Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
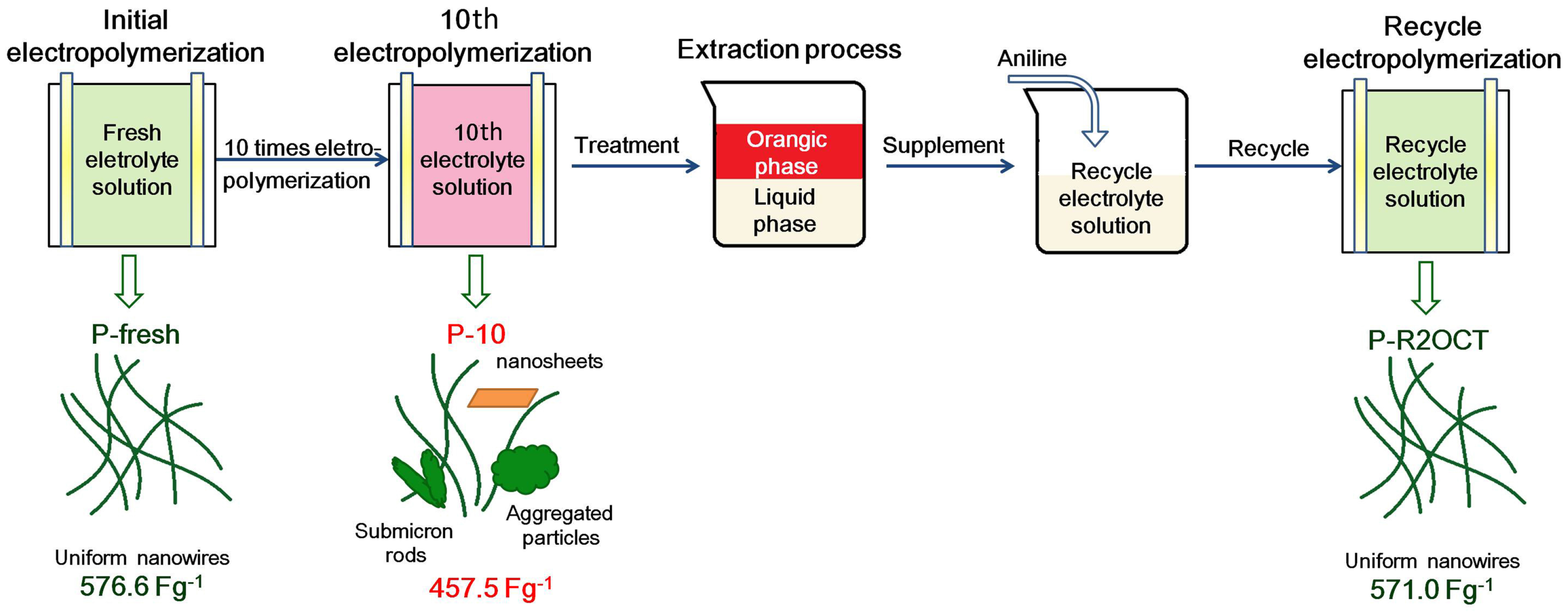
2.2.1. Preparation of the Used Electrolyte Solution
2.2.2. The Treatment and Recycling of the Used Electrolyte Solution
2.2.3. Preparation of PANI and PANI Electrode
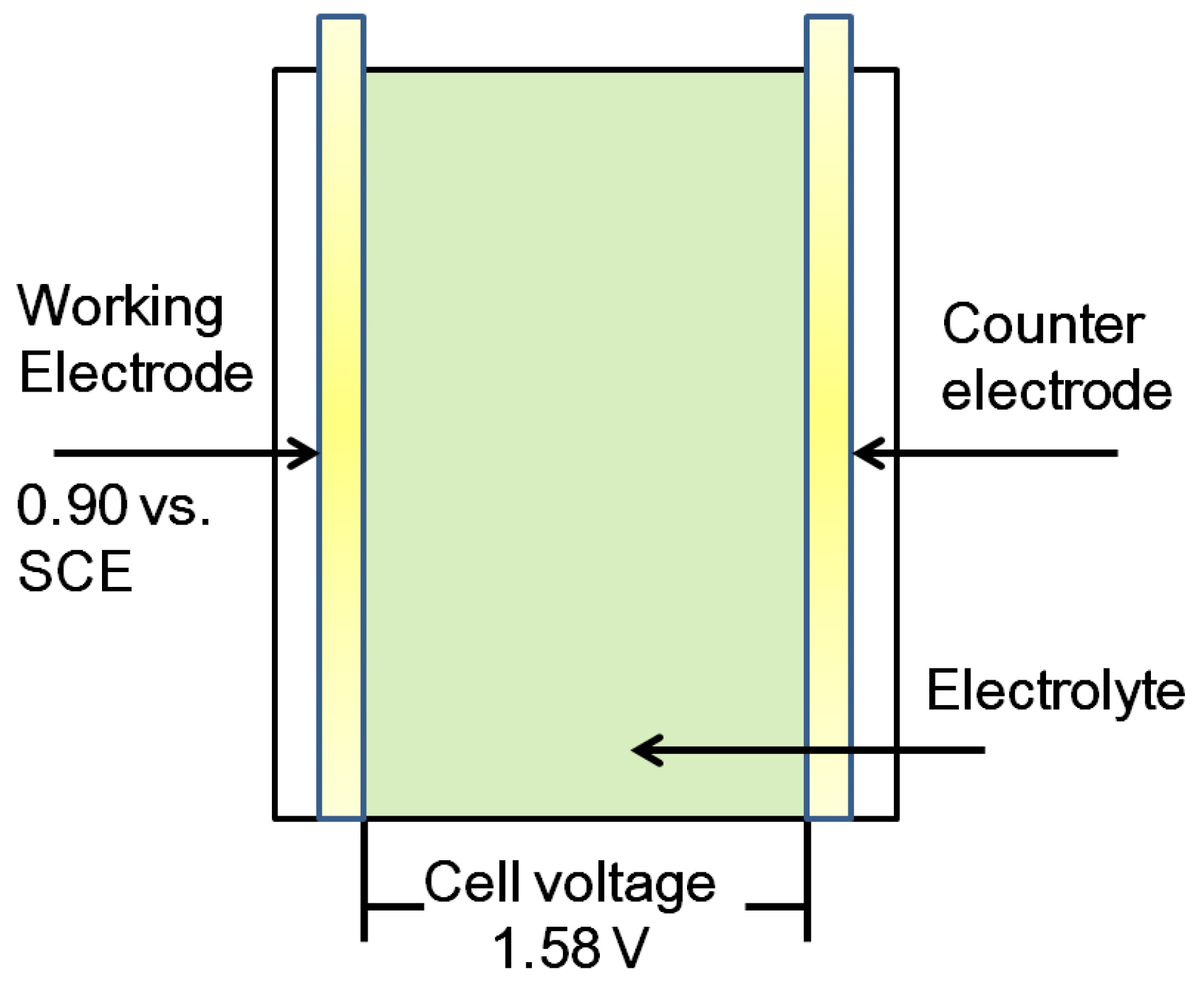
2.2.4. Characterization
3. Results and Discussion
3.1. The Composition of the Used Electrolyte Solution
3.2. Influence of BQ in the Electrolyte Solution on PANI
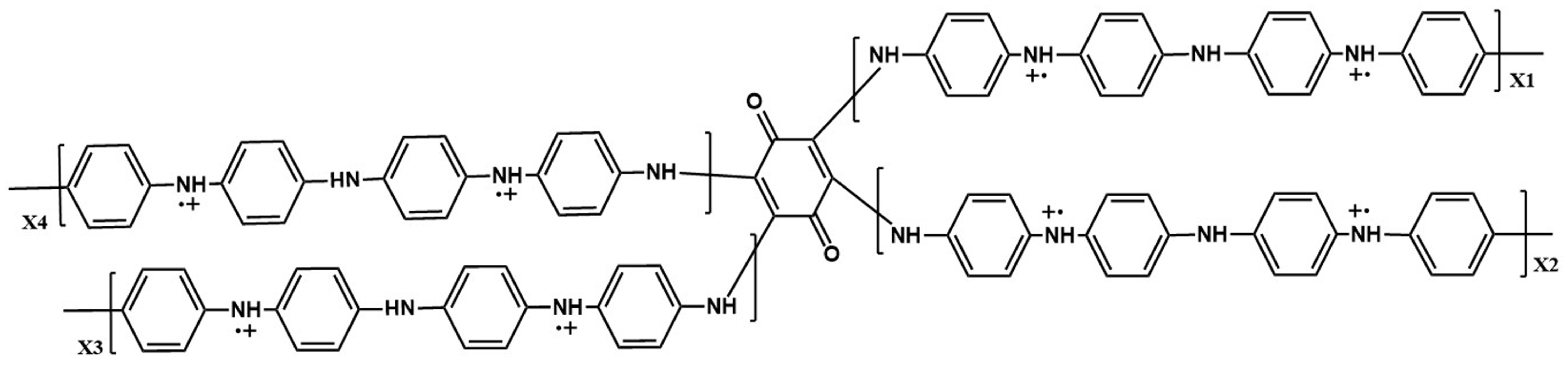
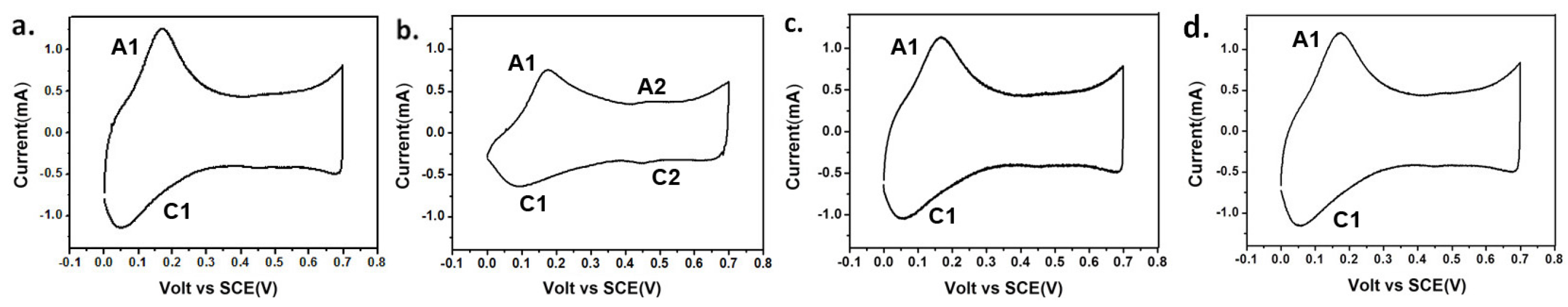
3.3. The Morphology and Electrochemical Deterioration of PANI through the Long-Term Electrochemical Polymerization Process
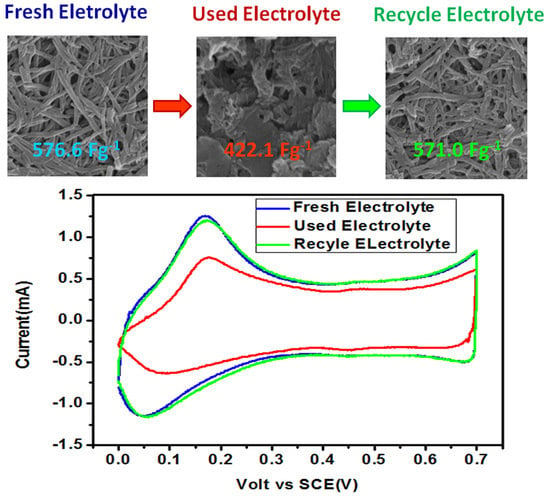
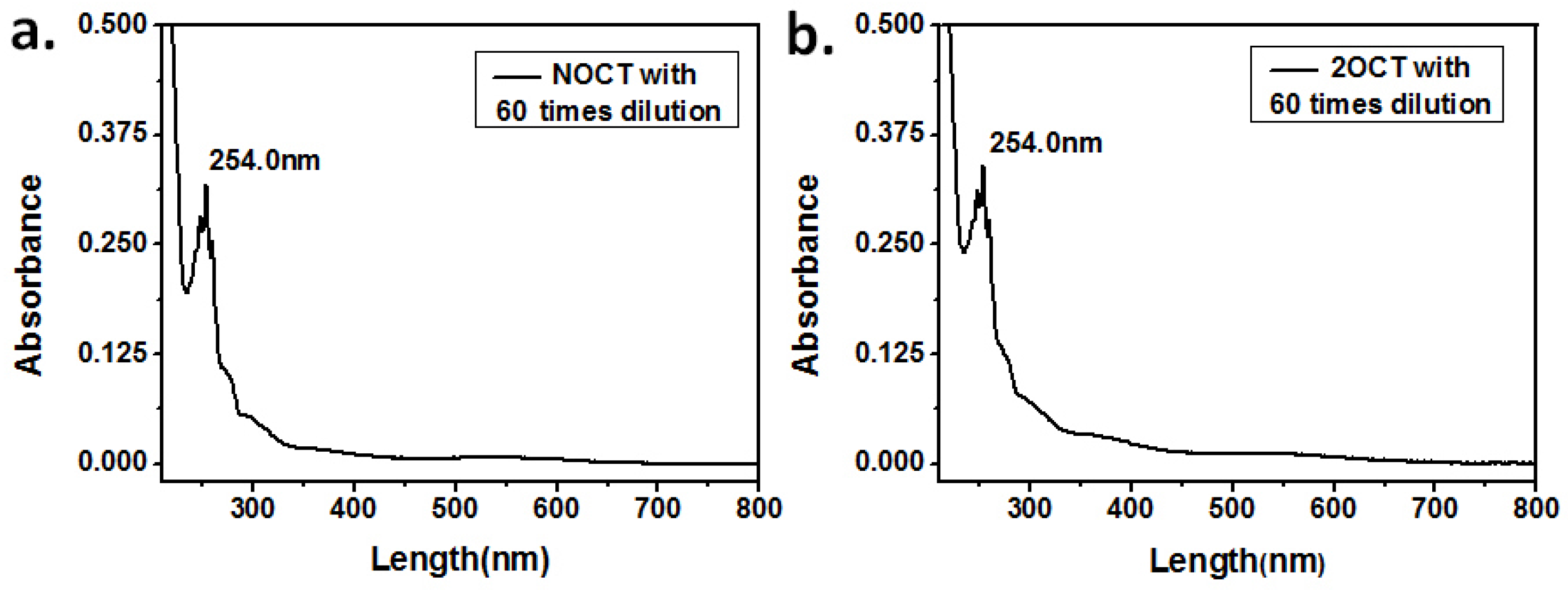
3.4. The Treatment and Recycling of Used Electrolyte Solution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A | ampere |
| V | volt |
| g | gram |
| PANI | polyaniline |
| HPLC-DAD | high performance liquid chromatography coupled with diode array detection |
| UV-Vis | ultraviolet and visible |
| HQ | p-hydroquinone |
| BQ | p-benzoquinone |
| CAB | co-oligomers of aniline and p-benzoquinone |
| ACN | acetonitrile |
| SS | stainless steel |
| SCE | saturated calomel electrode |
| CV | cyclic voltammetry |
| SEM | scanning electron microscope |
| n-th electrolyte solution | electrolyte solution after n repetitions of the electropolymerization of aniline |
| 10th electrolyte solution | electrolyte solution after 10 repetitions of the electropolymerization of aniline |
| NOCT | aqueous phase extracted by n-octanol, after 13 extractions of the 10th electrolyte |
| 2OCT | aqueous phase extracted by 2-octanone, after 13 extractions of the 10th electrolyte |
| ONOCT | n-octanol organic phase obtained by extracting the 10th electrolyte solution |
| O2OCT | 2-octanone organic phase obtained by extracting the 10th electrolyte solution |
| RNOCT | recycled electrolyze NOCT |
| R2OCT | recycled electrolyze 2OCT |
| P-Fresh | the PANI obtained by electropolymerization in fresh electrolyte solution |
| P-10 | the PANI obtained by electropolymerization in solution 10th electrolyte solution |
| P-RNOCT | the PANI obtained by electropolymerization in RNOCT |
| P-R2OCT | the PANI obtained by electropolymerization in R2OCT |
| P-BQ1 | the PANI obtained by fresh electrolyte solution with the addition of 0.001 M BQ |
| P-BQ2 | the PANI obtained by fresh electrolyte solution with the addition of 0.005 M BQ |
| P-BQ3 | the PANI obtained by fresh electrolyte solution with the addition of 0.01 M BQ |
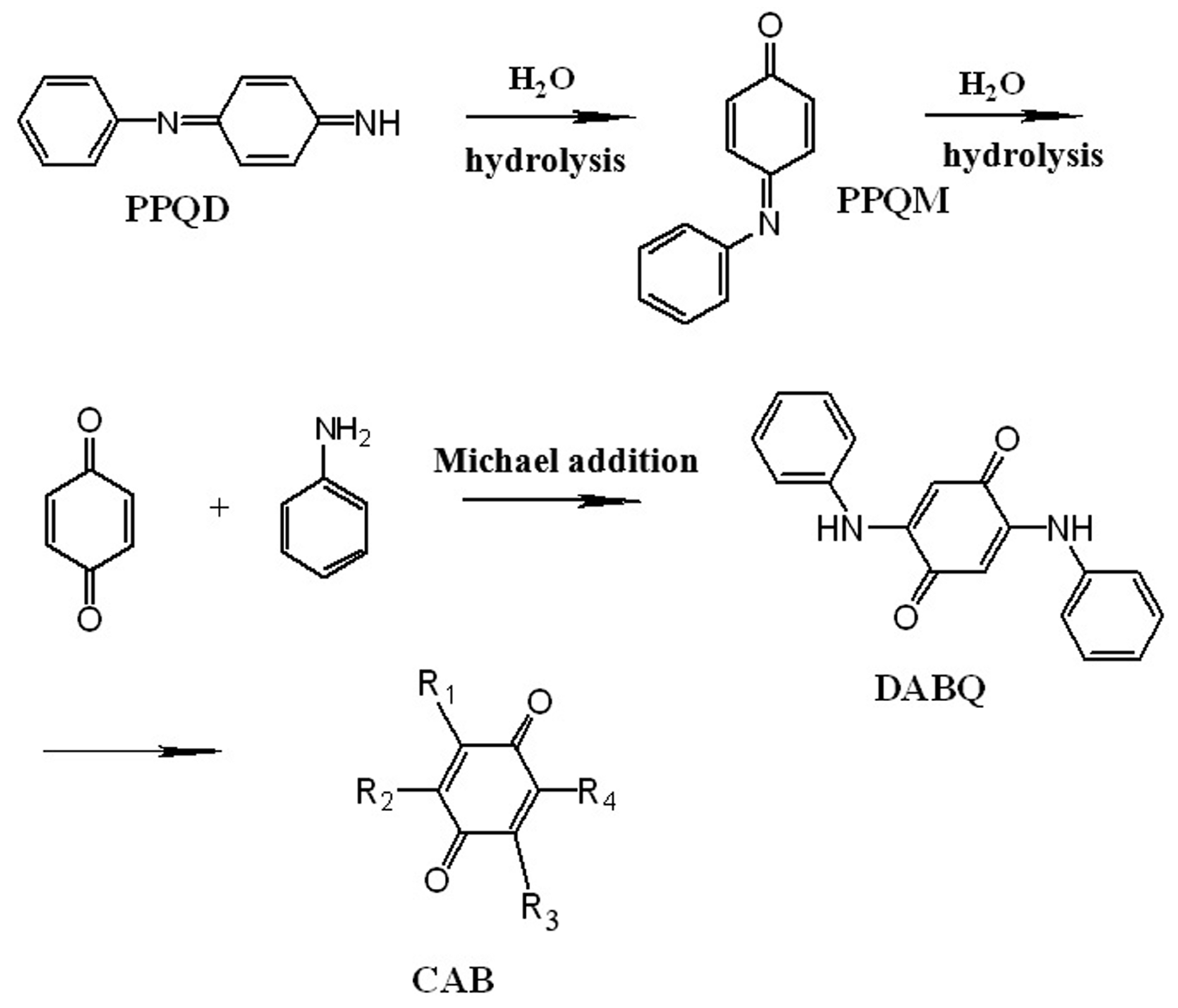
| PBQD | N-phenyl-1,4-benzoquinone diimine |
| PBQM | N-phenyl-1,4-benzoquinonemonoimine |
| PPD | N-phenyl-p-phenylene diamine |
| DABQ | 2,5-dianilino-p-benzoquinone |
References
- Sutar, D.S.; Padma, N.; Aswal, D.K.; Deshpande, S.K.; Gupta, S.K.; Yakhmi, J.V. Preparation of nanofibrous polyaniline films and their application as ammonia gas sensor. Sens. Actuators 2007, 128, 286–292. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Yan, X.; Xue, Q. NH3 and HCl sensing characteristics of polyaniline nanofibers deposited on commercial ceramic substrates using interfacial polymerization. Synth. Met. 2010, 160, 2452–2458. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, F.; Wang, J.; Wang, Z.; Wang, S. Polyaniline nanofibers prepared by a facile electrochemical approach and their supercapacitor performance. J. Mater. Res. 2008, 23, 2326–2332. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Oh, J.-S.; Kim, D.-W. Polyaniline nanofibres as a cathode material for rechargeable lithium-polymer cells assembled with gel polymer eletrolyte solution. J. Power Sources 2006, 163, 573–577. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Choi, J.-W. Conducting Polyaniline Nanowire and Its Applications in Chemiresistive Sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Performance evaluation and application of oxygen enriched waste rubber tire adsorbent for the removal of hazardous aniline derivatives from waste water. Chem. Eng. J. 2012, 203, 447–457. [Google Scholar] [CrossRef]
- Golovashin, V.L.; Lazarev, S.I.; Mamontov, V.V. Kinetic characteristics of reverse-osmosis separation of an aqueous solution of aniline in a flat-frame apparatus. Russ. J. Appl. Chem. 2005, 78, 1096–1100. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, J.; Zhong, H.; Li, X. Study of phenol removal using fluidized-bed Fenton process. Chem. Eng. Res. Des. 2012, 90, 377–382. [Google Scholar] [CrossRef]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.-C. Chemical Oxidation of 2,6-Dimethylaniline in the Fenton Process. Environ. Sci. Technol. 2009, 43, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ying, G.; Zhao, J.L.; Liu, S.; Zhou, L.J.; Chen, F. Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Res. 2012, 46, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.J.; Santos, V.; Ciriaco, L.; Lopes, A. Electrochemical degradation of aromatic amines on BDD electrodes. J. Hazard. Mater. 2011, 186, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.-C. Chemical oxidation of 2,6-dimethylaniline by electrochemically generated Fenton’s reagent. J. Hazard. Mater. 2010, 176, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Husain, Q. Redox-mediated oxidation and removal of aromatic amines from polluted water by partially purified bitter gourd (Momordica charantia) peroxidase. Int. Biodeterior. Biodegrad. 2009, 63, 587–593. [Google Scholar]
- Zhang, S.; Li, A.; Cui, D.; Yang, J.; Ma, F. Performance of enhanced biological SBR process for aniline treatment by mycelial pellet as biomass carrier. Bioresour. Technol. 2011, 102, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, J.; Lu, M.; Kan, Q.; Li, Z. Hemoglobin immobilized with modified “fish-in-net” approach for the catalytic removal of aniline. J. Hazard. Mater. 2012, 217, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, Y.; Ni, Y.; Kokot, S. Spectrophotometric analysis of phenols, which involves a hemin-graphene hybrid nanoparticles with peroxidase-like activity. J. Hazard. Mater. 2014, 266, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Zhao, Y.; Tomsik, E.; Wang, J.; Moravkova, Z.; Zhigunov, A.; Stejskal, J.; Trchova, M. Self-Assembly of Aniline Oligomers. CHEM-ASIAN J. 2013, 8, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.H.B.; Ferreira, D.C.; Constantino, V.R.L.; Temperini, M.L.A. Characterization of the products of aniline peroxydisulfate oligo/polymerization in media with different pH by resonance Raman spectroscopy at 413.1 and 1064 nm excitation wavelengths. J. Raman Spectrosc. 2011, 42, 1653–1659. [Google Scholar] [CrossRef]
- Johnson, B.J.; Park, S.M. Electrochemistry of Conductive Polymers: XX. Early Stages of Aniline Polymerization Studied by Spectroelectrochemical and Rotating Ring Disk Electrode Techniques. J. Raman Spectrosc. 1996, 143, 1277–1282. [Google Scholar]
- Hong, S.Y.; Jung, Y.M.; Kim, S.B.; Park, S.M. Electrochemistry of Conductive Polymers. 34. Two-Dimensional Correlation Analysis of Real-Time Spectroelectrochemical Data for Aniline Polymerization. J. Phys. Chem. B 2005, 109, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Balón, M.; Guardado, P.; Carmona, C.; Hidalgo, J.; Munoz, M.A. Influence of the acidity on the kinetics of diphenylamine oxidation by peroxodisulfate anions. Can. J. Chem. 1993, 71, 167–174. [Google Scholar] [CrossRef]
- Nayaki, S.K.; Swaminathan, M. Excited state solvatochromic and prototropic behaviour of 4-aminodiphenylamine and 4,4’-diaminodiphenylamine—A comparative study by electronic spectra. Spectrochim. Acta Part A Spectrosc. 2006, 64, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Bober, P.; Trchova, M.; Horsky, J.; Pilar, J.; Walterova, Z. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synth. Met. 2014, 192, 66–73. [Google Scholar] [CrossRef]
- Tandon, V.K.; Maurya, H.K. ‘On water’: Unprecedented nucleophilic substitution and addition reactions with 1,4-quinones in aqueous suspension. Tetrahedron Lett. 2009, 50, 5896–5902. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, H.; Arowo, M.; Yan, X.; Wu, W.; Chen, J.; Wang, Y.; Guo, Z. Electrochemical energy storage by polyaniline nanofibers: High gravity assisted oxidative polymerization vs. rapid mixing chemical oxidative polymerization. PCCP 2015, 17, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G.; Inganas, O. Renewable Cathode Materials from Biopolymer/Conjugated Polymer Interpenetrating Networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Chiou, N.R.; Epstein, A.J. A simple approach to control the growth of polyaniline nanofibers. Synth. Met. 2005, 153, 69–72. [Google Scholar] [CrossRef]
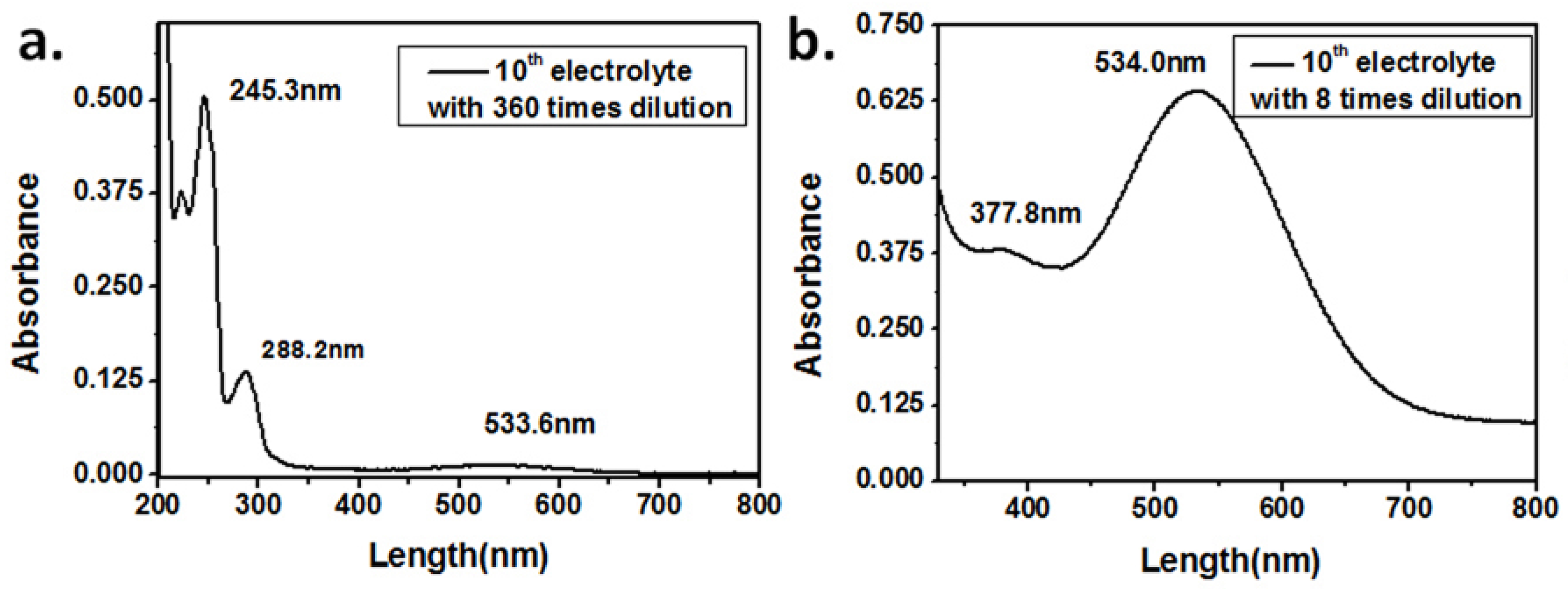

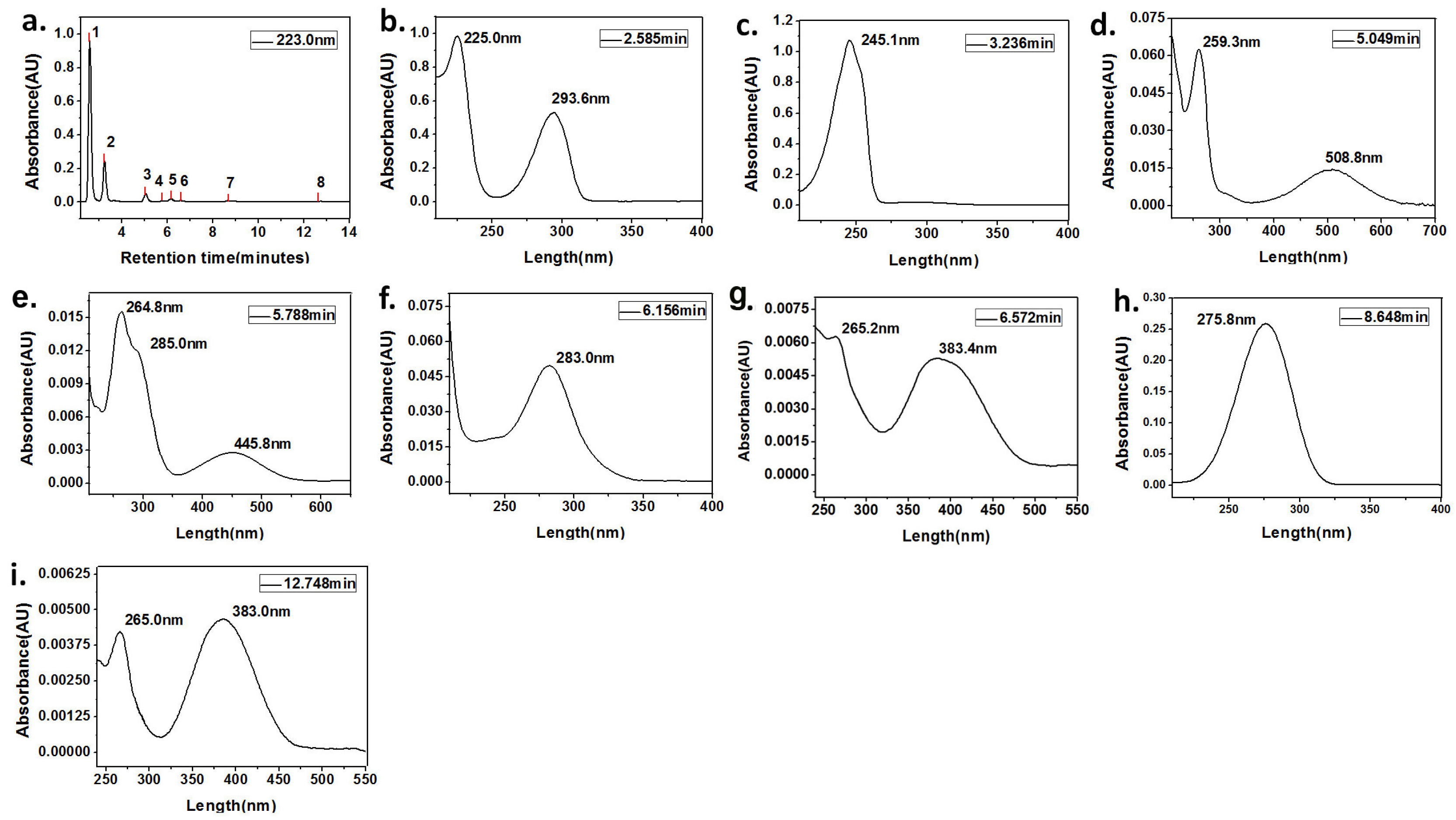



| Peaks Number | Retention Time (min) | Absorption Peaks (nm) | Compound |
|---|---|---|---|
| Peak 1 | 2.597 | 225.0, 293.6 | HQ |
| Peak 2 | 3.250 | 245.1 | BQ |
| Peak 3 | 3.795 | 234.4, 285.3 | aniline |
| Peak 4 | 5.078 | 259.3, 508.8 | PBQD |
| Peak 5 | 5.814 | 264.8, 285.0, 445.8 | PBQM |
| Peak 6 | 6.190 | 283.0 | PPD |
| Peak 7 | 6.572 | 265.2, 383.4 | DABQ |
| Peak 8 | 9.115 | 264.8, 367.4 | CAB |
| peak 9 | 12.901 | 265.0, 383.0 | CAB |
| Peaks Number | Retention Time (min) | Absorption Peaks (nm) | Compound |
|---|---|---|---|
| Peak 1 | 2.597 | 225.0, 293.6 | HQ |
| Peak 2 | 3.250 | 245.1 | BQ |
| Peak 3 | 5.049 | 259.3, 508.8 | PBQD |
| Peak 4 | 5.788 | 264.8, 285.0, 445.8 | PBQM |
| Peak 5 | 6.156 | 283.0 | PPD |
| Peak 6 | 6.572 | 265.2, 383.4 | DABQ |
| Peak 7 | 8.648 | 275.8 | 2-octanone |
| Peak 8 | 12.748 | 265.0, 383.0 | CAB |
| PANI Sample | Electrolyte Solution | Specific Capacitance (F/g) |
|---|---|---|
| P-Fresh | Fresh electrolyte solution | 576.6 |
| P-10 | 10th electrolyte solution | 457.5 |
| P-RNOCT | RNOCT | 555.8 |
| P-R2OCT | R2OCT | 571.0 |
| P-BQ1 | Fresh electrolyte solution 0.001 M BQ | 544.5 |
| P-BQ2 | Fresh electrolyte solution 0.005 M BQ | 524.7 |
| P-BQ3 | Fresh electrolyte solution 0.010 M BQ | 499.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z.; Wang, S. Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance. Nanomaterials 2018, 8, 103. https://doi.org/10.3390/nano8020103
Wu Y, Wang J, Ou B, Zhao S, Wang Z, Wang S. Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance. Nanomaterials. 2018; 8(2):103. https://doi.org/10.3390/nano8020103
Chicago/Turabian StyleWu, Ying, Jixiao Wang, Bin Ou, Song Zhao, Zhi Wang, and Shichang Wang. 2018. "Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance" Nanomaterials 8, no. 2: 103. https://doi.org/10.3390/nano8020103
APA StyleWu, Y., Wang, J., Ou, B., Zhao, S., Wang, Z., & Wang, S. (2018). Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance. Nanomaterials, 8(2), 103. https://doi.org/10.3390/nano8020103