Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies
Abstract
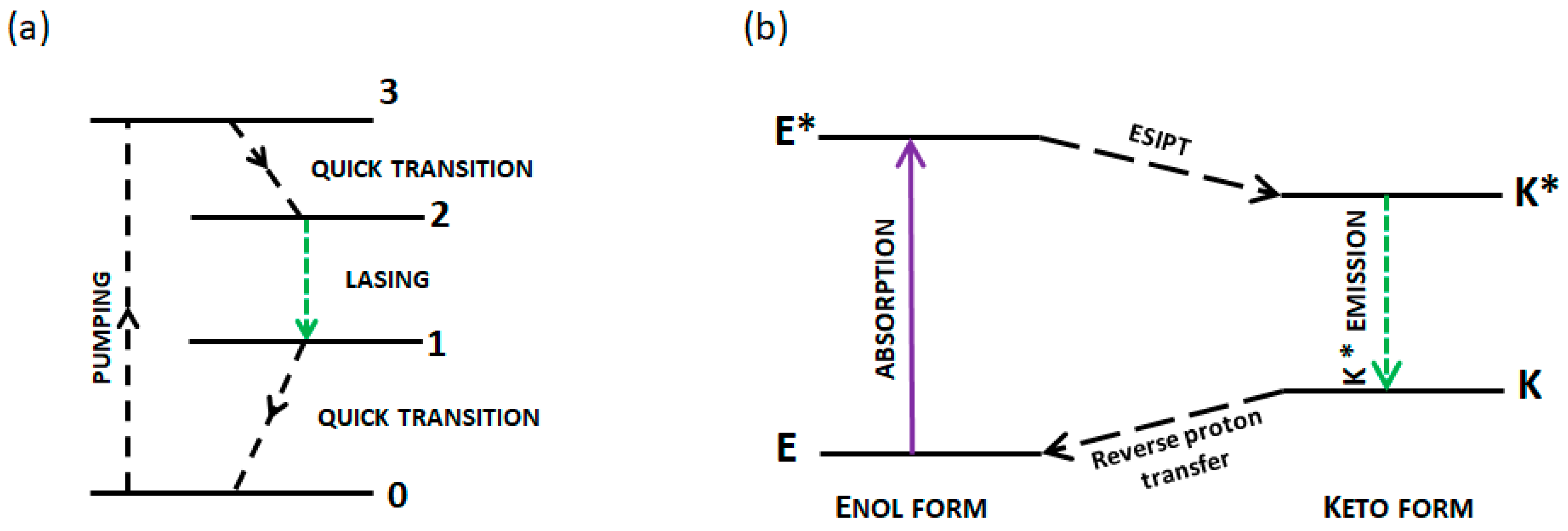
1. Introduction
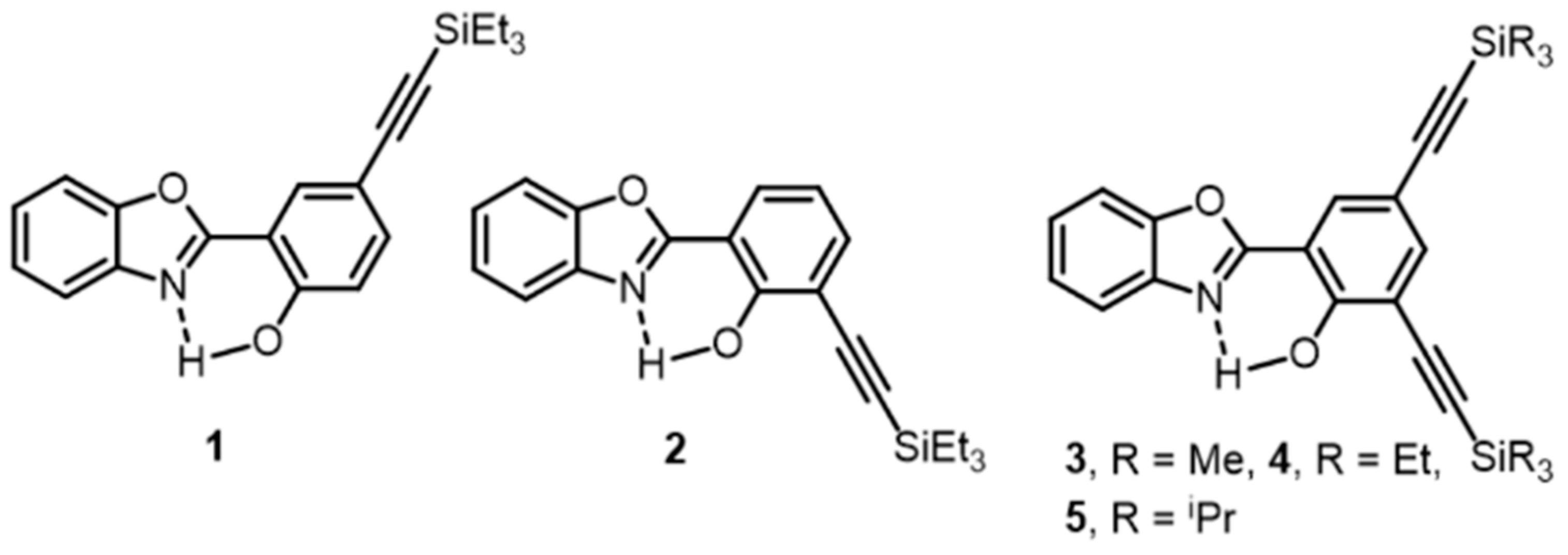
2. Materials
3. Results and Discussion
3.1. Photophysical Properties
3.2. Ab Initio Calculations
3.3. Random Lasing Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Massue, J.; Jacquemin, D.; Ulrich, G. Molecular engineering of excited-state intramolecular proton transfer (ESIPT) dual and triple emitters. Chem. Lett. 2018, 47, 1083–1089. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, Y.-T.; Demchenko, A.P.; Chou, P.-T. Amino proton donors in excited-state intramolecular proton-transfer reactions. Nat. Rev. Chem. 2018, 2, 131–143. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.E.; Kim, S.H.; Seo, J.; Chung, K.; Park, S.-Y.; Jang, D.-J.; Medina, B.M.; Gierschner, J.; Park, S.Y. A white-light-emitting molecule: Frustrated energy transfer between constituent emitting centers. J. Am. Chem. Soc. 2009, 131, 14043–14049. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.-C.; Chang, M.-J.; Lin, T.-Y.; Pan, H.-A.; Fang, T.-C.; Chen, K.-Y.; Hung, W.-Y.; Hsu, Y.-H.; Chou, P.-T. Fine tuning the energetics of excited-state intramolecular proton transfer (ESIPT): White light generation in a single ESIPT system. J. Am. Chem. Soc. 2011, 133, 17738–17745. [Google Scholar] [CrossRef]
- Klymchenko, A.; Duportail, G.; Mély, Y.; Demchenko, A.P. Ultrasensitive two-color fluorescence probes for dipole potential in phospholipid membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 11219–11224. [Google Scholar] [CrossRef]
- Sholokh, M.; Zamotaiev, O.M.; Das, R.; Postupalenko, V.Y.; Richert, L.; Dujardin, D.; Zaporozhets, O.A.; Pivovarenko, V.G.; Klymchenko, A.S.; Mély, Y. Fluorescent Amino Acid Undergoing Excited State Intramolecular Proton Transfer for Site-Specific Probing and Imaging of Peptide Interactions. J. Phys. Chem. B 2015, 119, 2585–2595. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Deng, G.-J.; Liu, Z.-X.; He, Y.-M.; Fan, Q.-H. Fluorescent Dendritic Organogels Based on 2-(2’-Hydroxyphenyl) benzoxazole: Emission Enhancement and Multiple Stimuli-Responsive Properties. Chem. Eur. J. 2015, 21, 11018–11028. [Google Scholar] [CrossRef]
- Jagadesan, P.; Eder, G.; McGrier, P.L. The excited-state intramolecular proton transfer properties of three imine-linked two-dimensional porous organic polymers. J. Mater. Chem. C 2017, 5, 5676–5679. [Google Scholar] [CrossRef]
- Ciuciu, A.I.; Flamigni, L.; Skonieczny, K.; Gryko, D.T. Blue-green emitting sulphonamido-imidazole derivatives: ESIPT based excited state dynamics. Phys. Chem. Chem. Phys. 2013, 15, 16907–16916. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwon, J.E.; Park, S.Y. Strategic emission color tuning of highly fluorescent imidazole-based excited-state intramolecular proton transfer molecules. Phys. Chem., Chem. Phys. 2012, 14, 8878–8884. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, X.; Zhang, H.; Wang, Y.; Zhang, H.; Yamaguchi, S. ESIPT-active organic compounds with white luminescence based on crystallization-induced keto emission (CIKE). Chem. Commun. 2017, 53, 7832–7835. [Google Scholar] [CrossRef] [PubMed]
- Rihn, S.; Retailleau, P.; De Nicola, A.; Ulrich, G.; Ziessel, R. Synthetic Routes to Fluorescent Dyes Exhibiting Large Stokes Shifts. J. Org. Chem. 2012, 77, 8851–8863. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Nastasi, F.; Retailleau, P.; Puntoriero, F.; Ziessel, R.; Campagna, S. Luminescent Excited-State Intramolecular Proton-Transfer (ESIPT) Dyes Based on 4-Alkyne-Functionalized [2,2′-Bipyridine]-3,3′-diol Dyes. Chem. Eur. J. 2008, 14, 4381–4392. [Google Scholar] [CrossRef] [PubMed]
- Benelhadj, K.; Muzuzu, W.; Massue, J.; Retailleau, P.; Charaf-Eddin, A.; Laurent, A.D.; Jacquemin, D.; Ulrich, G.; Ziessel, R. White Emitters by Tuning the Excited-State Intramolecular Proton-Transfer Fluorescence Emission in 2-(2′-Hydroxybenzofuran) benzoxazole Dyes. Chem. Eur. J. 2014, 20, 12843–12857. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Zhao, S.; Gao, J.; Zhang, Z.; Zhang, H.; Liu, Y.; Wang, Y. Hydroxyphenyl-benzothiazole based full color organic emitting materials generated by facile molecular modification. J. Mater. Chem. 2011, 21, 3568–3570. [Google Scholar] [CrossRef]
- Sakai, K.-I.; Ishikawa, T.; Akutagawa, T. A blue-white-yellow color-tunable excited state intramolecular proton transfer (ESIPT) fluorophore: Sensitivity to polar–nonpolar solvent ratios. J. Mater. Chem. C 2013, 1, 7866–7871. [Google Scholar] [CrossRef]
- Heyer, E.; Benelhadj, K.; Budzák, S.; Jacquemin, D.; Massue, J.; Ulrich, G. On the Fine-Tuning of the Excited-State Intramolecular Proton Transfer (ESIPT) Process in 2-(2′-Hydroxybenzofuran) benzazole (HBBX) Dyes. Chem. Eur. J. 2017, 23, 7324–7336. [Google Scholar] [CrossRef]
- Dobkowski, J.; Wnuk, P.; Buczynska, J.; Pszona, M.; Orzanowska, G.; Frath, D.; Ulrich, G.; Massue, J.; Mosquera-Vazquez, S.; Vauthey, E.; et al. Substituent and Solvent Effects on the Excited State Deactivation Channels in Anils and Boranils. Chem. Eur. J. 2015, 21, 1312–1357. [Google Scholar] [CrossRef] [PubMed]
- Skonieczny, K.; Yoo, J.; Larsen, J.M.; Espinoza, E.M.; Barbasiewicz, M.; Vullev, V.I.; Lee, C.-H.; Gryko, D.T. How to Reach Intense Luminescence for Compounds Capable of Excited-State Intramolecular Proton Transfer? Chem. Eur. J. 2016, 22, 7485–7496. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Suda, K.; Yokogawa, D.; Kitoh-Nishioka, H.; Irle, S.; Ando, A.; Abegao, L.M.G.; Kamada, K.; Fukazawa, A.; Yamaguchi, S. Near infrared two-photon-excited and -emissive dyes based on a strapped excited-state intramolecular proton-transfer (ESIPT) scaffold. Chem. Sci. 2018, 9, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Massue, J.; Felouat, A.; Curtil, M.; Vérité, P.M.; Jacquemin, D.; Ulrich, G. Solution and solid-state Excited-State Intramolecular Proton Transfer (ESIPT) emitters incorporating Bis-triethyl-or triphenylsilylethynyl units. Dyes Pigment. 2019, 160, 915–922. [Google Scholar] [CrossRef]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic. Nanolights Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hiyama, T. Organic Fluorophores Exhibiting Highly Efficient Photoluminescence in the Solid State. Chem. Asian J. 2010, 5, 1516–1531. [Google Scholar] [CrossRef]
- Park, S.; Kwon, O.-H.; Kim, S.; Park, S.; Choi, M.-G.; Cha, M.; Park, S.Y.; Jang, D.-J. Imidazole-Based Excited-State Intramolecular Proton-Transfer Materials: Synthesis and Amplified Spontaneous Emission from a Large Single Crystal. J. Am. Chem. Soc. 2005, 127, 10070–10074. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.-Y.; Pan, M.; Wu, K.; Wang, H.-P.; Fan, Y.-N.; Su, C.-Y. A naked eye colorimetric sensor for alcohol vapor discrimination and amplified spontaneous emission (ASE) from a highly fluorescent excited-state intramolecular proton transfer (ESIPT) molecule. J. Mater. Chem C 2016, 4, 6962–6966. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, K.; Huang, S.; Zhang, H.; Zhang, H.; Wang, Y. Organic Crystals with Near-Infrared Amplified Spontaneous Emissions Based on 2’-Hydroxychalcone Derivatives: Subtle Structure Modification but Great Property Change. Angew. Chem. Int. Ed. 2015, 54, 8369–8373. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Gu, J.; Yao, J.; Zhao, Y.S. Low-Threshold Wavelength-Switchable Organic Nanowire Lasers Based on Excited-State Intramolecular Proton Transfer. Angew. Chem. Int. Ed. 2015, 54, 7125–7129. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Han, S.; Li, F.; Zhang, H.; Wang, Y. Multicolor Amplified Spontaneous Emissions Based on Organic Polymorphs That Undergo Excited-State Intramolecular Proton Transfer. Chem. Eur. J. 2016, 22, 4899–4903. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.E.; Park, S.-Y.; Kwon, O.-H.; Kim, J.K.; Yoon, S.J.; Chung, J.W.; Whang, D.R.; Park, S.K.; Lee, D.K.; et al. Crystallization-Induced Emission Enhancement and Amplified Spontaneous Emission from a CF3-Containing Excited-State Intramolecular-Proton-Transfer Molecule. Adv. Opt. Mater. 2017, 5, 1700353. [Google Scholar] [CrossRef]
- Wiersma, D.S. The physics and applications of random lasers. Nat. Phys. 2008, 4, 359. [Google Scholar] [CrossRef]
- Cao, H. Lasing in random media. Waves Random Media 2003, 13, R1–R39. [Google Scholar] [CrossRef]
- Letokhov, V.S. Generation of Light by a Scattering Medium with Negative Resonance Absorption. Sov. J. Exp. Theor. Phys. 1968, 26, 835. [Google Scholar]
- Sznitko, L.; Mysliwiec, J.; Miniewicz, A. The role of polymers in random lasing. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 951. [Google Scholar] [CrossRef]
- Szukalski, A.; Sznitko, L.; Cyprych, K.; Miniewicz, A.; Mysliwiec, J. Light Amplification in Derivatives of Pyrazoline-Based Systems. J. Phys. Chem. C 2014, 118, 8102–8110. [Google Scholar] [CrossRef]
- Galisteo-Lopez, J.F.; Ibisate, M.; Lopez, C. FRET-Tuned Resonant Random Lasing. J. Phys. Chem. C 2014, 118, 9665–9669. [Google Scholar] [CrossRef]
- Ye, L.; Feng, Y.; Cheng, Z.; Wang, C.; Lu, C.; Lu, Y.; Cui, Y. Coherent Random Lasing from Dye Aggregates in Polydimethylsiloxane Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 27232–27238. [Google Scholar] [CrossRef]
- Massue, J.; Ulrich, G.; Ziessel, R. Effect of 3,5-Disubstitution on the Optical Properties of Luminescent 2-(2′-Hydroxyphenyl)benzoxazoles and Their Borate Complexes. Eur. J. Org. Chem. 2013, 25, 5701–5709. [Google Scholar] [CrossRef]
- Azarias, C.; Budzak, S.; Laurent, A.D.; Ulrich, G.; Jacquemin, D. Tuning ESIPT fluorophores into dual emitters. Chem. Sci. 2016, 7, 3763–3774. [Google Scholar] [CrossRef]
- Plasser, F.; Barbatti, M.; Aquino, A.J.A.; Lischka, H. Excited-State Diproton Transfer in [2,2′-Bipyridyl]-3,3′-diol: The Mechanism Is Sequential, Not Concerted. J. Phys. Chem. A 2009, 113, 8490–8499. [Google Scholar] [CrossRef]
- Omidyam, R.; Iravani, M. Excited State Proton Transfer and Deactivation Mechanism of 2-(4′-Amino-2′-hydroxyphenyl)-1H-imidazo-[4,5-c] pyridine and Its Analogues: A Theoretical Study. J. Phys. Chem. A 2016, 120, 1012–1019. [Google Scholar] [CrossRef]
- Massue, J.; Felouat, A.; Vérité, P.M.; Jacquemin, D.; Cyprych, K.; Durko, M.; Sznitko, L.; Mysliwiec, J.; Ulrich, G. An extended excited-state intramolecular proton transfer (ESIPT) emitter for random lasing applications. Phys. Chem. Chem. Phys. 2018, 20, 19958–19963. [Google Scholar] [CrossRef]
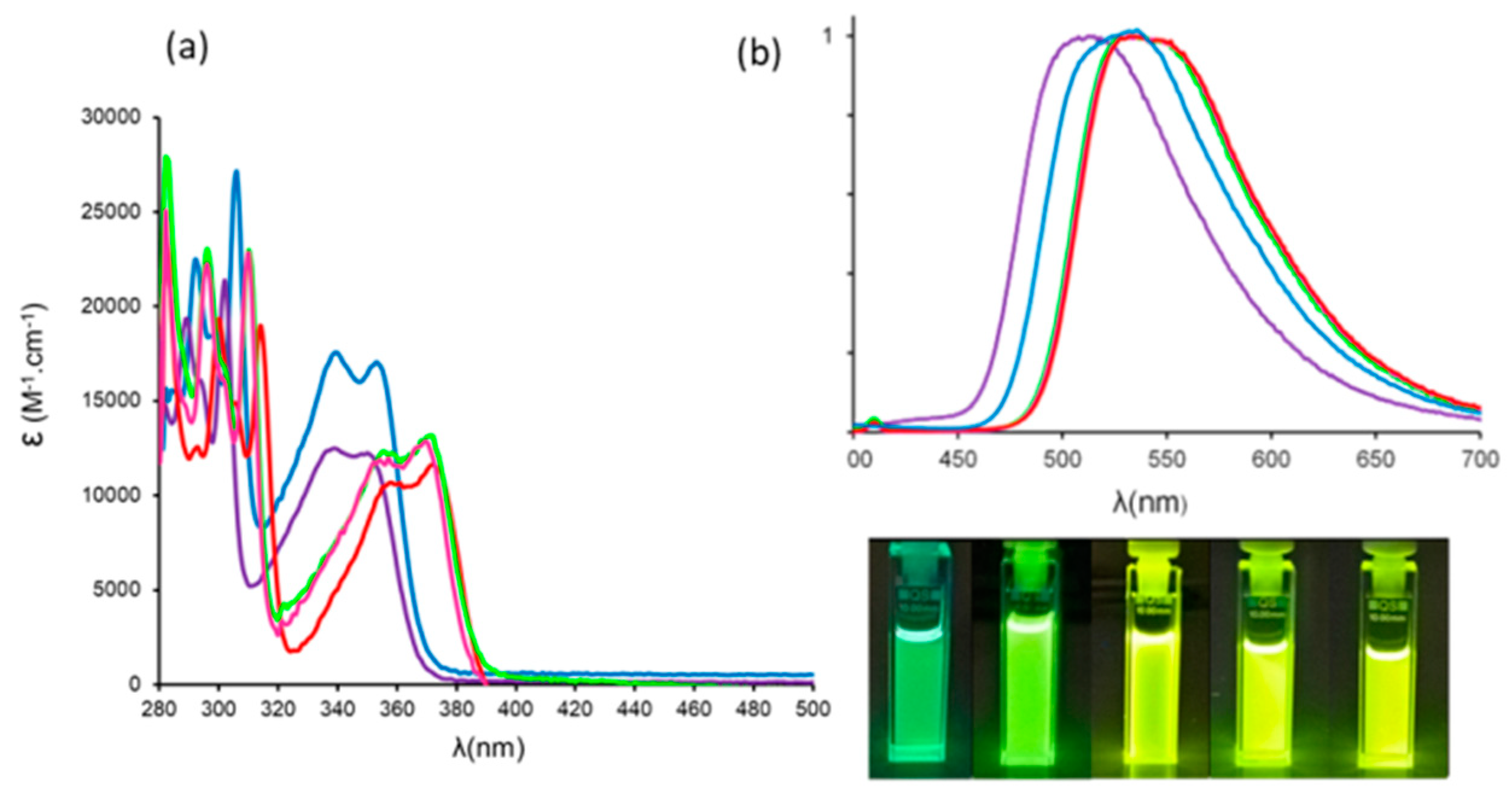

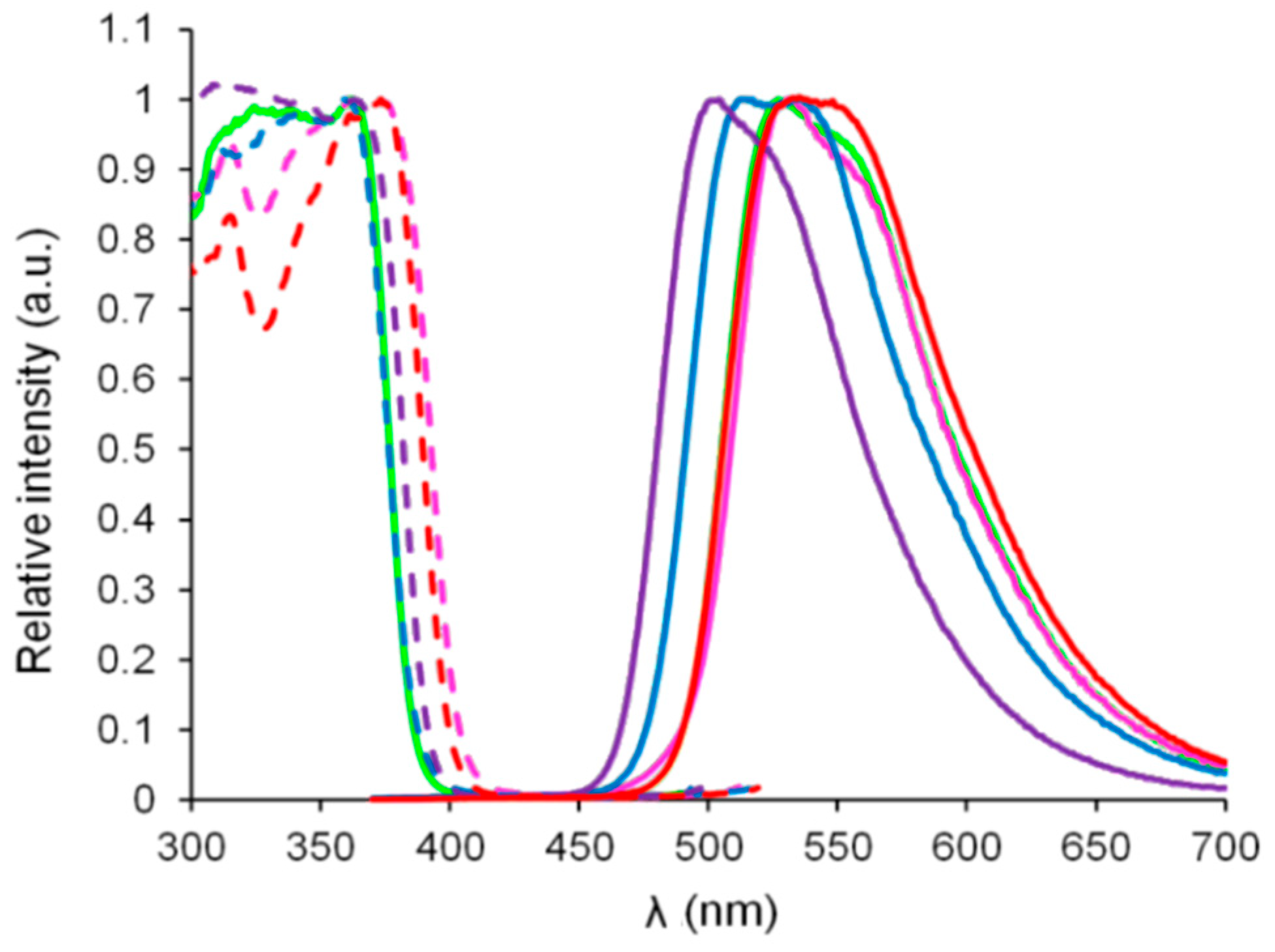
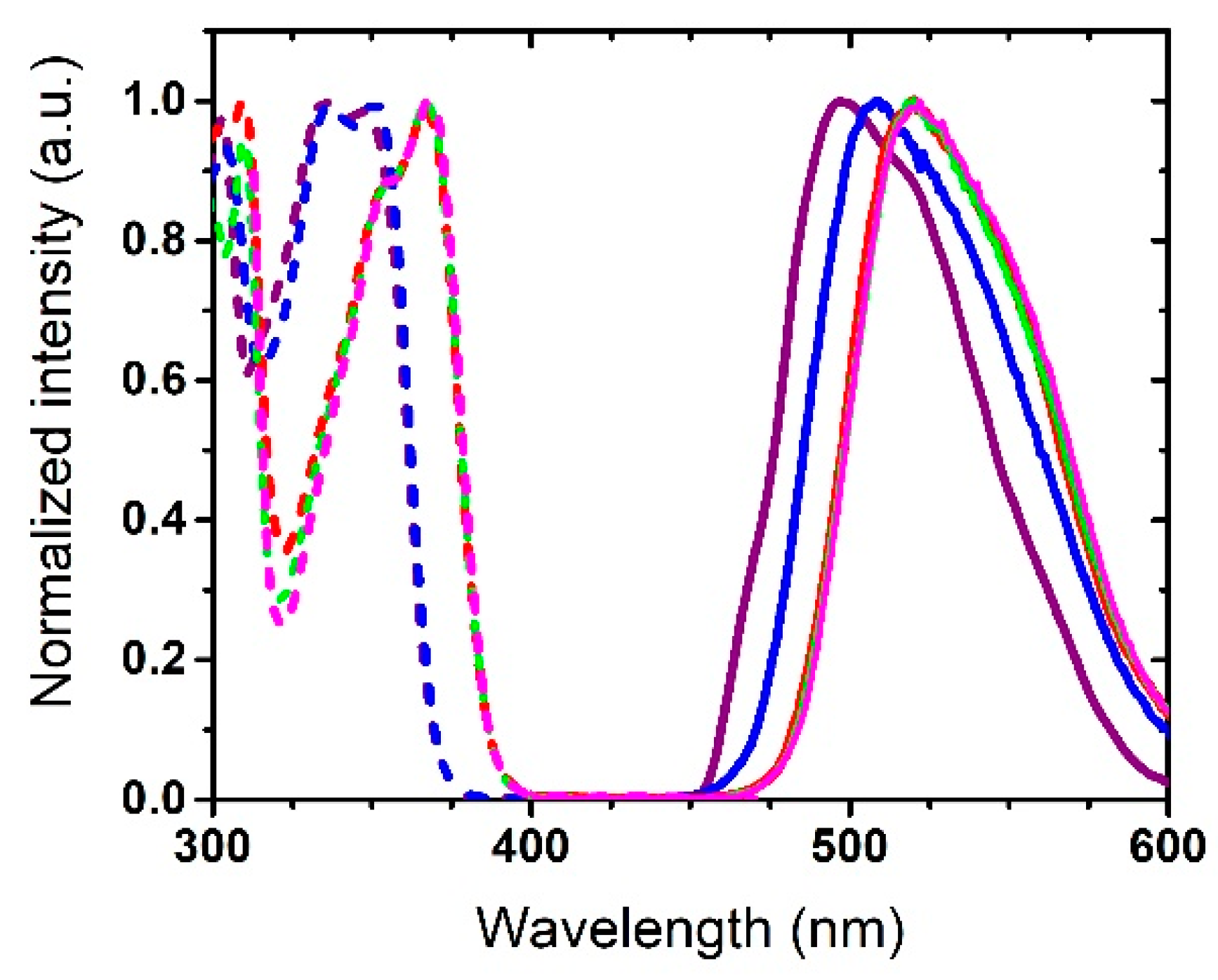
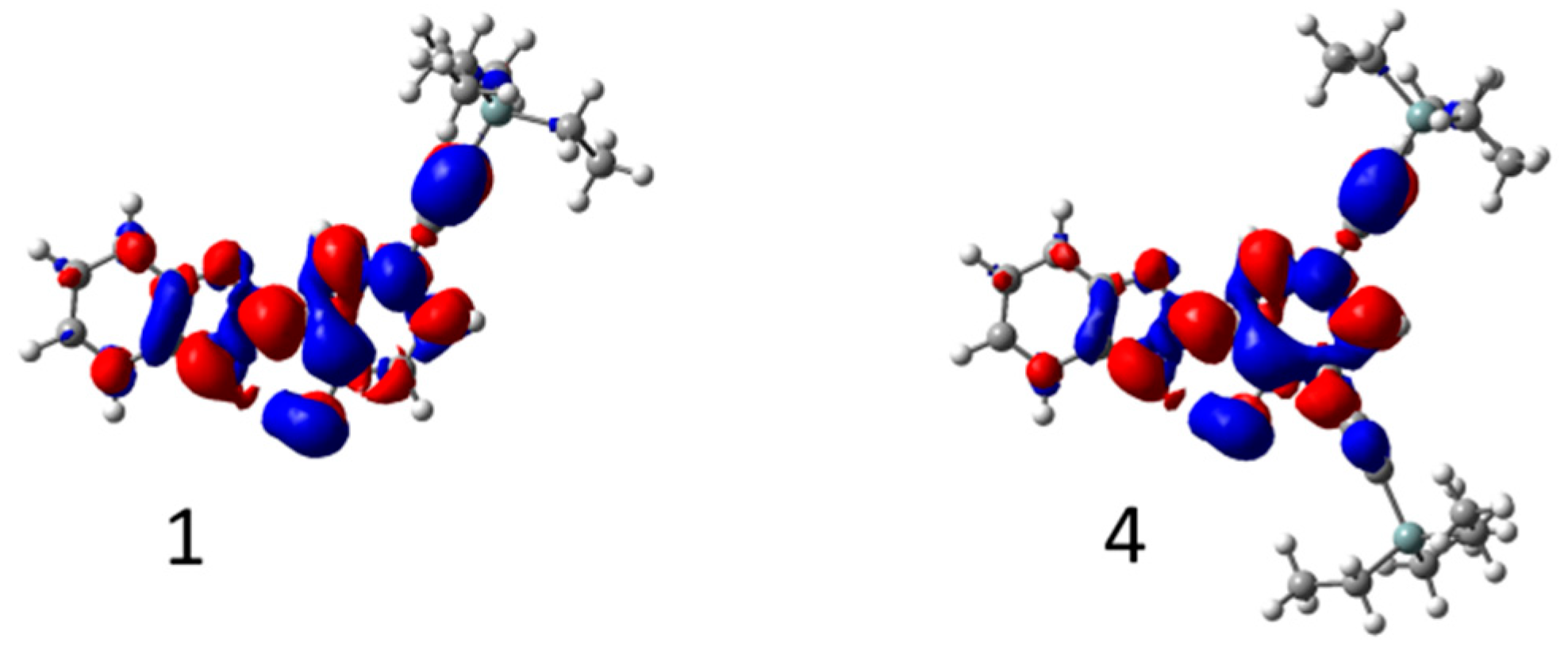
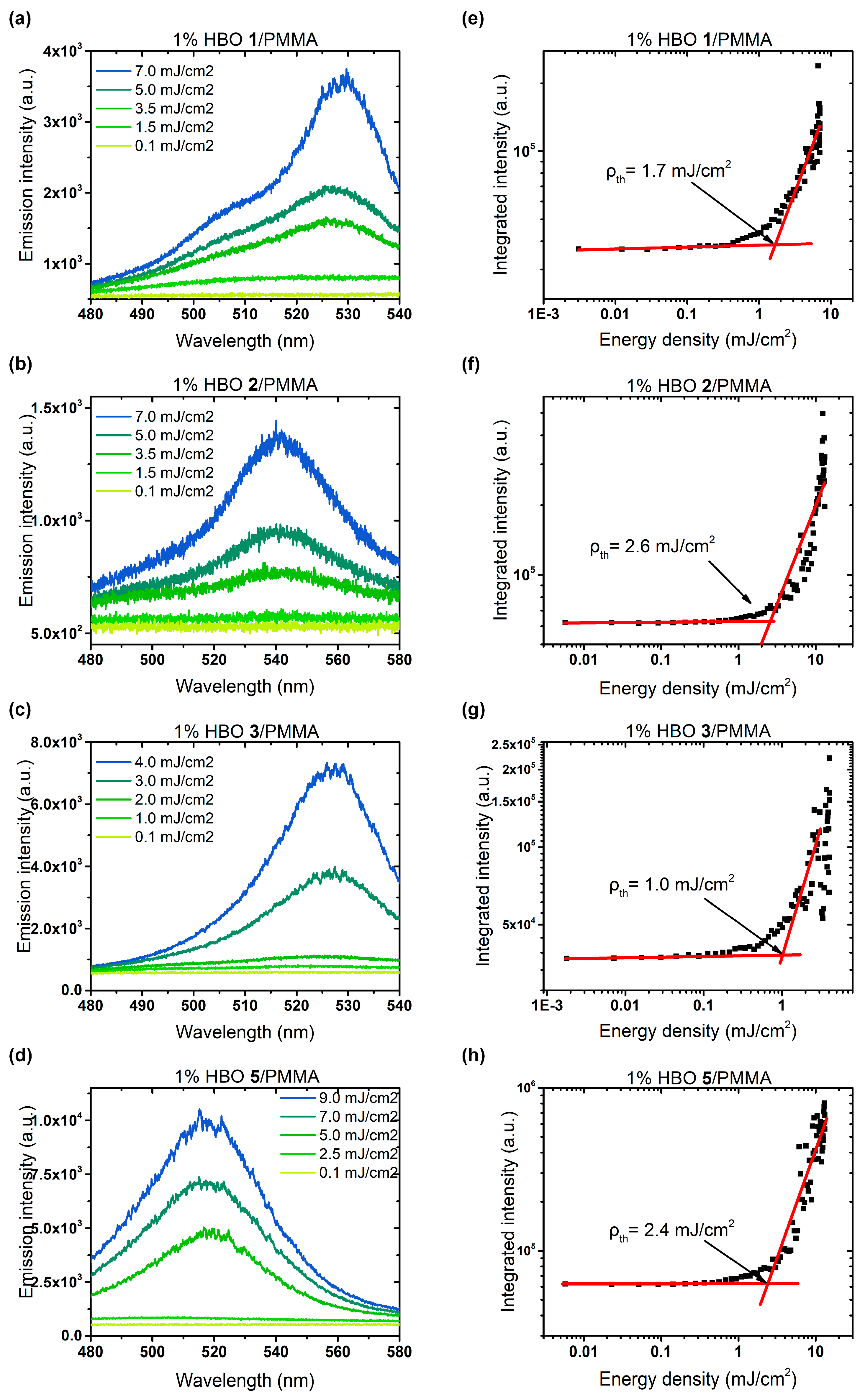
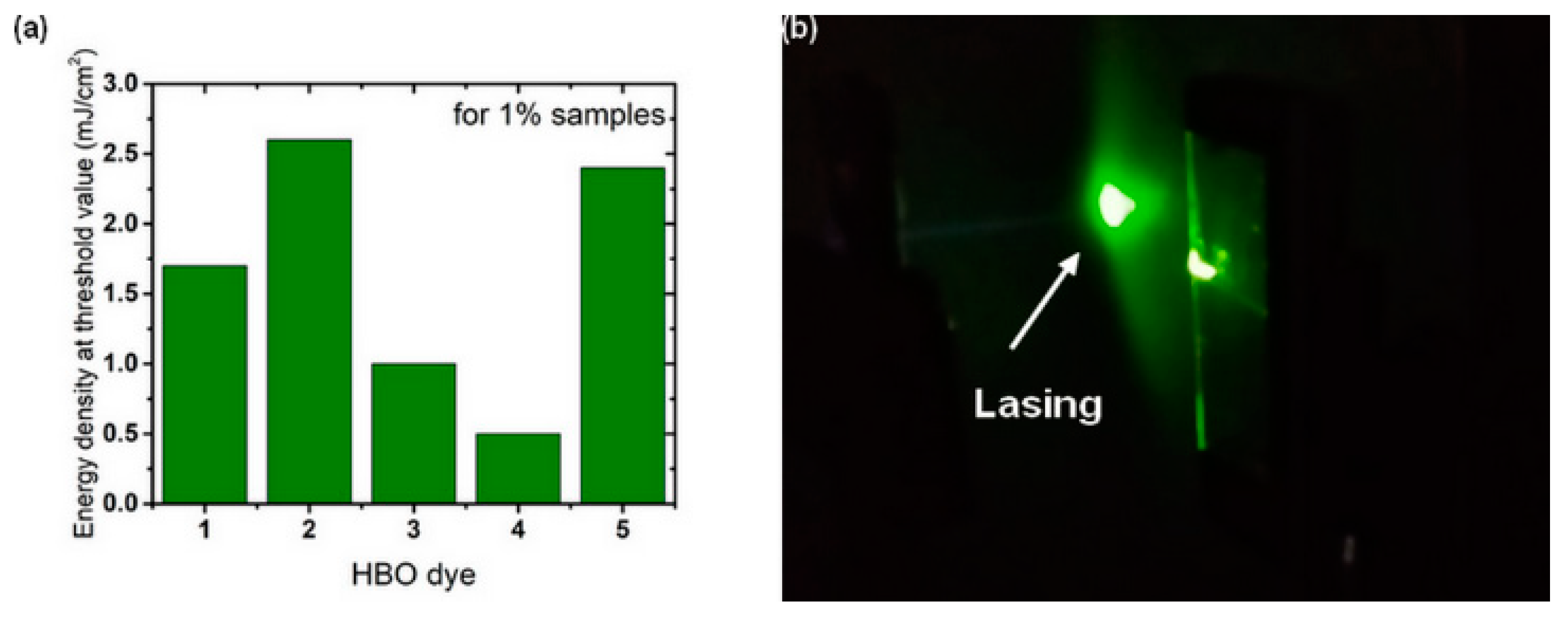
| Dye | λabs (nm) | ε (M−1·cm−1) | λem (nm) | ΔSS (cm−1) | ΦF [a] | τ (ns) | kr (108 s−1) [b] | knr (108 s−1) [b] | Solvent |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 345 | 11,900 | 513 | 9500 | 0.11 | 3.9 | 0.28 | 2.28 | Toluene |
| 1 | 339 | 11,700 | 407/504 | 4900 | 0.05 | 2.7 | 0.18 | 3.52 | EtOH |
| 2 | 340 | 17,000 | 538 | 10,800 | 0.28 | 2.9 | 0.97 | 2.48 | Toluene |
| 2 | 337 | 16,400 | 524 | 10,100 | 0.19 | 1.9 | 1.00 | 4.26 | EtOH |
| 3 | 368 | 12,800 | 539 | 8600 | 0.49 | 3.9 | 1.26 | 1.31 | Toluene |
| 3 | 367 | 8600 | 524 | 8200 | 0.33 | 3.0 | 1.10 | 2.23 | EtOH |
| 4 | 371 | 15,400 | 537 | 8300 | 0.38 | 3.7 | 1.41 | 2.30 | Toluene |
| 4 | 368 | 14,000 | 459/524 | 5400 | 0.28 | 3.2 | 0.88 | 2.25 | EtOH |
| 5 | 371 | 13,800 | 535 | 8300 | 0.32 | 3.8 | 0.84 | 1.79 | Toluene |
| 5 | 368 | 12,500 | 526 | 8200 | 0.31 | 3.0 | 1.03 | 2.30 | EtOH |
| λexc (nm) | λem (nm) | ΔSS (cm−1) | Φf [a] | Matrix | |
|---|---|---|---|---|---|
| 1 | 363 | 504 | 7600 | 0.66 | KBr |
| 1 | 337 | 430/516 | 5500 | 0.20 | PMMA |
| 2 | 359 | 514 | 8200 | 0.53 | KBr |
| 2 | 337 | 440/515 | 5800 | 0.22 | PMMA |
| 3 | 373 | 534 | 7800 | 0.70 | KBr |
| 3 | 363 | 450/526 | 5100 | 0.20 | PMMA |
| 4 | 363 | 527 | 7700 | 0.76 | KBr |
| 4 | 365 | 434/525 | 4200 | 0.49 | PMMA |
| 5 | 372 | 530 | 7900 | 0.82 | KBr |
| 5 | 364 | 457/527 | 5300 | 0.21 | PMMA |
| λabs (E) (nm) | λem (K*) (nm) | ΔGK*–E* (eV) | ΔGTS* (eV) | ΔGTS2* (eV) | |
|---|---|---|---|---|---|
| 1 | 317 | 510 | −0.25 | −0.15 | 0.06 |
| 2 | 317 | 532 | −0.28 | −0.10 | 0.12 |
| 3 | 332 | 547 | −0.26 | −0.07 | 0.16 |
| 4 | 334 | 550 | −0.26 | −0.11 | [a] |
| 5 | 335 | 552 | −0.28 | −0.09 | 0.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massue, J.; Pariat, T.; M. Vérité, P.; Jacquemin, D.; Durko, M.; Chtouki, T.; Sznitko, L.; Mysliwiec, J.; Ulrich, G. Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies. Nanomaterials 2019, 9, 1093. https://doi.org/10.3390/nano9081093
Massue J, Pariat T, M. Vérité P, Jacquemin D, Durko M, Chtouki T, Sznitko L, Mysliwiec J, Ulrich G. Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies. Nanomaterials. 2019; 9(8):1093. https://doi.org/10.3390/nano9081093
Chicago/Turabian StyleMassue, Julien, Thibault Pariat, Pauline M. Vérité, Denis Jacquemin, Martyna Durko, Tarek Chtouki, Lech Sznitko, Jaroslaw Mysliwiec, and Gilles Ulrich. 2019. "Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies" Nanomaterials 9, no. 8: 1093. https://doi.org/10.3390/nano9081093
APA StyleMassue, J., Pariat, T., M. Vérité, P., Jacquemin, D., Durko, M., Chtouki, T., Sznitko, L., Mysliwiec, J., & Ulrich, G. (2019). Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies. Nanomaterials, 9(8), 1093. https://doi.org/10.3390/nano9081093









