Abstract
Agricultural food crop plants interact with engineered nanomaterials (ENMs) from the application of agri-food nanotechnologies and from unintentional emissions originating from other nanotechnologies. Both types of exposure present implications for agricultural yield and quality, food chain transfer, and environmental and human health. In this review, the most recent findings from agricultural plant-ENM studies published in 2017 and 2018 are summarized. The aim of this is to identify the current hazard potential of ENMs for plants grown under typical field conditions that originate from both intentional and unintentional exposures and to contribute to knowledge-based decisions on the application of ENMs in food-agriculture. We also address recent knowledge on ENM adsorption, internalization, translocation, and bioaccumulation by plants, ENM impacts on agricultural crop yield and nutrition, and ENM biotransformation. Using adverse effect level concentrations and data on ENM accumulation in environmental matrices, the literature analyses revealed that C-, Ag-, Ce-, and Ti-based ENMs are unlikely to pose a risk to plants grown under typical field conditions, whereas Cu- and Zn-based ENMs require surveillance. Since multiple factors (e.g., ENM concentration, route of exposure, and plant type) influence the effects of ENMs on plants, biomonitoring is recommended for tracking ENM environmental exposure in the future.
1. Introduction
The 25th meeting of the Working Group for the Safety of Novel Foods and Feeds (WG-SNFF) in June 2018 recognized that the potential risks and benefits of nanotechnology-based products are examined on a case-by-case approach, as it is still a new field of application and research. However, it was recognized that new approaches may be necessary in the future to keep pace with the advances in this area [1]. At present, there is a lot of knowledge available to address the hazard and applications of nanomaterials (NMs) in the agri-food sector, however, it is scattered and limited to either particular applications or understandings of NM hazard. The aim of the present review is to integrate the applications and hazards of NMs to plants and critically address the safe use of NMs in the agri-food sector.
Potential applications of nanotechnologies in the agri-food sector include nanopesticides and nanofertilizers, nanozeolites and hydrogels to improve soil quality, NMs (SiO2, TiO2, and carbon nanotubes (CNTs)) to stimulate plant growth, and smart monitoring using nanosensors in connection with wireless communication devices. In addition, engineered NMs (ENMs) could be used for pesticide degradation, plant germination and growth, crop disease control, water purification, and pesticide residue detection [2]. While many agri-food nanotechnologies appear to be highly promising, they are not yet widely manufactured and implemented [3].
On the other hand, the unintentional emission of NMs due to increasing incorporation into consumer products has raised questions over the short- and long-term effects they may have on plants, i.e., food crop productivity, trophic transfer, and, ultimately, environmental and human health [4].
In this paper, we review recent scientific data on the application of ENMs in agriculture and data on the adverse effects of ENMs on plants in cases of unintentional exposures for some selected ENMs (C-, Ag-, Ce-, Ti-, and Zn-based ENMs). A literature search was made in the ACS, RSC, and Springer publication databases as well as in Google Scholar, using the search terms ‘plant’ AND ‘nanoparticle’ OR ‘nanomaterial’ for the years 2017 and 2018. Articles using non-agricultural and/or non-terrestrial plant species were excluded, along with papers whose topics did not align with those covered in this review. In the first part, we discuss the fate of NMs when interacting with plants (adsorption, internalization, translocation, and bioaccumulation), which is a major contributor to ENM effects, both beneficial and adverse. In the second part, we review adverse versus beneficial effects of some selected ENMs on plants. We address the question of whether the application of ENMs in the agri-food sector is justified from a nanosafety perspective and whether plants face a high hazard potential from ENMs during normal crop cultivation. Our primary aim is to support decision making for the application of ENMs to agricultural plants, including the Safety of Novel Foods and Feeds, which may include ENMs.
2. NM Interactions with Plants
A wide range of factors, including plant species, growth medium, exposure route and duration of exposure, abiotic and biotic stressors, and NM physicochemical properties affect plant–NM interactions [5,6]. These factors, along with NM adsorption, internalization, translocation, and bioaccumulation may be major contributors to their effects, both beneficial and adverse (i.e., agricultural crop yields, nutritional quality, NM transfer to human consumers, or plant nanotoxicity). Although plants have always been in contact with natural NMs (e.g., from forest fires and volcanic eruptions), there is significant interest in understanding their interactions with those originating from nanotechnologies [7]. In agricultural settings, plants are likely to be exposed to NMs through the application of biosolids and agricultural nanotechnologies (e.g., fertilizers, pesticides, and growth regulators) and through atmospheric deposition (especially in urban and industrial areas) [8,9]. Summaries of recent articles from 2017 and 2018, which address unintentional ENM exposures and their impacts on agricultural plants, are shown in Table 1.

Table 1.
Summaries of engineered nanomaterial (ENM) effects on agricultural plant yield and/or nutritional contents documented in recent papers from 2017 and 2018.
Recently, Drobne, et al. [10] published a review on the application of different microscopy and spectroscopy techniques for detecting and visualizing NMs in biological samples, which shows developments in the field of studying ENM loads and distributions in biota. The NM load can be transferred to higher levels of the food chain and also consumed by humans when adsorbed to or internalized by edible plants. Retention by leaves may occur by entrapment on the outermost cuticular wax layer and internalization through openings, such as stomata, that regulate gas and water balance. Translocation to roots may occur via phloem transport together with the products of photosynthesis [11]. In roots, NMs can also be internalized with water and nutrients in soil or hydroponic media, with uptake being highly modulated by external factors including the growth medium [12], pH [13,14], cation exchange capacity [15], root exudates [16,17], and mycorrhizal fungi [18,19]. In the event of uptake, translocation to leaves may be restricted by the Casparian strip, necessitating symplastic transport through cellular plasmodesmata to reach the xylem and phloem [4]. For both root and foliar exposures, NM uptake is highly dependent on the plant species and transpiration rate [9,11,20], and NM size [18,21], chemical composition [22,23], surface functionalization [24,25,26], age [27], and stability [28].
3. Unintentional NM Exposure and Impacts on Agricultural Crop Yield and Nutritional Value
A number of studies have shown that NMs may have adverse or beneficial effects on agricultural plant yield and nutritional value, which raise important implications for food quality. Summaries of both types of effects from recent literature (2017 and 2018) are shown in Table 1, while further elaboration of these effects is provided in Sections S1–S6 in the Supplementary Materials.
4. Co-Exposure to ENMs and Pollutants and Effects on Bioaccumulation and Phytotoxicity
Plant co-exposure to ENMs and environmental contaminants has been recently reviewed [47] and is a growing topic of interest in plant-ENM studies. Engineered NM co-exposures with organic and metal pollutants and other types of ENMs may affect the uptake and translocation of each material by plants, which ultimately affects NM interactions with plants, both adverse and beneficial. Summaries of some additional papers from 2017 and 2018 to those presented in a recent review by Deng et al. [47] are listed in Table 2.

Table 2.
Summaries of recent ENM co-exposure studies from 2017 and 2018.
Changes in plant susceptibility to pollutants when co-exposed with ENMs are often attributed to the high adsorption affinities of NMs which can enhance pollutant uptake and subsequent adverse effects. This was reported for rice co-exposure to graphene oxide (GO) and polycyclic aromatic hydrocarbons (PAHs) in hydroponic medium (26% and 92% higher PAH uptake at 0.01 and 0.1 mg GO/L, respectively) [48]. By the same mechanism, pollutant uptake and toxicity may also be decreased if the pollutant concentration and/or bioavailability are reduced due to its immobilization by surface adsorption to ENM surfaces. Deng et al. [25] reported reduced carbamazepine uptake by collard greens in soil and hydroponic medium with co-exposure to pristine and surface carboxylated CNTs, while Liu et al. [49] reported that CuO NMs (50 mg/kg soil) reduced As rice grain content by 35% relative to cultivation with As alone (10 mg/kg soil). In addition, the almost complete elimination of tetracycline uptake in rice was documented in the case of co-exposure with TiO2 NMs in hydroponic medium [50]. In soil-cultivated barley plants, SiO2 NMs alleviated the negative impacts of NiO NMs on plant biomass and antioxidant activity levels, and completely reversed its effects on photosynthetic parameters [51]. Very recently, Rossi et al. [17] reported that increased soybean root exudate excretion in response to polyvinylpyrrolidone (PVP)-CeO2 NMs and Cd2+ co-exposure led to binding between biomolecules in the root exudate and Cd2+, thus reducing shoot Cd content by 78% in hydroponic medium. In addition, shortened root apoplastic barrier structures in rapeseed following co-exposure to PVP-CeO2 NMs and NaCl in sand medium were attributed as the cause of altered Na concentrations in roots (−35%) and leaves (+30%) relative to the individual exposure treatments [52]. When assessing the effects of NMs on plants, it is necessary to take into consideration that co-exposure with other chemicals may significantly modify their effects.
5. NM Biotransformation in Plants
Nanomaterial biotransformations are a result of NM-biota interactions and alter the behavior and fate of ENMs in the environment. Nanomaterial biotransformations include dissolution, redox reactions, and chemical reactions with surrounding molecules which occur in contact with biological media and biological surfaces [58]. Some ENMs are generally recognized as stable under environmental and biological conditions, while others are prone to transformations. Table 3 provides summaries of ENM biotransformations recorded in recent literature from 2017 and 2018.

Table 3.
Summaries of ENM biotransformation studies in plants from 2017 and 2018.
From among the most recent literature on NM biotransformation, two points can be made. The first is that NM uptake and biotransformation are reported to follow dissolution outside the plant tissue. In other words, biotransformation of undissolved NMs does not appear to occur. This was reported for bean root exposure to ZnO NMs in hydroponic medium [59], rice root exposure to CuO NMs in soil [36], and lettuce root exposure to weathered CuO NMs, in which a 214% greater root Cu uptake was reported relative to plants exposed to unweathered CuO NMs [54].
The second point is that NM uptake and biotransformation occur more frequently in hydroponic medium, which is more conducive to NM aggregation and dissolution around roots than soil, indicating that NMs are more likely to exert effects on plants in hydroponic medium. No CeO2 NM biotransformation was detected following wheat [34] and tomato and fescue [60] root exposure to CeO2 NMs in soil, whereas studies conducted in hydroponic medium showed biotransformation of Ce(IV) to Ce(III) (15–20%) in cucumber [28] and wheat roots [61]. A recent study involving wheat root exposure to Ag NMs or Ag2S NMs in hydroponic medium reported complete biotransformation of both NM types, despite the fact that Ag2S NMs are reported to be highly stable. Ag2S NMs often constitute the final end product for Ag NMs, which may precipitate from Ag+ ions before sulfidation into Ag2S NMs. However, the lack of metallic Ag and the presence of Ag-thiol complexes (13%) in the secondary root tissue of Ag2S NM-exposed plants supported the conclusion that the Ag2S NMs dissolved prior to uptake, most likely due to the presence of root exudates [23]. Of relevance to toxicity, a bean seed germination study conducted with ZnO NMs in water reported that it was the amount of biotransformed Zn, rather than the total amount of Zn incorporated into the seedlings, that correlated with the severity of adverse effects (i.e., reduced weight gain) [62]. These findings suggest that under typical outdoor agricultural cultivation in soil, plants are more susceptible to NMs that dissolve easily (e.g., Ag, Cu, and Zn), with the main risk originating from the dissolved ions rather than from the NMs themselves.
6. Applications of ENMs in the Agri-Food Sector
There has been substantial interest in harnessing the intrinsic properties of NMs for agricultural applications, which has been the subject of several recent reviews [65,66,67,68]. It is now clear that there is not only one characteristic of NMs responsible for their biological effects, but rather that their interplay with environmental and biological media over time can result in beneficial or adverse effects for agri-food systems. Engineered NMs are used for targeted treatments as fertilizers, antimicrobial agents, and carrier systems for active ingredients (i.e., pesticides, herbicides, fertilizers, growth hormones, and metal nutrients). As carriers, ENMs (typically C-based) can increase the solubility, stability, and bioavailability of active ingredients, reducing field losses from runoff, degradation, and volatilization, and minimizing the extent of downstream environmental pollution [67,69]. Additional applications include seed coatings or soaks and hydroponic additives. A summary of recently published studies focusing on ENM applications in agriculture from 2017 and 2018 is shown in Table 4.

Table 4.
ENMs for agricultural applications described in recent literature from 2017 and 2018.
In the case of seed treatments [21,62,70,71,72,73,74,75,76] and early-stage root treatments in hydroponic media [56], ENM transfer into edible plant segments and to the environment is limited. In view of their reported benefits, their use appears to be justified. Relatively few studies investigated the addition of ENMs to soil, however, tested materials include those which are already used in outdoor settings (i.e., CB and hydroxyapatite) [55,77] and those which are prone to dissolution and are commonly used in conventional agriculture in dissolved form (i.e., Cu and Zn) [27,62,70,72,78]. This suggests that such uses are also justified from a nanosafety perspective. All foliar sprays, regardless of their intended function, directly expose plants and the environment to ENMs through drift. Borgatta et al. [78] found that dipping leaves into a NM-containing suspension provided superior results to spraying because it reduced drift while improving leaf exposure. There are no field studies on soil accumulation of these ENMs, but, with frequent use, in parallel environmental monitoring should be required.
7. Hazard Potential of ENMs to Plants: General Perspective
A summary of NOAEL (no observed adverse effect level) and LOAEL (lowest observed adverse effect level) values for plants exposed to the most frequently studied ENMs in the recent literature from 2017 and 2018 (C-, Ag-, Ce-, Cu-, Ti-, and Zn-based ENMs), together with their corresponding concentrations in relevant environmental matrices, are supplied in Table 5, while a graphical representation of the general hazard potential from these ENMs is available in Figure 1.

Table 5.
Plant NOAEL and LOAEL values for exposure to C-, Ag-, Ce-, Cu-, Ti-, and Zn-based ENMs with the recorded adverse physiological and/or biochemical effect(s).
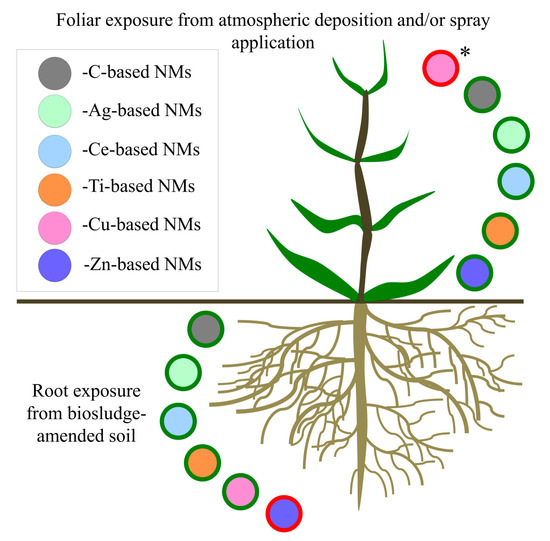
Figure 1.
Graphical representations of commonly investigated ENM types and their hazard potential to agricultural plants (green and red outlines represent low and high hazard potential, respectively). * Potential toxicity from foliar application of Cu-containing foliar sprays and atmospheric deposition in urban areas.
Risk analyses based on comparison of ENM LOAEL values with their concentrations in waste water treatment plant (WWTP) biosludge and biosludge-amended soil shows that, with a few exceptions which include dissolving ENMs, there is a low risk of plant phytotoxicity (Table 5). Mean concentrations of Ag- and Ce-based ENMs in biosludge-amended soil (assuming the unlikely scenario of 100% ENM persistence) were approximately 400–20,000 times lower and 200–11,000 times lower, respectively, than their lowest LOAEL values for root exposure in soil [84]. As TiO2 ENMs had no adverse plant effects up to a concentration of 750 mg/kg in soil, it is unlikely that a biosludge concentration of 170 mg/kg poses a risk to plants [85]. Among C-based ENMs, CNTs and CB (which exhibited an inverse dose-response relationship of greater toxicity at lower concentrations) [86] pose a low hazard potential, as they were measured at concentrations approximately 1.5 times and 5000–50,000 times above the LOAEL value of CNTs and CB in biosludge, respectively [85,87]. Even where adverse effects increased with increasing concentration in soil [88], the LOAEL concentration for CNTs is roughly 300 times below the concentration of CNTs measured in biosludge [85] and therefore would not have a high hazard potential. With Cu-based ENMs, their concentration measured in biosolids was ~200–5000 times lower than the lowest LOAEL value for root exposure in soil, indicating a nearly non-existent risk of phytotoxicity [87]. However, plants were adversely affected by short-term foliar exposures to CuO NMs [9] and a Cu(OH)2 pesticide spray, therefore this route of exposure could present a hazard to plants [38,89]. Only Zn-based ENMs were measured in biosludge at higher concentrations than the lowest level that induced toxicity from root exposure in soil (8 times higher) [84,85,87]. However, soil characteristics were shown to play a more dominant role than that of ZnO NM exposure in plant responses, and the reported adverse effects were changes to antioxidant enzyme activity levels rather than to physiological growth parameters [14].
There remains a lack of understanding about plant responses to long-term, low-dose exposures to ENMs, including exposures that occur over successive plant generations. Therefore, it is not possible to accurately model the future severity and types of effects that might occur as a result of current agricultural plant exposures to ENMs. Rather, we propose that environmental and biological monitoring (biomonitoring) would present an acceptable solution for recording plant exposures to ENMs before other regulatory requirements are elaborated and put into practice. Wastewater treatment plant biosludge and biosludge-amended soils are ideal matrices for environmental monitoring because they form the main point of agricultural plant exposure to ENMs originating from consumer and industrial sources [8,90]. Biomonitoring goes further by providing information on the bioavailability of a given substance through the measurement of specific biomarkers in target species [90]. A number of ENM-specific plant biomarkers have been identified in a recent meta-analysis of literature on omics-level plant responses to ENMs by Ruotolo et al. [91], indicating promising advances in this area.
8. Conclusions and Future Perspectives
Through a review of the recent literature, we have shown that agricultural plants do not currently face a high hazard potential from ENMs during crop cultivation. Many of the most frequently investigated ENMS (C-, Ce-, Ti-, and Ag-based) are present in environmental media at concentrations that are unlikely to pose a significant threat to agricultural plant safety, while Cu- and Zn-based ENMs may have the potential to exert adverse effects, depending on the mode of exposure and soil characteristics, respectively. A number of key points can be made:
- NMs do not pose risks to plant safety and agronomic characteristics, such as yield and nutritional quality, except at extremely high, environmentally unrealistic concentrations;
- NM dissolution appears to be a significant driver of toxicity due to the increased bioavailability of ions;
- NM co-exposures may enhance or diminish the risks posed by other toxic pollutants;
- NMs at low concentrations and/or applied during the early stages of plant growth (e.g., as seed coatings) provide beneficial effects with limited introduction into the environment or edible plant segments, justifying such uses from a nanosafety perspective.
In order to make progress in anticipating and responding to plant-ENM exposures and promote the responsible use of agricultural nanotechnologies, numerous data gaps must be addressed in future research. Multi-generational plant exposure studies that simulate realistic field exposure conditions and ENM types and doses are greatly needed, especially for evaluating ENM-based agricultural products which are already on the market. Likewise, ENM co-exposure and biotransformation studies remain needed to better understand the persistence and uptake of ENMs and other substances (e.g., nutrients and contaminants) which may be present in soil. While agri-food nanotechnologies have a high potential to reduce environmental pollution and ecosystem and human health risks associated with conventional agricultural practices while increasing food production and quality, the already-listed limitations and knowledge gaps make it difficult to compare the use of nanotechnologies with conventional practices in terms of these factors. Despite these informational gaps in the understanding of plant responses to ENMs, the implementation of effective monitoring for ENMs in the environment and plant responses to them (biomonitoring) could help to assure their beneficial use in the agri-food sector.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/9/8/1094/s1, Section S1: Carbon-based NM interactions with plants, Section S2: Silver-based NM interactions with plants, Section S3: Copper-based NM interactions with plants, Section S4: Cerium-based NM interactions with plants, Section S5: Titantium-based NM interactions with plants, and Section S6: Zinc-based NM interactions with plants.
Author Contributions
Conceptualization, E.K. and D.D.; methodology, E.K. and D.D.; data curation, E.K.; writing—original draft preparation, E.K.; writing—review and editing, E.K. and D.D.; visualization, E.K.; supervision, D.D.
Funding
This research was funded by the ISO-FOOD Project “ERA Chair for Isotope Techniques in Food Quality, Safety and Traceability” (grant agreement No. 621329).
Acknowledgments
We express our thanks to David Heath for providing the impetus for this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Organisation for Economic Co-operation and Development. Task Force on the Safety of Novel Foods and Feeds. In Proceedings of the Organisation for Economic Co-operation and Development 25th Meeting of the Working Group for the Safety of Novel Foods and Feeds, Paris, France, 26–27 June 2018. [Google Scholar]
- Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Zeng, Z.; Wang, A.; Liu, G.; Cui, H. The Application of Nano-TiO2 Photo Semiconductors in Agriculture. Nanoscale Res. Lett. 2016, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural Nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant. Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Morales-Díaz, A.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic Transfer, Transformation, and Impact of Engineered Nanomaterials in Terrestrial Environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper Oxide Nanoparticle Foliar Uptake, Phytotoxicity, and Consequences for Sustainable Urban Agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
- Drobne, D.; Novak, S.; Talaber, I.; Lynch, I.; Kokalj, A.; Drobne, D.; Novak, S.; Talaber, I.; Lynch, I.; Kokalj, A.J. The Biological Fate of Silver Nanoparticles from a Methodological Perspective. Materials 2018, 11, 957. [Google Scholar] [CrossRef]
- Kranjc, E.; Mazej, D.; Regvar, M.; Drobne, D.; Remškar, M. Foliar surface free energy affects platinum nanoparticle adhesion, uptake, and translocation from leaves to roots in arugula and escarole. Environ. Sci. Nano 2017, 5, 520–532. [Google Scholar] [CrossRef]
- Kořenková, L.; Šebesta, M.; Urík, M.; Kolenčík, M.; Kratošová, G.; Bujdoš, M.; Vávra, I.; Dobročka, E. Physiological response of culture media-grown barley (Hordeum vulgare L.) to titanium oxide nanoparticles. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2017, 67, 285–291. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Xie, Y.; Yin, Y.; Zhu, J.; Zhang, X.; Ji, R.; Gu, K.; Peralta-Videa, J.R.; Guo, H. Elevated CO2 levels modify TiO2 nanoparticle effects on rice and soil microbial communities. Sci. Total Environ. 2017, 578, 408–416. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Han, Y.; Tan, J.; Wang, Y.; Wang, G.; Wang, H. Effects of Nanochitin on the Enhancement of the Grain Yield and Quality of Winter Wheat. J. Agric. Food Chem. 2017, 66, 6637–6645. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, L.; Keller, A.A. Interactions, Transformations, and Bioavailability of Nano-Copper Exposed to Root Exudates. Environ. Sci. Technol. 2017, 51, 9774–9783. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.P.; Ma, X. Mutual effects and in planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max L. Merr). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Noori, A.; White, J.C.; Newman, L.A. Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J. Nanoparticle Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Siani, N.G.; Fallah, S.; Pokhrel, L.R.; Rostamnejadi, A. Natural amelioration of Zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant. Physiol. Biochem. 2017, 112, 227–238. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; White, J.C.; Dhankher, O.P.; Zhang, X.; Zhang, S.; Xing, B. Quantitative evaluation of multi-wall carbon nanotube uptake by terrestrial plants. Carbon 2017, 114, 661–670. [Google Scholar] [CrossRef]
- Duran, N.M.; Savassa, S.M.; Lima, R.G.; de Almeida, E.; Linhares, F.S.; van Gestel, C.A.M.; Pereira de Carvalho, H.W. X-ray Spectroscopy Uncovering the Effects of Cu Based Nanoparticle Concentration and Structure on Phaseolus vulgaris Germination and Seedling Development. J. Agric. Food Chem. 2017, 65, 7874–7884. [Google Scholar] [CrossRef]
- Pagano, L.; Pasquali, F.; Majumdar, S.; Torre-Roche, R.D.; Zuverza-Mena, N.; Villani, M.; Zappettini, A.; Marra, R.E.; Isch, S.M.; Marmiroli, M.; et al. Exposure of Cucurbita pepo to binary combinations of engineered nanomaterials: Physiological and molecular response. Environ. Sci. Nano 2017, 4, 1579–1590. [Google Scholar] [CrossRef]
- Pradas Del Real, A.E.; Vidal, V.; Carrière, M.; Castillo-Michel, H.; Levard, C.; Chaurand, P.; Sarret, G. Silver Nanoparticles and Wheat Roots: A Complex Interplay. Environ. Sci. Technol. 2017, 51, 5774–5782. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max L. Merr). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Deng, Y.; Eitzer, B.; White, J.C.; Xing, B. Impact of multiwall carbon nanotubes on the accumulation and distribution of carbamazepine in collard greens (Brassica oleracea). Environ. Sci. Nano 2017, 4, 149–159. [Google Scholar] [CrossRef]
- Medina-Velo, I.A.; Dominguez, O.E.; Ochoa, L.; Barrios, A.C.; Hernández-Viezcas, J.A.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Nutritional quality of bean seeds harvested from plants grown in different soils amended with coated and uncoated zinc oxide nanomaterials. Environ. Sci. Nano 2017, 4, 2336–2347. [Google Scholar] [CrossRef]
- Gao, X.; Avellan, A.; Laughton, S.N.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, X.; Zhang, P.; Zhang, Z.; Ding, Y.; Zhang, J.; Wang, G.; Xie, C.; Luo, W.; Zhang, J.; et al. Xylem and Phloem Based Transport of CeO2 Nanoparticles in Hydroponic Cucumber Plants. Environ. Sci. Technol. 2017, 51, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.M.; Bhati, A.; Singh, A.; Sonker, A.K.; Sarkar, S.; Sonkar, S.K. Sustainable Changes in the Contents of Metallic Micronutrients in First Generation Gram Seeds Imposed by Carbon Nano-onions: Life Cycle Seed to Seed Study. ACS Sustain. Chem. Eng. 2017, 5, 2906–2916. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2017, 66, 6654–6662. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Tang, X.; Yang, J.; Jiang, F.; Pan, Y.; Xiang, Z.; Hao, Y.; Rui, Y.; Cao, W.; et al. Phytotoxicity of Silver Nanoparticles to Peanut (Arachis hypogaea L.): Physiological Responses and Food Safety. ACS Sustain. Chem. Eng. 2017, 5, 6557–6567. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of Crop Yield and Quality of Wheat upon Exposure to Silver Nanoparticles in a Life Cycle Study. J. Agric. Food Chem. 2018, 66, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Rico, C.M.; Johnson, M.G.; Marcus, M.A.; Andersen, C.P. Intergenerational responses of wheat (Triticum aestivum L.) to cerium oxide nanoparticles exposure. Environ. Sci. Nano 2017, 4, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium Oxide Nanoparticles Decrease Drought-Induced Oxidative Damage in Sorghum Leading to Higher Photosynthesis and Grain Yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and Transformation of CuO Nanoparticles in the Soil–Rice System during the Life Cycle of Rice Plants. Environ. Sci. Technol. 2017, 51, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Pullagurala, V.L.R.; Hernandez-Molina, M.; Sun, Y.; Niu, G.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: A full life cycle study. Environ. Sci. Nano 2018, 5, 83–95. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Adeleye, A.S.; Keller, A.A. Metabolomics Reveals Cu(OH)2 Nanopesticide-Activated Anti-oxidative Pathways and Decreased Beneficial Antioxidants in Spinach Leaves. Environ. Sci. Technol. 2017, 51, 10184–10194. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.K.; Arshad, M. Growth and Metabolic Responses of Rice (Oryza sativa L.) Cultivated in Phosphorus-Deficient Soil Amended with TiO2 Nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Exposure to Weathered and Fresh Nanoparticle and Ionic Zn in Soil Promotes Grain Yield and Modulates Nutrient Acquisition in Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2018, 66, 9645–9656. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and Ionic Zn Promote Nutrient Loading of Sorghum Grain under Low NPK Fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jing, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased ZnO nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef] [PubMed]
- Medina-Velo, I.A.; Zuverza-Mena, N.; Tamez, C.; Ye, Y.; Hernandez-Viezcas, J.A.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Minimal Transgenerational Effect of ZnO Nanomaterials on the Physiology and Nutrient Profile of Phaseolus vulgaris. ACS Sustain. Chem. Eng. 2018, 6, 7924–7930. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; White, J.C.; Hao, Y.; Wang, Y.; Tang, X.; Yang, J.; Jiang, F.; Ali, A.; Rui, Y.; et al. Metal oxide nanoparticles alter peanut (Arachis hypogaea L.) physiological response and reduce nutritional quality: A life cycle study. Environ. Sci. Nano 2018, 5, 2088–2102. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, L.; Hu, X. Integrating proteomics, metabolomics and typical analysis to investigate the uptake and oxidative stress of graphene oxide and polycyclic aromatic hydrocarbons. Environ. Sci. Nano 2018, 5, 115–129. [Google Scholar] [CrossRef]
- Liu, J.; Simms, M.; Song, S.; King, R.S.; Cobb, G.P. Physiological Effects of Copper Oxide Nanoparticles and Arsenic on the Growth and Life Cycle of Rice (Oryza sativa japonica ‘Koshihikari’). Environ. Sci. Technol. 2018, 52, 13728–13737. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, H.; Chen, G.; Zhao, Q.; Eitzer, B.; Wang, Z.; Cai, W.; Newman, L.A.; White, J.C.; Dhankher, O.P.; et al. Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa. Environ. Sci. Nano 2017, 4, 1827–1839. [Google Scholar] [CrossRef]
- Soares, C.; Branco-Neves, S.; de Sousa, A.; Azenha, M.; Cunha, A.; Pereira, R.; Fidalgo, F. SiO2 nanomaterial as a tool to improve Hordeum vulgare L tolerance to nano-NiO stress. Sci. Total Environ. 2018, 622, 517–525. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Ma, X. Cerium oxide nanoparticles alter the salt stress tolerance of Brassica napus L. by modifying the formation of root apoplastic barriers. Environ. Pollut. Bark. Essex 1987 2017, 229, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Zhang, W.; Schwab, A.P.; Ma, X. Uptake, Accumulation, and in Planta Distribution of Coexisting Cerium Oxide Nanoparticles and Cadmium in Glycine max L Merr. Environ. Sci. Technol. 2017, 51, 12815–12824. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; Pagano, L.; Castillo-Michel, H.; De la Torre-Roche, R.; Hawthorne, J.; Hernandez-Viezcas, J.A.; Loredo-Portales, R.; Majumdar, S.; Gardea-Torresday, J.; Dhankher, O.P.; et al. Weathering in soil increases nanoparticle CuO bioaccumulation within a terrestrial food chain. Nanotoxicology 2017, 11, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yi, Y.; Fang, Z.; Tsang, E.P. Effects of biochar on phytotoxicity and translocation of polybrominated diphenyl ethers in Ni/Fe bimetallic nanoparticle-treated soil. Environ. Sci. Pollut. Res. 2018, 25, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.X.; Wang, G.Z.; Zhang, Y.X.; Zhao, H.J. Hydroxyapatite nanoparticles in root cells: Reducing the mobility and toxicity of Pb in rice. Environ. Sci. Nano 2018, 5, 398–407. [Google Scholar] [CrossRef]
- Jośko, I.; Oleszczuk, P.; Skwarek, E. Toxicity of combined mixtures of nanoparticles to plants. J. Hazard. Mater. 2017, 331, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of Engineered Nanoparticles in the Environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.N.M.; Savassa, S.M.; Gomes, M.H.F.; Rodrigues, E.S.; Duran, N.M.; Almeida, E.; Martinelli, A.P.; Carvalho, H.W.P. Shedding light on the mechanisms of absorption and transport of ZnO nanoparticles by plants via in vivo X-ray spectroscopy. Environ. Sci. Nano 2017, 4, 2367–2376. [Google Scholar] [CrossRef]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that Soil Properties and Organic Coating Drive the Phytoavailability of Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef] [PubMed]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G.V. Impact of Surface Charge on Cerium Oxide Nanoparticle Uptake and Translocation by Wheat (Triticum aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Savassa, S.M.; Duran, N.M.; Rodrigues, E.S.; de Almeida, E.; van Gestel, C.A.M.; Bompadre, T.F.V.; de Carvalho, H.W. Effects of ZnO Nanoparticles on Phaseolus vulgaris Germination and Seedling Development Determined by X-ray Spectroscopy. ACS Appl. Nano Mater. 2018, 1, 6414–6426. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef]
- Rico, C.M.; Johnson, M.G.; Marcus, M.A. Cerium oxide nanoparticles transformation at the root–soil interface of barley (Hordeum vulgare L.). Environ. Sci. Nano 2018, 5, 1807–1812. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; Medeiros, D.A.G.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in Agriculture: Which Innovation Potential Does It Have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 2517. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Baddar, Z.E.; Unrine, J.M. Functionalized-ZnO-Nanoparticle Seed Treatments to Enhance Growth and Zn Content of Wheat (Triticum aestivum) Seedlings. J. Agric. Food Chem. 2018, 66, 12166–12178. [Google Scholar] [CrossRef]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crop. Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Kumar, V.; Sachdev, D.; Pasricha, R.; Maheshwari, P.H.; Taneja, N.K. Zinc-Supported Multiwalled Carbon Nanotube Nanocomposite: A Synergism to Micronutrient Release and a Smart Distributor To Promote the Growth of Onion Seeds in Arid Conditions. ACS Appl. Mater. Interfaces 2018, 10, 36733–36745. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, D.; Cui, J. A graphene oxide/silver nanoparticle composite as a novel agricultural antibacterial agent against Xanthomonas oryzae pv. oryzae for crop disease management. New J. Chem. 2017, 41, 13692–13699. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Pasquoto, T.; Lima, R.; Grillo, R.; Andrade, D.J.; Santos, F.A.D.; Fraceto, L.F. Zein Nanoparticles as Eco-Friendly Carrier Systems for Botanical Repellents Aiming Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Coll. Surf. B Biointerfaces 2017, 150, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Barmota, H.; Bala, A. Antifungal evaluation studies of copper sulfide nano-aquaformulations and its impact on seed quality of rice (Oryzae sativa). Appl. Nanosci. 2017, 7, 681–689. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Plaza Pérez, C.D.; De La Torre-Roche, R.; Zuverza-Mena, N.; Haynes, C.L.; White, J.C.; Hamers, R.J. Copper Based Nanomaterials Suppress Root Fungal Disease in Watermelon (Citrullus lanatus): Role of Particle Morphology, Composition and Dissolution Behavior. ACS Sustain. Chem. Eng. 2018, 6, 14847–14856. [Google Scholar] [CrossRef]
- Ashfaq, M.; Verma, N.; Khan, S. Carbon nanofibers as a micronutrient carrier in plants: Efficient translocation and controlled release of Cu nanoparticles. Environ. Sci. Nano 2017, 4, 138–148. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Taran, N.; Storozhenko, V.; Svietlova, N.; Batsmanova, L.; Shvartau, V.; Kovalenko, M. Effect of Zinc and Copper Nanoparticles on Drought Resistance of Wheat Seedlings. Nanoscale Res. Lett. 2017, 12, 60. [Google Scholar] [CrossRef]
- Wang, X.; Cai, A.; Wen, X.; Jing, D.; Qi, H.; Yuan, H. Graphene oxide-Fe3O4 nanocomposites as high-performance antifungal agents against Plasmopara viticola. Sci. China Mater. 2017, 60, 258–268. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, W.; Xu, C.; Li, F.; Cao, L.; Huang, Q. Enhancement of Spirotetramat Transfer in Cucumber Plant Using Mesoporous Silica Nanoparticles as Carriers. J. Agric. Food Chem. 2018, 66, 11592–11600. [Google Scholar] [CrossRef] [PubMed]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. Bark. Essex 1987 2014, 185, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.H.; Ji, Z.; Bouchard, D.C.; Nisbet, R.M.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Agglomeration Determines Effects of Carbonaceous Nanomaterials on Soybean Nodulation, Dinitrogen Fixation Potential, and Growth in Soil. ACS Nano 2017, 11, 5753–5765. [Google Scholar] [CrossRef]
- Lazareva, A.; Keller, A.A. Estimating Potential Life Cycle Releases of Engineered Nanomaterials from Wastewater Treatment Plants. ACS Sustain. Chem. Eng. 2014, 2, 1656–1665. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Q.; Huang, Y.; Keller, A.A. Response at Genetic, Metabolic, and Physiological Levels of Maize (Zea mays) Exposed to a Cu(OH)2 Nanopesticide. ACS Sustain. Chem. Eng. 2017, 5, 8294–8301. [Google Scholar] [CrossRef]
- Paterson, G.; Macken, A.; Thomas, K.V. The need for standardized methods and environmental monitoring programs for anthropogenic nanoparticles. Anal. Methods 2011, 3, 1461–1467. [Google Scholar] [CrossRef]
- Ruotolo, R.; Maestri, E.; Pagano, L.; Marmiroli, M.; White, J.C.; Marmiroli, N. Plant response to metal-containing engineered nanomaterials: An omics-based perspective. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Liu, Y.; Mo, M.; Guan, X.; Huang, L.; Sun, C.; Yang, S.T.; Chang, X.L. Toxicity of graphene oxide to naked oats (Avena sativa L) in hydroponic and soil cultures. RSC Adv. 2018, 8, 15336–15343. [Google Scholar] [CrossRef]
- Zhang, H.; Du, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Keller, A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics Reveals How Cucumber (Cucumis sativus) Reprograms Metabolites To Cope with Silver Ions and Silver Nanoparticle-Induced Oxidative Stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, Q.; Huang, Y.; Fulton, A.N.; Hannah-Bick, C.; Adeleye, A.S.; Keller, A.A. Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a Cu(OH)2 nanopesticide. Environ. Sci. Nano 2017, 4, 1750–1760. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).