Abstract
That sulfide anions (S2−) in aquatic environments are produced by microorganisms through degrading sulfur-containing proteins and other organics are harmful to human health. Thus, it is of significance to develop a convenient method for the detection of S2− in water. Small molecular fluorescent probes are very popular for their advantages of visualization, real-time, high sensitivity, and convenience. However, low solubility in water limits the application of existing S2− probes. In this work, we found that our previously developed water-soluble glycosylated fluorescent bioprobe Cu[GluC] can achieve detection of S2− in water. Cu[GluC] can restore fluorescence within 20 s when it encounters S2− and shows good sensitivity towards S2− with a detection limit of 49.6 nM. Besides, Cu[GluC] derived fluorescent test strips were obtained by immersion and realized conveniently visual S2− detection in water by coupling with a UV lamp and a smartphone app. This work provides a fluorescent bioprobe with good water solubility as well as its derived fluorescent test strip for sensitive and simple detection of S2− in water, which shows good prospects in on-site water quality monitoring.
1. Introduction
S2− is a common environmental pollutant, which comes from the metabolites of microbial decomposition of organic matter [1,2]. S2− in environmental pollution can pose a threat to the health of animals, plants and even humans [3]. It is of significance to develop a convenient method for the detection of S2− in water.
Among different kinds of detection methods, small molecular fluorescent probes are very popular for their advantages of visualization, real-time, high sensitivity, and convenience [4,5]. At present, there are three main strategies to construct fluorescent probes for detecting S2−: the metal displacement strategy, azide reduction strategy, and nucleophilic reaction strategy [6,7,8]. Among them, fluorescent probes for the detection of S2− constructed by the metal ion displacement strategy have attracted much attention due to their high selectivity and short response time. Various fluorescent probes based on the Cu2+ displacement strategy have been reported successively. They are mostly derivatives of common fluorophores, such as coumarin, fluorescein, phthalazine-imidazole and BODIPY [6,9,10,11]. However, there is a problem that most fluorescent probes lack water solubility [12,13,14,15], which limits their application in S2− detection in water. A few water-soluble fluorescent probes have been reported by introducing hydrophilic groups, such as benzyl carbazate, phenolic, and trihydroxyl groups [6,11,16]. But they still have disadvantages of a narrow pH range, or complicated synthetic routes. Therefore, it is necessary to develop a water-soluble bioprobe for sensitive and rapid S2−detection in water.
Carbohydrates are abundant biological molecules in nature with good water solubility and biocompatibility. They are widely used in the modification of drugs, probes and biomaterials to enhance their water solubility [17,18,19,20,21]. Introducing carbohydrates may be a good choice for improving the water solubility of S2− fluorescent probes. Here, we found that our previously developed glycosylated fluorescent bioprobe Cu[GluC] can achieve S2− detection in water with a low detection limit. The fluorescence of Cu[GluC] can recover quickly within a few seconds when encountering S2− in water based on the metal ion displacement strategy. Fluorescent test strips produced by Cu[GluC] displayed obvious color changes under 365 nm UV light after dropping S2− aqueous solution with different concentrations, which can achieve simply S2− detection by coupling with a UV lamp and a color recognizer app in a smartphone (Scheme 1). It is important to emphasize that this bioprobe Cu[GluC] and its derived fluorescent test strips realize convenient and sensitive S2− detection in water, which shows application prospects for water quality monitoring. To the best of our knowledge, enhancing water solubility of the fluorescent probe by introducing a glucose unit for the detection of S2− has not been reported yet.
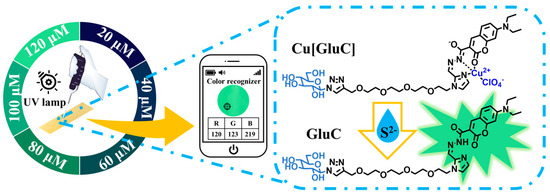
Scheme 1.
Detection of S2− in water by a glucose enhanced water-soluble fluorescent bioprobe.
2. Materials and Methods
2.1. Reagents and Instruments
Reagents related to the synthesis of Cu[GluC] were commercial reagents without further purification and were provided in the Supplementary Material. The water used in this work was ultrapure water. Sodium, potassium, and copper salts were purchased from Adamas Chemical Reagent Co. Stock solutions of these salts were prepared with ultrapure water, which was also used throughout the study. The synthetic compounds were characterized with a nuclear magnetic resonance (NMR) spectrometer (Bruker, Karlsruhe, Germany) where the residual signals from DMSO-d6 (1H: δ 2.50 ppm) or Chloroform-d (1H: δ 7.26 ppm) were used as internal standards and High-resolution ion mobility liquid chromatography–mass spectrometry (HRLCMS) (LC-30A + TripleTOF5600+, AB SCIEX, USA). The UV-vis spectra were measured with a 1750 UV-visible spectrophotometer (Shimadzu, Japan), and the fluorescence spectra were measured with an RF-6000 fluorescence spectrophotometer (Shimadzu, Kyoto, Japan).
2.2. Synthesis of Cu[GluC]
As shown in Scheme 2, compound g reacted with compound b to obtain compound h through a ‘click’ reaction and compound h reacted with compound d to obtain GluC through a Schiff base reaction. Finally, GluC reacted with Cu(ClO4)2·6H2O to obtain Cu[GluC]. The detailed synthesis route was provided in Supplementary Material according to our previous study [22] with some changes. 1H NMR and HRMS of the synthesized compounds were provided in Figures S1–S10.
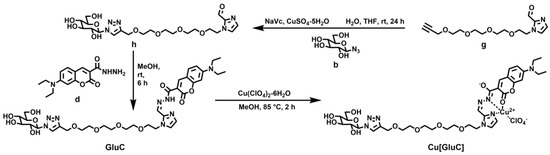
Scheme 2.
Synthesis of GluC and Cu[GluC].
2.3. Procedures of the Ion Sensing
The stock solution of GluC, Cu[GluC] and related substrates were prepared in ultrapure water. The samples were added in a 1 cm quartz cuvette and then measured with a UV-visible spectrophotometer and a fluorescence spectrophotometer. The excitation is carried out at 450 nm, and the slit width of emission and excitation is 5 nm. Besides, the calculation of quantum yield (Φ), binding constant (Kα), dissociation constant (Kd) in the experiment refers to the methods provided by our previous study [22]. Limit of detection (LOD) and limit of quantification (LOQ) are calculated as follows: “LOD = 3 σ/k”, “LOQ = 10 σ/k”, where σ is the standard deviation of blank and k is the linear correlation slope of fluorescence intensity at 494 nm relative to the concentrations of S2−.
2.4. Preparation of Test Strips
Filter papers were dipped in Cu[GluC] aqueous solution (100 μM) for 30 min and dried naturally to make fluorescent test strips. A procedure for fluorescent test strip analysis is presented. First, 10 μL S2− aqueous solution with different concentrations (20–120 μM) were dropped onto the fluorescent test strips that were prepared. After two minutes, photos of test strips were taken by a smartphone (PBBM30, OPPO, Dongguan, China) under a UV light of 365 nm. Then, RGB (red, green and blue) values of fluorescent test strips were obtained via a color recognizer app (V8.100, Xiyi Technology, Xiamen, China).
3. Results and Discussion
3.1. The Coordination Mode between GluC and Cu2+
The binding specificity of GluC to Cu2+ under the influence of different metal ions was first investigated. In comparison to other metal ions, only Cu2+ caused the maximum fluorescence quenching of GluC (Figure 1a,b). In addition, the complexation of GluC with Cu2+ was not affected by other metal ions. The Kα of GluC-Cu2+ (Cu[GluC]) was calculated to be 2.388 × 105 M−1 with a good linear relationship (R2 = 0.9989) according to Benesi–Hilderbrand equation (Figure 1c). The fluorescence quantum yield of Cu[GluC] before and after responding to S2− (2 equiv.) in an aqueous solution was 0.018 and 0.104. What is more, according to our previous study [22] and a Job’s plot (Figure S12), GluC and Cu2+ have formed the 1:1 complex. Besides, a peak of Cu[GluC] was found at 834.2602 in the mass spectrum (Figure S10), which is corresponding to [GluC + Cu2+]. Thus, all the above results suggest that the binding ratio of GluC and Cu2+ is 1:1. The Kd for Cu[GluC] was calculated to be 2.033 μM in our previous study [22], which indicates the good stability of Cu[GluC].
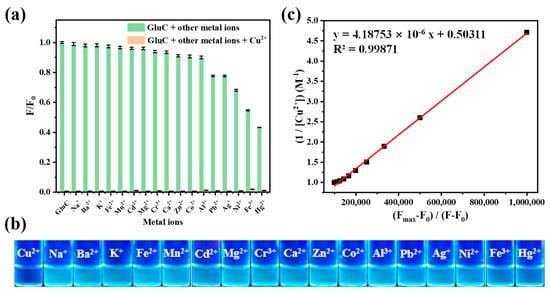
Figure 1.
(a) The influence of different metal ions (20.0 μM) to fluorescence intensity of GluC (10.0 μM) at 494 nm followed by adding Cu2+ (20.0 μM) in water (n = 3, “n” means number of experiment repeats); (b) Visible color changes of GluC (10.0 μM) under UV light (365 nm) when adding various metal ions (20.0 μM); (c) The Benesi-Hilderbrand plots of GluC with Cu2+.
3.2. S2− Responsiveness of Cu[GluC]
The spectroscopic responses of Cu[GluC] towards S2− were studied. As shown in Figure 2a, the fluorescence intensity of Cu[GluC] gradually increased with the addition of S2− and reached the plateau after adding 2.0 equiv of S2−. Fluorescence intensity shows a good linear relationship (R2 = 0.9941) towards the concentration of S2− ranging from 0–2 μM, according to which, the LOD and LOQ were calculated to be 49.6 nM and 165.3 nM, respectively (Figure 2b). Compared with other fluorescent probes [23,24,25,26], Cu[GluC] has a lower detection limit, indicating that Cu[GluC] has a sensitive detection ability toward S2− in water. The fluorescence responsive speed of Cu[GluC] (10.0 μM) towards S2− (30.0 μM) in water was further tested. As shown in Figure 2c, after adding S2− into aqueous solutions of Cu[GluC], the fluorescent intensity of Cu[GluC] increased quickly and reached the plateau within 20 s, which is faster than other S2− fluorescent probes [23,27,28,29], suggesting the quick S2− detection ability of Cu[GluC].
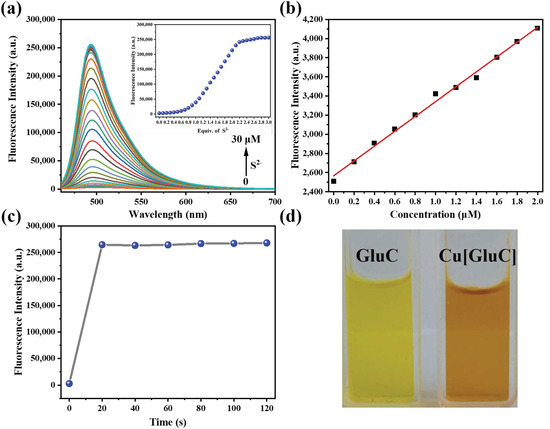
Figure 2.
(a) The fluorescence spectra titration of Cu[GluC] (10.0 μM) with increasing concentrations of S2− in aqueous solution. Inset: Fluorescence intensity changes of Cu[GluC] at 494 nm with the increasing of S2−concentrations−; (b) The linear relationship of the fluorescent intensity (494 nm) of Cu[GluC] (10 μM) relative to the concentrations of S2− in aqueous solution; (c) The fluorescence variation process (494 nm) of Cu[GluC] after adding S2− in aqueous solution within 120 s; (d) The aqueous solution of Cu[GluC] and GluC under visible light.
3.3. pH Stability and Water Solubility of Cu[GluC] and GluC
The stability of a fluorescent probe in working conditions with a wide range of pH is vital. Therefore, the fluorescence stability of Cu[GluC] and GluC were studied in water with different pHs. According to our previous study [22], both Cu[GluC] and GluC displayed excellent stability in the pH range of 6–11, which is better than other S2− fluorescent probes [23,28,30,31]. This result indicates that Cu[GluC] is suitable for the S2−detection in water with a wide pH range from 6 to 11. From the curve about pH influence on the fluorescence intensity of Cu[GluC], the pKa is calculated to be 4.18. In addition, the aqueous solution of GluC and Cu[GluC] was clear and transparent even if the concentrations were 1 mM (Figure 2d), which suggests that they have excellent water solubility. What is more, all the fluorescence intensity and UV absorbance of the Cu[GluC] aqueous solution before and after adding S2− were unchanged within 30 min (Figure S11), which suggests that Cu[GluC] has excellent solution stability in the presence/absence of the analyte.
3.4. Selectivity and Interference
As we all know, selectivity is an important criterion to evaluate the performance of fluorescent probes. Thus, the specific responsiveness of Cu[GluC] to S2− in water was studied via fluorescence and colorimetric method. As shown in Figure 3a,c, Cu[GluC] recovery its fluorescence only in the presence of S2−. Compared to adding other anions, Cu[GluC] aqueous solution (10 μM) shows light-blue under 365 nm UV lamp after adding S2−. What is more, other anions cannot show an effect on the fluorescence recovery of Cu[GluC] (Figure 3b). All these results demonstrate that Cu[GluC] possesses the ability of selective S2− detection in water.
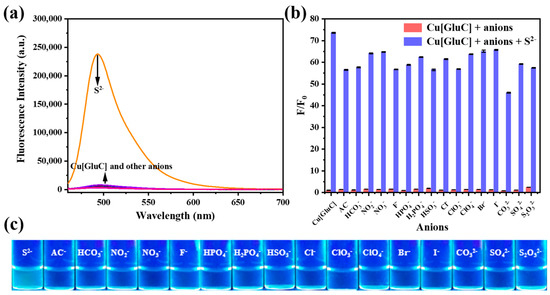
Figure 3.
(a) Fluorescence emission spectra of Cu[GluC] aqueous solution (10.0 μM) after adding different anions (30.0 μM); (b) Fluorescence intensity of Cu[GluC] aqueous solution (10.0 μM) at 494 nm in the presence of different anions (30.0 μM) and then adding S2− (30.0 μM) in water (n = 3); (c) The fluorescence of Cu[GluC] aqueous solution (10 μM) under UV light (365 nm) after adding different anions (30.0 μM).
3.5. Visual S2− Detection of by Fluorescent Test Strips
In order to make the S2− detection in water more convenient, fluorescent test strips were simply fabricated by soaking filter paper into Cu[GluC] aqueous solution (100 μM) for 30 min and then air drying. Surprisingly, under 365 nm UV light, the color of fluorescent test strips changed significantly from blue-black to light green after treatment with different concentrations of S2− (20–120 μM) (Figure 4a). Then, the RGB data of fluorescent test strips were acquired via a smartphone app (Figure 4b). As shown in Figure 4c, (R + G + B)/3 values had a good linear relationship with the concentrations of S2− (R2 = 0.99227). All results demonstrate that the fluorescent test strips coupled with a portable UV lamp and a smartphone app can achieve quantitative and convenient on-site S2− detection in water.
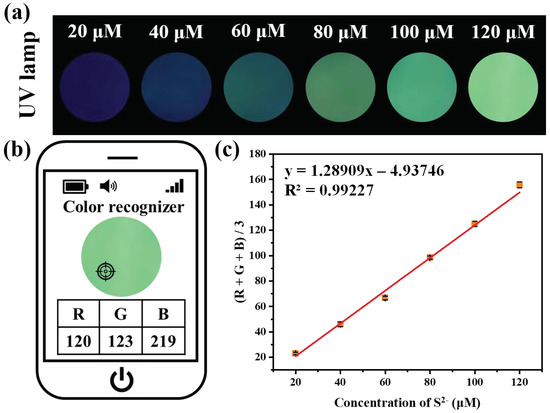
Figure 4.
(a) Color changes of fluorescent test strips prepared by filter papers embedded with Cu[GluC] after treated with different concentrations of S2− aqueous solution under UV lamp (365 nm) irradiation; (b) The RGB values of fluorescent test strips were obtained through a smartphone app; (c) The linear relationship of (R+G+B)/3 values of fluorescent test strips relative to the concentrations of S2− (n = 3).
3.6. Comparison with Other Fluorescent Probes for S2− Detection
A fluorescent probe for S2− detection based on the Cu2+ replacement strategy has a broad application prospect at present. Because of the need for practical detection, improving water solubility is an important development direction for fluorescent probes for S2− detection in water. The structures, properties, and highlights of some representative probes are listed in Table S1. Compared with other fluorescent probes for S2− detection [16,23,32,33], Cu[GluC] not only achieves sensitive and selective S2− detection in water with a wide pH range and short response time, but also its derived fluorescent test strips hold good potential in on-site water quality monitoring.
4. Conclusions
A small molecular fluorescent bioprobe Cu[GluC] with excellent water solubility endowed by a glucose unit was used for the detection of S2− in water. Cu[GluC] can quickly and selectively respond to S2− in water with a short response time, low detection limits and a wide pH range. Besides, fluorescent test strips can realize convenient on-site S2− detection in water by coupling a UV lamp and a smartphone app. Thus, this work provides a fluorescent bioprobe with good water solubility and its derived fluorescent test strips for sensitive and convenient S2− detection in water, which holds good potential in on-site water quality monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios12080600/s1, Scheme S1: Synthesis of bioprobe Cu[GluC]; Figure S1: 1H NMR spectrum of compound a in Chloroform-d; Figure S2: 1H NMR spectrum of compound b in DMSO-d6; Figure S3: 1H NMR spectrum of compound c in Chloroform-d. Figure S4: 1H NMR spectrum of compound d in Chloroform-d; Figure S5: 1H NMR spectrum of compound e in Chloroform-d; Figure S6: 1H NMR spectrum of compound f in Chloroform-d; Figure S7: 1H NMR spectrum of compound g in Chloroform-d; Figure S8: 1H NMR spectrum of compound h in DMSO-d6; Figure S9: 1H NMR spectrum of GluC in Chloroform-d; Figure S10: HRMS spectrum of Cu[GluC]; Figure S11: The fluorescence intensity and absorbance variation process of Cu[GluC] and Cu[GluC] + S2− in water within 30 min; Figure S12: Job’s plot for the stoichiometry of GluC and Cu2+ ions; Table S1: Some reported fluorescent probes for S2− detection in water.
Author Contributions
Conceptualization, X.A. and Y.W.; methodology, X.A. and Y.W.; validation, J.L.; investigation, X.A., Y.W. and J.L.; resources, Y.P.; data curation, X.A. and Y.W.; writing—original draft preparation, X.A. and Y.W.; writing—review and editing, Z.P. and Y.P.; visualization, X.A.; supervision, Z.P. and Y.P.; funding acquisition, Z.P. and Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [22171230 and 21877088] and the Project of Science and Technology of Social Development in Shaanxi Province [2021SF-120].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, F.; Cui, X.; Ren, J. A Novel QCM-based Biosensor for Detection of Microorganisms Producing Hydrogen Sulfide. Anal. Lett. 2008, 41, 2697–2709. [Google Scholar] [CrossRef]
- Thompson, R.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Rapid detection of hydrogen sulfide produced by pathogenic bacteria in focused growth media using SHS-MCC-GC-IMS. Microchem. J. 2018, 140, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Li, Z.; Deng, B.; Wang, L.; Lin, W. Single Fluorescent Probe Separately and Continuously Visualize H2S and HClO in Lysosomes with Different Fluorescence Signals. Anal. Chem. 2019, 91, 2932–2938. [Google Scholar] [CrossRef]
- Mao, G.J.; Wei, T.T.; Wang, X.X.; Huan, S.Y.; Lu, D.Q.; Zhang, J.; Zhang, X.B.; Tan, W.; Shen, G.L.; Yu, R.Q. High-sensitivity naphthalene-based two-photon fluorescent probe suitable for direct bioimaging of H2S in living cells. Anal. Chem. 2013, 85, 7875–7881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ning, L.; Zhou, X.; Song, Z.; Zhang, J.; Guan, F.; Yang, X.F. An Activatable Near-Infrared Fluorescence Hydrogen Sulfide (H2S) Donor for Imaging H2S Release and Inhibiting Inflammation in Cells. Anal. Chem. 2021, 93, 4894–4901. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Li, J.; Qin, Y.; Zhao, C.; Mi, Y.; Li, G.; Li, T.; Wu, Y. A new coumarin-based fluorescent probe for selective recognition of Cu2+ and S2− in aqueous solution and living cells. Tetrahedron 2019, 75, 3951–3957. [Google Scholar] [CrossRef]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Chapter Four—Azide-Based Fluorescent Probes: Imaging Hydrogen Sulfide in Living Systems. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 554, pp. 63–80. [Google Scholar]
- Peng, B.; Xian, M. Chapter Three—Hydrogen Sulfide Detection Using Nucleophilic Substitution–Cyclization-Based Fluorescent Probes. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 554, pp. 47–62. [Google Scholar]
- Hou, F.; Huang, L.; Xi, P.; Cheng, J.; Zhao, X.; Xie, G.; Shi, Y.; Cheng, F.; Yao, X.; Bai, D.; et al. A Retrievable and Highly Selective Fluorescent Probe for Monitoring Sulfide and Imaging in Living Cells. Inorg. Chem. 2012, 51, 2454–2460. [Google Scholar] [CrossRef]
- Rha, C.J.; Lee, H.; Kim, C. An effective phthalazine-imidazole-based chemosensor for detecting Cu2+, Co2+ and S2− via the color change. Inorg. Chim. Acta 2020, 511, 119788. [Google Scholar] [CrossRef]
- Wu, S.; Ma, X.; Wang, Y.; Zhou, J.; Li, X.; Wang, X. A novel fluorescent BODIPY-based probe for detection of Cu2+ and H2S based on displacement approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119330. [Google Scholar] [CrossRef]
- Qin, J.C.; Yang, Z.Y. Design of a novel coumarin-based multifunctional fluorescent probe for Zn(2)(+)/Cu(2)(+)/S(2)(-) in aqueous solution. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Shen, L.; Bao, H.; Fang, X.; Xu, J.; Zhao, Y.; Yang, W. A tricarbocyanine near-infrared fluorescent probe for sulfide through a copper displacement mechanism. Sensor. Actuator. B Chem. 2015, 220, 1361–1367. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Wang, Y.; Qiu, F.; Song, X.; Tang, X.; Zhang, G.; Liu, W. Dual-functional colorimetric fluorescent probe for sequential Cu2+ and S2− detection in bio-imaging. Sensor. Actuator. B Chem. 2019, 288, 27–37. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, J.; Zhou, P.; Han, X.; Sun, J.; Liu, S.; Zhang, L.; Fang, J. A selective colorimetric and red-emitting fluorometric probe for sequential detection of Cu2+ and H2S. Sensor. Actuator. B Chem. 2018, 255, 3155–3162. [Google Scholar] [CrossRef]
- Park, S.; Choe, D.; Lee, J.J.; Kim, C. A benzyl carbazate-based colorimetric chemosensor for relay detection of Cu2+ and S2− in near-perfect aqueous media. J. Mol. Struct. 2021, 1240, 130576. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, J.; Jung, D.W.; Williams, D.R. Visualizing sweetness: Increasingly diverse applications for fluorescent-tagged glucose bioprobes and their recent structural modifications. Sensors 2012, 12, 5005–5027. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.S.; Lee, H.Y.; Lim, C.S.; Park, J.; Kim, H.M.; Shin, Y.N.; Kim, E.S.; Jeon, H.J.; Park, S.B.; Cho, B.R. A two-photon tracer for glucose uptake. Angew. Chem. Int. Ed. 2009, 48, 8027–8031. [Google Scholar] [CrossRef]
- He, Q.L.; Minn, I.; Wang, Q.; Xu, P.; Head, S.A.; Datan, E.; Yu, B.; Pomper, M.G.; Liu, J.O. Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation. Angew. Chem. Int. Ed. 2016, 55, 12035–12039. [Google Scholar] [CrossRef]
- Ma, K.; Shi, J.; Pei, Y.; Pei, Z. A carrier-free supramolecular nanoprodrug based on lactose-functionalized dimeric camptothecin via self-assembly in water for targeted and fluorescence imaging-guided chemo-photodynamic therapy. J. Colloid Interface Sci. 2022, 609, 353–363. [Google Scholar] [CrossRef]
- Cao, S.P.; Pei, Z.C.; Xu, Y.Q.; Pei, Y.X. Glyco-Nanovesicles with Activatable Near-Infrared Probes for Real-Time Monitoring of Drug Release and Targeted Delivery. Chem. Mater. 2016, 28, 4501–4506. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Chen, Z.; Pu, L.; Pei, Z.; Pei, Y. A GLUTs/GSH cascade targeting-responsive bioprobe for the detection of circulating tumor cells. Chem. Commun. 2022, 58, 3945–3948. [Google Scholar] [CrossRef]
- Wang, H.; Shi, D.-L.; Li, J.; Tang, H.-Y.; Li, J.; Guo, Y. A facile fluorescent probe with a large Stokes shift for sequentially detecting copper and sulfide in 100% aqueous solution and imaging them in living cells. Sens. Actuators B Chem. 2018, 256, 600–608. [Google Scholar] [CrossRef]
- Gao, L.L.; Wang, B.B.; Chen, X.; Wang, Y.; Wu, W.N.; Zhao, X.L.; Yan, L.L.; Fan, Y.C.; Xu, Z.H. Hydrazone derivative bearing coumarin for the relay detection of Cu(2+) and H2S in an almost neat aqueous solution and bioimaging in lysosomes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 255, 119693. [Google Scholar] [CrossRef]
- Huang, J.; Qin, H.; Liang, H.; Lu, J. An AIE polymer prepared via aldehyde-hydrazine step polymerization and the application in Cu2+ and S2− detection. Polymer 2020, 202, 122663. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Chen, X.; Zhang, X.; Zhou, H.; Du, H.; Wang, M.; Ma, Y.; Jin, X. Detection of Cu(2+) and S(2-) with fluorescent polymer nanoparticles and bioimaging in HeLa cells. Anal. Bioanal. Chem. 2021, 413, 3945–3953. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu(2+) and S(2−) in 100% aqueous buffered solutions and living cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Z.; Zhou, D.; Wu, J.; Wang, P.; Yang, X.; Wen, S. A novel fluorescent probe for highly selective and sensitive detection of sulfur ions in real samples and living cells based on the tripeptide-Cu2+ ensemble system. Microchem. J. 2021, 169, 106612. [Google Scholar] [CrossRef]
- Shu, W.; Zang, S.; Wang, C.; Gao, M.; Jing, J.; Zhang, X. An Endoplasmic Reticulum-Targeted Ratiometric Fluorescent Probe for the Sensing of Hydrogen Sulfide in Living Cells and Zebrafish. Anal. Chem. 2020, 92, 9982–9988. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, S.; Wang, Y.; Song, X.; Cao, C.; Wang, K.; Jing, C.; Zhang, G.; Liu, W. A multifunctional fluorescent probe for visualizing H2S in wastewater with portable smartphone via fluorescent paper strip and sensing GSH in vivo. J. Hazard. Mater. 2021, 406, 124523. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Yang, X. Highly selective and sensitive detection of hydrogen sulfide in aqueous medium and live cells using peptide-based bioprobe to mimic the binding sites of the ceruloplasmin for Cu(II) ions. Biosens. Bioelectron. 2020, 163, 112283. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Di, C.; Zhou, R.; Zhang, H.; Su, P.; Xu, C.; Zhou, P.; Ge, Y.; Liu, D.; et al. A novel peptide-based fluorescence chemosensor for selective imaging of hydrogen sulfide both in living cells and zebrafish. Biosens. Bioelectron. 2017, 92, 602–609. [Google Scholar] [CrossRef]
- Li, K.-B.; Jia, W.-P.; Han, D.-M.; Liang, D.-X.; He, X.-P.; Chen, G.-R. Fluorogenic bis-triazolyl galactoprobe–metal complex for full-aqueous analysis of sulfide ion. Sens. Actuators B Chem. 2017, 246, 197–201. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).