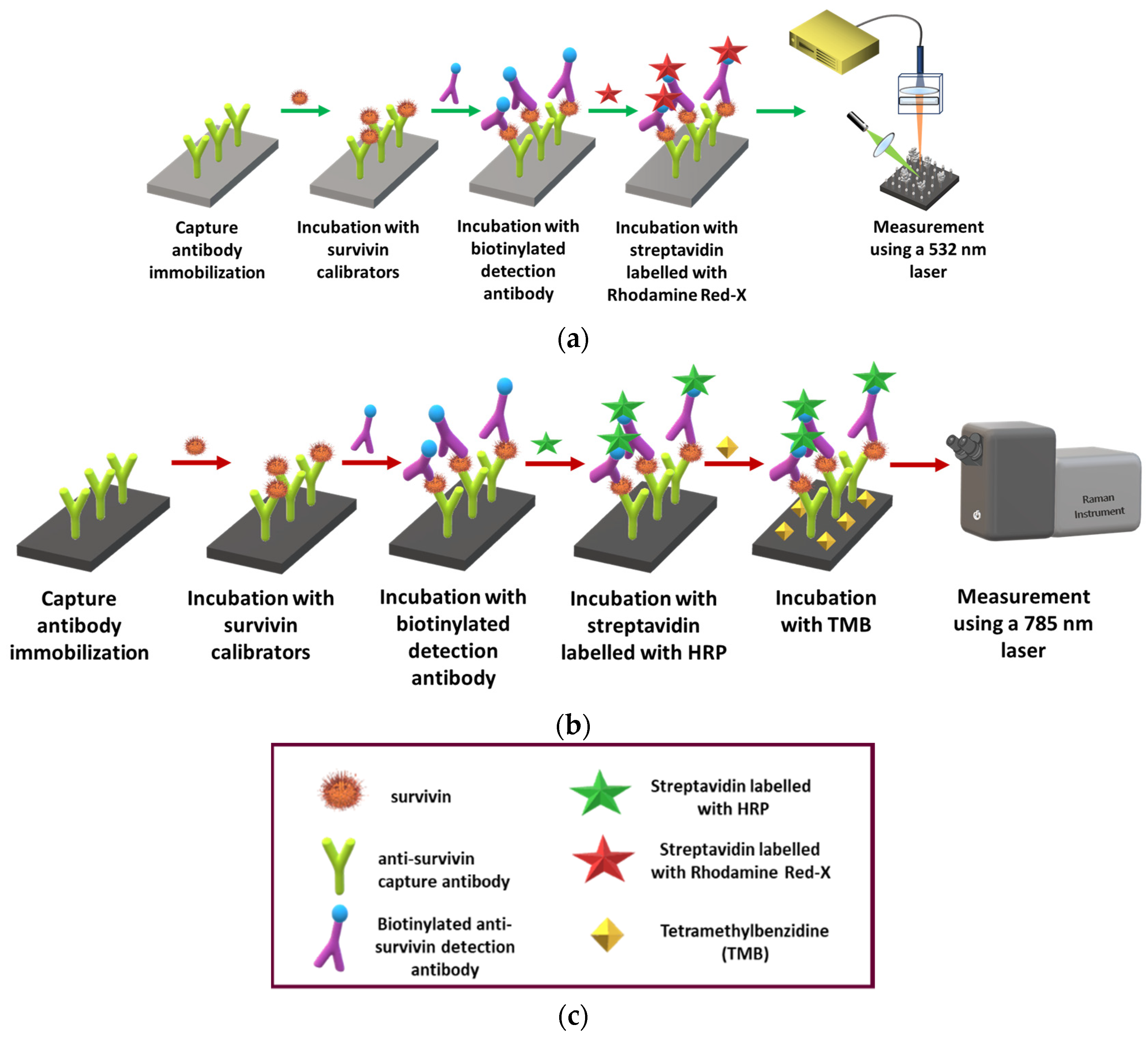
2.5. Survivin Immunoassay on Nanostructured Ag/Si Substrates
2.6. SEF Measurements
2.7. Raman Measurements
3. Results
3.1. Nanostructured Ag/Si Substrates
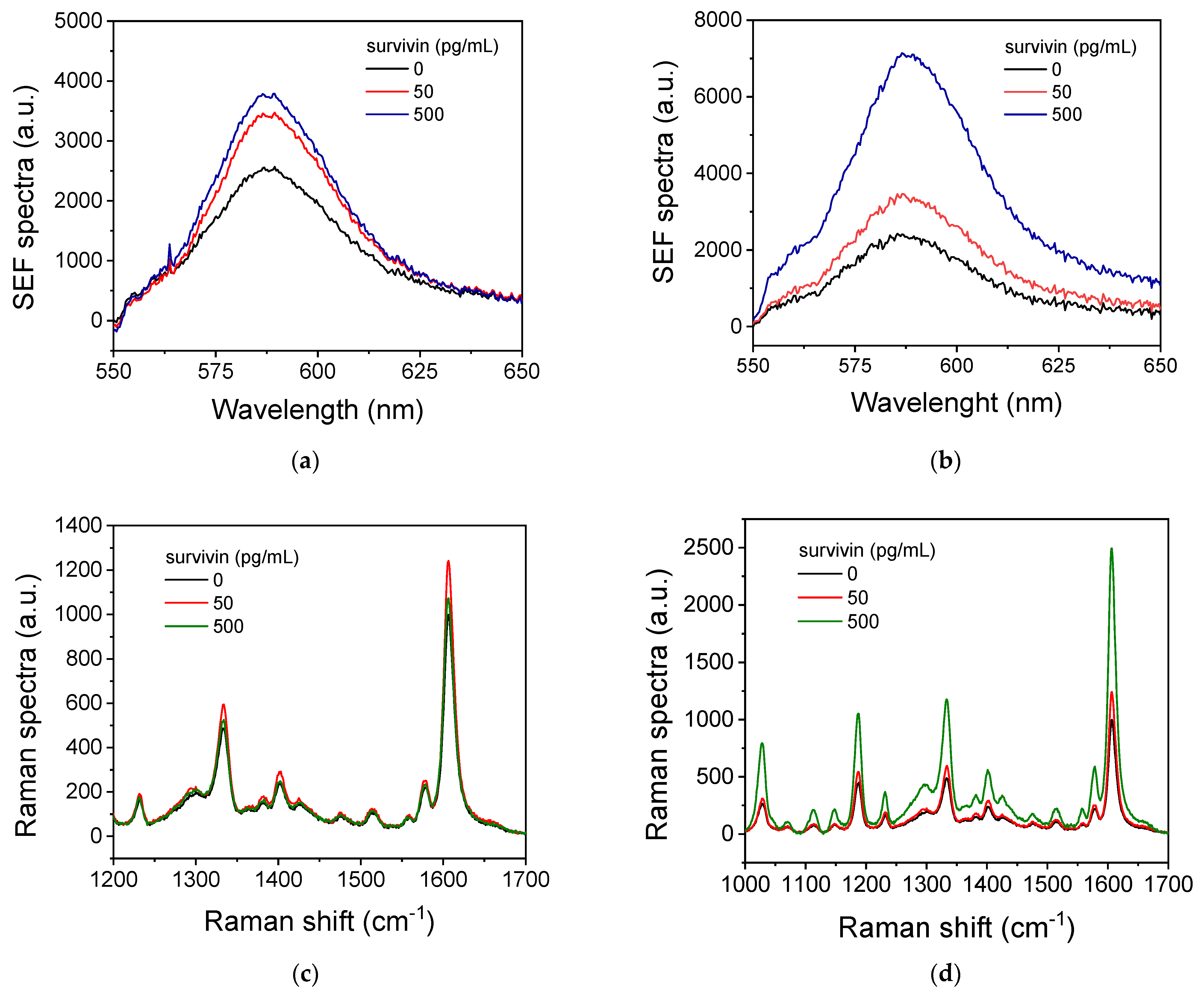
3.2. Selection of Survivin Immunoassay Configuration
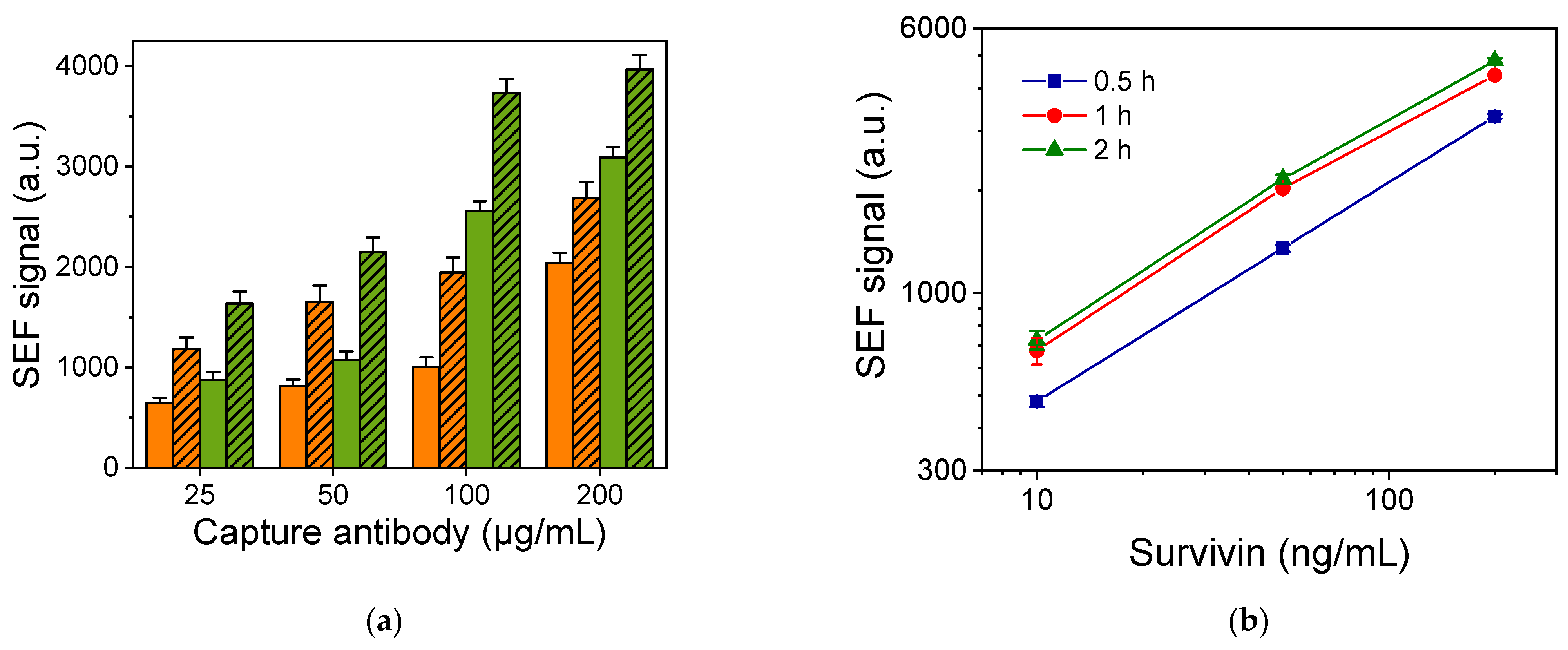
3.3. Survivin SEF Immunoassay Optimization
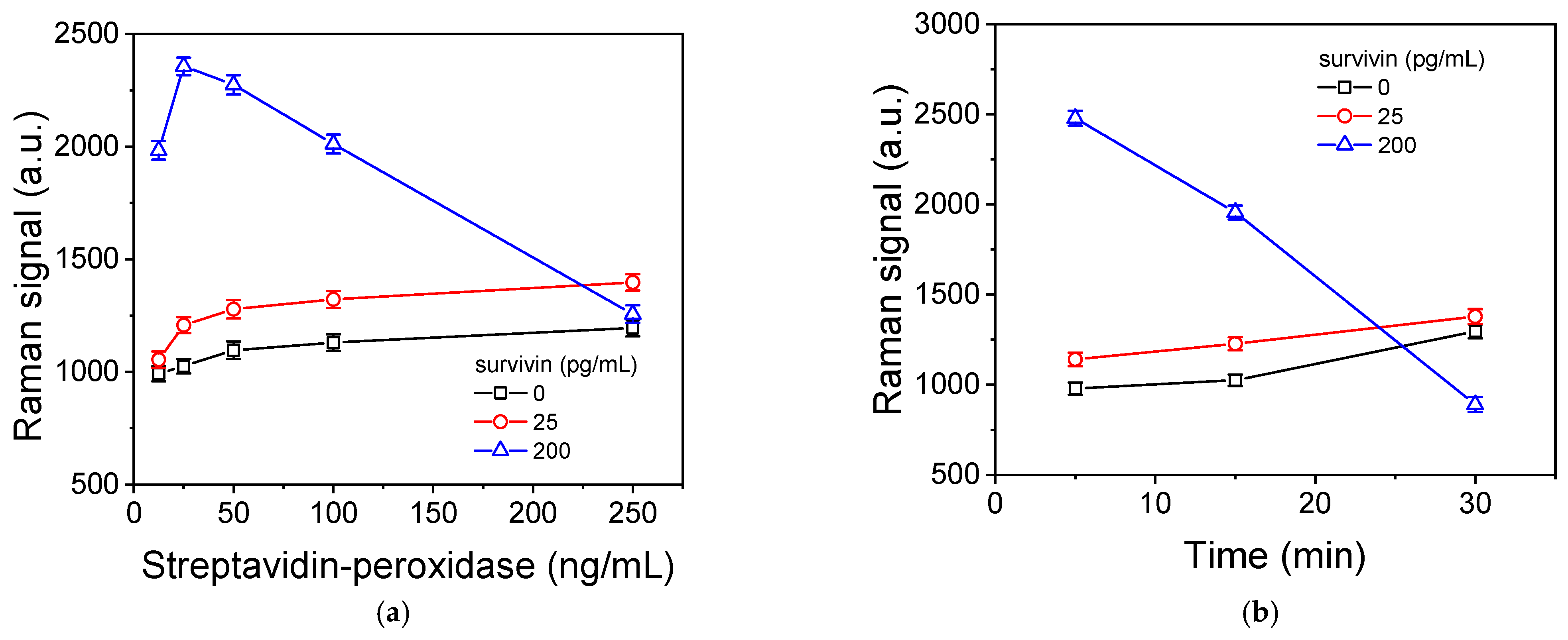
3.4. Optimization of Survivin SERS Immunoassay
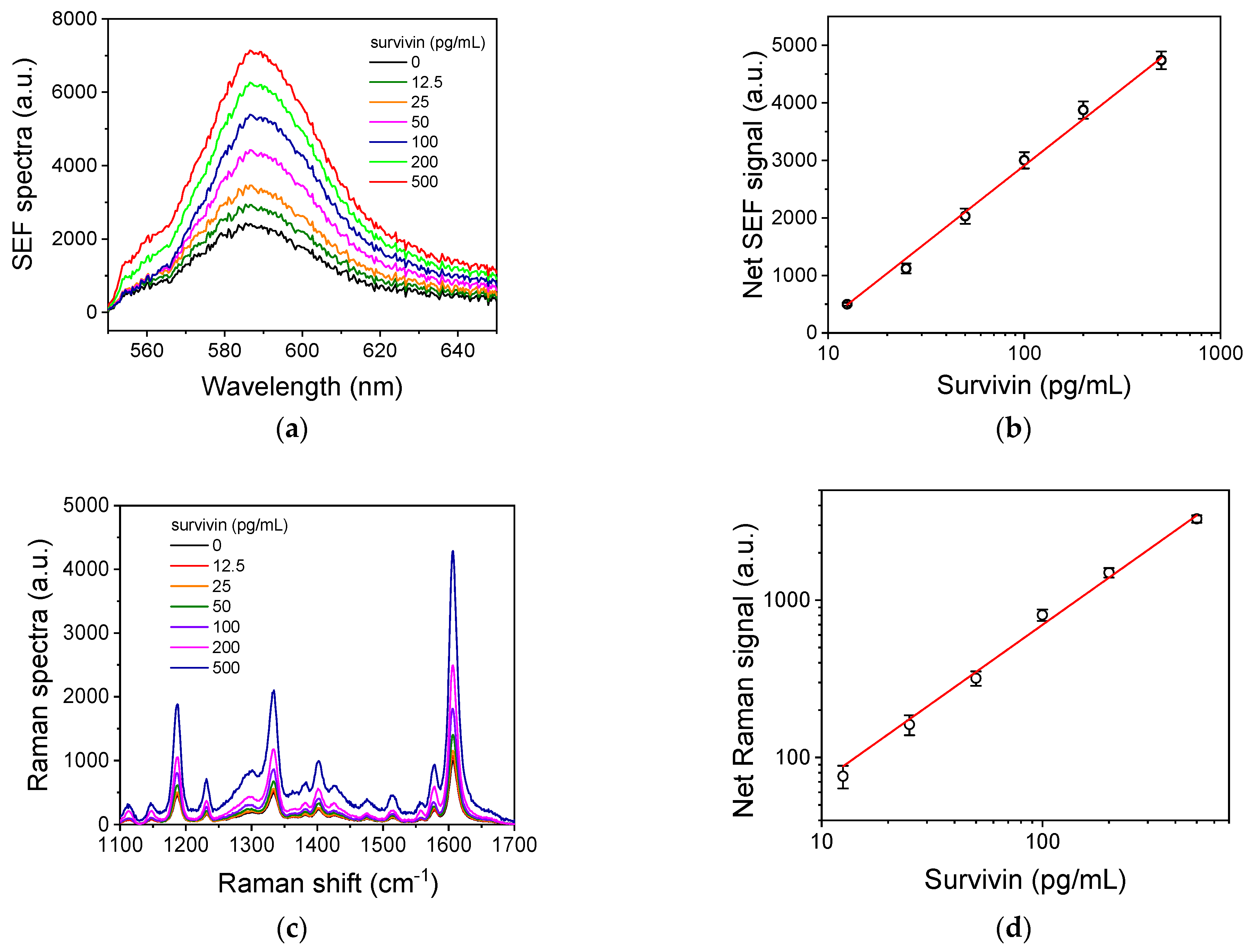
3.5. Analytical Characteristics of Survivin Assays
3.6. Comparison with Literature Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Lu, W.; Yang, J.; Edwards, M.; Jiang, S. Survivin as a biological biomarker for diagnosis and therapy. Expert Opin. Biol. Ther. 2021, 21, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian-Sani, M.R.; Alghasi, A.; Saeedi-Boroujeni, A.; Jalali, A.; Jamshidi, M.; Khodadadi, A. Survivin as a diagnostic and therapeutic marker for thyroid cancer. Pathol. Res. Pract. 2019, 215, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Colella, G.; Giannatempo, G.; Bambini, F.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Detection level of salivary survivin in patients with OSCC. J. Carcinog. Mutagen. 2013, S5, 4. [Google Scholar] [CrossRef]
- Meenakshi Warrier, N.; Agarwal, P.; Kumar, P. Emerging importance of survivin in stem cells and cancer: The development of new cancer therapeutics. Stem Cell Rev. Rep. 2020, 16, 828–852. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Aljahdali, I.; Ling, X. Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study. J. Experiment. Clin. Cancer Res. 2019, 38, 368. [Google Scholar] [CrossRef]
- Dobrzycka, B.; Mackowiak-Matejczyk, B.; Terlikowska, K.M.; Kulesza-Bronczyk, B.; Kinalski, M.; Terlikowski, S.J. Prognostic significance of pretreatment VEGF, survivin, and Smac/DIABLO serum levels in patients with serous ovarian carcinoma. Tumor Biol. 2015, 36, 4157–4165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunaldi, M.; Isiksacan, N.; Kocoglu, H.; Okuturlar, Y.; Gunaldi, O.; Topcu, T.O.; Karabulut, M. The value of serum survivin level in early diagnosis of cancer. J. Can. Res. Ther. 2018, 14, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Gleichenhagen, J.; Arndt, C.; Casjens, S.; Meinig, C.; Gerullis, H.; Raiko, I.; Brüning, T.; Ecke, T.; Johnen, G. Evaluation of a new survivin ELISA and UBC® rapid for the detection of bladder cancer in urine. Int. J. Mol. Sci. 2018, 19, 226. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Q. Microplate magnetic chemiluminescence immunoassay for detecting urinary survivin in bladder cancer. Oncol. Lett. 2017, 14, 4043–4052. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, J.; Zhang, Q. Detection of survivin expression in bladder cancer and renal cell carcinoma using specific monoclonal antibodies. Oncol. Rep. 2018, 39, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Gleichenhagen, J.; Arndt, C.; Casjens, S.; Topfer, C.; Gerullis, H.; Raiko, I.; Taeger, D.; Ecke, T.; Bruning, T.; Johnen, G. Exploring solid-phase proximity ligation assay for survivin detection in urine. PLoS ONE 2022, 17, e0270535. [Google Scholar] [CrossRef]
- Chatziharalambous, D.; Lygirou, V.; Latosinska, A.; Stravodimos, K.; Vlahou, A.; Jankowski, V.; Zoidakis, J. Analytical performance of ELISA assays in urine: One more bottleneck towards biomarker validation and clinical implementation. PLoS ONE 2016, 11, e0149471. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.C.; Shrivastava, S.; Saxena, S.; Kumar, N.; Maiti, S.K.; Mishra, B.P.; Singh, R.K. Surface plasmon resonance immunosensor for label-free detection of BIRC5 biomarker in spontaneously occurring canine mammary tumours. Sci. Rep. 2019, 9, 13485. [Google Scholar] [CrossRef]
- Pollap, A.; Swit, P. Recent advances in sandwich SERS immunosensors for cancer detection. Int. J. Mol. Sci. 2022, 23, 4740. [Google Scholar] [CrossRef]
- Ilyas, A.; Dyussupova, A.; Sultangaziyev, A.; Shevchenko, Y.; Filchakova, O.; Bukasov, R. SERS immuno- and apta-assays in biosensing/bio-detection: Performance comparison, clinical applications, challenges. Talanta 2023, 265, 124818. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, A.I.; Lyu, D.; Lu, Z.; Liu, G. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563. [Google Scholar] [CrossRef] [PubMed]
- Cutshaw, G.; Uthaman, S.; Hassan, N.; Kothadiya, S.; Wen, X.; Bardhan, R. The emerging role of Raman spectroscopy as an omics approach for metabolic profiling and biomarker detection toward precision medicine. Chem. Rev. 2023, 123, 8297–8346. [Google Scholar] [CrossRef] [PubMed]
- Sultangaziyev, A.; Bukasov, R. Review: Applications of surface-enhanced fluorescence (SEF) spectroscopy in bio-detection and biosensing. Sens. Biosens. Res. 2020, 30, 100382. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Peng, W.; Zhang, J.; Zhao, J.; Qi, J.; Yu, C.; Li, J.; Wei, Y. Surface-enhanced photoluminescence and Raman spectroscopy of single molecule confined in coupled Au bowtie nanoantenna. Nanotechnology 2024, 35, 155201. [Google Scholar] [CrossRef] [PubMed]
- Hawa, G.; Sonnleitner, L.; Missbichler, A.; Prinz, A.; Bauer, G.; Mauracher, C. Single step, direct fluorescence immunoassays based on metal enhanced fluorescence (MEF-FIA) applicable as micro plate, array-, multiplexing- or point of care-format. Anal. Biochem. 2018, 549, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Miranda, B.; Chu, K.Y.; Maffettone, P.L.; Shen, A.Q.; Funari, R. Metal-enhanced fluorescence immunosensor based on plasmonic arrays of gold nanoislands on an etched glass substrate. ACS Appl. Nano Mater. 2020, 3, 10470–10478. [Google Scholar] [CrossRef]
- Nebu, J.; Anslin, T.M. New trends in gold nanostructure-based SERS substrate: From fundamental to biomedical applications. Vib. Spec. 2023, 124, 103477. [Google Scholar] [CrossRef]
- Michalowska, A.; Kudelski, A. Plasmonic substrates for biochemical applications of surface-enhanced Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 308, 123786. [Google Scholar] [CrossRef]
- Yang Liu, Y.; Kim, M.; Cho, S.H.; Jung, Y.S. Vertically aligned nanostructures for a reliable and ultrasensitive SERS-active platform: Fabrication and engineering strategies. Nano Today 2021, 37, 101063. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Wang, F.; Jia, Z.; Zhou, J.; Jiang, T.; Petti, L.; Chen, Y.; Xiong, Q.; Wang, X. Classification analyses for prostate cancer, benign prostate hyperplasia and healthy subjects by SERS-based immunoassay of multiple tumour markers. Talanta 2018, 188, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, H.; Liu, Y.; Xu, B.; Song, L.; Han, X.; Liu, R.; He, C.; Cheng, Z.; Zhao, B. SERS-based biosensor for detection of f-PSA%: Implications for the diagnosis of prostate cancer. Talanta 2023, 261, 124654. [Google Scholar] [CrossRef]
- Phuc, L.G.; Do, P.Q.T.; Ta, H.K.T.; Dang, V.Q.; Joo, S.W.; Manh, D.H.; Bach, T.N.; Van, T.T.T.; Tran, N.H.T. Metal-enhanced fluorescence for alpha-fetoprotein detection and for SERS using hybrid nanoparticles of magnetic cluster core—plasmonic shell composite. Chemosensors 2023, 11, 56. [Google Scholar] [CrossRef]
- Han, C.; Chen, R.; Wu, X.; Shi, N.; Duan, T.; Xu, K.; Huang, T. Fluorescence turn-on immunosensing of HE4 biomarker and ovarian cancer cells based on target-triggered metal-enhanced fluorescence of carbon dots. Anal. Chim. Acta 2021, 1187, 339160. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, Z.; Qin, X.; Gu, Y.; Huang, Y.; Qian, Y.; Wang, Z.; Li, H.; Zhu, Q.; Wei, W. LoC-SERS platform for rapid and sensitive detection of colorectal cancer protein biomarkers. Talanta 2024, 270, 125563. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, L.; Li, L.; Zong, S.; Wang, Z.; Cui, Y. Simultaneous and highly sensitive detection of multiple breast cancer biomarkers in real samples using a SERS microfluidic chip. Talanta 2018, 188, 507–515. [Google Scholar] [CrossRef]
- Kochylas, I.; Gardelis, S.; Likodimos, V.; Giannakopoulos, K.P.; Falaras, P.; Nassiopoulou, A.G. Improved surface-enhanced-Raman scattering sensitivity using Si nanowires/silver nanostructures by a single step metal-assisted chemical etching. Nanomaterials 2021, 11, 1760. [Google Scholar] [CrossRef]
- Kochylas, I.; Dimitriou, A.; Apostolaki, M.A.; Skoulikidou, M.C.; Likodimos, V.; Gardelis, S.; Papanikolaou, N. Enhanced photoluminescence of R6G dyes from metal decorated silicon nanowires fabricated through metal assisted chemical etching. Materials 2023, 16, 1386. [Google Scholar] [CrossRef] [PubMed]
- Kanioura, A.; Geka, G.; Kochylas, I.; Likodimos, V.; Gardelis, S.; Dimitriou, A.; Papanikolaou, N.; Kakabakos, S.; Petrou, P. SERS determination of oxidative stress markers in saliva using substrates with silver nanoparticle-decorated silicon nanowires. Biosensors 2023, 13, 273. [Google Scholar] [CrossRef]
- Geka, G.; Kanioura, A.; Kochylas, I.; Likodimos, V.; Gardelis, S.; Dimitriou, A.; Papanikolaou, N.; Chatzantonaki, K.; Charvalos, E.; Economou, A.; et al. Cancer marker immunosensing through surface enhanced photoluminescence on nanostructured silver substrates. Nanomaterials 2023, 13, 3099. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, S. Au0.5Ag0.5 Alloy Nanolayer Deposited on Pyramidal Si Arrays as Substrates for Surface-Enhanced Raman Spectroscopy. ACS Appl. Nano Mater. 2020, 3, 7088–7095. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Li, J.; Tu, Z.; Gu, B.; Wang, S. Ultrasensitive and multiplex detection of four pathogenic bacteria on a bi-channel lateral flow immunoassay strip with three-dimensional membrane-like SERS nanostickers. Biosens. Bioelectron. 2022, 214, 114525. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Zhen, S.J.; Wan, X.Y.; Gao, P.F.; Huang, C.Z. A sensitive surface-enhanced Raman scattering enzyme-catalyzed immunoassay of respiratory syncytial virus. Talanta 2016, 148, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, M.; Sun, Y.; Fan, Y.; Wang, J.; Cao, X.; Lu, D. A sandwich SERS immunoassay platform based on a single-layer Au–Ag nanobox array substrate for simultaneous detection of SCCA and survivin in serum of patients with cervical lesions. RSC Adv. 2021, 11, 36734–36747. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Nedaeinia, R.; Manian, M.; Nickho, H. Rapid noninvasive detection of bladder cancer using survivin antibody-conjugated gold nanoparticles (GNPs) based on localized surface plasmon resonance (LSPR). Cancer Immunol. Immunother. 2020, 69, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Chalupa, A.; Dworakowska, B. Piezometric biosensors for anti-apoptotic protein survivin based on buried positive-potential barrier and immobilized monoclonal antibodies. Biosens. Bioelectron. 2016, 84, 37–43. [Google Scholar] [CrossRef] [PubMed]

| Substrate | Detection Principle | LOD (pg/mL) | Dynamic Range | Analysis Time (min) | Ref. |
|---|---|---|---|---|---|
| Au–Ag nanoboxes | SERS | 5.0 | 5 pg/mL–10 μg/mL | 240 | [40] |
| Au nanoparticles | LSPR | - | - | 15 | [41] |
| Au modified quartz crystal | piezoelectric | 28 | 28–500 pg/mL | 60 | [42] |
| SiNWs-dendrites/ SiNWs-aggregates | SEF SERS | 12.5 | 25–500 pg/mL | 150 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geka, G.; Kanioura, A.; Kochylas, I.; Likodimos, V.; Gardelis, S.; Dimitriou, A.; Papanikolaou, N.; Economou, A.; Kakabakos, S.; Petrou, P. Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates. Biosensors 2024, 14, 479. https://doi.org/10.3390/bios14100479
Geka G, Kanioura A, Kochylas I, Likodimos V, Gardelis S, Dimitriou A, Papanikolaou N, Economou A, Kakabakos S, Petrou P. Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates. Biosensors. 2024; 14(10):479. https://doi.org/10.3390/bios14100479
Chicago/Turabian StyleGeka, Georgia, Anastasia Kanioura, Ioannis Kochylas, Vlassis Likodimos, Spiros Gardelis, Anastasios Dimitriou, Nikolaos Papanikolaou, Anastasios Economou, Sotirios Kakabakos, and Panagiota Petrou. 2024. "Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates" Biosensors 14, no. 10: 479. https://doi.org/10.3390/bios14100479
APA StyleGeka, G., Kanioura, A., Kochylas, I., Likodimos, V., Gardelis, S., Dimitriou, A., Papanikolaou, N., Economou, A., Kakabakos, S., & Petrou, P. (2024). Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates. Biosensors, 14(10), 479. https://doi.org/10.3390/bios14100479










