Abstract
Imatinib mesylate, an anticancer drug, is prescribed to treat gastrointestinal stromal tumors and chronic myelogenous leukemia. A hybrid nanocomposite of N,S-doped carbon dots/carbon nanotube-poly(amidoamine) dendrimer (N,S-CDs/CNTD) was successfully synthesized and used as a significant modifier to design a new and highly selective electrochemical sensor for the determination of imatinib mesylate. A rigorous study with electrochemical techniques, such as cyclic voltammetry and differential pulse voltammetry, was performed to elucidate the electrocatalytic properties of the as-prepared nanocomposite and the preparation procedure of the modified glassy carbon electrode (GCE). A higher oxidation peak current was generated for the imatinib mesylate on a N,S-CDs/CNTD/GCE surface compared to the GCE and CNTD/GCE. The N,S-CDs/CNTD/GCE showed a linear relationship between the concentration and oxidation peak current of the imatinib mesylate in 0.01–100 μM, with a detection limit of 3 nM. Finally, the imatinib mesylate’s quantification in blood-serum samples was successfully performed. The N,S-CDs/CNTD/GCE’s reproducibility and stability were indeed excellent.
1. Introduction
Cancer, in which cells in the body proliferate uncontrollably and then spread throughout different parts of the body, is a fatal disease [1]. One of the drugs confirmed by the Food and Drug Administration for cancer treatment is imatinib mesylate (IMAT). With molecular structure of 4-[(4-methylpiperazin-1-yl)methyl]a-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl] benzamide, IMAT has been used to treat chronic melodic leukemia (CML) [2]. When this drug is administered, the activity of the tyrosine kinase enzyme, whose main function is to inhibit the Bcr-Abl fusion protein, is prevented, and the growth rate of cancer cells is reduced [3,4]. Specific dosages of IMAT lead to different concentrations in the bloodstreams of patients, which suggests a relationship between the concentration level of this drug and the clinical response [5]. Therefore, the progression of CML disease should be controlled by measuring the concentration of the IMAT drug at the plasma level. According to previous reports, monitoring IMB levels in real biological samples is of great environmental and clinical importance [6,7]. Various analyses, such as capillary electrophoresis, high-performance liquid chromatography, gas chromatography, and fluorescence spectroscopy, have been used to measure the concentration of IMAT [8,9,10,11,12,13]. Most of these methods, despite their high sensitivity and highly accurate measurements, are associated with limitations, such as high operating costs, long analysis responses, complex instrumentation, and the need for a large number of samples and skilled and capable operators. By contrast, electrochemical methods are cost-effective and sensitive, with short analytical responses and without the need for sophisticated instruments [14,15,16,17]. They involve the use of appropriate electrochemical sensors to perform the sensitive detection of electroactive substances [18,19,20]. Carbon electrodes have received more attention for use in electrochemical sensors than other electrodes due to their stability, non-reactivity with other materials, and vast electrochemical window [21,22,23,24]. Despite the noticeable features of carbon electrodes, they have a weak electrochemical signal, which is unsuitable for sensitive analyses. Therefore, the modification of carbon electrodes’ surfaces with potential materials will improve the electron transfer of the species in solution at the electrode surface, increase the electrocatalytic activity and, thus, improve the electrochemical responses of the electrode [25,26,27]. According to recent studies, the modification of the electrode surface with carbon nanomaterials, such as graphene, carbon nanotubes, graphene quantum dots, carbon quantum dots (CQDs), and carbon nanospheres, improves the electrochemical performance of the electrode [22,25,26,28,29,30].
Carbon dots (CDs), known as CQDs, are a new class of carbon-based nanomaterial with dimensions of less than 10 nm, which have been considered in many fields, such as electrochemical sensors, photocatalysis, and energy storage, due to their positive features, such as ease of synthesis, minor toxicity, high chemical stability, good dispersibility, biocompatibility, and outstanding optical properties [16,31,32,33,34]. Most of the early studies in this area were conducted on entirely carbon-containing CDs, and it was known that doping them with elements, such as nitrogen, sulfur, boron, and phosphorus, or their compounds, could be used to modulate their properties [35,36,37]. Nitrogen and sulfur atoms act as electron donors on the surfaces of CDs and, consequently, increase their ability to interact with various analyte species [38,39]. A small number of studies have been conducted on the use of CDs in electroanalysis. This intermittent use of CDs can be directly applied with the purpose of modifying electrodes alone or in combination with other compounds. Carbon dots are used in the electrochemical analysis of organic molecules more than other materials. In general, CDs may act as polyvalent redox species in these oxidative determinations, which can facilitate electron transfer and have catalytic benefits [40].
Chemical doping increases the density of charge carriers, resulting in increased electrical properties and catalytic activity. The combination of CDs with conductive nanomaterials, such as carbon nanotubes (CNTs) and graphene, is another solution to improve the dispersibility and electrocatalytic activity of these carbon nanomaterials [41,42,43,44]. Among carbon nanomaterials, CNTs have been considered in electrochemical sensors due to their outstanding optical, electrical, and mechanical properties, high charge-storage capacity, large surface area, excellent electrical conductivity, and chemical and mechanical stability [45,46]. One of the main issues with these nanomaterials is the ineffectiveness of their walls, which makes it difficult for other particles to deposit steadily and uniformly on their surface. The covalent or non-covalent functionalization of CNTs can improve the uniform distribution of nanoparticles on their surfaces, as well as improving their dispersibility in various solvents [47,48]. A suitable strategy for binding functional groups to CNT surfaces and enhancing their distribution and performance is the functionalization of CNT surfaces with nucleic acids, dendrimers, and polymers [16,49]. Multi-branched polymers or dendrimers with specific structures and architectures have been used to stabilize semiconductor nanoparticles [50]. In this study, a novel nanocomposite based on N,S-doped CDs/carbon nanotube-grafted poly(amidoamine) dendrimer (N,S-CDs/CNTD) was introduced as a potential modifier to construct an effective electrochemical sensor. Initially, carboxylic acid groups were created on the surfaces of the CNTs through interactions with nitric acid solution. Next, the CNT-NH2 structure was prepared through an amide reaction between the carboxylic acid and ethylene-diamine groups. Finally, the CNTD nanostructure was prepared through a two-step process, including (I) the preparation of the half-generation of dendrimer on a nanotube (CNTDG0.5) through the Michael-addition reaction between the amine groups on the CNT-NH2 and methyl acrylate, and (II) the synthesis of the first generation of dendrimer through the amidation between the CNTD0.5 and the ethylene diamine. Finally, as shown in Scheme 1, a one-pot synthesis using the hydrothermal method was used to prepare the CNTD decorated with N,S-CDs. In particular, thiourea, as a source of nitrogen and sulfur, can trap free citric acid on the surfaces and edges of CNTs.
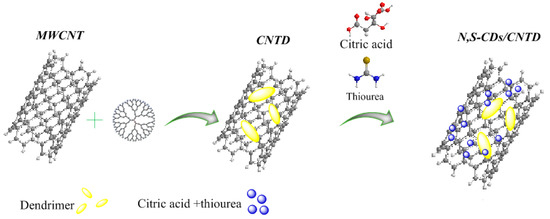
Scheme 1.
Schematic illustration of the synthesis of N,S-doped carbon dots/carbon nanotube-poly(amidoamine) dendrimer.
When comparing different materials, the effect of the nitrogen-doped CDs was more noticeable than that of the sulfur-doped CDs, but the doping of the CNTD with both nitrogen and sulfur had better responses than that of the unmodified CNTD on the electrochemical sensors. On the other hand, in the case of the CNTD nanostructure, the spatial barrier created by the dendrimer separated the CNTs from each other and improved the electrode wettability in the solution. The CNTD acted as a conductive substrate for the CDs, facilitated electron transfer and prevented the accumulation of CDs, thereby providing more electroactive locations. The superb applicability of the sensor design was shown for the electrochemical measurement of the IMAT anticancer drug.
2. Experimental Methods
2.1. Materials
All chemicals, including multi-walled carbon nanotube (MWCNTs), methyl acrylate (MA), ethylenediamine (EDA), nitric acid (HNO3), tetrahydrofuran (THF), N,N′-dicyclohexylcarbodiimide (DCC), citric acid, and thiourea were obtained from Shenzhen Nanoport Co., China, Sigma-Aldrich, and Merck.
2.2. Synthesis of Modifiers
2.2.1. Preparation of CNT-NH2
To create carboxylic acid groups on the surfaces of nanotubes, 100 mL of nitric acid (60%) was added to 2 g of MWCNTs and refluxed for 24 h. After the mixture was cooled to room temperature, it was filtered by a PTFE filter and rinsed several times with distilled water. Next, it was placed in an oven at 60 °C overnight. The CNT-NH2 was prepared via an amidation reaction between carboxyl groups of (CNT-COOH) and amino groups of ethylene diamine. First, 100 mL of THF was added to 4 g of nanotubes and stirred for 6 h, after which it was placed in an ultrasonic bath for 54 min. The mixture was added to a 250 round-bottomed flask, and after the addition of 0.5 g of DCC, 40 mL of ethylene diamine diluted with 40 mL of THF was added dropwise to the contents of the flask and stirred for 24 h. The reaction mixture was filtered through a PTFE-filter membrane and washed several times with THF. Finally, the black powder was dried at 60 °C for 24 h.
2.2.2. Preparation of CNTD
This approach involved a two-step process, including (I) preparation of the half-generation of dendrimer on nanotube (CNTDG0.5) through the Michael-addition reaction between amine groups on CNTNH2 and methyl acrylate and (II) synthesis of the first generation of dendrimer through amidation between CNTD0.5 and ethylene diamine.
Step I: in total, 2 g of CNTNH2 was added to the 100 mL of THF and stirred for 10 h in a two-necked round-bottomed flask, followed by sonication for 45 min. Next, the mixture was purged with nitrogen gas and sealed. Subsequently, 6.4 mL of methyl acrylate in 20 mL of THF was added dropwise to the reaction at 0 °C in an ice bath and stirred for three days in a dark room. The final product was filtered through a 0.2-µm-filter membrane and washed with THF several times, and the black solid (CNTD-G0.5) was dried at 55 °C in oven for 24 h.
Step II-1: in total, 6 g of as-prepared CNTD-G0.5 black powder was stirred in a laboratory reactor containing 160 mL of THF for one day. After probe sonication for 45 min, the reactor was degassed with N2 and then sealed. Subsequently, the reactor was placed in the ice bath, 38 mL of EDA in 40 mL THF was added dropwise to the mixture, and stirring was continued for three days at room temperature. The mixture was filtered by a 0.22-μm filter membrane, and the black solid was washed several times with THF and dried in an oven at 55 °C to obtain the final product.
2.2.3. Preparation of N,S-CDs/CNTD
The N,S-CDs/CNTD hybrid was prepared by the hydrothermal method. In brief, in a one-step synthesis, 0.03 g CNTD, 0.58 g citric acid, and 0.76 g thiourea were dispersed ultrasonically into 30.0 mL of double distilled water, successively. Next, the homogeneous mixture was transferred into a Teflon-lined hydrothermal autoclave (45 mL) and heated at 180 °C for 12 h. After this reaction, the autoclave was cooled to room temperature. The resultant sample was centrifuged at 100,000 rpm for 15 min and the sediment section of the sample containing N,S-CDs/CNTD was freeze-dried and stored at 4 °C.
2.3. Preparation of the Sensor
The glassy carbon electrode (GCE) was first polished using alumina powder. Next, the GCE surface was washed with water and ethanol, and after drying, it was activated by cyclic voltammetry technique in 0.1 M of sulfuric acid solution. Subsequently, 1 mg of the synthesized material was dispersed in 1 mL of DMF, and 2 μL of the material was placed on the electrode surface. After drying the electrode surface, IMAT measurement was performed.
2.4. Measurements and Analyses
All electrochemical analyses were performed using PSTrace software and a PalmSens EmStat3 + apparatus. The Fourier-transform infrared (FTIR) experiment was carried out in the spectral range of 400–4000 cm−1 using a PerkinElmer spectrometer. The X-ray diffraction (XRD) pattern was determined using a Siemens (Germany) X-ray diffractometer with Cu Kα radiation (λ = 0.1542 nm). The energy-dispersive X-ray spectroscopy (EDS) was carried out using a TESCAN MIRA3 SEM to confirm elemental composition of the samples. Elemental analysis (CHNS) was performed on a COSTEH elemental analyzer. The TEM images were obtained by a transmission-electron microscope (LEO 906 E, Germany) at an accelerator voltage of 100 kV. Photoluminescence (PL) spectra of CDs were measured with a JASCO FP-750 Spectrofluorometer.
3. Results
3.1. Characterization
The FTIR results of the CNT-NH2, CNTD, and N,S-CDs/CNTD are displayed in Figure 1. The CNTNH2 showed characteristic peaks at 1382 and 1633 cm−1, which were assigned to the stretching vibration of the C–N and the bending vibration of the N–H band, respectively. The stretching vibration of the C=O groups was at 1730 cm−1. Furthermore, the band observed at 3436 cm−1 was attributed to the stretching vibration of the N–H bands. In the case of the CNTD, the N–H bending vibration intensified at 1638 cm−1, and the stretching vibration of the carbonyl units of the methyl acrylate resulted in a peak at 1720 cm−1. The stretching vibration of the N–H units was also observed at 3430 cm−1. In the FTIR spectra of the N,S-CDs/CNTD, several characteristic peaks were identified. The sharp absorption peak at 3433 cm−1 corresponded to the stretching vibration of the N–H units. The stretching vibration of the C–H bond appeared at 2066 cm−1. The peak at 1702 cm–1 corresponds to the stretching vibration of the –C=O, and the peak at 1405 cm–1 was due to the C–N bending vibration. The C–OH stretching mode appeared at 1178 cm–1, and the peaks at 620 and 886 cm–1 corresponded to the C–S stretching vibration.
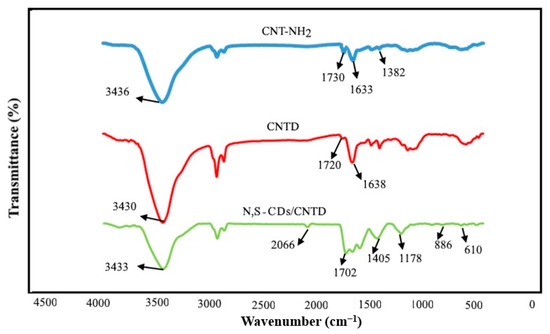
Figure 1.
FTIR spectra of the CNT-NH2, CNTD, and N,S-CDs/CNTD.
The elemental analysis of the N,S-CDs/CNTD was also performed by EDS. The amount of elements detected by the EDS is shown in Figure 2. Carbon (80.19 wt%), nitrogen (3.17 wt%), oxygen (7.13 wt%), and sulfur (8.84 wt%) were identified on the surfaces of the hybrid nanoparticles. Therefore, according to the EDS data, the presence of nitrogen and sulfur in the N, S-CDs confirmed its successful formation. The results of the CHNS elemental analysis provide robust support for the structural formation of the prepared N,S-CDs. According to the CHNS results, the percentages of carbon, nitrogen, and sulfur in the N,S-CDs/CNTD were determined as 77.70, 3.32, and 5.40 wt%, respectively, which are close to the results of the EDS analysis.
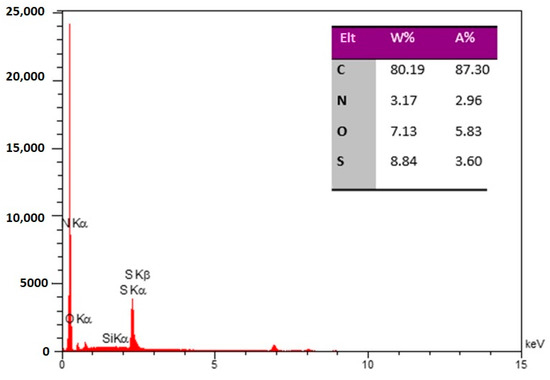
Figure 2.
EDS spectrum of the N, S-CDs/CNTD sample.
Figure 3 shows the typical X-ray diffraction (XRD) spectra of the CNT-NH2, CNTD, and N, S-CDs/CNTD powders. In the CNT-NH2 pattern, a peak represented at the diffraction angle of 25° was related to the X-ray reflection of the plate (002). After the modification of the CNT-NH2, the diffraction angle of the prepared CNTD and N, S-CDs/CNTD decreased from 25.3 to 24.7° due to the increase in the distance between the layers of nanotubes during the functionalization. The other two peaks, at 2θ = 39.4 and 44.6°, were related to crystal plates (100) and (101), respectively.
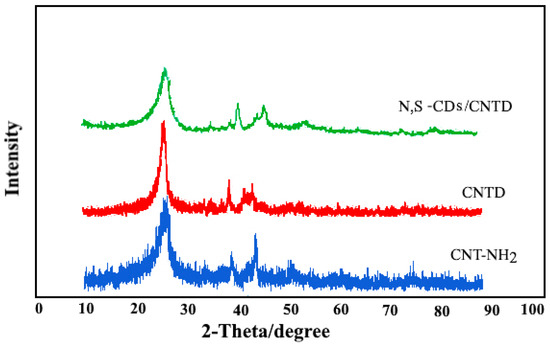
Figure 3.
XRD patterns of CNT-NH2, CNTD, and N,S-CDs/CNTD.
For an accurate study of the N,S-CDs/CNTD, the PL measurements were performed using various excitation wavelengths, as shown in Figure 4. By changing the wavelength of excitation from 280 to 380 nm, the N,S-CDs/CNTD showed PL-excitation intensity at 450 nm, and the PL intensity was maximized at the excitation wavelength of 380 nm. In this case, the PL peaks at 450 nm were related to the n→π* transition of the carboxyl and carbonyl groups. Moreover, when the excitation wavelength increased from 380 to 400 nm, the location of the spectral emission peak of the N, S-CDs/CNTD red-shifted and its intensity decreased. It was found that the N, S-CDs/CNTD showed photoemission corresponding to the excitation wavelength due to changes in the distribution of the particle size. In addition, various functional groups containing nitrogen, oxygen, and sulfur atoms at the edge of the N, S-CDs might have generated a trap state between the levels of HOMO and LUMO.
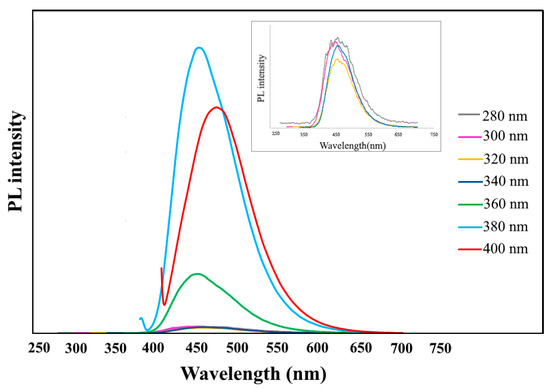
Figure 4.
PL spectra of N,S-CDs under different excitation wavelengths.
The nitrogen and sulfur atoms doped on the surfaces of the CDs acted as electron donors and increased their sensitivity to interactions with various analytical species [51]. To investigate the origin of these properties, the TEM analysis was performed to determine the size, morphology, and structure of the synthesized nanoparticles. Figure 5a,b show the representative TEM images of the CNTD and N, S-CDs/CNTD hybrid nanocomposites, respectively. Figure 5a shows carbon-nanotube surfaces grafted with layers of PAMAM dendrimers, which indicated the formation of the CNTD nanohybrids. Figure 5b demonstrates the formation of small-sized monodispersed N,S-CDs on dendrimer-modified carbon nanotubes.
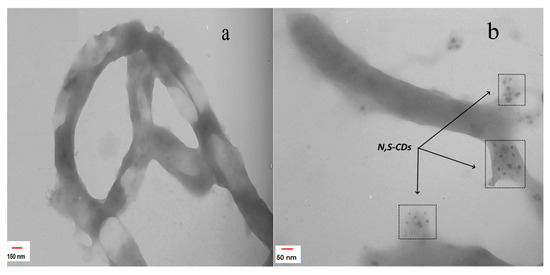
Figure 5.
TEM images of the (a) CNTD and (b) N, S-CDs/CNTD hybrids.
3.2. Electrochemical Analyses
The performance of the prepared electrodes in a probe solution containing 1 mM K4[Fe(CN)6]/K3[Fe(CN)6] and 0.1 M KCl was investigated by the cyclic voltammetry method, and the results are shown in Figure 6a. The differences between the oxidation and reduction peaks’ potential at GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE were 0.35, 0.17, and 0.13 V, respectively. The oxidation current of the electrochemical probe at the surface of the N,S-CDs/CNTD/GCE was greater than that of the GCE and CNTD/GCE, which indicates that the N,S-CDs/CNTD/GCE had better electrocatalytic properties. The electrochemical impedance spectroscopy results confirmed the better electrochemical performance of the N,S-CDs/CNTD/GCE. Figure 6b shows the Nyquist diagram of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE. The charge-transfer-resistance values of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE were 4.43, 1.25, and 0.43 Ω, respectively. The lower resistance on the surface of the N,S-CDs/CNTD/GCE indicates its better electrochemical behavior [52]. The surface area of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE was calculated using the Randles–Sevcik equation. The area obtained from the electrochemical response of the N,S-CDs/CNTD/GCE was greater than that obtained with the other electrodes, indicating its good capability as an electrochemical sensor.
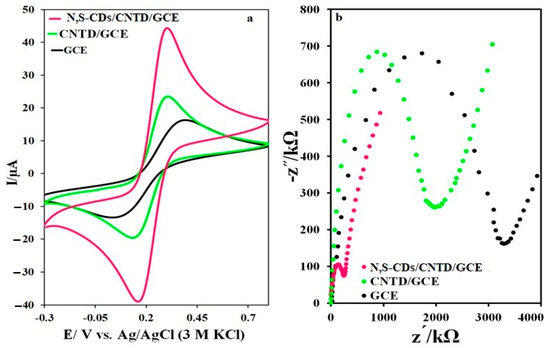
Figure 6.
(a) CVs and (b) Nyquist plot of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE in a 1-mM electrochemical probe solution.
The electrochemical behavior of the IMAT on the surfaces of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE was also investigated (Figure 7a). The oxidation potential of the IMAT on the GCE was 0.97 V without any reduction peaks, indicating an irreversible reaction. The oxidative peak current of the IMAT was equal to 2 μA on the GCE, which was about six and sixteen times less than the CNTD/GCE and N,S-CDs/CNTD/GCE, respectively. The oxidation-peak potential of the IMAT on the CNTD/GCE and N,S-CDs/CNTD/GCE was 0.93 V. Notably, the presence of dendrimer-containing CNTs improved the electrodes’ conductivity and extended their effective surface area. The synergistic effect of the N,S-CDs and CNTD facilitated the electron-transfer rate of the IMAT oxidation process on the N,S-CDs/CNTD/GCE and provided perfect capability for the IMAT analysis. No peaks were observed in the absence of the drug on the GCE, CNTD/GCE, or N,S-CDs/CNTD/GCE (Figure 7b).
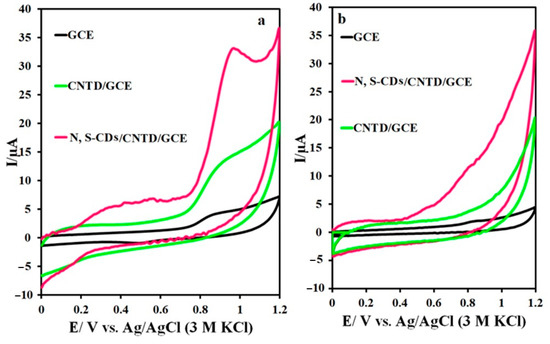
Figure 7.
(a) CVs of the GCE, CNTD/GCE, and N,S-CDs/CNTD/GCE in the (a) presence and (b) absence of IMAT.
An investigation of the effect of the pH of the buffer solution on the voltammetric response of the IMAT revealed effective pH behavior. Figure 8a shows the IMAT cyclic voltammograms at N,S-CDs/CNTD/GCE. Accordingly, the oxidation-peak potential of the IMAT shifted to negative values with increasing pH. The maximum peak current was related to pH 7, so this pH value was used as the optimum condition for further evaluations (Figure 8b). In addition, the peak-potential curve versus the pH for the IMAT oxidation process showed a slope value of −0.03 V/pH, which pointed to the double contribution of the electrons and protons in the process of IMAT oxidation (Figure 8c).
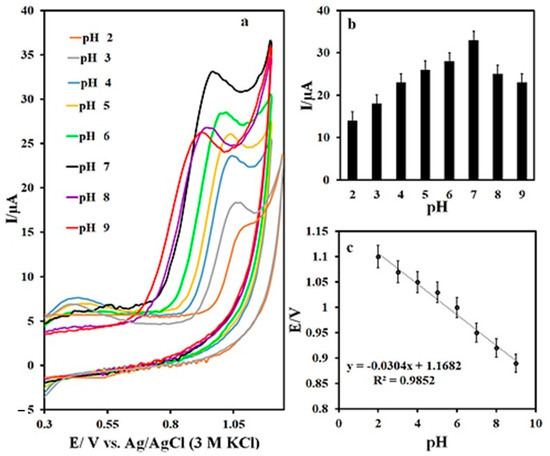
Figure 8.
(a) CVs of IMAT at different pH, (b) plot of Ipa against pH, and (c) plot of Epa against pH.
The scanning rate of the IMAT oxidation is shown in Figure 9a. The evaluation of the peak currents of the IMAT cyclic voltammograms recorded on the N,S-CDs/CNTD/GCE in a buffered solution (pH 7) revealed a linear relationship between the peak current and the square root of the potential scan rate, indicating a diffusion-controlled reaction for the IMAT oxidation (Figure 9b). The electron-transfer coefficient (α) was obtained by plotting the potential curve against the logarithm of the peak current at a scan speed of 10 mV/s (Figure 9c). The α = 0.63 was calculated by considering the slope value (0.1820) of the plotted curve, which was equal to 2.303 RT/(1-α)nF.
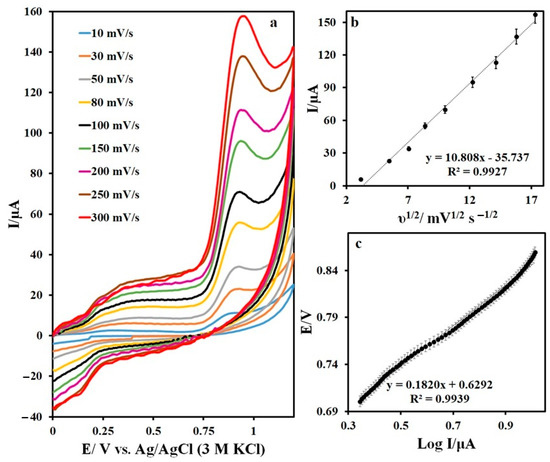
Figure 9.
(a) CVs of IMAT at different scan rates, (b) plot of Ipa against square of scan rate, and (c) plot of Epa against Log Ipa.
Different concentrations were measured for the N,S-CDs/CNTD/GCE at pH 7.0 by using the differential pulse voltammetry technique, as shown in Figure 10a. The linear relationship of the peak current with the IMAT concentration was obtained in the concentration range of 0.03–75 Μm (Figure 10b). The detection limit of the IMAT by the N,S-CDs/CNTD/GCE was calculated as LOD = 3 s/m equal to 0.01 μM. A comparison of the IMAT-measurement performance in previously reported methods with that of the N,S-CDs/CNTD/GCE sensor is given in Table 1. The results show that the N,S-CDs/CNTD/GCE produced a comparable LOD for IMAT analysis to the other methods and a more expansive concentration range. Therefore, the N,S-CDs/CNTD/GCE has outstanding capability in IMAT analysis.
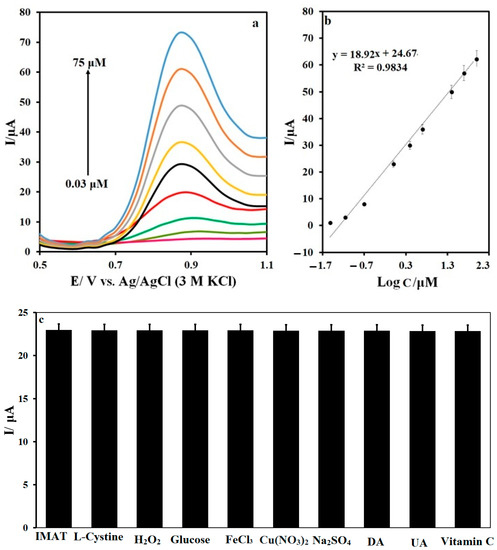
Figure 10.
(a) DPVs of different concentrations of IMAT, (b) the calibration curve (c), and the peak current of 5-μM IMAT recorded in the presence of 100 μM of some potential interferences.

Table 1.
Comparison of the performances of different analytical methods for IMAT measurement.
The reproducibility of the N,S-CDs/CNTD/GCE was evaluated by five consecutive measurements of the IMAT concentration. The results showed that the relative standard deviation obtained from the IMAT oxidation peak currents after five repetitions was equal to 3.45, which indicates the appropriate reproducibility of the N,S-CDs/CNTD/GCE. The stability test of the N,S-CDs/CNTD/GCE for 30 days showed that the IMAT’s peak current changed by about 2.4% by the thirtieth day compared to its value of the first day of the experiments. This indicates that the modified electrode had very good stability. The electrochemical measurements of the IMAT were investigated in the presence of various compounds using N,S-CDs/CNTD/GCE, as shown in Figure 10c. The results showed a slight change in the IMAT’s oxidation peak current in the presence of the evaluated compounds. Considering that the observed changes were less than 5%, it can be concluded that the N,S-CDs/CNTD/GCE has an appropriate selectivity for IMAT measurements. The IMAT measurement on the N,S-CDs/CNTD/GCE in the human-plasma samples was performed by using the standard addition method. The results are given in Table 2. After the standard addition of IMAT to the blood sample at 1, 3, and 10 μM, recovery percentages of about 109–92% were obtained when performing an analysis of the N,S-CDs/CNTD/GCE. These results confirmed the good ability of the N,S-CDs/CNTD/GCE to measure IMAT in a real sample.

Table 2.
The measurement of IMAT in human-blood serum.
4. Conclusions
An IMAT measurement was performed on the surface of N,S-CDs/CNTD/GCE. The N, S-CDs modifier composited with CNTD expanded the effective surface area of the sensor, and the conductivity and wettability of the electrode surface were boosted by the CNTD. On the other hand, the presence of CDS containing nitrogen and sulfur, which improved the performance of the carbon dot, showed very good results for IMAT analysis. The low detection limit, high selectivity, and sensitivity were among the most important features of this electrochemical sensor for measuring IMAT. In addition, the high interference tolerance of N,S-CDs/CNTD/GCE when measuring IMAT in real examples is an appreciable feature.
Author Contributions
Nano-hybrid synthesis, formal analysis, investigation, writing—original draft, M.S.M.; conceptualization, methodology, visualization, writing—revision and editing, M.G.; supervision and editing, S.H.; conceptualization, funding acquisition, project administration, H.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Iran National Science Foundation (INSF) (grant no.: 99021685).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support for this work provided by the Shahid Rajaee Teacher Training University, Tehran, Iran.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghalkhani, M.; Sohouli, E. Synthesis of the decorated carbon nano onions with aminated MCM-41/Fe3O4 NPs: Morphology and electrochemical sensing performance for methotrexate analysis. Microporous Mesoporous Mater. 2021, 331, 111658. [Google Scholar] [CrossRef]
- Cahill, K.N.; Katz, H.R.; Cui, J.; Lai, J.; Kazani, S.; Crosby-Thompson, A.; Garofalo, D.; Castro, M.; Jarjour, N.; DiMango, E. KIT inhibition by imatinib in patients with severe refractory asthma. N. Engl. J. Med. 2017, 376, 1911–1920. [Google Scholar] [CrossRef]
- Bende, G.; Kollipara, S.; Movva, S.; Moorthy, G.; Saha, R. Validation of an HPLC method for determination of imatinib mesylate in rat serum and its application in a pharmacokinetic study. J. Chromatogr. Sci. 2010, 48, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Al-nayili, A.; Rzoqy, M. Local silica sand as a silica source in the synthesis of Y zeolite. Asia-Pac. J. Chem. Eng. 2022, 17, e2824. [Google Scholar] [CrossRef]
- Buclin, T.; Thoma, Y.; Widmer, N.; André, P.; Guidi, M.; Csajka, C.; Decosterd, L.A. The steps to therapeutic drug monitoring: A structured approach illustrated with imatinib. Front. Pharmacol. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Mariño-Enríquez, A.; Tao, D.L.; Ketzer, J.; Eilers, G.; Zhu, M.; Yu, C.; Mannan, A.M.; Rubin, B.P.; Demetri, G.D. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer 2019, 120, 612–620. [Google Scholar] [CrossRef]
- Ajel, M.K.; Al-Nayili, A. Synthesis, characterization of Ag-WO3/bentonite nanocomposites and their application in photocatalytic degradation of humic acid in water. Environ. Sci. Pollut. Res. 2023, 30, 20775–20789. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Huang, L.; Ye, L.; Zhou, Z.; Xiang, G.; Xu, L. Determination of imatinib mesylate and related compounds by field amplified sample stacking with large volume sample injection capillary electrophoresis. J. Pharm. Biomed. Anal. 2012, 70, 26–31. [Google Scholar] [CrossRef]
- Roth, O.; Spreux-Varoquaux, O.; Bouchet, S.; Rousselot, P.; Castaigne, S.; Rigaudeau, S.; Raggueneau, V.; Therond, P.; Devillier, P.; Molimard, M. Imatinib assay by HPLC with photodiode-array UV detection in plasma from patients with chronic myeloid leukemia: Comparison with LC-MS/MS. Clin. Chim. Acta 2010, 411, 140–146. [Google Scholar] [CrossRef]
- Karim, S.E.A.; El-Nashar, R.M.; Abadi, A.H. Potentiometric determination of imatinib under batch and flow injection analysis conditions. Int. J. Electrochem. Sci. 2012, 7, 9668–9681. [Google Scholar]
- Karim, S.S.E.A.; Farghaly, R.A.; El-Nashar, R.M.; Abadi, A.H. Spectrophotometric Determination of Imatinib Mesylate using Charge Transfer Complexs in Pure Form and Pharmaceutical Formulation. Chem. Rapid Commun. 2014, 2, 55–63. [Google Scholar]
- Rahimi Kakavandi, N.; Asadi, T.; Jannat, B.; Abdi, K.; Ghazi-Khansari, M.; Shahali, H.; Naraki, K. Method development for determination of imatinib and its major metabolite, N-desmethyl imatinib, in biological and environmental samples by SA–SHS–LPME and HPLC. Biomed. Chromatogr. 2021, 35, e5088. [Google Scholar] [CrossRef] [PubMed]
- Al-nayili, A.; Muhammad, N.J. Perovskite’s LaNiMnO6/montmorillonite K10 nanocomposites: Synthesis and enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2023, 155, 107254. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Gharagozlou, M.; Sohouli, E.; Khosrowshahi, E.M. Preparation of an electrochemical sensor based on a HKUST-1/CoFe2O4/SiO2-modified carbon paste electrode for determination of Azaperone. Microchem. J. 2022, 175, 107199. [Google Scholar] [CrossRef]
- Sohouli, E.; Keihan, A.H.; Shahdost-fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A glassy carbon electrode modified with carbon nanoonions for electrochemical determination of fentanyl. Mater. Sci. Eng. C 2020, 110, 110684. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B. Stimuli-chromism of photoswitches in smart polymers: Recent advances and applications as chemosensors. Prog. Polym. Sci. 2019, 98, 101149. [Google Scholar] [CrossRef]
- Dezhakam, E.; Khalilzadeh, B.; Mahdipour, M.; Isildak, I.; Yousefi, H.; Ahmadi, M.; Naseri, A.; Rahbarghazi, R. Electrochemical biosensors in exosome analysis; a short journey to the present and future trends in early-stage evaluation of cancers. Biosens. Bioelectron. 2022, 222, 114980. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Liang, M.; Zheng, H.; Zhu, C.; Wang, Y. Detection of imatinib based on electrochemical sensor constructed using biosynthesized graphene-silver nanocomposite. Front. Chem. 2021, 9, 670074. [Google Scholar] [CrossRef]
- Zaidan, B.A.H.; Sohouli, E.; Mazaheri, S. A Novel Capping Agent in Preparation and Characterization of CuAl2O4/CuO Nanocomposite and its Application for Electrochemical Detection of Dopamine. Anal. Bioanal. Electrochem. 2019, 11, 108–122. [Google Scholar]
- Gbair, G.A.; Alshamsi, H.A. Facile green synthesis of CuO-ZnO nanocomposites from Argyreia nervosa leaves extract for photocatalytic degradation of Rhodamine B dye. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. Highly sensitive electrochemical sensor based on La3+-doped Co3O4 nanocubes for determination of sudan I content in food samples. Food Chem. 2019, 286, 191–196. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: A docking/experimental investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Albdiry, M.; Al-Nayili, A. Ternary sulfonated graphene/polyaniline/carbon nanotubes nanocomposites for high performance of supercapacitor electrodes. Polym. Bull. 2022, 1–14. [Google Scholar] [CrossRef]
- Al-Abidy, M.; Al-Nayili, A. Enhancement of photocatalytic activities of ZnFe2O4 composite by incorporating halloysite nanotubes for effective elimination of aqueous organic pollutants. Environ. Monit. Assess. 2023, 195, 190. [Google Scholar] [CrossRef]
- Alam, A.U.; Clyne, D.; Jin, H.; Hu, N.-X.; Deen, M.J. Fully integrated, simple, and low-cost electrochemical sensor array for in situ water quality monitoring. ACS Sens. 2020, 5, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Albdiry, M. Identification of active structure and catalytic efficiency of MCM-22 zeolite detemplated by two different processes. J. Porous Mater. 2021, 28, 1439–1448. [Google Scholar] [CrossRef]
- Alwan, S.H.; Salem, K.H.; Alshamsi, H.A. Visible light-driven photocatalytic degradation of Rhodamine B dye onto TiO2/rGO nanocomposites. Mater. Today Commun. 2022, 33, 104558. [Google Scholar] [CrossRef]
- Dayekh, N.S.; Al-Nayili, A. Heterogeneous photocatalytic degradation of phenol over Pd/rGO sheets. AIP Conf. Proc. 2022, 2398, 030010. [Google Scholar]
- Karami, M.; Ghanbari, M.; Alshamsi, H.A.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. The effect of CuI–PbI2 nanocomposite fabricated by the sonochemical route on electrochemical hydrogen storage characteristics. Int. J. Hydrogen Energy 2021, 46, 19074–19084. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Saleh-Mohammadnia, M.; Roghani-Mamaqani, H.; Mardani, H.; Rezvani-Moghaddam, A.; Hemmati, S.; Salami-Kalajahi, M. Fluorescent cellulosic composites based on carbon dots: Recent advances, developments, and applications. Carbohydr. Polym. 2022, 294, 119768. [Google Scholar] [CrossRef] [PubMed]
- Abdulhusain, Z.H.; Alshamsi, H.A.; Salavati-Niasari, M. Silver and zinc oxide decorated on reduced graphene oxide: Simple synthesis of a ternary heterojunction nanocomposite as an effective visible-active photocatalyst. Int. J. Hydrogen Energy 2022, 47, 34036–34047. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, X.; Ma, J.; Gu, Y.; Qian, Z.; Chen, J.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Albo Hay Allah, M.A.; Alshamsi, H.A. Green synthesis of ZnO NPs using Pontederia crassipes leaf extract: Characterization, their adsorption behavior and anti-cancer property. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Dong, Y.; Xie, P.; Zhang, Y.; Cai, Z. Nitrogen and sulfur co-doped carbon-dot-assisted laser desorption/ionization time-of-flight mass spectrometry imaging for profiling bisphenol S distribution in mouse tissues. Anal. Chem. 2018, 90, 10872–10880. [Google Scholar] [CrossRef]
- Dara, M.; Hassanpour, M.; Alshamsi, H.A.; Baladi, M.; Salavati-Niasari, M. Green sol–gel auto combustion synthesis and characterization of double perovskite Tb2ZnMnO6 nanoparticles and a brief study of photocatalytic activity. RSC Adv. 2021, 11, 8228–8238. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Nguyen, A.T.N.; Shim, J.H. All carbon hybrid N-doped carbon dots/carbon nanotube structures as an efficient catalyst for the oxygen reduction reaction. RSC Adv. 2021, 11, 12520–12530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, J.; Xiao, H.; Hu, T.; Jia, J.; Wu, H. Facile in situ synthesis of a carbon quantum dot/graphene heterostructure as an efficient metal-free electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tian, Z.; Li, J.; Ruan, H.; Ma, Y.; Yang, Z.; Qu, Y. Synergistically enhanced activity of graphene quantum dot/multi-walled carbon nanotube composites as metal-free catalysts for oxygen reduction reaction. Nanoscale 2014, 6, 2603–2607. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A. In situ synthesis NiO/F-MWCNTs nanocomposite for adsorption of malachite green dye from polluted water. Carbon Lett. 2022, 32, 1073–1084. [Google Scholar] [CrossRef]
- Senokos, E.; Reguero, V.; Palma, J.; Vilatela, J.; Marcilla, R. Macroscopic fibres of CNTs as electrodes for multifunctional electric double layer capacitors: From quantum capacitance to device performance. Nanoscale 2016, 8, 3620–3628. [Google Scholar] [CrossRef]
- Pour, B.H.; Haghnazari, N.; Keshavarzi, F.; Ahmadi, E.; Zarif, B.R. High sensitive electrochemical sensor for imatinib based on metal-organic frameworks and multiwall carbon nanotubes nanocomposite. Microchem. J. 2021, 165, 106147. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Ghaedi, M.; Kokhdan, S.N.; Vashaee, D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic Fe–Ag/TiO2 nanoparticles. Dalton Trans. 2019, 48, 898–907. [Google Scholar] [CrossRef]
- Li, F.; Peng, J.; Zheng, Q.; Guo, X.; Tang, H.; Yao, S. Carbon nanotube-polyamidoamine dendrimer hybrid-modified electrodes for highly sensitive electrochemical detection of microRNA24. Anal. Chem. 2015, 87, 4806–4813. [Google Scholar] [CrossRef]
- Trigueiro, J.P.C.; Figueiredo, R.C.; Rojo, J.; Viana, R.M.; Schnitzler, M.C.; Silva, G.G. Carbon nanotube/dendrimer hybrids as electrodes for supercapacitors. J. Solid State Electrochem. 2016, 20, 1991–2000. [Google Scholar] [CrossRef]
- Sun, J.-T.; Hong, C.-Y.; Pan, C.-Y. Surface modification of carbon nanotubes with dendrimers or hyperbranched polymers. Polym. Chem. 2011, 2, 998–1007. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; Algarra, M.; Petković, M.; Del Valle, M. Modification of electrodes with N-and S-doped carbon dots. Evaluation of the electrochemical response. Talanta 2020, 212, 120806. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, E.; Ghalkhani, M.; Zargar, T.; Joseph, Y.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Plonska-Brzezinska, M.E.; Ehrlich, H. A new electrochemical aptasensor based on gold/nitrogen-doped carbon nano-onions for the detection of Staphylococcus aureus. Electrochim. Acta 2022, 403, 139633. [Google Scholar] [CrossRef]
- Vivekanand, V.; Rao, D.S.; Vaidyanathan, G.; Sekhar, N.; Kelkar, S.A.; Puranik, P.R. A validated LC method for imatinib mesylate. J. Pharm. Biomed. Anal. 2003, 33, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmad, S.; Kohli, K.; Ali, J.; Khar, R. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2005, 39, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, L.; Wu, Z.; Chang, C.; Yang, Z. Determination of sulfonate ester genotoxic impurities in imatinib mesylate by gas chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 3558–3563. [Google Scholar] [CrossRef]
- Tan, K.L.; Ankathil, R.; Gan, S.H. Method development and validation for the simultaneous determination of imatinib mesylate and N-desmethyl imatinib using rapid resolution high performance liquid chromatography coupled with UV-detection. J. Chromatogr. B 2011, 879, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Castañeda, G.; Lizcano, I. Electrochemical sensor for leukemia drug imatinib determination in urine by adsorptive striping square wave voltammetry using modified screen-printed electrodes. Electrochim. Acta 2018, 269, 668–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).